Copper Sources for Sod1 Activation
Abstract
1. Introduction
2. Discussion
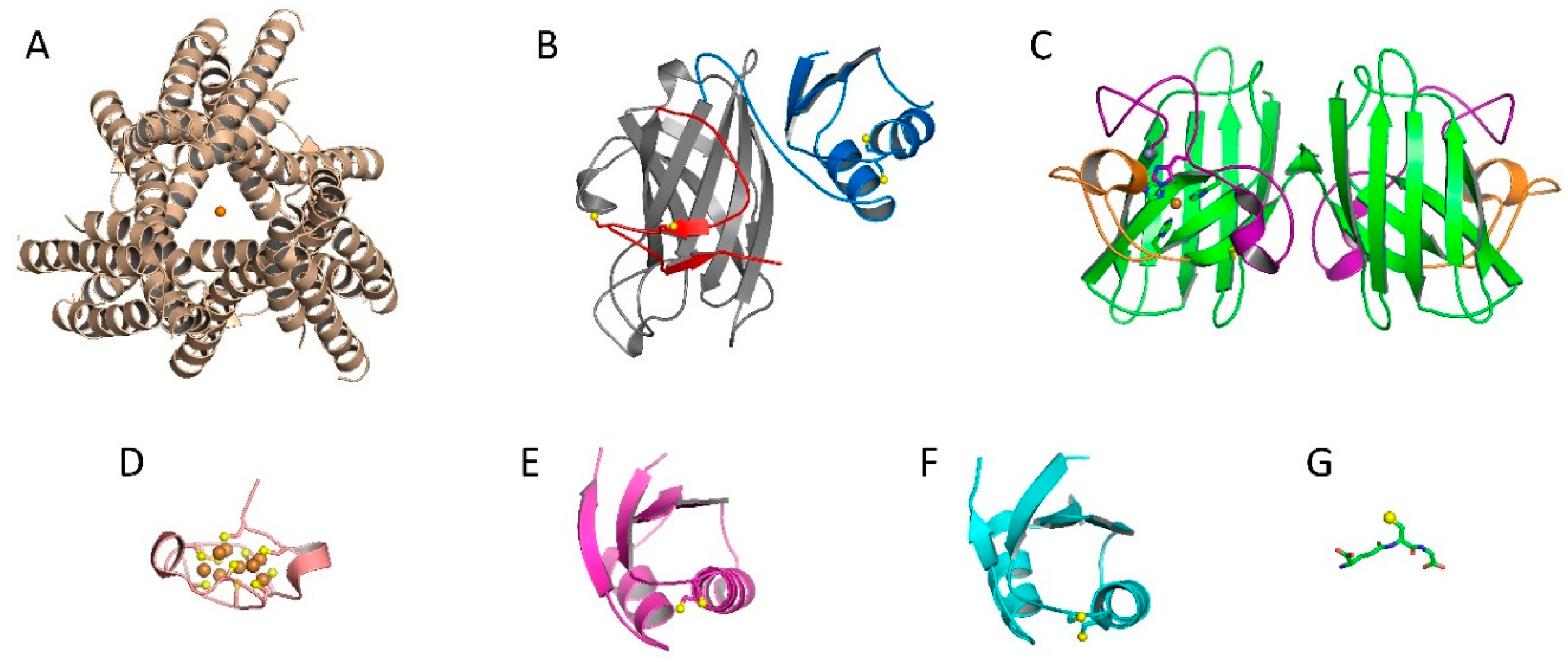
2.1. Cu, Zn Superoxide Dismutase (Sod1) and Copper Chaperone for Sod1 (Ccs1)
2.2. Ccs-Mediated Sod1 Maturation
2.3. Ccs-Independent Sod1 Maturation
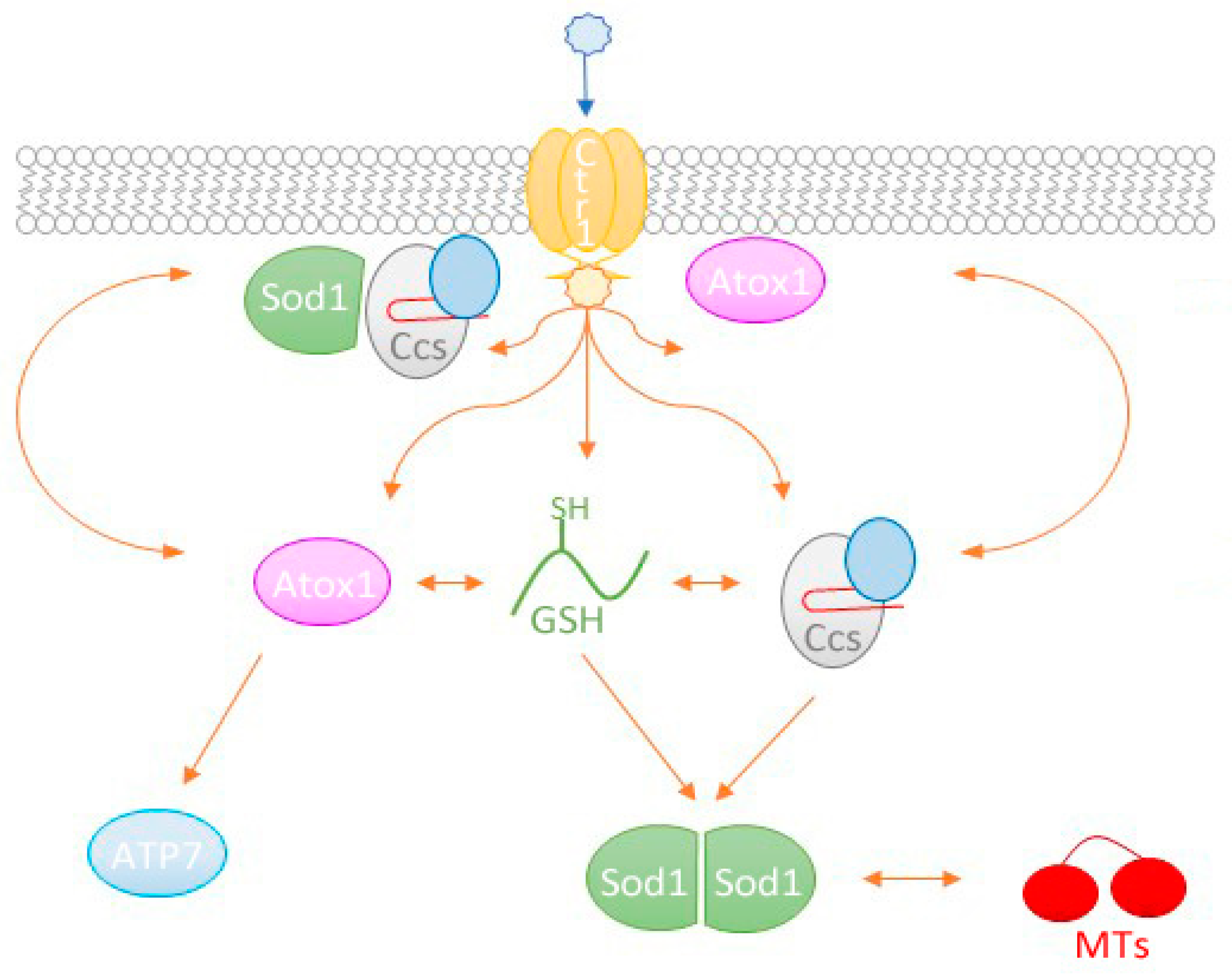
2.4. Copper Acquisition by Ccs via Ctr1
2.5. Involvement of other Chaperones and Metalloproteins
2.5.1. Atox1
2.5.2. MTs
2.5.3. Glutathione
3. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Nevitt, T.; Ohrvik, H.; Thiele, D.J. Charting the travels of copper in eukaryotes from yeast to mammals. Biochim. et Biophys. Acta (BBA) Bioenerg. 2012, 1823, 1580–1593. [Google Scholar] [CrossRef]
- Boal, A.K.; Rosenzweig, A.C. Structural Biology of Copper Trafficking. Chem. Rev. 2009, 109, 4760–4779. [Google Scholar] [CrossRef]
- Cox, D.W. Disorders of copper transport. Br. Med. Bull. 1999, 55, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Fetherolf, M.M.; Boyd, S.D.; Winkler, D.D.; Winge, D.R. Oxygen-dependent activation of Cu,Zn-superoxide dismutase-1. Metallomics 2017, 9, 1047–1059. [Google Scholar] [CrossRef]
- Mccord, J.M.; Fridovich, I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Boil. Chem. 1969, 244, 6049–6055. [Google Scholar]
- Banci, L.; Bertini, I.; Cantini, F.; Kozyreva, T.; Massagni, C.; Palumaa, P.; Rubino, J.T.; Zovo, K. Human superoxide dismutase 1 (hSOD1) maturation through interaction with human copper chaperone for SOD1 (hCCS). Proc. Natl. Acad. Sci. USA 2012, 109, 13555–13560. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.C.; Waggoner, D.; Subramaniam, J.R.; Tessarollo, L.; Bartnikas, T.B.; Culotta, V.C.; Price, N.L.; Rothstein, J.; Gitlin, J.D. Copper chaperone for superoxide dismutase is essential to activate mammalian Cu/Zn superoxide dismutase. Proc. Natl. Acad. Sci. USA 2000, 97, 2886–2891. [Google Scholar] [CrossRef] [PubMed]
- Culotta, V.C.; Yang, M.; O’Halloran, T.V. Activation of superoxide dismutases: Putting the metal to the pedal. Biochim. et Biophys. Acta (BBA) Bioenerg. 2006, 1763, 747–758. [Google Scholar] [CrossRef]
- Harrison, M.; Jones, C.E.; Dameron, C.T. Copper chaperones: Function, structure and copper-binding properties. JBIC J. Boil. Inorg. Chem. 1999, 4, 145–153. [Google Scholar] [CrossRef]
- Stadtman, E.R.; Berlett, B.S. Fenton chemistry. Amino acid oxidation. J. Boil. Chem. 1991, 266, 17201–17211. [Google Scholar]
- Okado-Matsumoto, A.; Fridovich, I. Subcellular Distribution of Superoxide Dismutases (SOD) in Rat Liver: Cu, Zn-SOD In Mitochondria. J. Boil. Chem. 2001, 276, 38388–38393. [Google Scholar] [CrossRef]
- Sturtz, L.A.; Diekert, K.; Jensen, L.T.; Lill, R.; Culotta, V.C. A fraction of yeast Cu,Zn-superoxide dismutase and its metallochaperone, CCS, localize to the intermembrane space of mitochondria. A physiological role for SOD1 in guarding against mitochondrial oxidative damage. J. Boil. Chem. 2001, 276, 38084–38089. [Google Scholar]
- Gleason, J.E.; Galaleldeen, A.; Peterson, R.L.; Taylor, A.B.; Holloway, S.P.; Waninger-Saroni, J.; Cormack, B.P.; Cabelli, D.E.; Hart, P.J.; Culotta, V.C. Candida albicans SOD5 represents the prototype of an unprecedented class of Cu-only superoxide dismutases required for pathogen defense. Proc. Natl. Acad. Sci. USA 2014, 111, 5866–5871. [Google Scholar] [CrossRef] [PubMed]
- Gunther, M.R.; Vangilder, R.; Fang, J.; Beattie, D.S. Expression of a familial amyotrophic lateral sclerosis-associated mutant human superoxide dismutase in yeast leads to decreased mitochondrial electron transport. Arch. Biochem. Biophys. 2004, 431, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Culotta, V.C.; Klomp, L.W.J.; Strain, J.; Casareno, R.L.B.; Krems, B.; Gitlin, J.D. The Copper Chaperone for Superoxide Dismutase. J. Boil. Chem. 1997, 272, 23469–23472. [Google Scholar] [CrossRef] [PubMed]
- Hamza, I.; Prohaska, J.; Gitlin, J.D. Essential role for Atox1 in the copper-mediated intracellular trafficking of the Menkes ATPase. Proc. Natl. Acad. Sci. USA 2003, 100, 1215–1220. [Google Scholar] [CrossRef]
- Banci, L.; Bertini, I.; Ciofi-Baffoni, S.; Hadjiloi, T.; Martinelli, M.; Palumaa, P. Mitochondrial copper(I) transfer from Cox17 to Sco1 is coupled to electron transfer. Proc. Natl. Acad. Sci. USA 2008, 105, 6803–6808. [Google Scholar] [CrossRef]
- Horng, Y.-C.; Cobine, P.A.; Maxfield, A.B.; Carr, H.S.; Winge, D.R. Specific Copper Transfer from the Cox17 Metallochaperone to Both Sco1 and Cox11 in the Assembly of Yeast Cytochrome c Oxidase. J. Boil. Chem. 2004, 279, 35334–35340. [Google Scholar] [CrossRef] [PubMed]
- Rae, T.D. Undetectable Intracellular Free Copper: The Requirement of a Copper Chaperone for Superoxide Dismutase. Science 1999, 284, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Kawamata, H.; Manfredi, G. Import, Maturation, and Function of SOD1 and Its Copper Chaperone CCS in the Mitochondrial Intermembrane Space. Antioxid. Redox Signal. 2010, 13, 1375–1384. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Wedd, A.G. A C-terminal domain of the membrane copper pump Ctr1 exchanges copper(I) with the copper chaperone Atx1. Chem. Commun. 2002, 2002, 588–589. [Google Scholar] [CrossRef] [PubMed]
- Freedman, J.H.; Ciriolo, M.R.; Peisach, J. The role of glutathione in copper metabolism and toxicity. J. Boil. Chem. 1989, 264, 5598–5605. [Google Scholar]
- Presta, A. Incorporation of copper into the yeast saccharomyces cerevisiae. Identification of Cu(I)-metallothionein in intact yeast cells. J. Inorg. Biochem. 1997, 66, 231–240. [Google Scholar] [CrossRef]
- Blair, B.G.; Larson, C.A.; Adams, P.L.; Abada, P.B.; Pesce, C.E.; Safaei, R.; Howell, S.B. Copper transporter 2 regulates endocytosis and controls tumor growth and sensitivity to cisplatin in vivo. Mol. Pharmacol. 2010, 79, 157–166. [Google Scholar] [CrossRef]
- Knight, S.; Labbé, S.; Kwon, L.F.; Kosman, D.J.; Thiele, D.J. A widespread transposable element masks expression of a yeast copper transport gene. Genome Res. 1996, 10, 1917–1929. [Google Scholar] [CrossRef] [PubMed]
- Eisses, J.F.; Kaplan, J. Molecular Characterization of hCTR1, the Human Copper Uptake Protein. J. Boil. Chem. 2002, 277, 29162–29171. [Google Scholar] [CrossRef] [PubMed]
- Klomp, A.E.M.; Tops, B.; Van DenBerg, I.E.T.; Berger, R.; Klomp, L.W.J. Biochemical characterization and subcellular localization of human copper transporter 1 (hCTR1). Biochem. J. 2002, 364, 497–505. [Google Scholar] [CrossRef]
- Lee, J.; Peña, M.M.O.; Nose, Y.; Thiele, D.J. Biochemical Characterization of the Human Copper Transporter Ctr1. J. Boil. Chem. 2001, 277, 4380–4387. [Google Scholar] [CrossRef]
- Ramos, D.; Mar, D.; Ishida, M.; Vargas, R.; Gaite, M.; Montgomery, A.; Linder, M.C. Mechanism of Copper Uptake from Blood Plasma Ceruloplasmin by Mammalian Cells. PLoS ONE 2016, 11, e0149516. [Google Scholar] [CrossRef]
- Shenberger, Y.; Shimshi, A.; Ruthstein, S. EPR Spectroscopy Shows that the Blood Carrier Protein, Human Serum Albumin, Closely Interacts with the N-Terminal Domain of the Copper Transporter, Ctr1. J. Phys. Chem. B 2015, 119, 4824–4830. [Google Scholar] [CrossRef]
- Stefaniak, E.; Płonka, D.; Drew, S.C.; Bossak-Ahmad, K.; Haas, K.L.; Pushie, M.J.; Faller, P.; Wezynfeld, N.E.; Bal, W. The N-terminal 14-mer model peptide of human Ctr1 can collect Cu(ii) from albumin. Implications for copper uptake by Ctr1. Metallomics 2018, 10, 1723–1727. [Google Scholar] [CrossRef]
- De Feo, C.J.; Aller, S.G.; Siluvai, G.S.; Blackburn, N.J.; Unger, V.M. Three-dimensional structure of the human copper transporter hCTR1. Proc. Natl. Acad. Sci. USA 2009, 106, 4237–4242. [Google Scholar] [CrossRef] [PubMed]
- Ohrvik, H.; Thiele, D.J. How copper traverses cellular membranes through the mammalian copper transporter 1, Ctr1. Ann. N. Y. Acad. Sci. 2014, 1314, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Skopp, A.; Boyd, S.D.; Ullrich, M.S.; Liu, L.; Winkler, D.D. Copper–zinc superoxide dismutase (Sod1) activation terminates interaction between its copper chaperone (Ccs) and the cytosolic metal-binding domain of the copper importer Ctr1. BioMetals 2019, 32, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.R.; De Feo, C.J.; Unger, V.M. Cellular distribution of copper to superoxide dismutase involves scaffolding by membranes. Proc. Natl. Acad. Sci. USA 2013, 110, 20491–20496. [Google Scholar] [CrossRef] [PubMed]
- Linz, R.; Lutsenko, S. Copper-transporting ATPases ATP7A and ATP7B: Cousins, not twins. J. Bioenerg. Biomembr. 2007, 39, 403–407. [Google Scholar] [CrossRef]
- La Fontaine, S.; Mercer, J.F. Trafficking of the copper-ATPases, ATP7A and ATP7B: Role in copper homeostasis. Arch. Biochem. Biophys. 2007, 463, 149–167. [Google Scholar] [CrossRef]
- Hamer, D.H. Metallothionein. Ann. Rev. Biochem. 1986, 55, 913–951. [Google Scholar] [CrossRef]
- Vašák, M.; Meloni, G. Chemistry and biology of mammalian metallothioneins. JBIC J. Boil. Inorg. Chem. 2011, 16, 1067–1078. [Google Scholar] [CrossRef]
- Calvo, J.S.; Lopez, V.M.; Meloni, G. Non-coordinative metal selectivity bias in human metallothioneins metal-thiolate clusters. Metallomics 2018, 10, 1777–1791. [Google Scholar] [CrossRef]
- Fetherolf, M.M.; Boyd, S.D.; Taylor, A.B.; Kim, H.J.; Wohlschlegel, J.A.; Blackburn, N.J.; Hart, P.J.; Winge, D.R.; Winkler, D.D. Copper-zinc superoxide dismutase is activated through a sulfenic acid intermediate at a copper ion entry site. J. Boil. Chem. 2017, 292, 12025–12040. [Google Scholar] [CrossRef]
- Furukawa, Y.; Torres, A.S.; O’Halloran, T.V. Oxygen-induced maturation of SOD1: A key role for disulfide formation by the copper chaperone CCS. EMBO J. 2004, 23, 2872–2881. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, M.; Tokuda, E.; Nakagome, K.; Wu, Z.; Nagano, I.; Furukawa, Y. An essential role of N-terminal domain of copper chaperone in the enzymatic activation of Cu/Zn-superoxide dismutase. J. Inorg. Biochem. 2017, 175, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Keele, B.B.; Mccord, J.M.; Fridovich, I. Further characterization of bovine superoxide dismutase and its isolation from bovine heart. J. Boil. Chem. 1971, 246, 2875–2880. [Google Scholar]
- Fisher, C.L.; Cabelli, D.E.; Tainer, J.; Hallewell, R.A.; Getzoff, E.D. The role of arginine 143 in the electrostatics and mechanism of Cu, Zn superoxide dismutase: Computational and experimental evaluation by mutational analysis. Proteins Struct. Funct. Bioinform. 1994, 19, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Luchinat, E.; Barbieri, L.; Rubino, J.T.; Kozyreva, T.; Cantini, F.; Banci, L. In-cell NMR reveals potential precursor of toxic species from SOD1 fALS mutants. Nat. Commun. 2014, 5, 5502. [Google Scholar] [CrossRef] [PubMed]
- Leitch, J.M.; Yick, P.J.; Culotta, V.C. The Right to Choose: Multiple Pathways for Activating Copper, Zinc Superoxide Dismutase. J. Boil. Chem. 2009, 284, 24679–24683. [Google Scholar] [CrossRef]
- Lamb, A.L.; Wernimont, A.K.; Pufahl, R.A.; O’Halloran, T.V.; Rosenzweig, A.C. Crystal Structure of the Second Domain of the Human Copper Chaperone for Superoxide Dismutase. Biochemistry 2000, 39, 1589–1595. [Google Scholar] [CrossRef]
- Rosenzweig, A. Structure and chemistry of the copper chaperone proteins. Curr. Opin. Chem. Boil. 2000, 4, 140–147. [Google Scholar] [CrossRef]
- Furukawa, Y.; O’Halloran, T.V. Posttranslational Modifications in Cu,Zn-Superoxide Dismutase and Mutations Associated with Amyotrophic Lateral Sclerosis. Antioxid. Redox Signal. 2006, 8, 847–867. [Google Scholar] [CrossRef]
- Ogihara, N.L.; Parge, H.E.; Hart, P.J.; Weiss, M.S.; Goto, J.J.; Crane, B.R.; Tsang, J.; Slater, K.; Roe, J.A.; Valentine, J.S.; et al. Unusual Trigonal-Planar Copper Configuration Revealed in the Atomic Structure of Yeast Copper−Zinc Superoxide Dismutase. Biochemistry 1996, 35, 2316–2321. [Google Scholar] [CrossRef] [PubMed]
- Hart, P.J.; Balbirnie, M.M.; Ogihara, N.L.; Nersissian, A.M.; Weiss, M.S.; Valentine, J.S.; Eisenberg, D. A Structure-Based Mechanism for Copper−Zinc Superoxide Dismutase. Biochemistry 1999, 38, 2167–2178. [Google Scholar] [CrossRef] [PubMed]
- Tainer, J.; Getzoff, E.D.; Richardson, J.S.; Richardson, D.C. Structure and mechanism of copper, zinc superoxide dismutase. Nature 1983, 306, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Cudd, A.; Fridovich, I. Electrostatic interactions in the reaction mechanism of bovine erythrocyte superoxide dismutase. J. Boil. Chem. 1982, 257, 11443–11447. [Google Scholar]
- Klug, D.; Rabani, J.; Fridovich, I. A direct demonstration of the catalytic action of superoxide dismutase through the use of pulse radiolysis. J. Boil. Chem. 1972, 247, 4839–4842. [Google Scholar]
- Schmidt, P.J.; Ramos-Gomez, M.; Culotta, V.C. A Gain of Superoxide Dismutase (SOD) Activity Obtained with CCS, the Copper Metallochaperone for SOD1. J. Boil. Chem. 1999, 274, 36952–36956. [Google Scholar] [CrossRef] [PubMed]
- Casareno, R.L.B.; Waggoner, D.; Gitlin, J.D. The copper chaperone CCS directly interacts with copper/zinc superoxide dismutase. J. Boil. Chem. 1998, 273, 23625–23628. [Google Scholar] [CrossRef]
- Rae, T.D.; Torres, A.S.; Pufahl, R.A.; O’Halloran, T.V. Mechanism of Cu,Zn-Superoxide Dismutase Activation by the Human Metallochaperone hCCS. J. Boil. Chem. 2000, 276, 5166–5176. [Google Scholar] [CrossRef]
- Wright, G.S.A.; Hasnain, S.S.; Grossmann, J.G. The structural plasticity of the human copper chaperone for SOD1: Insights from combined size-exclusion chromatographic and solution X-ray scattering studies. Biochem. J. 2011, 439, 39–44. [Google Scholar] [CrossRef]
- Boyd, S.D.; Calvo, J.S.; Liu, L.; Ullrich, M.S.; Skopp, A.; Meloni, G.; Winkler, D.D. The yeast copper chaperone for copper-zinc superoxide dismutase (CCS1) is a multifunctional chaperone promoting all levels of SOD1 maturation. J. Boil. Chem. 2018, 294, 1956–1966. [Google Scholar] [CrossRef]
- Boyd, S.D.; Liu, L.; Bulla, L.; Winkler, D.D. Quantifying the Interaction between Copper-Zinc Superoxide Dismutase (Sod1) and its Copper Chaperone (Ccs1). J. Proteom. Bioinform. 2018, 11, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Boyd, S.; Ullrich, M.; Calvo, J.S.; Behnia, F.; Meloni, G.; Winkler, D.D. Mutations in Superoxide Dismutase 1 (Sod1) Linked to Familial Amyotrophic Lateral Sclerosis Can Disrupt High-Affinity Zinc-Binding Promoted by the Copper Chaperone for Sod1 (Ccs). Molecules 2020, 25, 1086. [Google Scholar] [CrossRef] [PubMed]
- Luchinat, E.; Barbieri, L.; Banci, L. A molecular chaperone activity of CCS restores the maturation of SOD1 fALS mutants. Sci. Rep. 2017, 7, 17433. [Google Scholar] [CrossRef] [PubMed]
- Rothstein, J.D.; Dykes-Hoberg, M.; Corson, L.B.; Becker, M.; Cleveland, D.W.; Price, N.L.; Culotta, V.C.; Wong, P.C. The copper chaperone CCS is abundant in neurons and astrocytes in human and rodent brain. J. Neurochem. 1999, 72, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Carroll, M.C.; Girouard, J.B.; Ulloa, J.L.; Subramaniam, J.R.; Wong, P.C.; Valentine, J.S.; Culotta, V.C. Mechanisms for activating Cu- and Zn-containing superoxide dismutase in the absence of the CCS Cu chaperone. Proc. Natl. Acad. Sci. USA 2004, 101, 5964–5969. [Google Scholar] [CrossRef]
- Leitch, J.M.; Jensen, L.T.; Bouldin, S.D.; Outten, C.E.; Hart, P.J.; Culotta, V.C. Activation of Cu,Zn-Superoxide Dismutase in the Absence of Oxygen and the Copper Chaperone CCS. J. Boil. Chem. 2009, 284, 21863–21871. [Google Scholar] [CrossRef]
- Jensen, L.T.; Culotta, V.C. Activation of CuZn Superoxide Dismutases from Caenorhabditis elegans Does Not Require the Copper Chaperone CCS. J. Boil. Chem. 2005, 280, 41373–41379. [Google Scholar] [CrossRef]
- Petzoldt, S.; Kahra, D.; Kovermann, M.; Dingeldein, A.P.G.; Niemiec, M.S.; Ådén, J.; Wittung-Stafshede, P. Human cytoplasmic copper chaperones Atox1 and CCS exchange copper ions in vitro. Biometals 2015, 28, 577–585. [Google Scholar] [CrossRef]
- Miyayama, T.; Ishizuka, Y.; Iijima, T.; Hiraoka, D.; Ogra, Y. Roles of copper chaperone for superoxide dismutase 1 and metallothionein in copper homeostasis. Metallomics 2011, 3, 693. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhou, H.; Thiele, D.J. Identification of a Novel High Affinity Copper Transport Complex in the Fission YeastSchizosaccharomyces pombe. J. Boil. Chem. 2001, 276, 20529–20535. [Google Scholar] [CrossRef]
- Sancenón, V.; Puig, S.; Mira, H.; Thiele, D.J.; Peñarrubia, L. Identification of a copper transporter family in Arabidopsis thaliana. Plant Mol. Boil. 2003, 51, 577–587. [Google Scholar] [CrossRef]
- Kampfenkel, K.; Kushnir, S.; Babiychuk, E.; Inzé, D.; Van Montagu, M. Molecular characterization of a putative Arabidopsis thaliana copper transporter and its yeast homologue. J. Boil. Chem. 1995, 270, 28479–28486. [Google Scholar]
- MacKenzie, N.C.; Brito, M.; Reyes, A.E.; Allende, M.L. Cloning, expression pattern and essentiality of the high-affinity copper transporter 1 (ctr1) gene in zebrafish. Gene 2004, 328, 113–120. [Google Scholar] [CrossRef]
- Haremaki, T.; Weinstein, D. Xmc mediates Xctr1-independent morphogenesis inXenopus laevis. Dev. Dyn. 2009, 238, 2382–2387. [Google Scholar] [CrossRef] [PubMed]
- Riggio, M.; Lee, J.; Scudiero, R.; Parisi, E.; Thiele, D.J.; Filosa, S. High affinity copper transport protein in the lizard Podarcis sicula: Molecular cloning, functional characterization and expression in somatic tissues, follicular oocytes and eggs. Biochim. et Biophys. Acta (BBA) Gene Struct. Expr. 2002, 1576, 127–135. [Google Scholar] [CrossRef]
- Lee, J.; Prohaska, J.R.; Dagenais, S.L.; Glover, T.W.; Thiele, D.J. Isolation of a murine copper transporter gene, tissue specific expression and functional complementation of a yeast copper transport mutant. Gene 2000, 254, 87–96. [Google Scholar] [CrossRef]
- Dancis, A.; Haile, D.; Yuan, D.S.; Klausner, R.D. The Saccharomyces cerevisiae copper transport protein (Ctr1p). Biochemical characterization, regulation by copper, and physiologic role in copper uptake. J. Boil. Chem. 1994, 269, 25660–25667. [Google Scholar]
- Waterman, S.R.; Hacham, M.; Hu, G.; Zhu, X.; Park, Y.; Shin, S.; Panepinto, J.; Valyi-Nagy, T.; Beam, C.; Husain, S.; et al. Role of a CUF1/CTR4 copper regulatory axis in the virulence of Cryptococcus neoformans. J. Clin. Investig. 2007, 117, 794–802. [Google Scholar] [CrossRef]
- Ding, C.; Yin, J.; Tovar, E.M.M.; Fitzpatrick, D.A.; Higgins, D.; Thiele, D.J. The copper regulon of the human fungal pathogen Cryptococcus neoformans H99. Mol. Microbiol. 2011, 81, 1560–1576. [Google Scholar] [CrossRef]
- Bertinato, J.; Swist, E.; Plouffe, L.J.; Brooks, S.P.; L’Abbé, M.R. Ctr2 is partially localized to the plasma membrane and stimulates copper uptake in COS-7 cells. Biochem. J. 2008, 409, 731–740. [Google Scholar] [CrossRef]
- Puig, S.; Lee, J.; Lau, M.; Thiele, D.J. Biochemical and Genetic Analyses of Yeast and Human High Affinity Copper Transporters Suggest a Conserved Mechanism for Copper Uptake. J. Boil. Chem. 2002, 277, 26021–26030. [Google Scholar] [CrossRef]
- Klomp, A.E.M.; Juijn, J.A.; Van Der Gun, L.T.M.; Berg, I.E.T.V.D.; Berger, R.; Klomp, L.W.J. The N-terminus of the human copper transporter 1 (hCTR1) is localized extracellularly, and interacts with itself. Biochem. J. 2003, 370, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Smith, K.; Lee, J.; Thiele, D.J.; Petris, M.J. Identification of Methionine-rich Clusters That Regulate Copper-stimulated Endocytosis of the Human Ctr1 Copper Transporter. J. Boil. Chem. 2004, 279, 17428–17433. [Google Scholar] [CrossRef]
- Aller, S.G.; Eng, E.T.; De Feo, C.J.; Unger, V.M. Eukaryotic CTR Copper Uptake Transporters Require Two Faces of the Third Transmembrane Domain for Helix Packing, Oligomerization, and Function. J. Boil. Chem. 2004, 279, 53435–53441. [Google Scholar] [CrossRef] [PubMed]
- De Feo, C.J.; Mootien, S.; Unger, V.M. Tryptophan Scanning Analysis of the Membrane Domain of CTR-Copper Transporters. J. Membr. Boil. 2010, 234, 113–123. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nose, Y.; Wood, L.K.; Kim, B.-E.; Prohaska, J.R.; Fry, R.S.; Spears, J.W.; Thiele, D.J. Ctr1 Is an Apical Copper Transporter in Mammalian Intestinal Epithelial Cells in Vivo That Is Controlled at the Level of Protein Stability. J. Boil. Chem. 2010, 285, 32385–32392. [Google Scholar] [CrossRef] [PubMed]
- Maryon, E.B.; Molloy, S.A.; Kaplan, J. O-Linked Glycosylation at Threonine 27 Protects the Copper Transporter hCTR1 from Proteolytic Cleavage in Mammalian Cells. J. Boil. Chem. 2007, 282, 20376–20387. [Google Scholar] [CrossRef]
- Eisses, J.F.; Kaplan, J.H. The Mechanism of Copper Uptake Mediated by Human CTR1 a Mutational Analysis. J. Biol. Chem. 2005, 280, 37159–37168. [Google Scholar] [CrossRef]
- Hassett, R.; Kosman, D.J. Evidence for Cu(II) Reduction as a Component of Copper Uptake bySaccharomyces cerevisiae. J. Boil. Chem. 1995, 270, 128–134. [Google Scholar] [CrossRef]
- Georgatsou, E.; Mavrogiannis, L.A.; Fragiadakis, G.S.; Alexandraki, D. The Yeast Fre1p/Fre2p Cupric Reductases Facilitate Copper Uptake and Are Regulated by the Copper-modulated Mac1p Activator. J. Boil. Chem. 1997, 272, 13786–13792. [Google Scholar] [CrossRef]
- Kahra, D.; Kovermann, M.; Wittung-Stafshede, P. The C-Terminus of Human Copper Importer Ctr1 Acts as a Binding Site and Transfers Copper to Atox1. Biophys. J. 2016, 110, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Maryon, E.B.; Molloy, S.A.; Kaplan, J. Cellular glutathione plays a key role in copper uptake mediated by human copper transporter 1. Am. J. Physiol. 2013, 304, C768–C779. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Petris, M.J.; Thiele, D.J. Characterization of Mouse Embryonic Cells Deficient in the Ctr1 High Affinity Copper Transporter. J. Boil. Chem. 2002, 277, 40253–40259. [Google Scholar] [CrossRef] [PubMed]
- Simon, I.; Schaefer, M.; Reichert, J.; Stremmel, W. Analysis of the human Atox 1 homologue in Wilson patients. World J. Gastroenterol. 2008, 14, 2383–2387. [Google Scholar] [CrossRef]
- Huffman, D.L.; O’Halloran, T.V. Function, Structure, and Mechanism of Intracellular Copper Trafficking Proteins. Annu. Rev. Biochem. 2001, 70, 677–701. [Google Scholar] [CrossRef]
- Arnesano, F.; Banci, L.; Bertini, I.; Ciofi-Baffoni, S.; Molteni, E.; Huffman, D.L.; O’Halloran, T.V. Metallochaperones and Metal-Transporting ATPases: A Comparative Analysis of Sequences and Structures. Genome Res. 2002, 12, 255–271. [Google Scholar] [CrossRef]
- Hellman, N.E.; Gitlin, J.D. Ceruloplasmin metabolism and function. Annu. Rev. Nutr. 2002, 22, 439–458. [Google Scholar] [CrossRef]
- Flores, A.G.; Unger, V.M. Atox1 contains positive residues that mediate membrane association and aid subsequent copper loading. J. Membr. Boil. 2013, 246, 903–913. [Google Scholar] [CrossRef]
- Xiao, Z.; Brose, J.; Schimo, S.; Ackland, S.M.; La Fontaine, S.; Wedd, A.G. Unification of the Copper(I) Binding Affinities of the Metallo-chaperones Atx1, Atox1, and Related Proteins. J. Boil. Chem. 2011, 286, 11047–11055. [Google Scholar] [CrossRef]
- Puig, S.; Thiele, D.J. Molecular mechanisms of copper uptake and distribution. Curr. Opin. Chem. Boil. 2002, 6, 171–180. [Google Scholar] [CrossRef]
- Salgado, M.T.; Stillman, M.J. Cu+ distribution in metallothionein fragments. Biochem. Biophys. Res. Commun. 2004, 318, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Krężel, A.; Hao, Q.; Maret, W. The zinc/thiolate redox biochemistry of metallothionein and the control of zinc ion fluctuations in cell signaling. Arch. Biochem. Biophys. 2007, 463, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.T.; Kuroda, T. Transfer of copper and zinc from ionic and metallothionein-bound forms to Cu, Zn—Superoxide dismutase. Res. Commun. Mol. Pathol. Pharmacol. 1995, 87, 287–296. [Google Scholar] [PubMed]
- Lutsenko, S. Human copper homeostasis: A network of interconnected pathways. Curr. Opin. Chem. Boil. 2010, 14, 211–217. [Google Scholar] [CrossRef]
- Brouwer, M.; Brouwer-Hoexum, T. Glutathione-mediated transfer of copper (I) into American lobster apohemocyanin. Biochemistry 1992, 31, 4096–4102. [Google Scholar] [CrossRef]
- Deng, H.-X.; Hentati, A.; Tainer, J.; Iqbal, Z.; Cayabyab, A.; Hung, W.; Getzoff, E.; Hu, P.; Herzfeldt, B.; Roos, R.; et al. Amyotrophic lateral sclerosis and structural defects in Cu, Zn superoxide dismutase. Science 1993, 261, 1047–1051. [Google Scholar] [CrossRef]
- Gurney, M.; Pu, H.; Chiu, A.; Canto, M.D.; Polchow, C.; Alexander, D.; Caliendo, J.; Hentati, A.; Kwon, Y.; Deng, H.-X.; et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science 1994, 264, 1772–1775. [Google Scholar] [CrossRef]
- Watanabe, M.; Dykes-Hoberg, M.; Culotta, V.C.; Price, D.L.; Wong, P.C.; Rothstein, J.D. Histological Evidence of Protein Aggregationin Mutant SOD1 Transgenic Mice and inAmyotrophic Lateral Sclerosis Neural Tissues. Neurobiol. Dis. 2001, 8, 933–941. [Google Scholar] [CrossRef]
- Kuo, M.T.; Beckman, J.S.; Shaw, C.A. Neuroprotective effect of CuATSM on neurotoxin-induced motor neuron loss in an ALS mouse model. Neurobiol. Dis. 2019, 130, 104495. [Google Scholar] [CrossRef]
- Kaplan, J.; Maryon, E.B. How Mammalian Cells Acquire Copper: An Essential but Potentially Toxic Metal. Biophys. J. 2016, 110, 7–13. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boyd, S.D.; Ullrich, M.S.; Skopp, A.; Winkler, D.D. Copper Sources for Sod1 Activation. Antioxidants 2020, 9, 500. https://doi.org/10.3390/antiox9060500
Boyd SD, Ullrich MS, Skopp A, Winkler DD. Copper Sources for Sod1 Activation. Antioxidants. 2020; 9(6):500. https://doi.org/10.3390/antiox9060500
Chicago/Turabian StyleBoyd, Stefanie D., Morgan S. Ullrich, Amelie Skopp, and Duane D. Winkler. 2020. "Copper Sources for Sod1 Activation" Antioxidants 9, no. 6: 500. https://doi.org/10.3390/antiox9060500
APA StyleBoyd, S. D., Ullrich, M. S., Skopp, A., & Winkler, D. D. (2020). Copper Sources for Sod1 Activation. Antioxidants, 9(6), 500. https://doi.org/10.3390/antiox9060500




