Ethosomes for Coenzyme Q10 Cutaneous Administration: From Design to 3D Skin Tissue Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. CoQ10 Solubility Evaluation
2.3. Production of Ethosomes
2.4. Characterization of Ethosomes
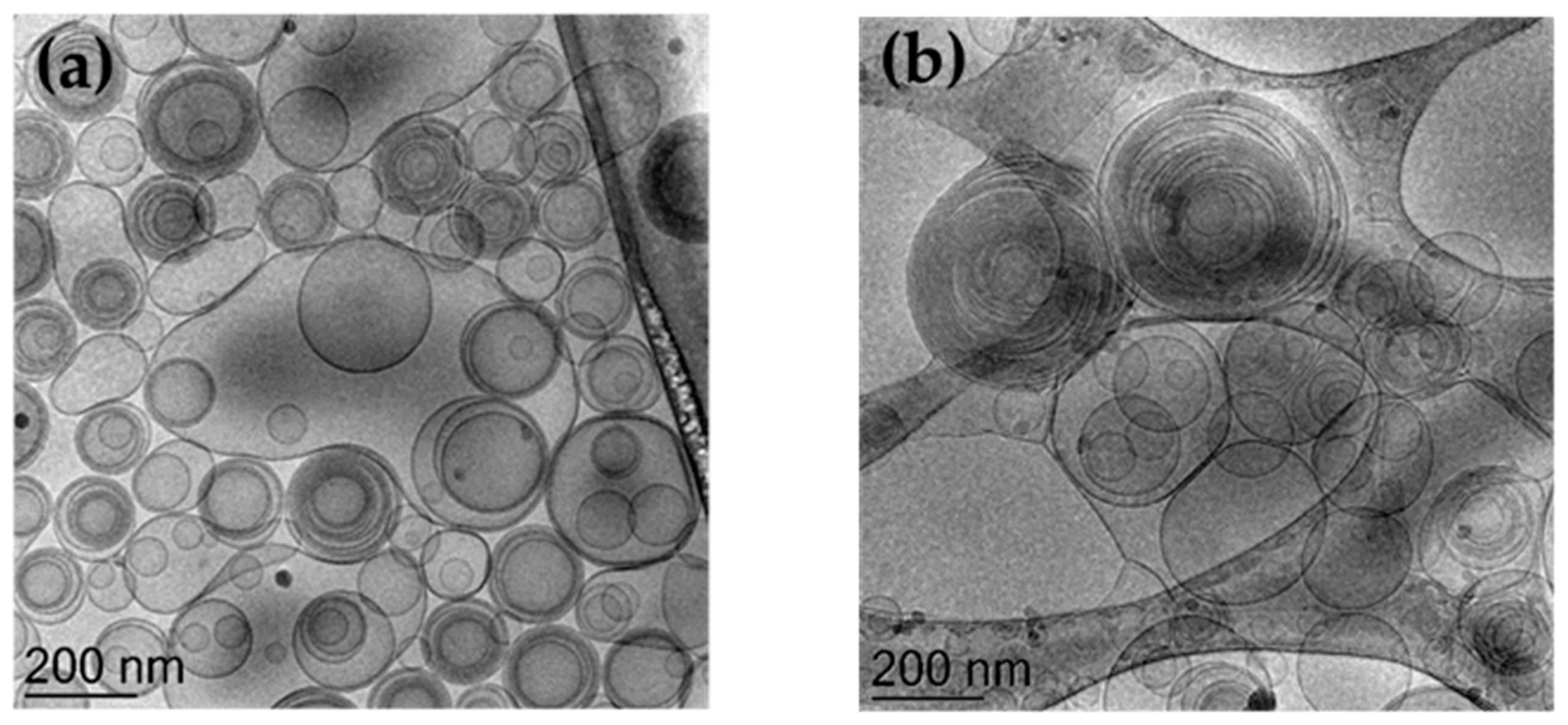
2.4.1. Cryo-Transmission Electron Microscopy (Cryo-TEM)
2.4.2. Photon Correlation Spectroscopy (PCS)
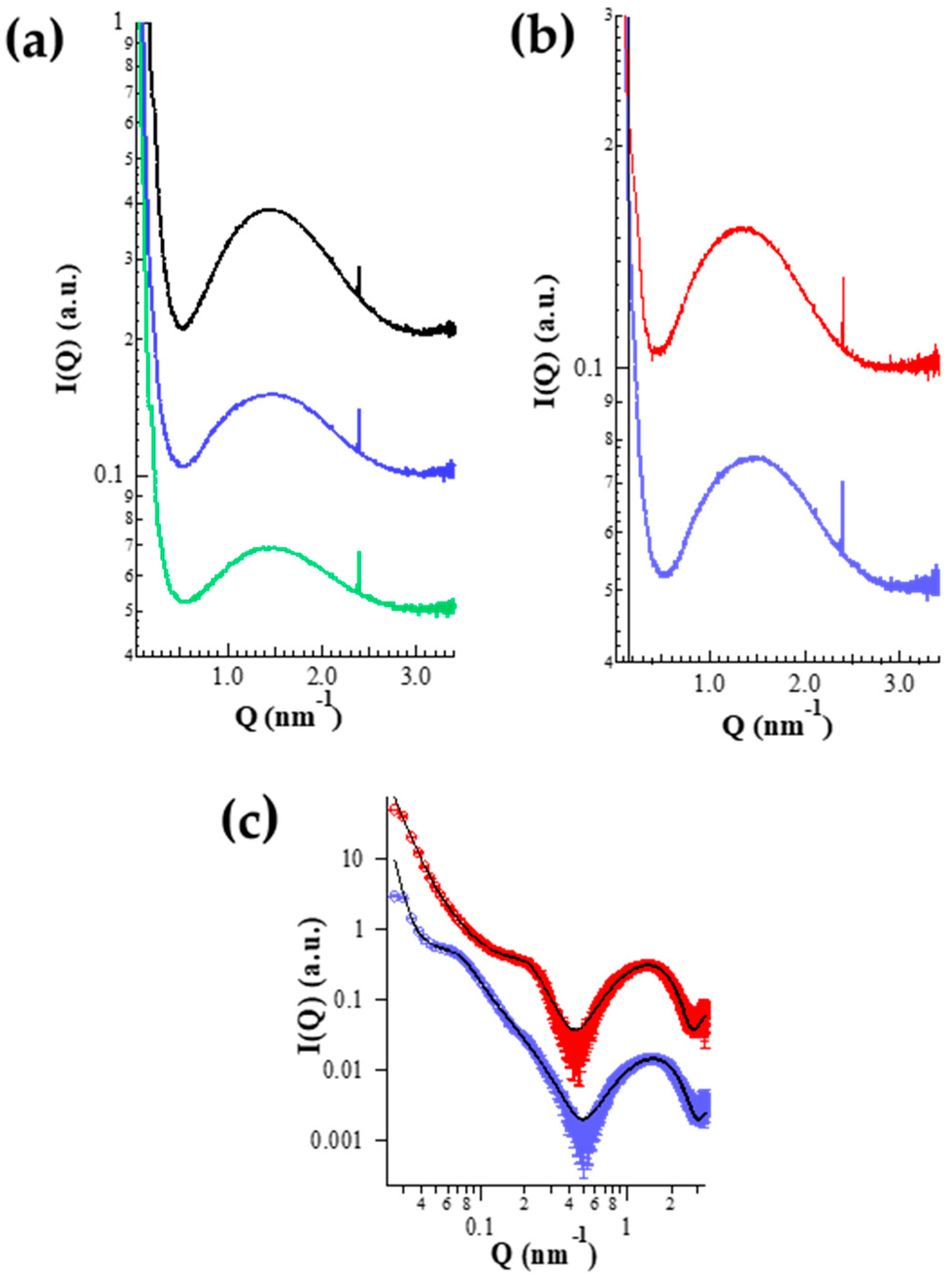
2.4.3. X-ray Diffraction
2.5. CoQ10 Content of Ethosomes
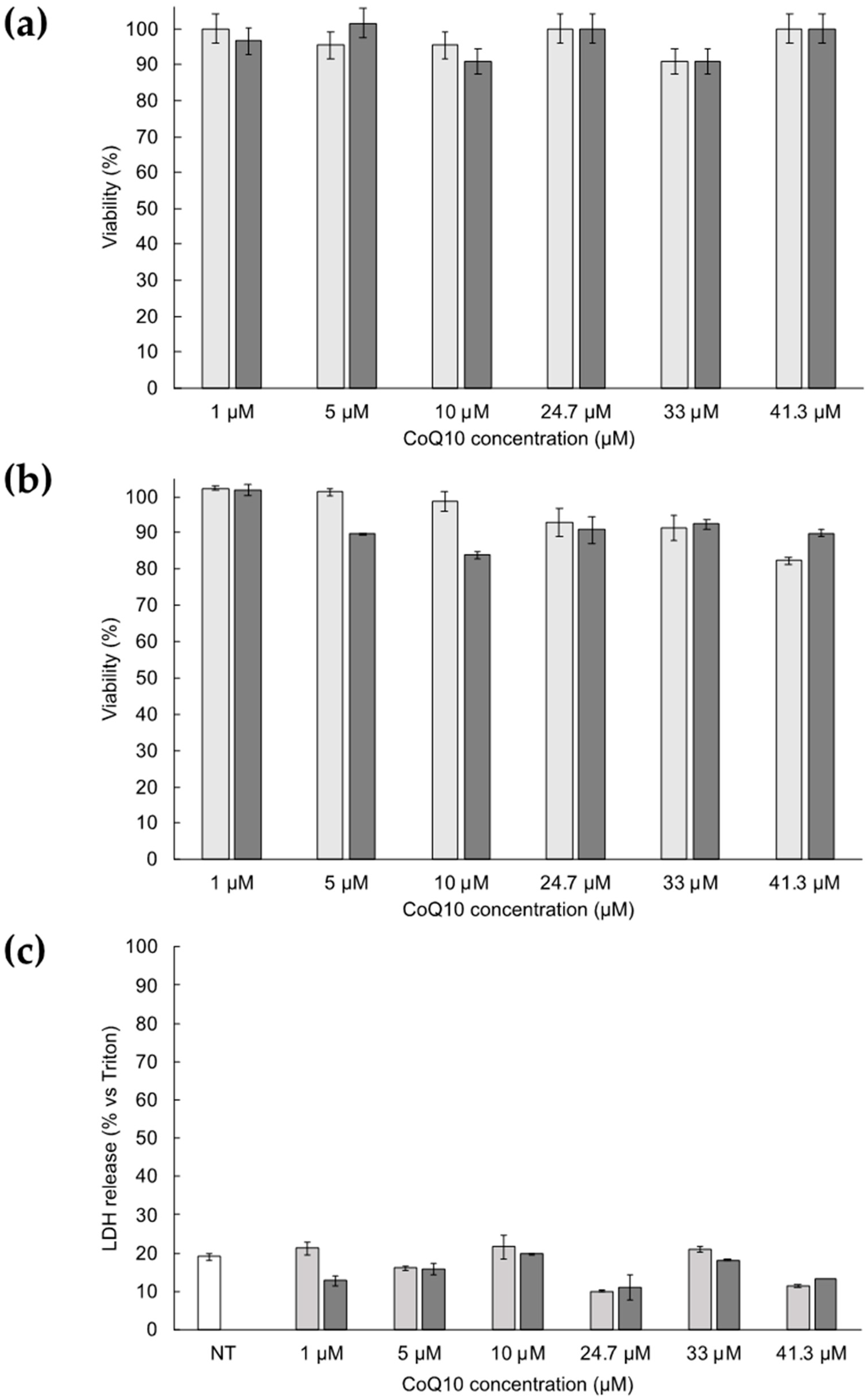
2.6. Cell Culture and Cytotoxicity Studies
2.7. Reconstitution of Human Epidermal Equivalents
2.8. Transmission Electron Microscopy (TEM) Analyses
2.9. Immunocytochemistry
2.10. Immunohistochemistry
2.11. Statistical Analysis
3. Results
3.1. Solubility Study
3.2. Preparation and Characterization of Ethosomes
X-ray Diffraction
3.3. Entrapment Capacity of CoQ10 in ETHO
3.4. Cytotoxicity of ETHO Containing CoQ10
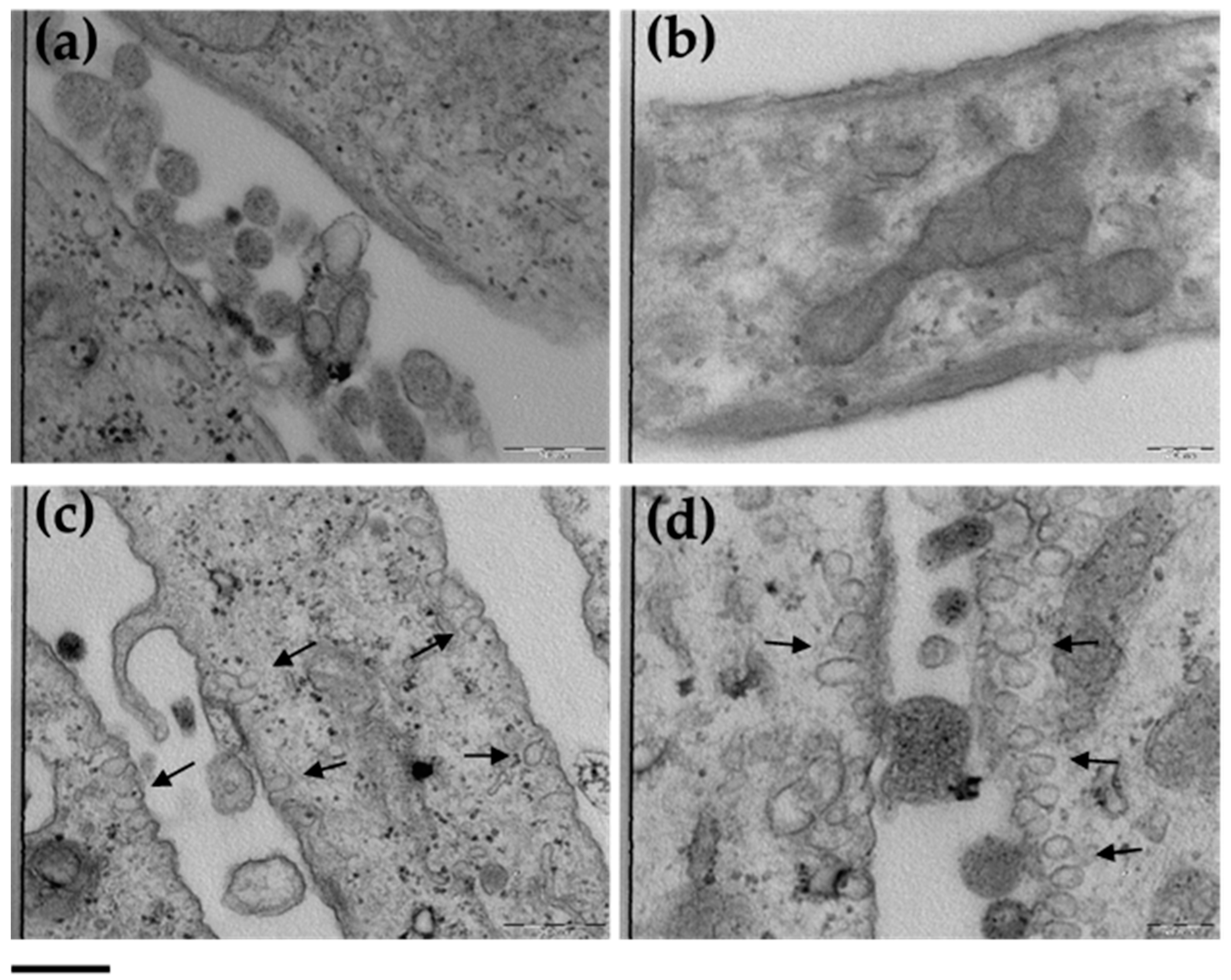
3.5. ETHO Uptake in Fibroblasts Detected by TEM
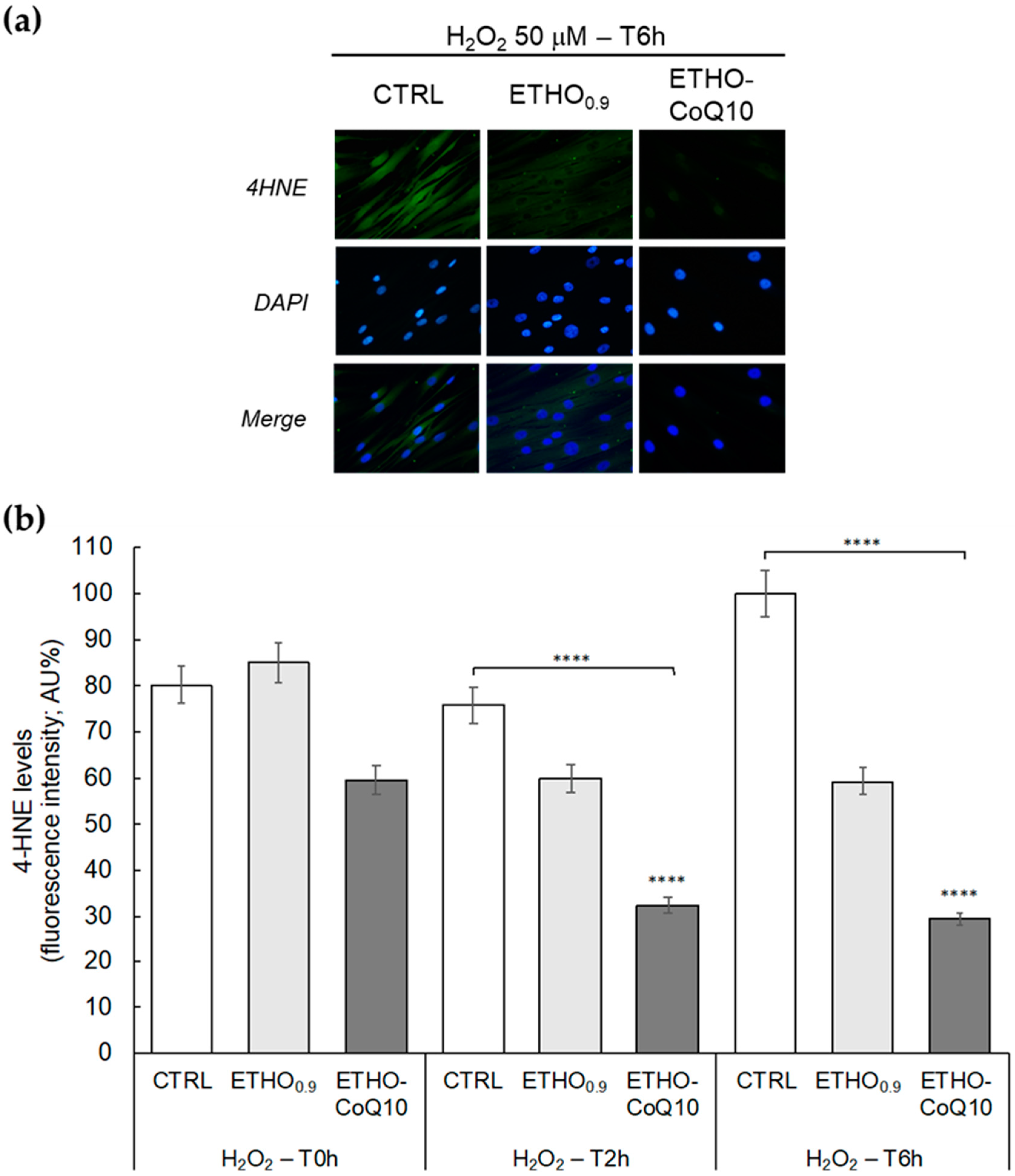
3.6. Evaluation of 4HNE Protein Adducts Levels in Fibroblasts Treated with ETHO-CoQ10
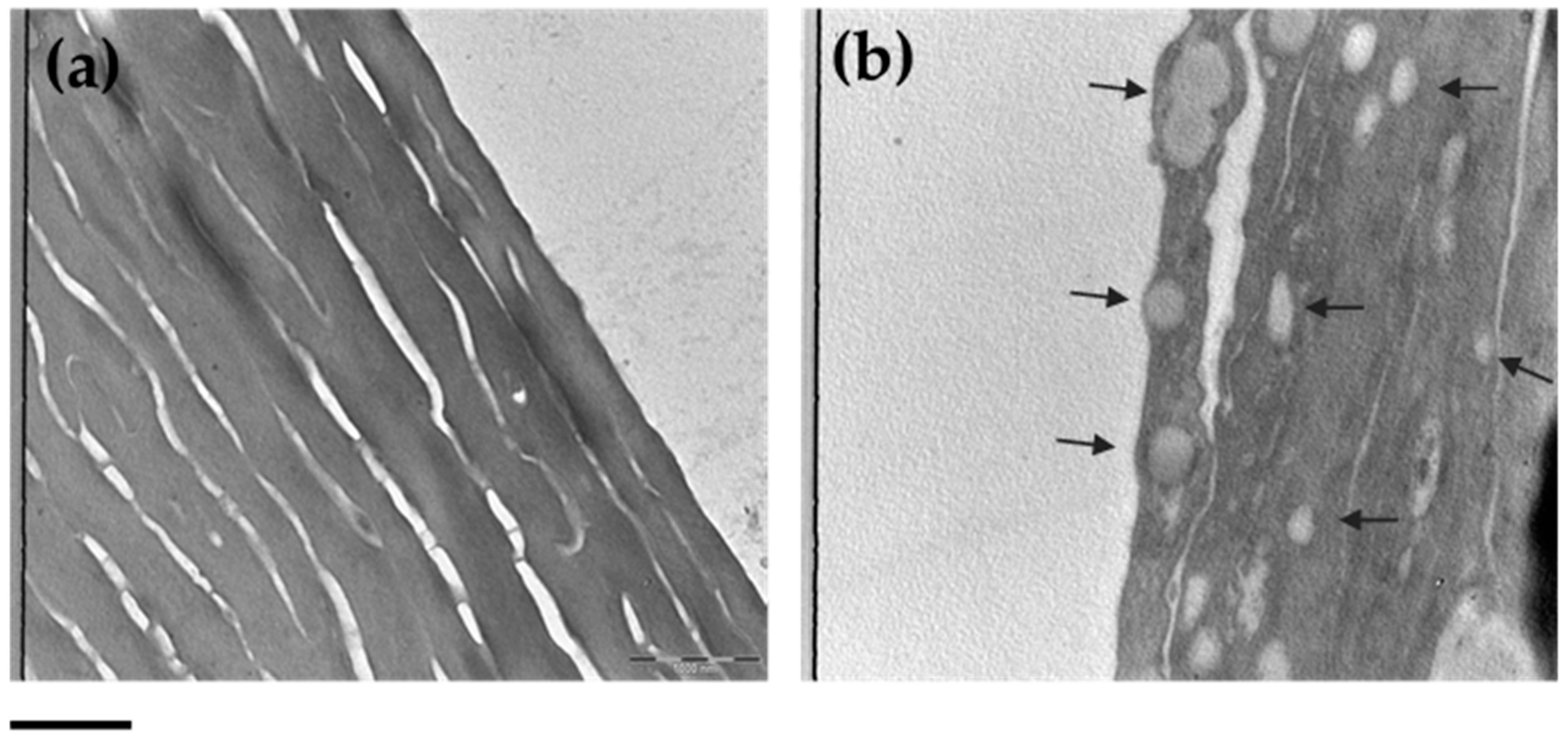
3.7. ETHO Skin Uptake in Reconstituted Human Epidermis (RHE) Detected by TEM
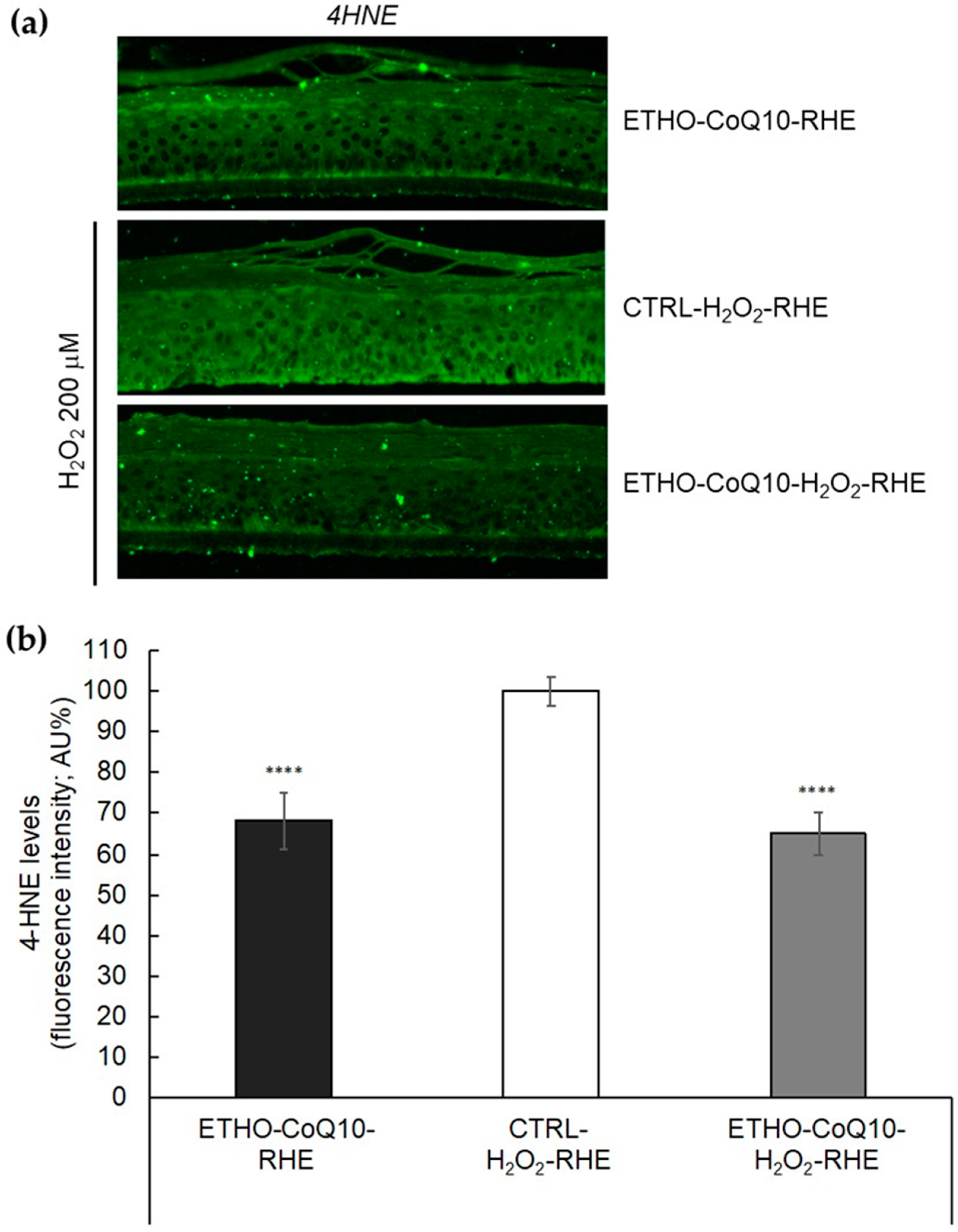
3.8. Evaluation of 4HNE Protein Adducts Levels in RHE Treated with ETHO-CoQ10
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Genova, M.L.; Lenaz, G. New developments on the functions of coenzyme Q in mitochondria. BioFactors 2011, 37, 330–354. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Maraver, J.; Cordero, M.D.; Oropesa-Ávila, M.; Fernández Vega, A.; De la Mata, M.; Delgado Pavón, A.; De Miguel, M.; Pérez Calero, C.; Villanueva Paz, M.; Cotán, D.; et al. Coenzyme Q10 therapy. Mol. Syndromol. 2014, 5, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Saini, R. Coenzyme Q10: The essential nutrient. J. Pharm. Bioallied Sci. 2011, 3, 466–467. [Google Scholar] [CrossRef] [PubMed]
- Fotino, A.D.; Thompson-Paul, A.M.; Bazzano, L.A. Effect of coenzyme Q10 supplementation on heart failure: A meta-analysis. Am. J. Clin. Nutr. 2013, 97, 268–275. [Google Scholar] [CrossRef]
- Singh, U.; Devaraj, S.; Jialal, I. Coenzyme Q10 supplementation and heart failure. Nutr. Rev. 2007, 65, 286–293. [Google Scholar] [CrossRef]
- Hernández-Camacho, J.D.; Bernier, M.; López-Lluch, G.; Navas, P. Coenzyme Q10 supplementation in aging and disease. Front. Physiol. 2018, 9, 44. [Google Scholar] [CrossRef]
- Folkers, K.; Hanioka, T.; Xia, L.-J.; McRee, J.T.; Langsjoen, P. Coenzyme Q10 increases T4/T8 ratios of lymphocytes in ordinary subjects and relevance to patients having the aids related complex. Biochem. Biophys. Res. Commun. 1991, 176, 786–791. [Google Scholar] [CrossRef]
- Thapa, M.; Dallmann, G. Role of coenzymes in cancer metabolism. Semin. Cell Dev. Biol. 2020, 98, 44–53. [Google Scholar] [CrossRef]
- Knott, A.; Achterberg, V.; Smuda, C.; Mielke, H.; Sperling, G.; Dunckelmann, K.; Vogelsang, A.; Krüger, A.; Schwengler, H.; Behtash, M.; et al. Topical treatment with coenzyme Q10-containing formulas improves skin’s Q10 level and provides antioxidative effects: Topical Treatment with Coenzyme Q10. BioFactors 2015, 41, 383–390. [Google Scholar] [CrossRef]
- Prahl, S.; Kueper, T.; Biernoth, T.; Wöhrmann, Y.; Münster, A.; Fürstenau, M.; Schmidt, M.; Schulze, C.; Wittern, K.-P.; Wenck, H.; et al. Aging skin is functionally anaerobic: Importance of coenzyme Q10 for anti aging skin care. Biofactors 2008, 32, 245–255. [Google Scholar] [CrossRef]
- Ashida, Y.; Yamanishi, H.; Terada, T.; Oota, N.; Sekine, K.; Watabe, K. CoQ10 supplementation elevates the epidermal CoQ10 level in adult hairless mice. Biofactors 2005, 25, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Rusciani, L.; Proietti, I.; Rusciani, A.; Paradisi, A.; Sbordoni, G.; Alfano, C.; Panunzi, S.; De Gaetano, A.; Lippa, S. Low plasma coenzyme Q10 levels as an independent prognostic factor for melanoma progression. J. Am. Acad. Dermatol. 2006, 54, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Hseu, Y.-C.; Ho, Y.-G.; Mathew, D.C.; Yen, H.-R.; Chen, X.-Z.; Yang, H.-L. The in vitro and in vivo depigmenting activity of Coenzyme Q10 through the down-regulation of α-MSH signaling pathways and induction of Nrf2/ARE-mediated antioxidant genes in UVA-irradiated skin keratinocytes. Biochem. Pharmacol. 2019, 164, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Pegoraro, N.S.; Mattiazzi, J.; Da Silveira, E.F.; Azambuja, J.H.; Braganhol, E.; Cruz, L. Improved photostability and cytotoxic effect of coenzyme Q10 by its association with vitamin E acetate in polymeric nanocapsules. Pharm. Dev. Technol. 2018, 23, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Zhou, H.; Liu, G.; Li, Y.; Yan, Z.; Duan, M. The advantages of a novel CoQ10 delivery system in skin photo-protection. Int. J. Pharm. 2010, 392, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Gokce, E.H.; Korkmaz, E.; Tuncay-Tanrıverdi, S.; Dellera, E.; Sandri, G.; Bonferoni, M.C.; Ozer, O. A comparative evaluation of coenzyme Q10-loaded liposomes and solid lipid nanoparticles as dermal antioxidant carriers. Int. J. Nanomed. 2012, 7, 5109–5117. [Google Scholar] [CrossRef][Green Version]
- Wang, J.; Wang, H.; Xia, Q. Ubidecarenone-loaded nanostructured lipid carrier (UB-NLC): Percutaneous penetration and protective effects against hydrogen peroxide-induced oxidative stress on HaCaT cells. IJMS 2018, 19, 1865. [Google Scholar] [CrossRef]
- Shaheen, M.A.; Elmeadawy, S.H.; Bazeed, F.B.; Anees, M.M.; Saleh, N.M. Innovative coenzyme Q10-loaded nanoformulation as an adjunct approach for the management of moderate periodontitis: Preparation, evaluation, and clinical study. Drug Deliv. Transl. Res. 2020, 10, 548–564. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Zhou, X.; Tang, Z.; Liu, G.; Liu, G.; Xia, Q. Physicochemical characterization, photo-stability and cytotoxicity of coenzyme Q10-loading nanostructured lipid carrier. J. Nanosci. Nanotechnol. 2012, 12, 2136–2148. [Google Scholar] [CrossRef]
- Nanjwade, B.K.; Kadam, V.T.; Manvi, F.V. Formulation and characterization of nanostructured lipid carrier of ubiquinone (coenzyme Q10). J. Biomed. Nanotechnol. 2013, 9, 450–460. [Google Scholar] [CrossRef]
- Abdulbaqi, I.M.; Darwis, Y.; Khan, N.A.K.; Assi, R.A.; Khan, A.A. Ethosomal nanocarriers: The impact of constituents and formulation techniques on ethosomal properties, in vivo studies, and clinical trials. Int. J. Nanomed. 2016, 11, 2279–2304. [Google Scholar] [CrossRef] [PubMed]
- Natsheh, H.; Vettorato, E.; Touitou, E. Ethosomes for dermal administration of natural active molecules. CPD 2019, 25, 2338–2348. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.; Mandal, U.K.; Chatterjee, B. Transdermal delivery of raloxifene HCl via ethosomal system: Formulation, advanced characterizations and pharmacokinetic evaluation. Int. J. Pharm. 2018, 542, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.D.; Patel, C.N.; Shah, D.R. Formulation and optimization of ethosomes for transdermal delivery of ropinirole hydrochloride. Curr. Drug Deliv. 2013, 10, 500–516. Available online: http://www.eurekaselect.com/115015/article (accessed on 29 April 2020). [CrossRef]
- Shen, L.-N.; Zhang, Y.-T.; Wang, Q.; Xu, L.; Feng, N.-P. Enhanced in vitro and in vivo skin deposition of apigenin delivered using ethosomes. Int. J. Pharm. 2014, 460, 280–288. [Google Scholar] [CrossRef]
- Zhang, Y.-T.; Shen, L.-N.; Zhao, J.-H.; Feng, N.-P. Evaluation of psoralen ethosomes for topical delivery in rats by using in vivo microdialysis. Int. J. Nanomed. 2014, 9, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H.; Cho, S.-H.; Yun, J.-J.; Yu, Y.-B.; Cho, C.-W. Ethosomes and transfersomes for topical delivery of ginsenoside Rhl from red ginseng: Characterization and in vitro evaluation. J. Nanosci. Nanotechnol. 2015, 15, 5660–5662. [Google Scholar] [CrossRef]
- Bodade, S.S.; Shaikh, K.S.; Kamble, M.S.; Chaudhari, P.D. A study on ethosomes as mode for transdermal delivery of an antidiabetic drug. Drug Deliv. 2013, 20, 40–46. [Google Scholar] [CrossRef]
- Yu, X.; Du, L.; Li, Y.; Fu, G.; Jin, Y. Improved anti-melanoma effect of a transdermal mitoxantrone ethosome gel. Biomed. Pharmacother. 2015, 73, 6–11. [Google Scholar] [CrossRef]
- Tian, W.; Hu, Q.; Xu, Y.; Xu, Y. Effect of soybean-lecithin as an enhancer of buccal mucosa absorption of insulin. Bio Med Mater. Eng. 2012, 22, 171–178. [Google Scholar] [CrossRef]
- Lachenmeier, D.W. Safety evaluation of topical applications of ethanol on the skin and inside the oral cavity. J. Occup. Med. Toxicol. 2008, 3, 26. [Google Scholar] [CrossRef]
- Esposito, E.; Mariani, P.; Ravani, L.; Contado, C.; Volta, M.; Bido, S.; Drechsler, M.; Mazzoni, S.; Menegatti, E.; Morari, M.; et al. Nanoparticulate lipid dispersions for bromocriptine delivery: Characterization and in vivo study. Eur. J. Pharm. Biopharm. 2012, 80, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Pecora, R. Dynamic light scattering measurement of nanometer particles in liquids. J. Nanopart. Res. 2000, 2, 123–131. [Google Scholar] [CrossRef]
- Barbosa, L.R.S.; Ortore, M.G.; Spinozzi, F.; Mariani, P.; Bernstorff, S.; Itri, R. The importance of protein-protein interactions on the pH-induced conformational changes of bovine serum albumin: A small-angle X-ray scattering study. Biophys. J. 2010, 98, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Touitou, E.; Dayan, N.; Bergelson, L.; Godin, B.; Eliaz, M. Ethosomes—Novel vesicular carriers for enhanced delivery: Characterization and skin penetration properties. J. Control. Release 2000, 65, 403–418. [Google Scholar] [CrossRef]
- Esposito, E.; Sticozzi, C.; Ravani, L.; Drechsler, M.; Muresan, X.M.; Cervellati, F.; Cortesi, R.; Valacchi, G. Effect of new curcumin-containing nanostructured lipid dispersions on human keratinocytes proliferative responses. Exp. Dermatol. 2015, 24, 449–454. [Google Scholar] [CrossRef]
- Valacchi, G.; Sticozzi, C.; Belmonte, G.; Cervellati, F.; Demaude, J.; Chen, N.; Krol, Y.; Oresajo, C. Vitamin C compound mixtures prevent ozone-induced oxidative damage in human keratinocytes as initial assessment of pollution protection. PLoS ONE 2015, 10, e0131097. [Google Scholar] [CrossRef]
- Sguizzato, M.; Valacchi, G.; Pecorelli, A.; Boldrini, P.; Simelière, F.; Huang, N.; Cortesi, R.; Esposito, E. Gallic acid loaded poloxamer gel as new adjuvant strategy for melanoma: A preliminary study. Colloids Surf. B Biointerfaces 2020, 185, 110613. [Google Scholar] [CrossRef]
- Dijkhoff, I.M.; Petracca, B.; Prieux, R.; Valacchi, G.; Rothen-Rutishauser, B.; Eeman, M. A Reliable Procedure to Cultivate Three-dimensional Human Epidermal Equivalents at Large Scale. Submitted in J Vis Exp.
- Pecorelli, A.; Cervellati, F.; Belmonte, G.; Montagner, G.; Waldon, P.; Hayek, J.; Gambari, R.; Valacchi, G. Cytokines profile and peripheral blood mononuclear cells morphology in Rett and autistic patients. Cytokine 2016, 77, 180–188. [Google Scholar] [CrossRef]
- Canella, R.; Benedusi, M.; Martini, M.; Cervellati, F.; Cavicchio, C.; Valacchi, G. Role of Nrf2 in preventing oxidative stress induced chloride current alteration in human lung cells. J. Cell Physiol. 2018, 233, 6018–6027. [Google Scholar] [CrossRef]
- Sticozzi, C.; Belmonte, G.; Pecorelli, A.; Cervellati, F.; Leoncini, S.; Signorini, C.; Ciccoli, L.; De Felice, C.; Hayek, J.; Valacchi, G. Scavenger receptor B1 post-translational modifications in Rett syndrome. FEBS Lett. 2013, 587, 2199–2204. [Google Scholar] [CrossRef] [PubMed]
- Fuller, B. Role of PGE-2 and other inflammatory mediators in skin aging and their inhibition by topical natural anti-inflammatories. Cosmetics 2019, 6, 6. [Google Scholar] [CrossRef]
- Esposito, E.; Drechsler, M.; Huang, N.; Pavoni, G.; Cortesi, R.; Santonocito, D.; Puglia, C. Ethosomes and organogels for cutaneous administration of crocin. Biomed. Microdevices 2016, 18, 108. [Google Scholar] [CrossRef] [PubMed]
- Puri, R.; Jain, S. Ethogel topical formulation for increasing the local bioavailability of 5-fluorouracil: A mechanistic study. Anticancer Drugs 2012, 23, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Zhaowu, Z.; Xiaoli, W.; Yangde, Z.; Nianfeng, L. Preparation of matrine ethosome, its percutaneous permeation in vitro and anti-inflammatory activity in vivo in rats. J. Liposome Res. 2009, 19, 155–162. [Google Scholar] [CrossRef]
- Touitou, E.; Godin, B. Dermal drug delivery with ethosomes: Therapeutic potential. Therapy 2007, 4, 465–472. [Google Scholar] [CrossRef]
- Spinozzi, F.; Paccamiccio, L.; Mariani, P.; Amaral, L.Q. Melting regime of the anionic phospholipid DMPG: New lamellar phase and porous bilayer model. Langmuir 2010, 26, 6484–6493. [Google Scholar] [CrossRef]
- Andreozzi, P.; Funari, S.S.; La Mesa, C.; Mariani, P.; Ortore, M.G.; Sinibaldi, R.; Spinozzi, F. Multi- to unilamellar transitions in catanionic vesicles. J. Phys. Chem. B 2010, 114, 8056–8060. [Google Scholar] [CrossRef][Green Version]
- Spinozzi, F.; Ferrero, C.; Ortore, M.G.; De Maria Antolinos, A.; Mariani, P. GENFIT: Software for the analysis of small-angle X-ray and neutron scattering data of macro molecules in solution. J. Appl. Crystallogr. 2014, 47, 1132–1139. [Google Scholar] [CrossRef]
- Godin, B.; Touitou, E. Ethosomes: New prospects in transdermal delivery. Crit. Rev. Ther. Drug Carr. Syst. 2003, 20, 63–102. [Google Scholar] [CrossRef]
- Zhang, Y.-T.; Shen, L.-N.; Wu, Z.-H.; Zhao, J.-H.; Feng, N.-P. Evaluation of skin viability effect on ethosome and liposome-mediated psoralen delivery via cell uptake. J. Pharm. Sci. 2014, 103, 3120–3126. [Google Scholar] [CrossRef] [PubMed]
- Touitou, E.; Godin, B.; Dayan, N.; Weiss, C.; Piliponsky, A.; Levi-Schaffer, F. Intracellular delivery mediated by an ethosomal carrier. Biomaterials 2001, 22, 3053–3059. [Google Scholar] [CrossRef]
- Jain, S.; Tiwary, A.K.; Sapra, B.; Jain, N.K. Formulation and evaluation of ethosomes for transdermal delivery of lamivudine. Aaps Pharmscitech 2007, 8, 249. [Google Scholar] [CrossRef]
- Castro, J.P.; Jung, T.; Grune, T.; Siems, W. 4-Hydroxynonenal (HNE) modified proteins in metabolic diseases. Free Radic. Biol. Med. 2017, 111, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Mine, Y.; Okamoto, T. Extracellular coenzyme Q10 (CoQ10) is reduced to ubiquinol-10 by intact Hep G2 cells independent of intracellular CoQ10 reduction. Arch. Biochem. Biophys. 2019, 672, 108067. [Google Scholar] [CrossRef] [PubMed]
- Palmer, B.; DeLouise, L. Nanoparticle-enabled transdermal drug delivery systems for enhanced dose control and tissue targeting. Molecules 2016, 21, 1719. [Google Scholar] [CrossRef]
- Prieux, R.; Eeman, M.; Rothen-Rutishauser, B.; Valacchi, G. Mimicking cigarette smoke exposure to assess cutaneous toxicity. Toxicol. Vitr. 2020, 62, 104664. [Google Scholar] [CrossRef]
| Ethosome | PC 1 | Ethanol % w/w | CoQ10 % w/w | Water % w/w |
|---|---|---|---|---|
| ETHO0.6 | 0.6 | 29.4 | n.p. | 70.0 |
| ETHO0.9 | 0.9 | 29.1 | n.p. | 70.0 |
| ETHO1.5 | 1.5 | 28.5 | n.p. | 70.0 |
| ETHO-CoQ10 | 0.9 | 29.0 | 0.1 | 70.0 |
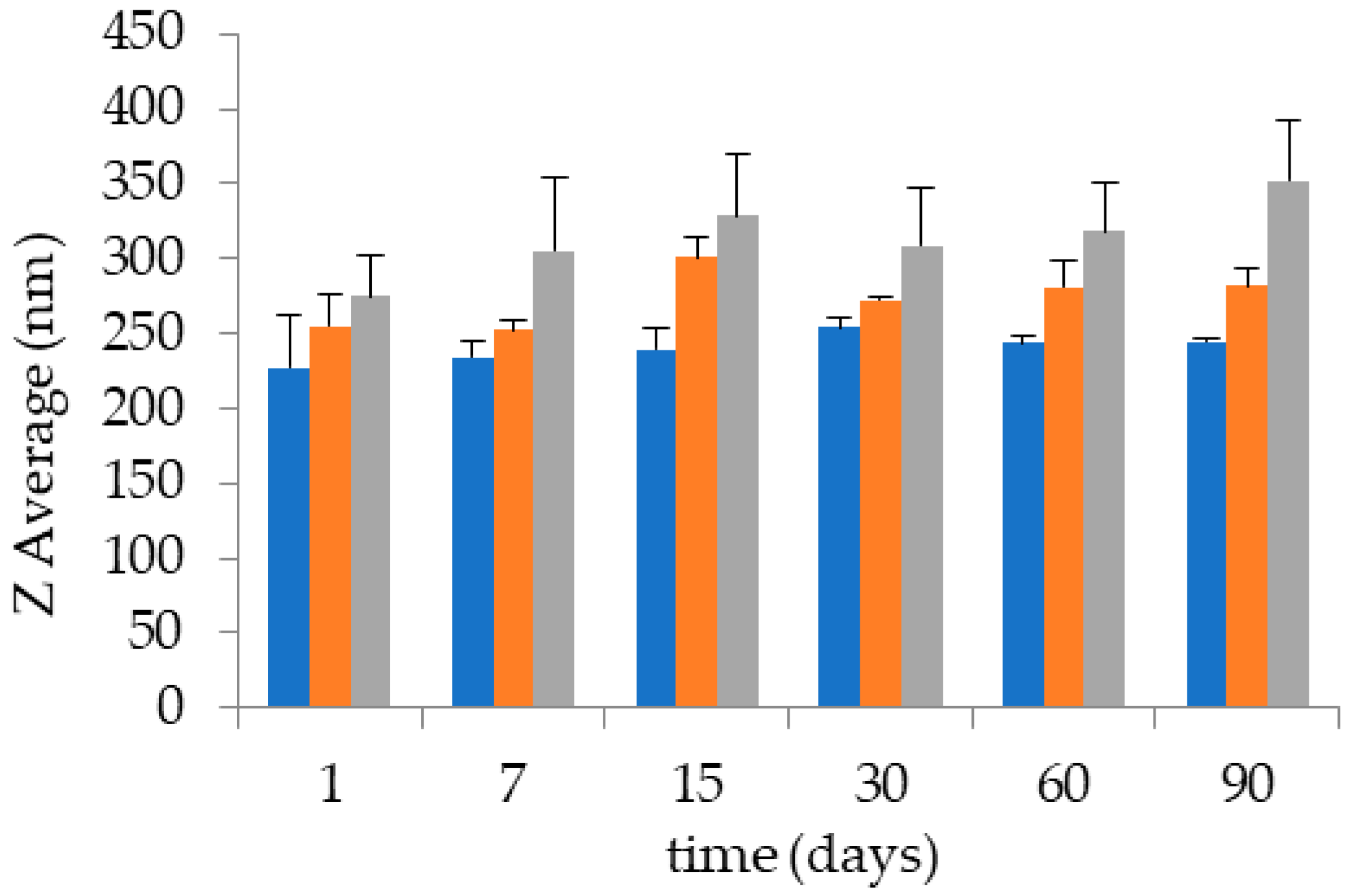
| Ethosome | Days | Z Average ± s.d. (nm) | Typical Intensity Distribution | Dispersity Index ± s.d. | |
|---|---|---|---|---|---|
| nm | Area % | ||||
| ETHO0.6 | 0 | 227.45 ± 34.10 | 237.55 | 100 | 0.12 ± 0.02 |
| 90 | 244.60 ± 5.30 | 242.11 | 100 | 0.14 ± 0.03 | |
| ETHO0.9 | 0 | 254.73 ± 21.0 | 244.68 | 100 | 0.10 ± 0.02 |
| 90 | 282.04 ± 10.01 | 275.34 | 100 | 0.12 ± 0.03 | |
| ETHO1.5 | 0 | 274.60 ± 32.10 | 266.51 | 100 | 0.13 ± 0.02 |
| 90 | 352.15 ± 22.01 | 327.53 | 100 | 0.22 ± 0.03 | |
| ETHO-CoQ10 | 0 | 271.20 ± 5.56 | 277.30 | 100 | 0.14 ± 0.01 |
| 90 | 293.05 ± 17.18 | 278.42 | 100 | 0.16 ± 0.03 | |
| Ethosome | R1 (Å) | R2 (Å) | R3 (Å) | ρ1 e/Å3 | ρ2 e/Å3 | ρ3 e/Å3 | N | c (Å) | gc | Ro (Å) | ξRo | ρet e/Å3 | dlip (Å) | Rtot (Å) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ETHO0.9 | 5.2 | 10.3 | 2.3 | 0.43 | 0.31 | 0.29 | 3 ± 2 | 560 | 0.33 | 133 | 0.33 | 0.29 | 35.4 | 270 |
| ETHO-CoQ10 | 6.9 | 11.9 | 1.2 | 0.42 | 0.30 | 0.29 | 9 ± 1 | 115 | 0.40 | 199 | 0.40 | 0.29 | 39.8 | 296 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sguizzato, M.; Mariani, P.; Spinozzi, F.; Benedusi, M.; Cervellati, F.; Cortesi, R.; Drechsler, M.; Prieux, R.; Valacchi, G.; Esposito, E. Ethosomes for Coenzyme Q10 Cutaneous Administration: From Design to 3D Skin Tissue Evaluation. Antioxidants 2020, 9, 485. https://doi.org/10.3390/antiox9060485
Sguizzato M, Mariani P, Spinozzi F, Benedusi M, Cervellati F, Cortesi R, Drechsler M, Prieux R, Valacchi G, Esposito E. Ethosomes for Coenzyme Q10 Cutaneous Administration: From Design to 3D Skin Tissue Evaluation. Antioxidants. 2020; 9(6):485. https://doi.org/10.3390/antiox9060485
Chicago/Turabian StyleSguizzato, Maddalena, Paolo Mariani, Francesco Spinozzi, Mascia Benedusi, Franco Cervellati, Rita Cortesi, Markus Drechsler, Roxane Prieux, Giuseppe Valacchi, and Elisabetta Esposito. 2020. "Ethosomes for Coenzyme Q10 Cutaneous Administration: From Design to 3D Skin Tissue Evaluation" Antioxidants 9, no. 6: 485. https://doi.org/10.3390/antiox9060485
APA StyleSguizzato, M., Mariani, P., Spinozzi, F., Benedusi, M., Cervellati, F., Cortesi, R., Drechsler, M., Prieux, R., Valacchi, G., & Esposito, E. (2020). Ethosomes for Coenzyme Q10 Cutaneous Administration: From Design to 3D Skin Tissue Evaluation. Antioxidants, 9(6), 485. https://doi.org/10.3390/antiox9060485












