Characterization and Modulation of Systemic Inflammatory Response to Exhaustive Exercise in Relation to Oxidative Stress
Abstract
1. Introduction
2. Background Information of Cytokines
3. Dynamics and Sources of Cytokines in Response to Exercise
4. The Exercise-Induced Endotoxemia and Systemic Inflammation
5. The Exercise-Induced Inflammation and Organ Damage
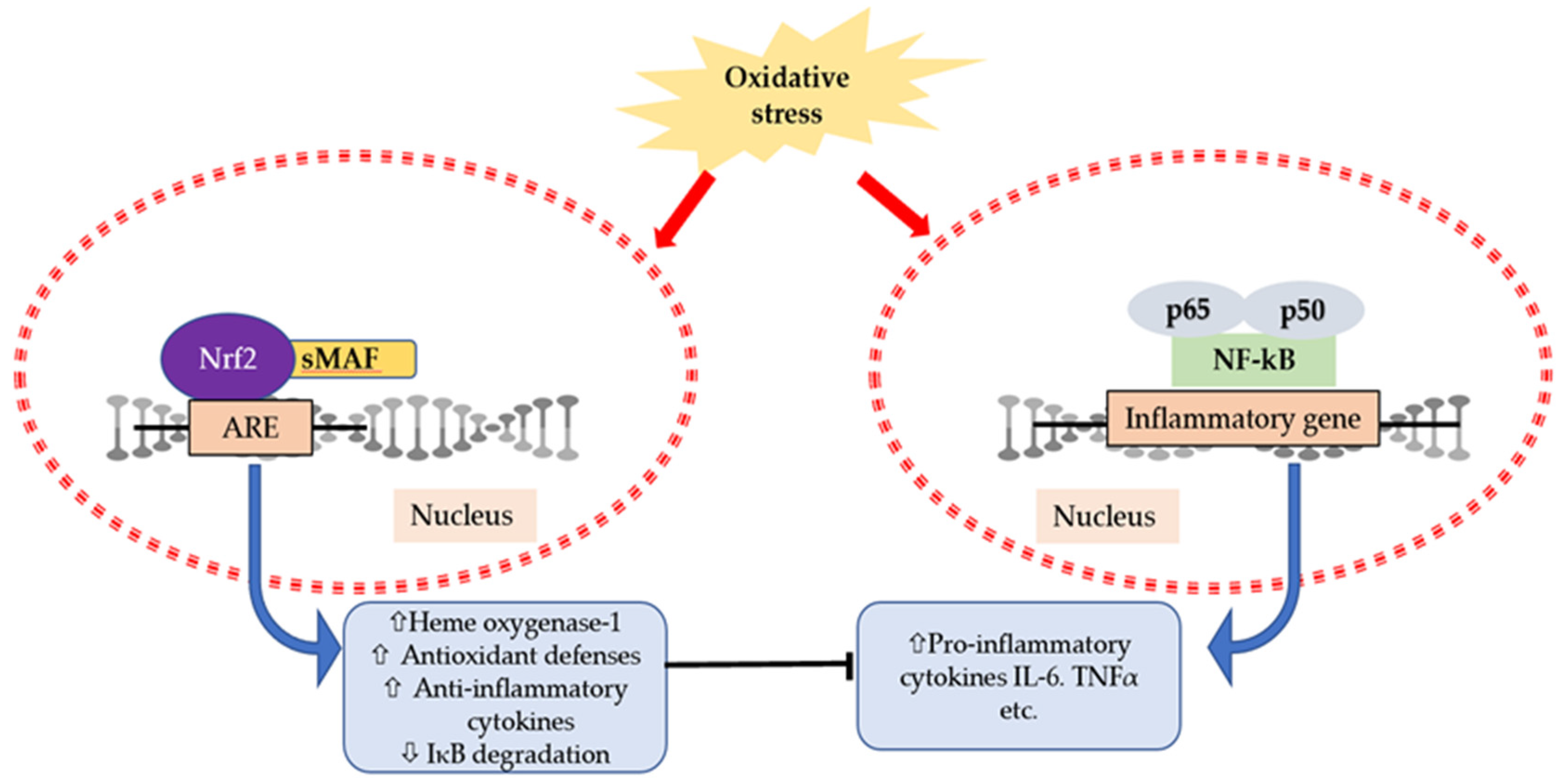
6. Interaction of Cytokines and Oxidative Stress
7. Potential Strategy/Countermeasures to Reduce Exercise-Induced Inflammation and Oxidative Stress
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hung, Y.-L.; Suzuki, K. The pattern recognition receptors and lipopolysaccharides (LPS)-induced systemic inflammation. Int. J. Res. Stud. Med. Health Sci. 2017, 2, 1–7. [Google Scholar]
- Suzuki, K. Involvement of neutrophils in exercise-induced muscle damage. Gen. Intern. Med. Clin. Innov. 2018, 3, 1–8. [Google Scholar] [CrossRef]
- Ma, S.; Suzuki, K. Toll-like receptor 4: Target of lipotoxicity and exercise-induced anti-inflammatory effect? Ann. Nutr. Food Sci. 2018, 2, 1027. [Google Scholar]
- Suzuki, K. Characterization of exercise-induced cytokine release, the impacts on the body, the mechanisms and modulations. Int. J. Sports Exerc. Med. 2019, 5, 122. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an endocrine organ: Focus on muscle-derived interleukin-6. Physiol. Rev. 2008, 88, 1379–1406. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K. Chronic inflammation as an immunological abnormality and effectiveness of exercise. Biomolecules 2019, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Torma, F.; Berkes, I.; Goto, S.; Mimura, T.; Posa, A.; Balogh, L.; Boldogh, I.; Suzuki, K.; Higuchi, M.; et al. Exercise effects on physiological function during aging. Free Radic. Biol. Med. 2019, 132, 33–41. [Google Scholar] [CrossRef]
- Aw, N.H.; Canetti, E.; Suzuki, K.; Goh, J. Monocyte subsets in atherosclerosis and modification with exercise in humans. Antioxidants 2018, 7, 196. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization “Global strategy on diet, physical activity and health”. Available online: https://www.who.int/dietphysicalactivity/pa/en/ (accessed on 14 April 2020).
- Cerqueira, E.; Marinho, D.A.; Neiva, H.P.; Lourenco, O. Inflammatory effects of high and moderate intensity exercise—A systematic review. Front. Physiol. 2020, 10, 1550. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K. Cytokine response to exercise and its modulation. Antioxidants 2018, 7, 17. [Google Scholar] [CrossRef]
- Peake, J.; Suzuki, K. Neutrophil activation, antioxidant supplements and exercise-induced oxidative stress. Exerc. Immunol. Rev. 2004, 10, 129–141. [Google Scholar] [PubMed]
- Suzuki, K.; Nakaji, S.; Yamada, M.; Totsuka, M.; Sato, K.; Sugawara, K. Systemic inflammatory response to exhaustive exercise: Cytokine kinetics. Exerc. Immunol. Rev. 2002, 8, 6–48. [Google Scholar] [PubMed]
- Sugama, K.; Suzuki, K.; Yoshitani, K.; Shiraishi, K.; Kometani, T. IL-17, neutrophil activation and muscle damage following endurance exercise. Exerc. Immunol. Rev. 2012, 18, 116–127. [Google Scholar] [PubMed]
- Suzuki, K.; Nakaji, S.; Kurakake, S.; Totsuka, M.; Sato, K.; Kuriyama, T.; Fujimoto, H.; Shibusawa, K.; Machida, K.; Sugawara, K. Exhaustive exercise and type-1/type-2 cytokine balance in special focus on interleukin-12 p40/p70. Exerc. Immunol. Rev. 2003, 9, 48–57. [Google Scholar]
- Sugama, K.; Suzuki, K.; Yoshitani, K.; Shiraishi, K.; Miura, S.; Yoshioka, H.; Mori, Y.; Kometani, T. Changes of thioredoxin, oxidative stress markers, inflammation and muscle/renal damage following intensive endurance exercise. Exerc. Immunol. Rev. 2015, 21, 130–142. [Google Scholar]
- Sugama, K.; Suzuki, K.; Yoshitani, K.; Shiraishi, K.; Kometani, T. Urinary excretion of cytokines versus their plasma levels after endurance exercise. Exerc. Immunol. Rev. 2013, 19, 29–48. [Google Scholar]
- Suzuki, K.; Nakaji, S.; Yamada, M.; Liu, Q.; Kurakake, S.; Okamura, N.; Kumae, T.; Umeda, T.; Sugawara, K. Impact of a competitive marathon race on systemic cytokine and neutrophil responses. Med. Sci. Sports Exerc. 2003, 35, 348–355. [Google Scholar] [CrossRef]
- Suzuki, K.; Shiraishi, K.; Yoshitani, K.; Sugama, K.; Kometani, T. The effect of a sports drink based on highly branched cyclic dextrin on cytokine responses to exhaustive endurance exercise. J. Sports Med. Phys. Fit. 2014, 54, 622–630. [Google Scholar]
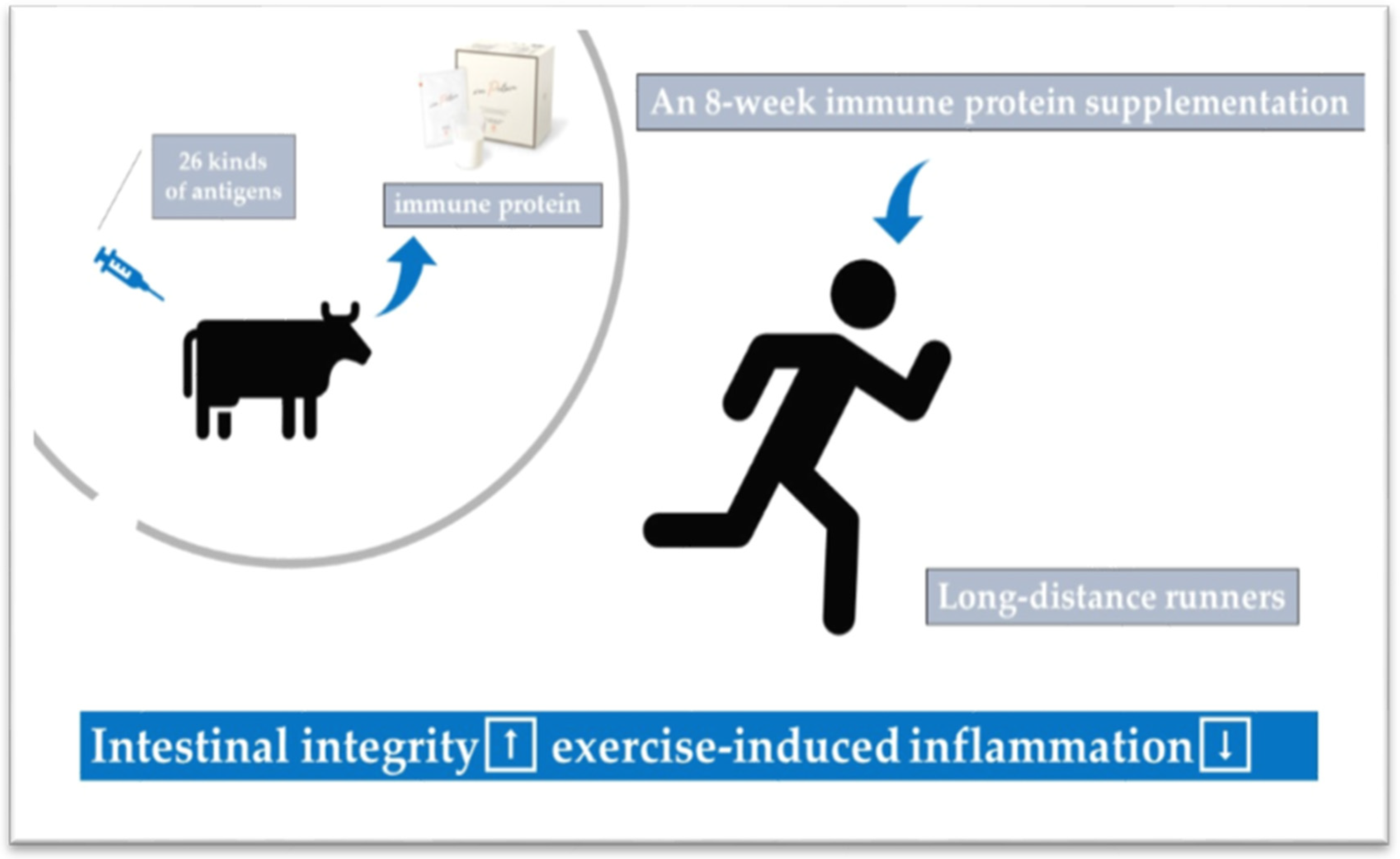
- Ma, S.; Tominaga, T.; Kanda, K.; Sugama, K.; Omae, C.; Hashimoto, S.; Aoyama, K.; Yoshikai, Y.; Suzuki, K. Effects of an 8-week protein supplementation regimen with hyperimmunized cow milk on exercise-induced organ damage and inflammation in male runners: A randomized, placebo controlled, cross-over study. Biomedicines 2020, 8, 51. [Google Scholar] [CrossRef]
- Suzuki, K.; Naganuma, S.; Totsuka, M.; Suzuki, K.J.; Mochizuki, M.; Shiraishi, M.; Nakaji, S.; Sugawara, K. Effects of exhaustive endurance exercise and its one-week daily repetition on neutrophil count and functional status in untrained men. Int. J. Sports Med. 1996, 17, 205–212. [Google Scholar] [CrossRef]
- Suzuki, K.; Yamada, M.; Kurakake, S.; Okamura, N.; Yamaya, K.; Liu, Q.; Kudoh, S.; Kowatari, K.; Nakaji, S.; Sugawara, K. Circulating cytokines and hormones with immunosuppressive but neutrophil-priming potentials rise after endurance exercise in humans. Eur. J. Appl. Physiol. 2000, 81, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Goh, J.M.; Lim, C.L.; Suzuki, K. Effects of endurance-, strength-, and concurrent training on cytokines and inflammation. In Concurrent Aerobic and Strength Training: Scientific Basics and Practical Applications; Schumann, M., Ronnestad, B., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 125–138. ISBN 978-3-319-75547-2. [Google Scholar]
- Suzuki, K.; Peake, J.; Nosaka, K.; Okutsu, M.; Abbiss, C.R.; Surriano, R.; Bishop, D.; Quod, M.J.; Lee, H.; Martin, D.T.; et al. Changes in markers of muscle damage, inflammation and HSP70 after an Ironman triathlon race. Eur. J. Appl. Physiol. 2006, 98, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Peake, J.; Della Gatta, P.; Suzuki, K.; Nieman, D. Cytokine expression and secretion by skeletal muscle cells: Regulatory mechanisms and exercise effects. Exerc. Immunol. Rev. 2015, 21, 8–25. [Google Scholar] [PubMed]
- Suzuki, K.; Totsuka, M.; Nakaji, S.; Yamada, M.; Kudoh, S.; Liu, Q.; Sugawara, K.; Yamaya, K.; Sato, K. Endurance exercise causes interaction among stress hormones, cytokines, neutrophil dynamics, and muscle damage. J. Appl. Physiol. 1999, 87, 1360–1367. [Google Scholar] [CrossRef]
- Kim, H.K.; Konishi, M.; Takahashi, M.; Tabata, H.; Endo, N.; Numao, S.; Lee, S.K.; Suzuki, K.; Kim, Y.H.; Sakamoto, S. Effects of acute endurance exercise performed in the morning and evening on inflammatory cytokine and metabolic hormone responses. PLoS ONE 2015, 10, e0137567. [Google Scholar] [CrossRef]
- Suzuki, K.; Sato, H.; Kikuchi, T.; Abe, T.; Nakaji, S.; Sugawara, K.; Totsuka, M.; Sato, K.; Yamaya, K. Capacity of circulating neutrophils to produce reactive oxygen species after exhaustive exercise. J. Appl. Physiol. 1996, 81, 1213–1222. [Google Scholar] [CrossRef]
- Nieman, D.C.; Davis, J.M.; Henson, D.A.; Walberg-Rankin, J.; Shute, M.; Dumke, C.L.; Utter, A.C.; Vinci, D.M.; Carson, J.A.; Brown, A.; et al. Carbohydrate ingestion influences skeletal muscle cytokine mRNA and plasma cytokine levels after a 3-h run. J. Appl. Physiol. 2003, 94, 1917–1925. [Google Scholar] [CrossRef]
- Nieman, D.C.; Davis, J.M.; Henson, D.A.; Gross, S.J.; Dumke, C.L.; Utter, A.C.; Vinci, D.M.; Carson, J.A.; Brown, A.; McAnulty, S.R.; et al. Muscle cytokine mRNA changes after 2.5 h of cycling: Influence of carbohydrate. Med. Sci. Sports Exerc. 2005, 37, 1283–1290. [Google Scholar] [CrossRef]
- Nieman, D.C.; Henson, D.A.; Davis, J.M.; Dumke, C.L.; Gross, S.J.; Jenkins, D.P.; Murphy, E.A.; Carmichael, M.D.; Quindry, J.C.; McAnulty, S.R.; et al. Quercetin ingestion does not alter cytokine changes in athletes competing in the Western States Endurance Run. J. Interf. Cytokine Res. 2007, 27, 1003–1011. [Google Scholar] [CrossRef]
- Suzuki, K. Exhaustive exercise-induced neutrophil-associated tissue damage and possibility of its prevention. J. Nanomed. Biother. Discov. 2017, 7, 156. [Google Scholar] [CrossRef]
- Peake, J.M.; Suzuki, K.; Wilson, G.; Hordern, M.; Nosaka, K.; Mackinnon, L.; Coombs, J. Exercise-induced muscle damage, plasma cytokines, and markers of neutrophil activation. Med. Sci. Sports Exerc. 2005, 37, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, H.; Shimura, M.; Sugama, K.; Kanda, K.; Suzuki, K. Exercise-induced inflammation during different phases of the menstrual cycle. Physiother. Rehabil. 2016, 1, 4. [Google Scholar] [CrossRef]
- Peake, J.; Peiffer, J.J.; Abbiss, C.R.; Nosaka, K.; Okutsu, M.; Laursen, P.B.; Suzuki, K. Body temperature and its effect on leukocyte mobilization, cytokines and markers of neutrophil activation during and after exercise. Eur. J. Appl. Physiol. 2008, 102, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Peake, J.; Peiffer, J.J.; Abbiss, C.R.; Nosaka, K.; Laursen, P.B.; Suzuki, K. Carbohydrate gel ingestion and immunoendocrine responses to cycling in temperate and hot conditions. Int. J. Sport Nutr. Exerc. Metab. 2008, 18, 229–246. [Google Scholar] [CrossRef]
- Yamada, M.; Suzuki, K.; Kudo, S.; Totsuka, M.; Nakaji, S.; Sugawara, K. Raised plasma G-CSF and IL-6 after exercise may play a role in neutrophil mobilization into the circulation. J. Appl. Physiol. 2002, 92, 1789–1794. [Google Scholar] [CrossRef]
- Peake, J.; Wilson, G.; Hordern, M.; Suzuki, K.; Yamaya, K.; Nosaka, K.; Mackinnon, L.; Coombes, J.S. Changes in neutrophil surface receptor expression, degranulation, and respiratory burst activity after moderate- and high-intensity exercise. J. Appl. Physiol. 2004, 97, 612–618. [Google Scholar] [CrossRef]
- Mezil, Y.A.; Allison, D.; Kish, K.; Ditor, D.; Ward, W.E.; Tsiani, E.; Klentrou, P. Response of bone turnover markers and cytokines to high-intensity low-impact exercise. Med. Sci. Sports Exerc. 2015, 47, 1495–1502. [Google Scholar] [CrossRef]
- Lira, F.S.; dos Santos, T.; Caldeira, R.S.; Inoue, D.S.; Panissa, V.L.G.; Cabral-Santos, C.; Campos, E.Z.; Rodrigues, B.; Monteiro, P.A. Short-term high- and moderate-intensity training modifies inflammatory and metabolic factors in response to acute exercise. Front. Physiol. 2017, 8, 856. [Google Scholar] [CrossRef]
- Brenner, I.K.; Natale, V.M.; Vasiliou, P.; Moldoveanu, A.I.; Shek, P.N.; Shephard, R.J. Impact of three different types of exercise on components of the inflammatory response. Eur. J. Appl. Physiol. Occup. Physiol. 1999, 80, 452–460. [Google Scholar] [CrossRef]
- Kanda, K.; Sugama, K.; Hayashida, H.; Sakuma, J.; Kawakami, Y.; Miura, S.; Yoshioka, H.; Mori, Y.; Suzuki, K. Eccentric exercise-induced delayed-onset muscle soreness and changes in markers of muscle damage and inflammation. Exerc. Immunol. Rev. 2013, 19, 74–87. [Google Scholar]
- Kanda, K.; Sugama, K.; Sakuma, J.; Kawakami, Y.; Suzuki, K. Evaluation of serum leaking enzymes and investigation into new biomarkers for exercise-induced muscle damage. Exerc. Immunol. Rev. 2014, 20, 39–54. [Google Scholar] [PubMed]
- Peake, J.M.; Roberts, L.A.; Figueiredo, V.C.; Egner, I.; Krog, S.; Aas, S.N.; Suzuki, K.; Markworth, J.F.; Coombes, J.S.; Cameron-Smith, D.; et al. The effects of cold water immersion and active recovery on inflammation and cell stress responses in human skeletal muscle after resistance exercise. J. Physiol. 2017, 595, 695–711. [Google Scholar] [CrossRef] [PubMed]
- Galvão, D.A.; Nosaka, K.; Taaffe, D.R.; Spry, N.; Kristjanson, L.J.; McGuigan, M.R.; Suzuki, K.; Yamaya, K.; Newton, R.U. Resistance training and reduction of treatment side effects in prostate cancer patients. Med. Sci. Sports Exerc. 2006, 38, 2045–2052. [Google Scholar] [CrossRef] [PubMed]
- Galvão, D.A.; Nosaka, K.; Taaffe, D.R.; Peake, J.; Spry, N.; Suzuki, K.; Yamaya, K.; McGuigan, M.R.; Kristjanson, L.J.; Newton, R.U. Endocrine and immune responses to resistance training in prostate cancer patients. Prostate Cancer Prostatic Dis. 2008, 11, 160–165. [Google Scholar] [CrossRef]
- Ross, M.L.; Halson, S.L.; Suzuki, K.; Garnham, A.; Hawley, J.A.; Cameron-Smith, D.; Peake, J.M. Cytokine responses to carbohydrate ingestion during recovery from exercise-induced muscle injury. J Interf. Cytokine Res. 2010, 30, 329–337. [Google Scholar] [CrossRef]
- Scott, J.P.R.; Sale, C.; Greeves, J.P.; Casey, A.; Dutton, J.; Fraser, W.D. Effect of exercise intensity on the cytokine response to an acute bout of running. Med. Sci. Sports Exerc. 2011, 43, 2297–2306. [Google Scholar] [CrossRef]
- Starkie, R.L.; Arkinstall, M.J.; Koukoulas, I.; Hawley, J.A.; Febbraio, M.A. Carbohydrate ingestion attenuates the increase in plasma interleukin-6, but not skeletal muscle interleukin-6 mRNA, during exercise in humans. J. Physiol. 2001, 533, 585–591. [Google Scholar] [CrossRef]
- Keller, C.; Keller, P.; Marshal, S.; Pedersen, B.K. IL-6 gene expression in human adipose tissue in response to exercise--effect of carbohydrate ingestion. J. Physiol. 2003, 550, 927–931. [Google Scholar] [CrossRef]
- Febbraio, M.A.; Steensberg, A.; Keller, C.; Starkie, R.L.; Nielsen, H.B.; Krustrup, P.; Ott, P.; Secher, N.H.; Pedersen, B.K. Glucose ingestion attenuates interleukin-6 release from contracting skeletal muscle in humans. J. Physiol. 2003, 549, 607–612. [Google Scholar] [CrossRef]
- Hashimoto, H.; Ishijima, T.; Hayashida, H.; Suzuki, K.; Higuchi, M. Menstrual cycle phase and carbohydrate ingestion alter immune response following endurance exercise and high intensity time trial performance test under hot conditions. J. Int. Soc. Sports Nutr. 2014, 11, 39. [Google Scholar] [CrossRef]
- Gudiksen, A.; Schwartz, C.L.; Bertholdt, L.; Joensen, E.; Knudsen, J.G.; Pilegaard, H. Lack of skeletal muscle IL-6 affects pyruvate dehydrogenase activity at rest and during prolonged exercise. PLoS ONE 2016, 11, e0156460. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Zwetsloot, K.A.; Lomiwes, D.D.; Meaney, M.P.; Hurst, R.D. Muscle glycogen depletion following 75-km of cycling is not linked to increased muscle IL-6, IL-8, and MCP-1 mRNA expression and protein content. Front. Physiol. 2016, 7, 431. [Google Scholar] [CrossRef] [PubMed]
- Mansour, S.G.; Verma, G.; Pata, R.W.; Martin, T.G.; Perazella, M.A.; Parikh, C.R. Kidney injury and repair biomarkers in marathon runners. Am. J. Dis. 2017, 70, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, N.; Mizokami, T.; Niihara, H.; Yada, K.; Suzuki, K. Macrophage depletion by clodronate liposome attenuates muscle injury and inflammation following exhaustive exercise. Biochem. Biophys. Rep. 2016, 5, 146–151. [Google Scholar] [CrossRef]
- Kawanishi, N.; Mizokami, T.; Niihara, H.; Yada, K.; Suzuki, K. Neutrophil depletion attenuates muscle injury after exhaustive exercise. Med. Sci. Sports Exerc. 2016, 48, 1917–1924. [Google Scholar] [CrossRef]
- Tsuchiya, M.; Sekiai, S.; Hatakeyama, H.; Koide, M.; Chaweewannakorn, C.; Yaoita, F.; Tan-No, K.; Sasaki, K.; Watanabe, M.; Sugawara, S.; et al. Neutrophils provide a favorable IL-1-mediated immunometabolic niche that primes GLUT4 translocation and performance in skeletal muscles. Cell Rep. 2018, 23, 2354–2364. [Google Scholar] [CrossRef] [PubMed]
- Steensberg, A.; Van Hall, G.; Osada, T.; Sacchetti, M.; Saltin, B.; Pedersen, B.K. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J. Physiol. 2000, 529, 237–242. [Google Scholar] [CrossRef]
- Hiscock, N.; Chan, M.H.S.; Bisucci, T.; Darby, I.A.; Febbraio, M.A. Skeletal myocytes are a source of interleukin-6 mRNA expression and protein release during contraction: Evidence of fiber type specificity. FASEB J. 2004, 18, 992–994. [Google Scholar] [CrossRef]
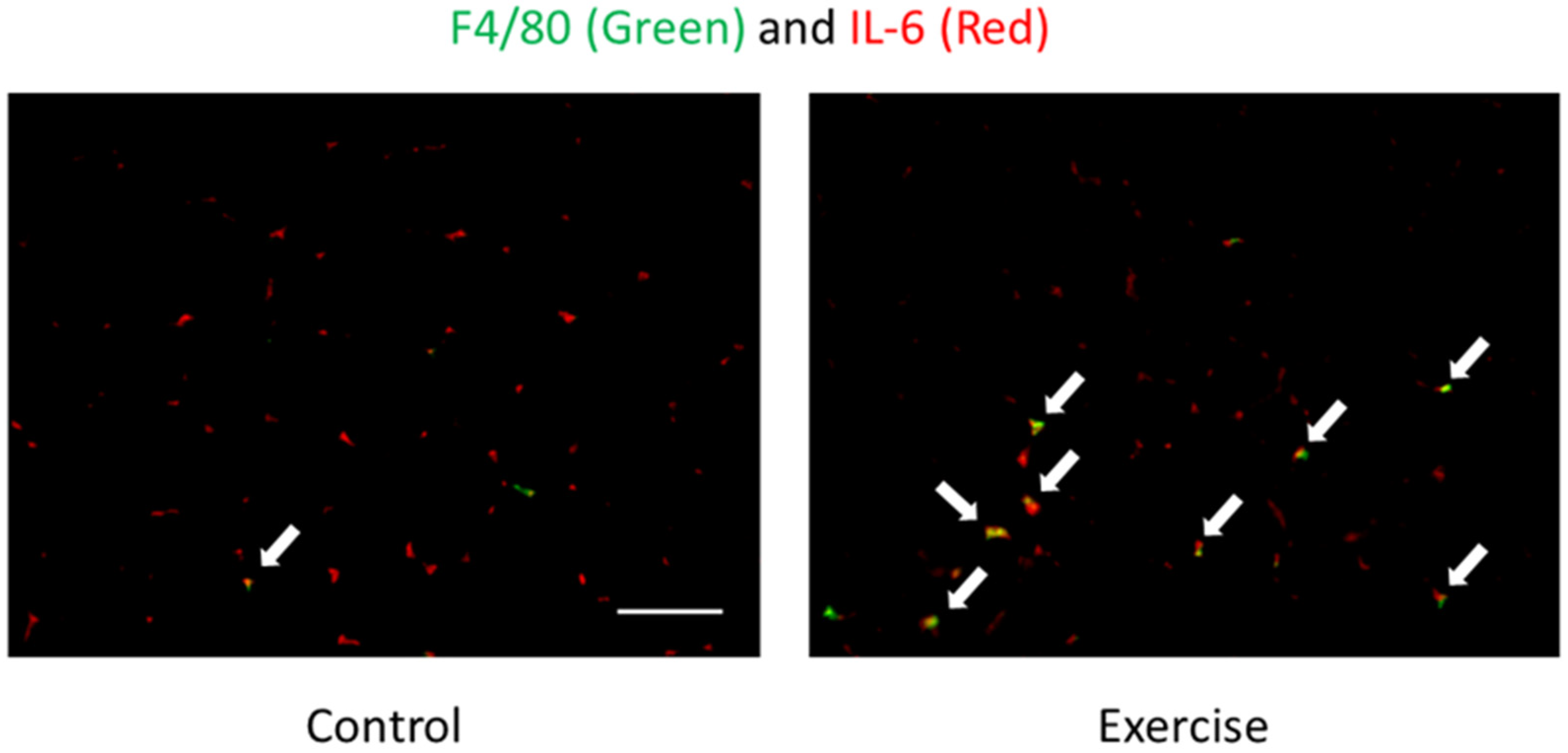
- Tominaga, T.; Ma, S.; Saitou, K.; Suzuki, K. Glucose ingestion inhibits endurance exercise-induced IL-6 producing macrophage infiltration in mice muscle. Nutrients 2019, 11, 1496. [Google Scholar] [CrossRef]
- Banzet, S.; Koulmann, N.; Simler, N.; Birot, O.; Sanchez, H.; Chapot, R.; Peinnequin, A.; Bigard, X. Fibre-type specificity of interleukin-6 gene transcription during muscle contraction in rat: Association with calcineurin activity. J. Physiol. 2005, 566, 839–847. [Google Scholar] [CrossRef]
- Ma, S.; Huang, Q.; Tominaga, T.; Liu, C.; Suzuki, K. An 8-week ketogenic diet alternated interleukin-6, ketolytic and lipolytic gene expression, and enhanced exercise capacity in mice. Nutrients 2018, 10, 1696. [Google Scholar] [CrossRef] [PubMed]
- Moreau, D.; Dubots, P.; Boggio, V.; Guilland, J.C.; Cometti, G. Effects of electromyostimulation and strength training on muscle soreness, muscle damage and sympathetic activation. J. Sports Sci. 1995, 13, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, T.; Machida, K.; Suzuki, K. Importance of correlations between phagocytic activity and superoxide production of neutrophils under conditions of voluntary exercise and stress. J. Clin. Lab. Anal. 1996, 10, 458–464. [Google Scholar] [CrossRef]
- Peake, J.M.; Suzuki, K.; Coombes, J.S. The influence of antioxidant supplementation on markers of inflammation and the relationship to oxidative stress after exercise. J. Nutr. Biochem. 2007, 18, 357–371. [Google Scholar] [CrossRef]
- Suzuki, K. Inflammatory response to exercise and its prevention. Curr. Top. Biochem. Res. 2018, 19, 37–42. [Google Scholar]
- Suzuki, K.; Ohno, S.; Suzuki, Y.; Ohno, Y.; Okuyama, R.; Aruga, A.; Yamamoto, M.; Ishihara, K.O.; Nozaki, T.; Miura, S.; et al. Effect of green tea extract on reactive oxygen species produced by neutrophils from cancer patients. Anticancer Res. 2012, 32, 2369–2375. [Google Scholar]
- Ohno, S.; Ohno, Y.; Suzuki, Y.; Miura, S.; Yoshioka, H.; Mori, Y.; Suzuki, K. Ingestion of Tabebuia avellanedae (Taheebo) inhibits production of reactive oxygen species from human peripheral blood neutrophils. Int. J. Food Sci. Nutr. Diet. 2015, S6, 1–4. [Google Scholar]
- Hung, Y.L.; Miyazaki, H.; Fang, S.H.; Li, C.; Suzuki, K. The structural characteristics of green tea polyphenols on lipopolysaccharide-stimulated RAW cells. J. Nutr. Biol. 2018, 2, 151–157. [Google Scholar] [CrossRef]
- Kawanishi, N.; Kato, K.; Takahashi, M.; Mizokami, T.; Otsuka, Y.; Imaizumi, A.; Shiva, D.; Yano, H.; Suzuki, K. Curcumin attenuates oxidative stress following downhill running-induced muscle damage. Biochem. Biophys. Res. Commun. 2013, 441, 573–578. [Google Scholar] [CrossRef]
- Takahashi, M.; Suzuki, K.; Kim, H.K.; Otsuka, Y.; Imaizumi, A.; Miyashita, M.; Sakamoto, S. Effects of curcumin supplementation on exercise-induced oxidative stress in humans. Int. J. Sports Med. 2013, 34, 1–7. [Google Scholar] [CrossRef]
- Li, C.Y.; Suzuki, K.; Hung, Y.L.; Yang, M.S.; Yu, C.P.; Lin, S.P.; Hou, Y.C.; Fang, S.H. Aloe metabolites prevent LPS-induced sepsis and inflammatory response by inhibiting mitogen-activated protein kinase activation. Am. J. Chin. Med. 2017, 45, 847–861. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.L.; Fang, S.H.; Wang, S.C.; Cheng, W.C.; Liu, P.L.; Su, C.C.; Chen, C.S.; Huang, M.Y.; Hua, K.F.; Shen, K.H.; et al. Corylin protects LPS-induced sepsis and attenuates LPS-induced inflammatory response. Sci. Rep. 2017, 7, 46299. [Google Scholar] [CrossRef]
- Ma, S.; Yada, K.; Lee, H.; Fukuda, Y.; Iida, A.; Suzuki, K. Taheebo polyphenols attenuate FFA-induced inflammation in murine and human macrophage cell lines as inhibitor of COX-2. Front. Nutr. 2017, 4, 63. [Google Scholar] [CrossRef] [PubMed]
- Ruhee, R.T.; Ma, S.; Suzuki, K. Sulforaphane protects cells against lipopolysaccharide-stimulated inflammation in murine macrophages. Antioxidants 2019, 8, 577. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, T.; Suzuki, K.; Takahashi, M.; Tomari, M.; Hara, R.; Gando, Y.; Muraoka, I. Involvement of neutrophil dynamics and function in exercise-induced muscle damage and delayed onset muscle soreness: Effect of hydrogen bath. Antioxidants 2018, 7, 127. [Google Scholar] [CrossRef]
- Hirata, N.; Ichimaru, R.; Tominari, T.; Matsumoto, C.; Watanabe, K.; Taniguchi, K.; Hirata, M.; Ma, S.; Suzuki, K.; Grundler, F.M.W.; et al. Beta-cryptoxanthin inhibits lipopolysaccharide-induced osteoclast differentiation and bone resorption via the suppression of inhibitor of NF-κB kinase activity. Nutrients 2019, 11, 368. [Google Scholar] [CrossRef] [PubMed]
- Yada, K.; Suzuki, K.; Oginome, N.; Ma, S.; Fukuda, Y.; Iida, A.; Radak, Z. Single dose administration of taheebo polyphenol enhances endurance capacity in mice. Sci. Rep. 2018, 8, 14625. [Google Scholar] [CrossRef] [PubMed]
- Ruhee, R.T.; Ma, S.; Suzuki, K. Protective effects of sulforaphane on exercise-induced organ damage via inducing antioxidant defense responses. Antioxidants 2020, 9, 136. [Google Scholar] [CrossRef]
- Petersen, A.M.W.; Pedersen, B.K. The Anti-inflammatory effect of exercise. J. Appl. Physiol. 2005, 98, 1154–1162. [Google Scholar] [CrossRef]
- Takahashi, M.; Miyashita, M.; Kawanishi, N.; Park, J.H.; Hayashida, H.; Kim, H.S.; Nakamura, Y.; Sakamoto, S.; Suzuki, K. Low-volume exercise training attenuates oxidative stress and neutrophils activation in older adults. Eur. J. Appl. Physiol. 2013, 113, 1117–1126. [Google Scholar] [CrossRef]
- Ogawa, K.; Sanada, K.; Machida, S.; Okutsu, M.; Suzuki, K. Resistance exercise training-induced muscle hypertrophy was associated with reduction of inflammatory markers in elderly women. Mediat. Inflamm. 2010, 2010, 171023. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, H.; Shimura, M.; Sugama, K.; Kanda, K.; Suzuki, K. Effects of the menstrual cycle and acute aerobic exercise on cytokine levels. J. Sports Med. Doping Stud. 2015, 6, 173. [Google Scholar] [CrossRef]
- van Wijck, K.; Lenaerts, K.; van Loon, L.J.; Peters, W.H.; Buurman, W.A.; Dejong, C.H. Exercise-induced splanchnic hypoperfusion results in gut dysfunction in healthy men. PLoS ONE 2011, 6, e22366. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Hashimoto, H.; Oh, T.; Ishijima, T.; Mitsuda, H.; Peake, J.M.; Sakamoto, S.; Muraoka, I.; Higuchi, M. The effects of sports drink osmolality on fluid intake and immunoendocrine responses to cycling in hot conditions. J. Nutr. Sci. Vitaminol. 2013, 59, 206–212. [Google Scholar] [CrossRef][Green Version]
- Tanisawa, K.; Suzuki, K.; Ma, S.; Kondo, S.; Okugawa, S.; Higuchi, M. Effects of ingestion of different amounts of carbohydrate after endurance exercise on circulating cytokines and markers of neutrophil activation. Antioxidants 2018, 7, 51. [Google Scholar] [CrossRef]
- Lim, C.L.; Pyne, D.B.; Horn, P.; Kalz, A.; Saunders, P.; Peake, J.; Suzuki, K.; Wilson, G.; Mackinnon, L.T. The effects of increased endurance training load on biomarkers of heat tolerance during intense exercise in the heat. Appl. Physiol. Nutr. Metab. 2009, 34, 616–624. [Google Scholar] [CrossRef]
- Lim, C.L.; Suzuki, K. Systemic inflammation mediates the effects of endotoxemia in the mechanisms of heat stroke. Biol. Med. 2017, 9, 1000376. [Google Scholar] [CrossRef]
- Leon, L.R.; Helwig, B.G. Heat stroke: Role of the systemic inflammatory response. J. Appl. Physiol. 2010, 109, 1980–1988. [Google Scholar] [CrossRef]
- Kawanishi, N.; Mizokami, T.; Yada, K.; Suzuki, K. Exercise training suppresses scavenger receptor CD36 expression in kupffer cells of nonalcoholic steatohepatitis model mice. Physiol. Rep. 2018, 6, e13902. [Google Scholar] [CrossRef]
- Kawanishi, N.; Niihara, H.; Mizokami, T.; Yada, K.; Suzuki, K. Exercise training attenuates neutrophil infiltration and elastase expression in adipose tissue of high-fat-diet-induced obese mice. Physiol. Rep. 2015, 3, e12534. [Google Scholar] [CrossRef]
- Kawanishi, N.; Niihara, H.; Mizokami, T.; Yano, H.; Suzuki, K. Exercise training attenuates adipose tissue fibrosis in diet-induced obese mice. Biochem. Biophys. Res. Commun. 2013, 440, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, N.; Mizokami, T.; Yano, H.; Suzuki, K. Exercise attenuates M1 macrophages and CD8+ T cells in the adipose tissue of obese mice. Med. Sci. Sports Exerc. 2013, 45, 1684–1693. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, N.; Yano, H.; Mizokami, T.; Takahashi, M.; Oyanagi, E.; Suzuki, K. Exercise training attenuates hepatic inflammation, fibrosis and macrophage infiltration during diet induced-obesity in mice. Brain Behav. Immun. 2012, 26, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, N.; Kato, Y.; Yokozeki, K.; Sawada, S.; Sakurai, R.; Fujiwara, Y.; Shinkai, S.; Goda, N.; Suzuki, K. Effects of aging on serum levels of lipid molecular species as determined by lipidomics analysis in Japanese men and women. Lipids Health Dis. 2018, 17, 135. [Google Scholar] [CrossRef]
- Sell, H.; Habich, C.; Eckel, J. Adaptive immunity in obesity and insulin resistance. Nat. Rev. Endocrinol. 2012, 8, 709–716. [Google Scholar] [CrossRef]
- Costa, R.J.S.; Snipe, R.M.J.; Kitic, C.M.; Gibson, P.R. Systematic review: Exercise-induced gastrointestinal syndrome—implications for health and intestinal disease. Aliment. Pharmacol. Ther. 2017, 46, 246–265. [Google Scholar] [CrossRef]
- Pires, W.; Veneroso, C.E.; Wanner, S.P.; Pacheco, D.A.S.; Vaz, G.C.; Amorim, F.T.; Tonoli, C.; Soares, D.D.; Coimbra, C.C. Association between exercise-induced hyperthermia and intestinal permeability: A systematic review. Sports Med. 2017, 47, 1389–1403. [Google Scholar] [CrossRef]
- van Wijck, K.; Lenaerts, K.; Grootjans, J.; Wijnands, K.A.; Poeze, M.; van Loon, L.J.; Dejong, C.H.; Buurman, W.A. Physiology and pathophysiology of splanchnic hypoperfusion and intestinal injury during exercise: Strategies for evaluation and prevention. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G155–G168. [Google Scholar] [CrossRef]
- Jaeschke, H. Mechanisms of liver injury. II. Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia-reperfusion and other acute inflammatory conditions. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G1083–G1088. [Google Scholar] [CrossRef]
- Sharfuddin, A.A.; Molitoris, B.A. Pathophysiology of ischemic acute kidney injury. Nat. Rev. Nephrol. 2011, 7, 189–200. [Google Scholar] [CrossRef]
- Luissint, A.-C.; Parkos, C.A.; Nusrat, A. Inflammation and the intestinal barrier: Leukocyte-epithelial cell interactions, cell junction remodeling, and mucosal repair. Gastroenterology 2016, 151, 616–632. [Google Scholar] [CrossRef]
- Wu, G.L.; Chen, Y.S.; Huang, X.D.; Zhang, L.X. Exhaustive swimming exercise related kidney injury in rats-protective effects of acetylbritannilactone. Int. J. Sports Med. 2012, 33, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Godínez-Victoria, M.; Drago-Serrano, M.E.; Reyna-Garfias, H.; Viloria, M.; Lara-Padilla, E.; Resendiz-Albor, A.A.; Sánchez-Torres, L.E.; Cruz-Hernández, T.R.; Campos-Rodriguez, R. Effects on secretory IgA levels in small intestine of mice that underwent moderate exercise training followed by a bout of strenuous swimming exercise. Brain Behav. Immun. 2012, 26, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.; Zhou, X.; Yu, L.; Yao, Y.; Zhang, Y.; Huang, Y.; Chen, M.; Yi, L.; Mi, M. Exhaustive exercise induces gastrointestinal syndrome through reduced ILC3 and IL-22 in mouse model. Med. Sci. Sports Exerc. 2020. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, M.H.; Davis, M.J.; Secher, N.H.; van Lieshout, J.J.; Arce-Esquivel, A.A.; Simmons, G.H.; Bender, S.B.; Padilla, J.; Bache, R.J.; Merkus, D.; et al. Peripheral circulation. Compr. Physiol. 2012, 2, 321–447. [Google Scholar] [CrossRef]
- Snipe, R.M.J.; Khoo, A.; Kitic, C.M.; Gibson, P.R.; Costa, R.J.S. The impact of exertional-heat stress on gastrointestinal integrity, gastrointestinal symptoms, systemic endotoxin and cytokine profile. Eur. J. Appl. Physiol. 2018, 118, 389–400. [Google Scholar] [CrossRef]
- Sureda, A.; Mestre-Alfaro, A.; Banquells, M.; Riera, J.; Drobnic, F.; Camps, J.; Joven, J.; Tur, J.A.; Pons, A. Exercise in a hot environment influences plasma anti-inflammatory and antioxidant status in well-trained athletes. J. Therm. Biol. 2015, 47, 91–98. [Google Scholar] [CrossRef]
- Steinberg, J.G.; Ba, A.; Bregeon, F.; Delliaux, S.; Jammes, Y. Cytokine and oxidative responses to maximal cycling exercise in sedentary subjects. Med. Sci. Sports Exerc. 2007, 39, 964–968. [Google Scholar] [CrossRef]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Nemes, R.; Koltai, E.; Taylor, A.W.; Suzuki, K.; Gyori, F.; Radak, Z. Reactive oxygen and nitrogen species regulate key metabolic, anabolic, and catabolic pathways in skeletal muscle. Antioxidants 2018, 7, 85. [Google Scholar] [CrossRef]
- Albensi, B.C. What is nuclear factor kappa B (NF-κB) doing in and to the mitochondrion? Front. Cell Dev. Biol. 2019, 7, 154. [Google Scholar] [CrossRef] [PubMed]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef]
- Jin, W.; Wang, H.; Yan, W.; Xu, L.; Wang, X.; Zhao, X.; Yang, X.; Chen, G.; Ji, Y. Disruption of Nrf2 enhances upregulation of nuclear factor-B activity, proinflammatory cytokines, and intercellular adhesion molecule-1 in the brain after traumatic brain injury. Mediat. Inflamm. 2008, 2008, 725174. [Google Scholar] [CrossRef]
- Mao, L.; Wang, H.; Qiao, L.; Wang, X. Disruption of Nrf2 enhances the upregulation of nuclear factor-kappaB activity, tumor necrosis factor, and matrix metalloproteinase-9 after spinal cord injury in mice. Mediat. Inflamm. 2010, 2010, 238321. [Google Scholar] [CrossRef] [PubMed]
- Bellezza, I.; Mierla, A.L.; Minelli, A. Nrf2 and NF-κB and their concerted modulation in cancer pathogenesis and progression. Cancers 2010, 2, 483–497. [Google Scholar] [CrossRef]
- Baeuerle, P.A.; Henkel, T. Function and activation of NF-kappaB in the immune system. Ann. Rev Immunol. 1994, 12, 141–179. [Google Scholar] [CrossRef]
- Ji, L.L.; Gomez-Cabrera, M.C.; Steinhafel, N.; Vina, J. Acute exercise activates nuclear factor (NF)-κB signaling pathway in rat skeletal muscle. FASEB J. 2004, 18, 1499–1506. [Google Scholar] [CrossRef]
- Bulua, A.C.; Simon, A.; Maddipati, R.; Pelletier, M.; Park, H.; Kim, K.Y.; Sack, M.N.; Kastner, D.L.; Siegel, R.M. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). J. Exp. Med. 2011, 208, 519–533. [Google Scholar] [CrossRef]
- Cleto, L.S.; Oleto, A.; Sousa, L.; Barreto, T.O.; Cruz, J.d.S.; Penaforte, C.L.; Magalhães, J.C.d.; Franco, J.d.S.; Pinto, K.M.d.C.; Azevedo, A.C.C. Plasma cytokine response, lipid peroxidation and NF-κB activation in skeletal muscle following maximum progressive swimming. Braz. J. Med. Biol. Res. 2011, 44, 546–552. [Google Scholar] [CrossRef][Green Version]
- Ma, S.; Suzuki, K. Potential application of ketogenic diet to metabolic status and exercise performance: A review. EC Nutr. 2018, 13, 496–499. [Google Scholar]
- Ma, S.; Suzuki, K. Keto-adaptation and endurance exercise capacity, fatigue recovery, and exercise-induced muscle and organ damage prevention. Sports 2019, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Huang, Q.; Yada, K.; Liu, C.; Suzuki, K. An 8-week ketogenic low carbohydrate, high fat diet enhanced exhaustive exercise capacity in mice. Nutrients 2018, 10, 673. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Komine, S.; Warabi, E.; Akiyama, K.; Ishii, A.; Ishige, K.; Mizokami, Y.; Kuga, K.; Horie, M.; Miwa, Y. Nuclear factor (erythroid derived 2)-like 2 activation increases exercise endurance capacity via redox modulation in skeletal muscles. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Malaguti, M.; Angeloni, C.; Garatachea, N.; Baldini, M.; Leoncini, E.; Collado, P.S.; Teti, G.; Falconi, M.; Gonzalez-Gallego, J.; Hrelia, S. Sulforaphane treatment protects skeletal muscle against damage induced by exhaustive exercise in rats. J. Appl. Physiol. 2009, 107, 1028–1036. [Google Scholar] [CrossRef]
- de Figueiredo, S.M.; Binda, N.S.; Nogueira-Machado, J.A.; Vieira-Filho, S.A.; Caligiorne, R.B. The antioxidant properties of organosulfur compounds (sulforaphane). Recent Pat. Endocr. Metab. Immune Drug Discov. 2015, 9, 24–39. [Google Scholar] [CrossRef]
- Jeffery, E.H.; Araya, M. Physiological effects of broccoli consumption. Phytochem. Rev. 2009, 8, 283–298. [Google Scholar] [CrossRef]
- Sun, C.-C.; Li, S.-J.; Yang, C.-L.; Xue, R.-L.; Xi, Y.-Y.; Wang, L.; Zhao, Q.-L.; Li, D.-J. Sulforaphane attenuates muscle inflammation in dystrophin-deficient Mdx mice via Nrf2-mediated inhibition of NF-κB signaling pathway. J. Biol. Chem. 2015, 290, 17784–17795. [Google Scholar] [CrossRef]
- Pal, S.; Konkimalla, V.B. Sulforaphane regulates phenotypic and functional switching of both induced and spontaneously differentiating human monocytes. Int. Immunopharmacol. 2016, 35, 85–98. [Google Scholar] [CrossRef]
- Shing, C.M.; Peake, J.; Suzuki, K.; Okutsu, M.; Pereira, R. Effects of bovine colostrum supplementation on immune variables in highly trained cyclists. J. Appl. Physiol. 2007, 102, 1113–1122. [Google Scholar] [CrossRef]
- Shing, C.M.; Peake, J.M.; Suzuki, K.; Jenkins, D.G.; Coombes, J.S. Bovine colostrum modulates cytokine production in human peripheral blood mononuclear cells stimulated with lipopolysaccharide and phytohemagglutinin. J. Interf. Cytokine Res. 2009, 29, 37–44. [Google Scholar] [CrossRef]
- Abraham, D.; Feher, J.; Scuderi, G.L.; Szabo, D.; Dobolyi, A.; Cservenak, M.; Juhasz, J.; Ligeti, B.; Pongor, S.; Gomez-Cabrera, M.C.; et al. Exercise and probiotics attenuate the development of Alzheimer’s disease in transgenic mice: Role of microbiome. Exp. Gerontol. 2019, 115, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Asadi, A.; Arazi, H.; Suzuki, K. Effects of β-hydroxy-β-methylbutyrate-free acid supplementation on strength, power and hormonal adaptations following resistance training. Nutrients 2017, 9, 1316. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Takahashi, M.; Li, C.Y.; Lin, S.P.; Tomari, M.; Shing, C.M.; Fang, S.H. The acute effects of green tea and carbohydrate co-ingestion on systemic inflammation and oxidative stress during sprint cycling. Appl. Physiol. Nutr. Metab. 2015, 40, 997–1003. [Google Scholar] [CrossRef]
- March, D.S.; Marchbank, T.; Playford, R.J.; Jones, A.W.; Thatcher, R.; Davison, G. Intestinal fatty acid-binding protein and gut permeability responses to exercise. Eur. J. Appl. Physiol. 2017, 117, 931–941. [Google Scholar] [CrossRef]
- Ogden, H.B.; Child, R.B.; Fallowfield, J.L.; Delves, S.K.; Westwood, C.S.; Layden, J.D. The gastrointestinal exertional heat stroke paradigm: Pathophysiology, assessment, severity, aetiology and nutritional countermeasures. Nutrients 2020, 12, 537. [Google Scholar] [CrossRef]
- Shing, C.M.; Ogawa, K.; Zhang, X.; Nagatomi, R.; Peake, J.M.; Suzuki, K.; Jenkins, D.G.; Coombes, J.S. Reduction in resting plasma granulysin as a marker of increased training load. Exerc. Immunol. Rev. 2007, 13, 89–99. [Google Scholar]
- Tsukamoto, K.; Suzuki, K.; Machida, K.; Saiki, C.; Murayama, R.; Sugita, M. Relationships between lifestyle factors and neutrophil functions in the elderly. J. Clin. Lab. Anal. 2002, 16, 266–272. [Google Scholar] [CrossRef]
- Ogawa, K.; Suzuki, K.; Okutsu, M.; Yamazaki, K.; Shinkai, S. The association of elevated reactive oxygen species levels from neutrophils with low-grade inflammation in the elderly. Immun. Ageing 2008, 5, 13. [Google Scholar] [CrossRef]
- Roberts, L.; Suzuki, K. Exercise and Inflammation. Antioxidants 2019, 8, 155. [Google Scholar] [CrossRef]
- Special Issue “Anti-Inflammatory and Antioxidant Effects of Dietary Supplementation and Lifestyle Factors”. Available online: https://www.mdpi.com/journal/antioxidants/special_issues/anti-inflammatory_antioxidant_effects (accessed on 25 March 2020).

| References | Subjects | Exercise Protocols | Exercise Duration | Time Points | Measured Substances | The Changes of Substances |
|---|---|---|---|---|---|---|
| Suzuki, et al. [13] | 10 male athletic students | Maximal exercise test by treadmill running | 10.2 ± 1.7 min | IM, Post 1 h, Post 2 h | TNF-α, IL-1β, IL-2, IL-12, IFN-ɤ, IL-1ra, IL-4, IL-10, IL-6, G-CSF, GM-CSF, M-CSF, IL-8, MCP-1 | IM: G-CSF, GM-CSF, M-CSF, MCP-1↑ Post 1 h: IL-1ra, IL-6, G-CSF, GM-CSF↑ Post 2 h: IL-1β, IL-4, G-CSF↑ |
| Sugama, et al. [14,17] | 14 male triathletes | Duathlon race (5-km running, 40-km cycling, 5-km running) | mean time; approx. 2 h | IM, Post 1.5 h, Post 3 h | TNF-α, IL-1β, IL-1ra, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, MCP-1, IL-17, IL-23, MPO, IL-12p40 | IM: IL-1ra, IL-6, IL-8, IL-10, IL-12p40, MCP-1, MPO↑ IL-17, IL-23↓ Post 1.5 h: IL-1β, IL-1ra, IL-6, IL-8, MCP-1, MPO↑ Post 3 h: IL-1ra,↑ |
| Suzuki, et al. [18] | 10 male runners | Full marathon race | mean time; 2.62 h (rang, 2.55–68 h) | IM | TNF-α, IL-1β, IL-6, IL-8, IL-10, G-CSF, M-CSF, GM-CSF, MCP-1 | IM: IL-6, IL-8, IL-10, G-CSF, M-CSF, MCP-1, MPO↑ |
| Suzuki, et al. [19] | 7 male triathletes | Duathlon race (5-km running, 40-km cycling, 5-km running) | mean time; approx. 2 h | IM | IL-6, IL-8, IL-10, IL-1ra, MCP-1 | IM: IL-6, IL-8, IL-10, MCP-1↑ |
| Suzuki, et al. [22] | 16 male runners | Full marathon race | mean time; 2 h 34 min (rang, 2 h 25 min-2 h 40 min) | IM | IL-1β, IL-1ra, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, TNF-α, IFN-α, IFN-ɤ, G-CSF, GM-CSF, TGF-β1 | IM: IL-1ra, IL-6, IL-8, IL-10, G-CSF↑ IL-4↓ |
| Suzuki, et al. [24] | 9 male triathletes | Ironman triathlon race (3.8-km swim, 180-km cycling, 42.2-km running) | mean time; 9 h 59 min | IM | IL-1ra, IL-6, IL-10, G-CSF, IL-12p40, IL-4, IL-1β | IM: IL-1ra, IL-6, IL-10, IL-12p40, G-CSF↑ Post 1 d: IL-1ra, IL-6, G-CSF↑ |
| Suzuki, et al. [26] | 8 male athletic students | Cycling with 90W power output | 90 mim | Ex 30 min, 60 min, IM, Post 1 h, 3 h, 12 h | IL-1β, IL-6, IL-8, TNF-α, IFN-ɤ | IM: IL-6↑ Post 3 h: IL-6 Post 12 h: IL-6↑ |
| Kim, et al. [27] | 14 males with no regular exercise training | 60% VO2max walking | 60 min | IM, Post 2 h | IL-6, TNF-α, IL-1β | IM: IL-6↑ |
| Nieman, et al. [29] | 12 male and 4 female marathon runners | Treadmill running | 3 h | IM | IL-6, IL-8, IL-10, IL-1ra | IM: IL-6, IL-8, IL-10, IL-1ra↑ |
| Nieman, et al. [30] | 15 trained male cyclists | 75% VO2max cycling | 2.5 h | IM, Post 12 h | IL-6, IL-8, IL-10, IL-1ra | IM: IL-6, IL-8, IL-10, IL-1ra↑ |
| Nieman, et al. [31] | 18 male and 3 female ultramarathon runners as the placebo group | 160-km Western States Endurance Run | 27.5 ± 0.6 h | IM | IL-6, IL-8, IL-10, IL-1ra, G-CSF, MCP-1, MIP-1β, TNF-α, MIF-1 | IM: IL-6, IL-8, IL-10, IL-1ra, G-CSF, MCP-1, MIP-1β, TNF-α, MIF-1↑ |
| Peake, et al. [33] | 10 well-trained male runners | Running at 60% VO2max. | 45 min | IM, Post 1 h, 24 h | IL-6, IL-8 | IM: IL-6↑ Post 1 h:IL-6↑ |
| Hayashida, et al. [34] | 10 healthy sedentary females | Cycling at 75% of their individual anaerobic threshold | 60 min | IM, Post 30 min | IL-6, Calprotectin, MPO | IM: IL-6↑ Post 30 min: Calprotectin, MPO↑ |
| Peake, et al. [35] | 10 well-trained male cyclists | Cycling at 60% VO2max + 16.1-km time trial | 90 min | IM, R1: Post 35-40 min; R2: Post 80-85 min | Calprotectin, G-CSF, MPO, TNF-α, IL-1ra, IL-6, IL-8, IL-10 | IM, R1 and R2: G-CSF, IL-8, Calprotectin, MPO, IL-10↑ |
| Peake, et al. [36] | 10 male cyclists | ① 18.1 +/− 0.4 degrees C, 58% +/− 8% relative humidity, 90 min at approximately 60% VO2max and then completed a 16.1-km time trial②32.2 +/− 0.7 degrees C, 55% +/− 2% relative humidity, 90 min at approximately 60% VO2max and then completed a 16.1-km time trial | Cycling for 90 min and a time trial | IM | IL-6, IL-8, IL-10, G-CSF, Calprotectin, MPO | ① and ②: IL-6, IL-8, IL-10, G-CSF, Calprotectin, MPO↑ |
| Yamada, et al. [37] | 12 male winter-sports athletes | A maximal exercise test on a treadmill (started at 220 m/min for the first 2 min and 220 m/min at a 4% grade for the next 2 min) | Mean running time: 10.3 ± 2.3 min | IM, Post 1 h, 2 h | G-CSF, IL-6 | IM: G-CSF↑Post 1 h: IL-6↑ |
| Mezil, et al. [39] | 23 males | High intensity interval exercise | Total 6 min | Post 5 min, 1 h, 24 h | IL-1α, IL-1β, IL-6, TNF-α | Post 5 min: IL-1α, IL-1β, IL-6, TNF-α↑ |
| Lira, et al. [40] | 10 active males | ① High intensity intermittent training ② Running at 70% maximal aerobic speed | Not described (total 5 km running) | IM, Post 1 h | IL-6, IL-10, TNF-α | Not changed |
| Brenner, et al. [41] | 8 males | ① All out cycling (equivalent to 90% VO2 max) ② Standard circuit-training routine ③ Cycling at 60–65% VO2 max | ① 5 min ② Not described ③ 2 h | IM, Post 3 h, 24 h, 72 h | IL-6, TNF-α, IL-10 | ① Post 3 h: IL-10↓ Post 24 h: IL-10↓ Post 72 h: IL-10↓ ② Not changed ③ IM: IL-6↑, Post 3 h: IL-6, TNF-α↑ Post 24 h: TNF-α↑ Post 72 h: TNF-α↑ |
| Kanda, et al. [42,43] | 9 healthy males | 10 sets of 40 repetitions of exercise at 0.5 Hz by the load corresponding to the half of body weight | Not described | Post 2 h, 4 h, 24 h, 48 h, 72 h, 96 h | TNF-α, IL-1β, IL-1ra, IL-2, IL-4, IL-6, IL-8,IL-10, IL-12, MCP-1, IL-17, IL-23,MPO, IL-12p40, IL-12 p70, IFN-γ, MCP-1, G-CSF, Calprotectin, C5a | Not changed |
| Scott, et al. [48] | 10 active males | ① Running at 55% VO2 max ② Running at 65% VO2 max ③ Running at 75% VO2 max | 60 min | Ex 20 min, Ex 40 min, IM, Post 0.5 h, 1 h, 2 h, Post 3 h, 1 d, 2 d, 3 d | TNF-α, IL-6, IL-1ra | ① Ex 40 min~Post 3 h: IL-6↑ ② Ex 40 min~Post 3 h: IL-6↑ Ex 40 min~Post: 1 h IL-1ra↑ ③ Ex 40 min~Post 3 h: IL-6↑ Ex 20 min~Post: 3 h IL-1ra↑ |
| Nieman, et al. [54] | 20 male cyclists | 75 km cycling time trial | 168 ± 26.0 min | IM | IL-6, IL-8, MCP-1 | IM: IL-6, IL-8, MCP-1↑ |
| van Wijck, et al. [85] | 20 males | Cycling at 70% maximal workload | 60 min | IM | MPO, Calprotectin | IM: MPO, Calprotectin↑ |
| Suzuki, et al. [86] | 6 well-trained male cyclists | Cycling at 60% VO2max | 90 min | IM, Post 30 min | IL-1ra, MCP-1, IL-6, Calprotectin, MPO, IL-8, IL-10, IL-12p40 | IM: IL-6↑; Post 30 min: Calprotectin↑ |
| Tanisawa, et al. [87] | 9 healthy males | Cycling at 70% VO2max | 60 min | IM, Post 30 min, 1 h, 2 h | IL-6, G-CSF, MCP-1, IL-8, C5a, MPO, Calprotectin, Elastase | IM: IL-6, IL-8, MPO, Calprotectin, Elastase↑ Post 30 min: IL-6, G-CSF, MCP-1, IL-8, Calprotectin↑ Post 1 h: IL-6, G-CSF, Calprotecin↑ Post 2 h: IL-6, Calprotectin↑ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, K.; Tominaga, T.; Ruhee, R.T.; Ma, S. Characterization and Modulation of Systemic Inflammatory Response to Exhaustive Exercise in Relation to Oxidative Stress. Antioxidants 2020, 9, 401. https://doi.org/10.3390/antiox9050401
Suzuki K, Tominaga T, Ruhee RT, Ma S. Characterization and Modulation of Systemic Inflammatory Response to Exhaustive Exercise in Relation to Oxidative Stress. Antioxidants. 2020; 9(5):401. https://doi.org/10.3390/antiox9050401
Chicago/Turabian StyleSuzuki, Katsuhiko, Takaki Tominaga, Ruheea Taskin Ruhee, and Sihui Ma. 2020. "Characterization and Modulation of Systemic Inflammatory Response to Exhaustive Exercise in Relation to Oxidative Stress" Antioxidants 9, no. 5: 401. https://doi.org/10.3390/antiox9050401
APA StyleSuzuki, K., Tominaga, T., Ruhee, R. T., & Ma, S. (2020). Characterization and Modulation of Systemic Inflammatory Response to Exhaustive Exercise in Relation to Oxidative Stress. Antioxidants, 9(5), 401. https://doi.org/10.3390/antiox9050401








