Effect of Antioxidants on High-Temperature Stability of Renewable Bio-Oils Revealed by an Innovative Method for the Determination of Kinetic Parameters of Oxidative Reactions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Oil Characterization
2.3. Oxidative Stability Index
2.4. Measure of Oxygen Consumption
3. Results
3.1. Analysis of the O2 Consumption Plots
3.2. Inhibition Period (Type B oils)
3.3. Steady Oxygen Consumption (Rst) in Type A–C Oils
3.4. Standard Addition of Propyl Gallate
3.5. Initial Fast O2 Consumption (Type C Oils)
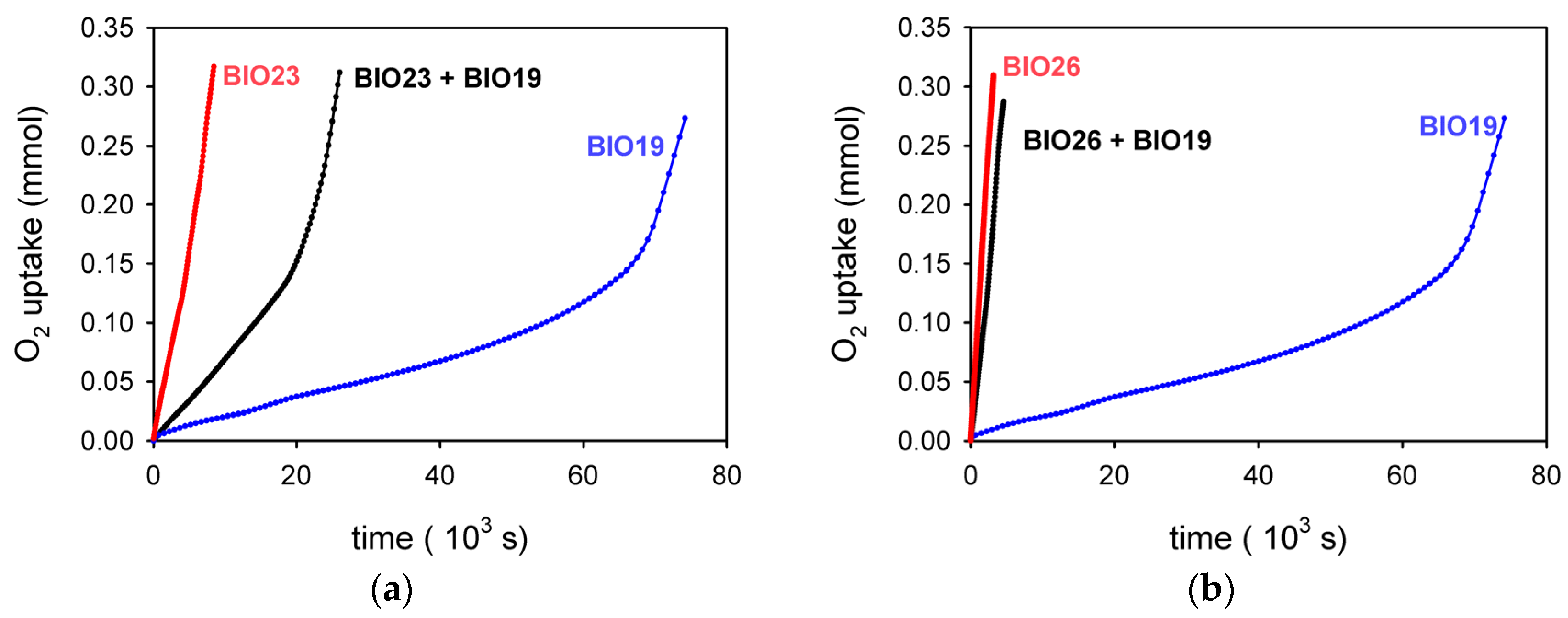
3.6. Oil Mixtures
4. Discussion
- Equation (11) describes the oxygen consumption in the steady state regime (Rst), after the conclusion of the inhibition period or in case the oil does not contain antioxidants. In these conditions, the rate of oil oxidation depends on the concentration of the oxidizable groups, the facility of oxidation of fatty acids (i.e., on kp/√2kt), and Ri. As we found linear O2 consumption, we deduced that in our system the initiation rate (Ri) is constant. The effect of oil acidity may influence kp, kt or both.
- The presence of an inhibition period is clear experimental evidence of the presence of a naturally occurring antioxidant in the oil. It finishes when the antioxidant is consumed. The inhibition period is characterized by its duration and by the slope (−d[O2]/dt) that is associated to the effectiveness of the antioxidant in the inhibition of the O2 consumption. The slope and duration of oxygen consumption during the inhibition period are described by Equations (12) and (13). From Equation (12), −d[O2]/dt = Rin depends on the concentration of the oxidizable groups, the nature of the oil (kp), the antioxidant (n and kinh) and the rate of initiation (Ri).
- The duration of the inhibited period (Equation (13)) depends not only on the type and concentration of antioxidants present in the oil but also it is inversely proportional to the rate of initiation Ri. For this reason, the addition of a known fixed quantity of a specific antioxidant provides an estimate of the Ri value of the oil, as we did with the addition of propyl gallate (PG). As the n value for PG at 130 °C is not known, we could obtain only the Ri / n ratio. Interestingly, when excluding oils with high acidity, iron content, and a few other outliers in which PG exerted little or no inhibiting activity, there is a good inverse correlation between τPG-τ and the concentration of bis-allylic groups in the oils with y-intercept close to zero. Therefore, it can be inferred that Ri is directly proportional to the concentration of bis-allylic groups. This result confirms the hypothesis that the initiation of oil oxidation at 130 °C is mainly due to the direct reaction of activated C-H bonds with O2 [11].
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Singh, D.; Sharma, D.; Soni, S.L.; Sharma, S.; Sharma, P.K.; Jhalani, A. A review on feedstocks, production processes, and yield for different generations of biodiesel. Fuel 2020, 262, 116553. [Google Scholar] [CrossRef]
- Liu, S.; Simonetti, T.; Zheng, W.; Saha, B. Selective hydrodeoxygenation of vegetable oils and waste cooking oils to green diesel using a silica-supported Ir–ReOx bimetallic catalyst. Chem. Sus. Chem. 2018, 11, 1446–1454. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Luo, X.; Jin, Y.; Li, J.; Zhang, H.; Zhang, A.; Xie, J. Heterogeneous sulfur-free hydrodeoxygenation catalysts for selectively upgrading the renewable bio-oils to second generation biofuels. Renew. Sustain. Energy Rev. 2018, 82, 3762–3797. [Google Scholar] [CrossRef]
- Xin, J.; Imahara, H.; Saka, S. Kinetics on the oxidation of biodiesel stabilized with antioxidant. Fuel 2009, 88, 282–286. [Google Scholar] [CrossRef]
- Farhoosh, R.; Nyström, L. Antioxidant potency of gallic acid, methyl gallate and their combinations in sunflower oil triacylglycerols at high temperature. Food Chem. 2018, 244, 29–35. [Google Scholar] [CrossRef]
- Tavakoli, J.; Hashemi, S.M.B.; Khaneghah, A.M.; Barba, F.J.; Amorati, R.; Kenari, R.E.; Amarowicz, R. Improving the frying performance and oxidative stability of refined soybean oil by tocotrienol-rich unsaponifiable matters of kolkhoung (Pistacia khinjuk) hull oil. J. Am. Oil Chem. Soc. 2018, 95, 619–628. [Google Scholar] [CrossRef]
- Sazzad, B.S.; Fazal, M.A.; Haseeb, A.S.M.A.; Masjuki, H.H. Retardation of oxidation and material degradation in biodiesel. RSC Adv. 2016, 6, 60244–60263. [Google Scholar] [CrossRef]
- Gertz, C.; Kochhar, S.P. A new method to determine oxidative stability of vegetable fats and oils at simulated frying temperature. OCL 2001, 8, 82–88. [Google Scholar] [CrossRef][Green Version]
- McCormick, R.L.; Ratcliff, M.; Moens, L.; Lawrence, R. Several factors affecting the stability of biodiesel in standard accelerated tests. Fuel Process. Technol. 2007, 88, 651–657. [Google Scholar] [CrossRef]
- Denisov, E.T.; Khudyakov, I.V. Mechanisms of action and reactivities of the free radicals of inhibitors. Chem. Rev. 1987, 87, 1313–1357. [Google Scholar] [CrossRef]
- Jensen, R.K.; Korcek, S.; Zinbo, M.; Johnson, M.D. Initiation in hydrocarbon autoxidation at elevated temperatures. Int. J. Chem. Kinet. 1990, 22, 1095–1107. [Google Scholar] [CrossRef]
- Harrison, K.A.; Haidasz, E.A.; Griesser, M.; Pratt, D.A. Inhibition of hydrocarbon autoxidation by nitroxide-catalyzed cross-dismutation of hydroperoxyl and alkylperoxyl radicals. Chem. Sci. 2018, 9, 6068–6079. [Google Scholar] [CrossRef] [PubMed]
- Cedrowski, J.; Litwinienko, G.; Baschieri, A.; Amorati, R. Hydroperoxyl Radicals (HOO•): Vitamin E regeneration and H-bonde Effects on the hydrogen atom transfer. Chem. Eur. J. 2016, 22, 16441–16445. [Google Scholar] [CrossRef] [PubMed]
- Korcek, S.; Chenier, J.H.B.; Howard, J.A.; Ingold, K.U. Absolute rate constants for hydrocarbon autoxidation. XXI. Activation energies for propagation and the correlation of propagation rate constants with carbon–hydrogen bond strengths. Can. J. Chem. 1972, 50, 2285–2297. [Google Scholar] [CrossRef]
- Burton, G.W.; Ingold, K.U. Autoxidation of biological molecules. 1. Antioxidant activity of vitamin E and related chain-breaking phenolic antioxidants in vitro. J. Am. Chem. Soc. 1981, 103, 6472–6477. [Google Scholar] [CrossRef]
- Baschieri, A.; Pizzol, R.; Guo, Y.; Amorati, R.; Valgimigli, L. Calibration of Squalene, p-Cymene, and Sunflower Oil as Standard Oxidizable Substrates for Quantitative Antioxidant Testing. J. Agric. Food Chem. 2019, 67, 6902–6910. [Google Scholar] [CrossRef]
- Dunn, R.O. Oxidative Stability of Soybean Oil Fatty Acid Methyl Esters by Oil Stability Index (OSI). J. Am. Oil Chem. Soc. 2005, 82, 381–387. [Google Scholar] [CrossRef]
- Zahira, Y.; Binitha, N.; Silija, P.; Surya, U.K.; Mohammed, A.P. A Review on the Oxidation Stability of Biodiesel. Renew. Sustain. Energy Rev. 2014, 35, 136–153. [Google Scholar]
- Redondo-Cuevasa, L.; Castellano, G.; Torrens, F.; Raikos, V. Revealing the relationship between vegetable oil composition and oxidative stability: A multifactorial approach. J. Food Compos. Anal. 2018, 66, 221–229. [Google Scholar] [CrossRef]
- Yang, J.; He, Q.; Corscadden, K.; Caldwell, C. Improvement on oxidation and storage stability of biodiesel derived from an emerging feedstock Camelina sativa. Fuel Process. Technol. 2017, 157, 90–98. [Google Scholar] [CrossRef]
- Supriyono, S.; Sulistyo, H.; Almeida, M.F.; Dias, J.M. Influence of synthetic antioxidants on the oxidation stability of biodiesel produced from acid raw Jatropha curcas oil. Fuel Process. Technol. 2015, 132, 133–138. [Google Scholar] [CrossRef]
- Nieva-Echevarría, B.; Goicoechea, E.; Manzanos, M.J.; Guillén, M.D. A method based on 1H NMR spectral data useful to evaluate the hydrolysis level in complex lipid mixtures. Food Res. Int. 2014, 66, 379–387. [Google Scholar] [CrossRef]
- UOP 389-15. Trace Metals in Organics by ICP OES; UOP: A Honeywell Company: Des Plaines, IL, USA, 2015. [Google Scholar]
- Knothe, G. Analyzing biodiesel: Standards and other methods. J. Am. Oil Chem. Soc. 2006, 83, 823–833. [Google Scholar] [CrossRef]
- Mittelbach, M.; Gangl, S. Long storage stability of biodiesel made from rapeseed and used frying oil. J. Am. Oil Chem. Soc. 2001, 78, 573–577. [Google Scholar] [CrossRef]
- I.S. EN 14112. Fat and Oil Derivatives-Fatty Acid Methyl Esters (FAME) Determination of Oxidation Stability; NSAI: Dublin, Ireland, 2003. [Google Scholar]
- Pullen, J.; Saeed, K. Experimental study of the factors affecting the oxidation stability of biodiesel FAME fuels. Fuel Process. Technol. 2014, 125, 223–235. [Google Scholar] [CrossRef]
- Larson, R.A.; Marley, K.A. Optimized Antioxidants for Biodiesel; Illinois Sustainable Technology Center: Champaign, IL, USA, 2011. [Google Scholar]
- Wang, J.; Lu, Y.; Zheng, T.; Sang, S.; Lv, L. Scavenging of acrolein by food-grade antioxidant propyl gallate in a model reaction system and cakes. J. Agric. Food Chem. 2019, 67, 8520–8526. [Google Scholar] [CrossRef]
- Varatharajan, K.; Pushparani, D.S. Screening of antioxidant additives for biodiesel fuels. Renew. Sustain. Energy Rev. 2018, 82, 2017–2028. [Google Scholar] [CrossRef]
- Da Silva, J.C.M.; Nicolau, C.L.; Cabral, M.R.P.; Costa, E.R.; Stropa, J.M.; Silva, C.A.A.; Scharf, D.; Simionatto, E.L.; Fiorucci, A.R.; de Oliveira, L.C.S.; et al. Thermal and oxidative stabilities of binary blends of esters from soybean oil and non-edible oils (Aleurites moluccanus, Terminalia catappa, and Scheelea phalerata). Fuel 2020, 262, 116644. [Google Scholar] [CrossRef]
- Moser, B.R. Influence of blending canola, palm, soybean, and sunflower oil methyl esters on fuel properties of biodiesel. Energy Fuels 2008, 22, 4301–4306. [Google Scholar] [CrossRef]
- Torres, M.; Lloret, C.; Sosa, M.; Maestri, D. Composition and oxidative stability of soybean oil in mixtures with jojoba oil. Eur. J. Lipid Sci. Technol. 2006, 108, 513–520. [Google Scholar] [CrossRef]
- Ingold, K.U.; Pratt, D.A. Advances in Radical-Trapping Antioxidant Chemistry in the 21st Century: A Kinetics and Mechanisms Perspective. Chem. Rev. 2014, 114, 9022. [Google Scholar] [CrossRef] [PubMed]
- Haidasz, E.A.; Meng, D.; Amorati, R.; Baschieri, A.; Ingold, K.U.; Valgimigli, L.; Pratt, D.A. Acid Is Key to the Radical-Trapping Antioxidant Activity of Nitroxides. J. Am. Chem. Soc. 2016, 138, 5290. [Google Scholar] [CrossRef] [PubMed]
- Baschieri, A.; Amorati, R.; Benelli, T.; Mazzocchetti, L.; D’Angelo, E.; Valgimigli, L. Enhanced Antioxidant Activity under Biomimetic Settings of Ascorbic Acid Included in Halloysite Nanotubes. Antioxidants 2019, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Konopko, A.; Kusio, J.; Litwinienko, G. Antioxidant Activity of Metal Nanoparticles Coated with Tocopherol-Like Residues—The Importance of Studies in Homo-and Heterogeneous Systems. Antioxidants 2020, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Félix, R.; Valentão, P.; Andrade, P.B.; Félix, C.; Novais, S.C.; Lemos, M.F.L. Evaluating the In Vitro Potential of Natural Extracts to Protect Lipids from Oxidative Damage. Antioxidants 2020, 9, 231. [Google Scholar] [CrossRef] [PubMed]


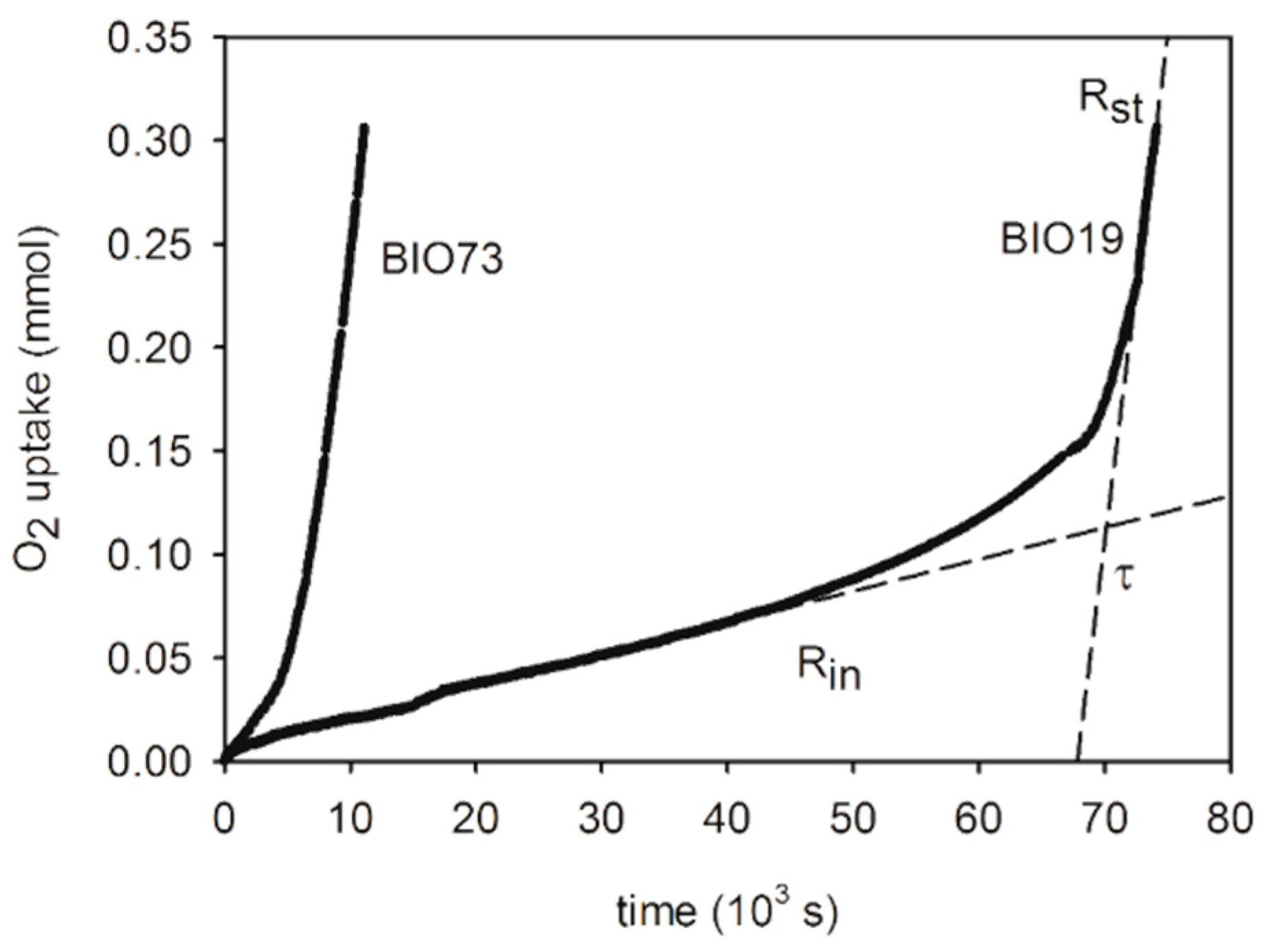
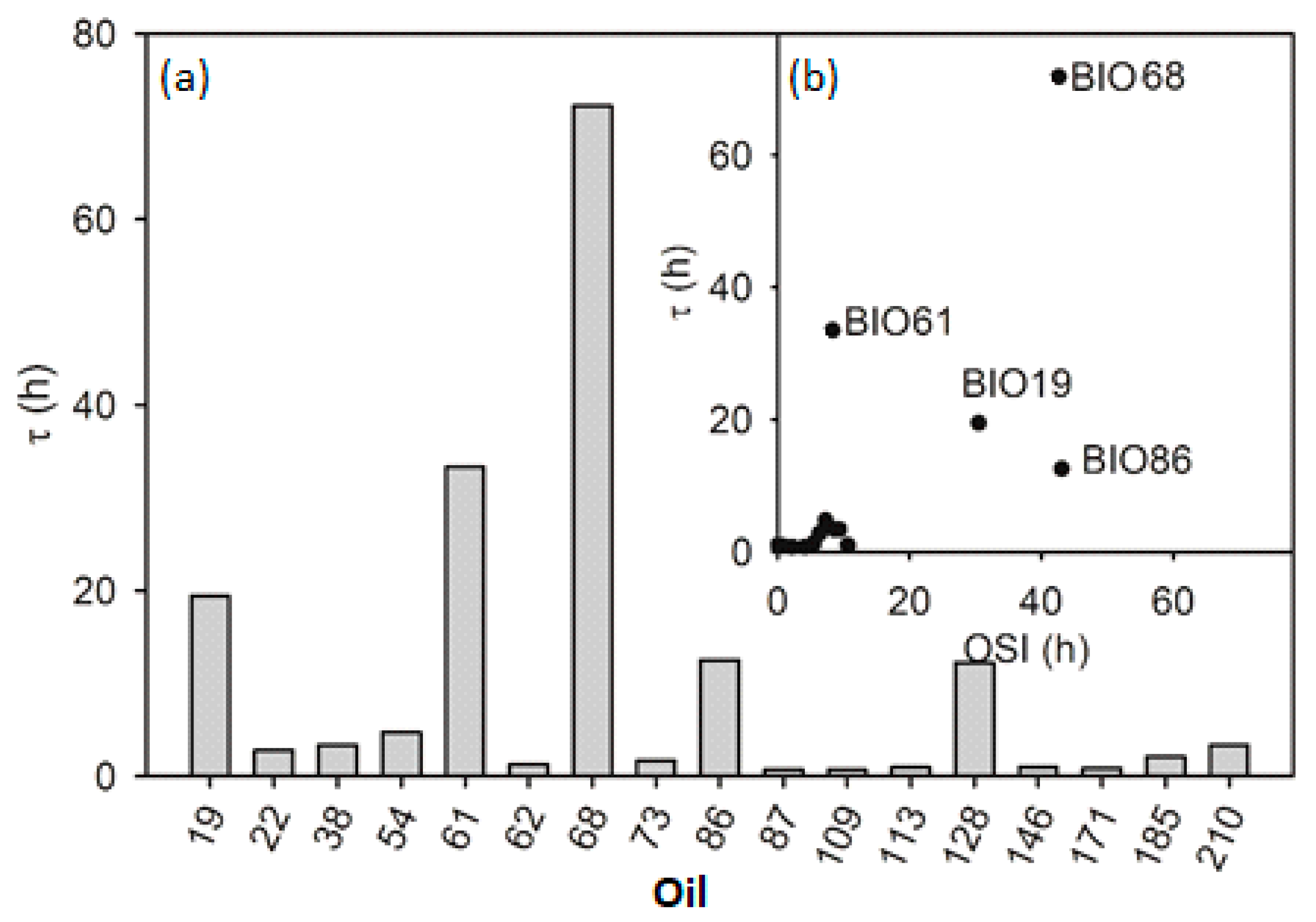
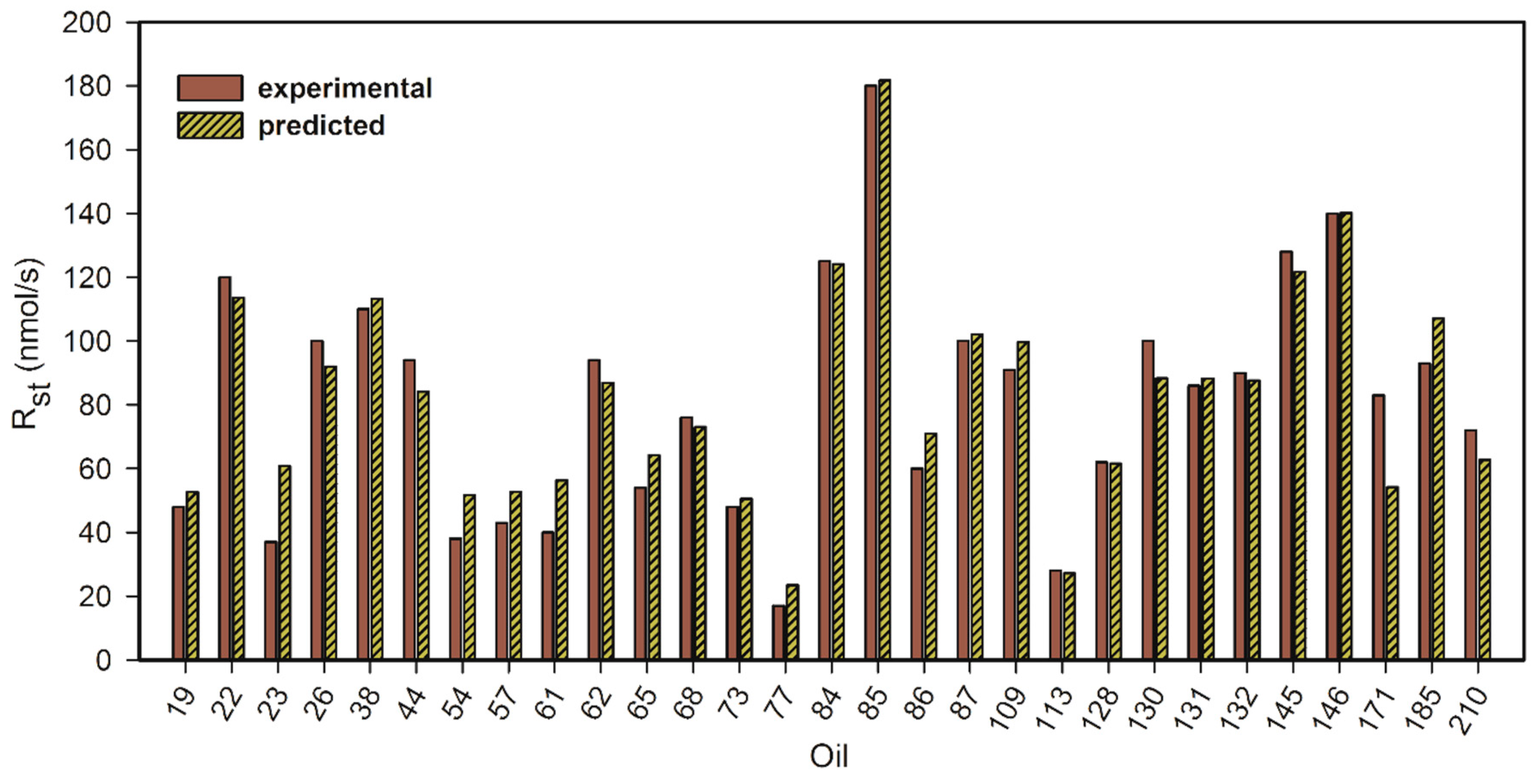
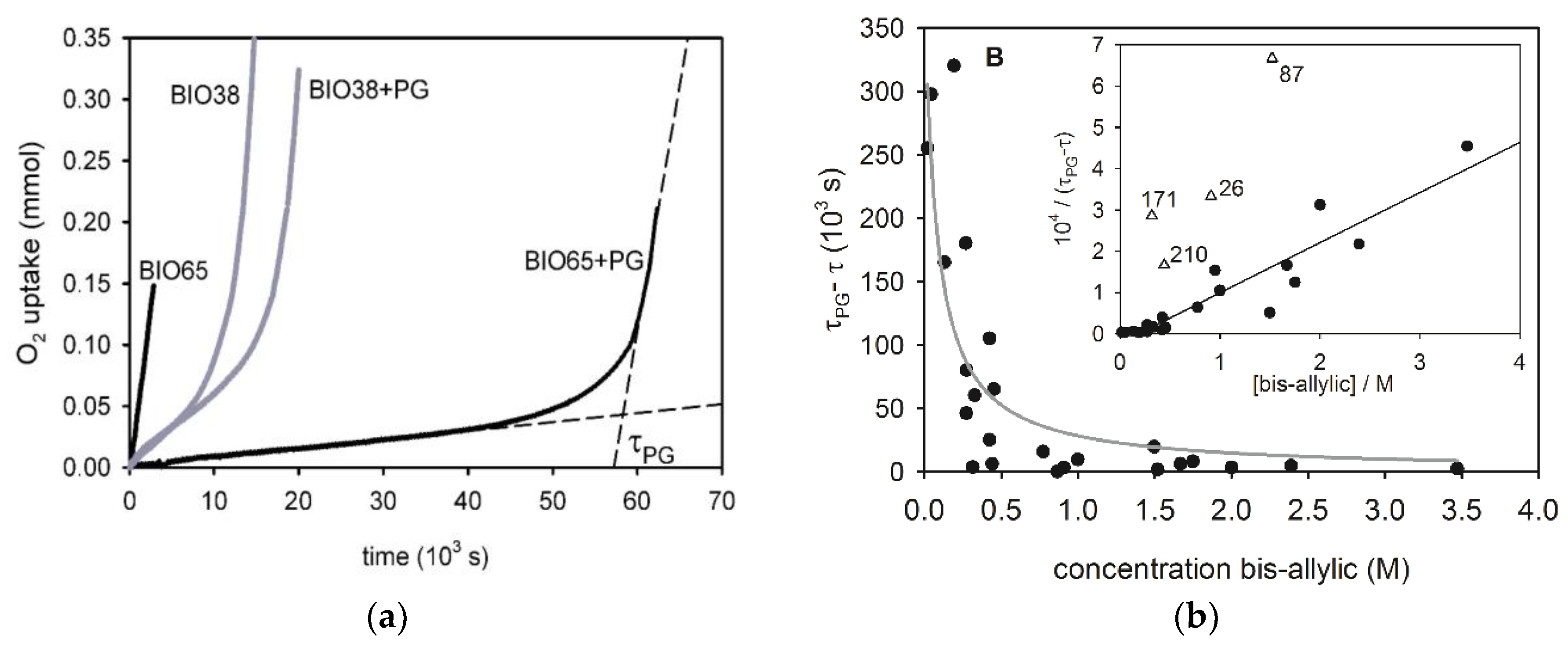
| Oil | Description [a] | Rin nmol/s | τ 103 s | Rst nmol/s | τ-τPG 103 s | Fe ppm | H+ mg KOH/g | OSI [b] h | SA [c] | MO [c] | DI [c] (TRI) [c] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Type A | |||||||||||
| BIO26 | Refined Used Cooking Oil (T) | 0 | <1 | 100 | 3.0 | 3 | 8.55 | 1.00 | 19.05 | 52.72 | 27.10 (1.13) |
| BIO44 | Used Cooking Oil (T) | 0 | <1 | 94 | 15.5 | 4.3 | 8.62 | 0 | 26.30 | 50.60 | 21.20 (1.90) |
| BIO57 | Oil Distillation pitch (T) | 0 | <1 | 38 | 0 | 1472 | 33.79 | 0.70 | 60.05 | 35.86 | 0.68 (3.41) |
| BIO65 | Vegetable oil fraction (T) | 0 | <1 | 54 | 60 | 3.7 | 31.07 | 0.83 | 42.59 | 46.82 | 10.59 (0) |
| BIO84 | Safflawer oil (T) | 0 | <1 | 120 | 3.2 | <0.5 | 0.96 | 2.01 | 18.30 | 17.16 | 64.54 (0) |
| BIO85 | Linseed oil (T) | 0 | <1 | 180 | 2.2 | <0.5 | 1.20 | 0.10 | 14.00 | 22.00 | 16.00 (48) |
| BIO130 | Tall oil (1) (A) [d] | 0 | <1 | 100 | 0 | 31.3 | 159.63 | 2.77 | 52.40 | 27.80 | 16.90 (2.90) |
| BIO131 | Tall oil (2) (A) [d] | 0 | <1 | 86 | 0 | 67.9 | 155.14 | 0.10 | 51.10 | 28.90 | 17.40 (2.60) |
| BIO132 | Tall oil (3) (A) [d] | 0 | <1 | 90 | 0 | 35.01 | 155.43 | 0.10 | 52.20 | 28.00 | 17.10 (2.70) |
| BIO145 | Tall oil fatty acid (A) | 0 | <1 | 128 | 0 | 0.6 | 193.27 | 0.10 | 30.60 | 30.94 | 36.10 (2.36) |
| Type B | |||||||||||
| BIO19 | Palm oil (T) | 1,5 | 70 | 48 | 80 | 5.4 | 8.48 | 30.50 | 55.06 | 36.22 | 8.56 (0.16) |
| BIO22 | Soybean oil (T) | 8.9 | 10.0 | 120 | 8 | 0.5 | 2.24 | 6.38 | 25.17 | 21.76 | 49.70 (3.36) |
| BIO23 | Fractioned Seed rape oil (M,D,T) | 36 | 5.5 | 49 | 65 | 9.1 | 1.39 | 0.10 | 48.56 | 37.49 | 13.24 (0.70) |
| BIO38 | Corn oil (T) | 6.0 | 12.0 | 110 | 6.0 | <0.5 | 0.11 | 9.40 | 19.65 | 27.20 | 52.50 (0.65) |
| BIO54 | Animal fat (T) | 2.2 | 17.0 | 38 | 320 | 1.1 | 8.62 | 7.28 | 51.63 | 42.63 | 5.22 (0.52) |
| BIO61 | Empty fruit bunch (T) | 0.88 | 120 | 40 | 180 | 7.8 | 33.76 | 9.12 | 54.32 | 37.12 | 8.44 (0.12) |
| BIO62 | Carinata oil (T) | 11 | 4,5 | 94 | 9,5 | 0.8 | 0.10 | 5.53 | 33.00 | 40.50 | 20.75 (5.75) |
| BIO68 | Castor oil (D,T) | 0.28 | 260 | 76 | 165 | 2.1 | 0.32 | 44.69 | 8.17 | 87.58 | 4.25 (0) |
| BIO73 | RBD Palm oil (T) | 8.25 | 6 | 48 | 46 | <0.5 | 0.21 | na | 56.8 | 34.4 | 8.8 (0) |
| BIO86 | Jojoba Oil | 0.24 | 45 | 60 | 255 | <0.5 | 0.47 | 43.06 | 5.81 | 93.55 | 0.64 (0) |
| BIO87 | Cotton Oil (T) | 21 | 2.5 | 100 | 1.5 | <0.5 | 0.10 | 4.18 | 31.70 | 19.25 | 49.05 (0) |
| BIO109 | Canapa Oil (T) | 18.0 | 2.5 | 90 | 19.5 | 2.4 | 27.61 | 2.20 | 44.20 | 14.10 | 34.90 (6.70) |
| BIO113 | Palm kernel oil (T) | 5.4 | 3.5 | 27 | 297.5 | 1 | 3.94 | 10.68 | 87.59 | 10.93 | 1.47 (0) |
| BIO128 | Animal fat (ME) | 23.0 | 44 | 60 | 25 | <0.5 | 3.12 | na | 40.2 | 46.40 | 11.80 (1.70) |
| BIO146 | Tobacco oil (T) | 23 | 3.2 | 140 | 4.6 | 1.36 | 7.72 | 0.10 | 12.72 | 11.57 | 74.34 (1.36) |
| BIO171 | Fatty acids (ME) | 36 | 2.5 | 85 | 3.5 | 4.21 | 4.96 | 0.80 | 53.70 | 36.10 | 10.20 (0) |
| BIO185 | Palm oil mill effluent (A) | 11 | 7.5 | 93 | 6.5 | 126 | 132.02 | 0.95 | 23.89 | 45.46 | 30.65 (0) |
| BIO210 | Fatty acids (ME) | 8.3 | 12 | 72 | 0.6 | 0.8 | 0.18 | 8.69 | 43.9 | 41.8 | 14.3 (0) |
| TYPE C | |||||||||||
| BIO77 | C10SE1 (BE) [e] | 43 | 1.0 | 17 | 0 | 3.7 | 4.57 | 0.20 | 92.00 | 8.00 | 0 (0) |
| BIO69 | C10SE2 (BE) [e] | 54 | 2.5 | 18 | 0 | 14.3 | 4.57 | 0.20 | 92.00 | 8.00 | 0 (0) |
| Oil or Mixture | τ (103 s) | τPG-τ (103 s) |
|---|---|---|
| BIO19 | 70 | 80 |
| BIO23 | 5.5 | 65 |
| BIO26 | 0 | 3.0 |
| BIO44 | 0 | 16 |
| BIO86 | 45 | 255 |
| BIO19 + BIO23 | 23 | - |
| BIO19 + BIO26 | 0 | - |
| BIO19 + BIO44 | 4.3 | - |
| BIO86 + BIO23 | 23 | - |
| BIO86 + BIO44 | 3.8 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mollica, F.; Lucarini, M.; Passerini, C.; Carati, C.; Pavoni, S.; Bonoldi, L.; Amorati, R. Effect of Antioxidants on High-Temperature Stability of Renewable Bio-Oils Revealed by an Innovative Method for the Determination of Kinetic Parameters of Oxidative Reactions. Antioxidants 2020, 9, 399. https://doi.org/10.3390/antiox9050399
Mollica F, Lucarini M, Passerini C, Carati C, Pavoni S, Bonoldi L, Amorati R. Effect of Antioxidants on High-Temperature Stability of Renewable Bio-Oils Revealed by an Innovative Method for the Determination of Kinetic Parameters of Oxidative Reactions. Antioxidants. 2020; 9(5):399. https://doi.org/10.3390/antiox9050399
Chicago/Turabian StyleMollica, Fabio, Marco Lucarini, Cinzia Passerini, Claudio Carati, Silvia Pavoni, Lucia Bonoldi, and Riccardo Amorati. 2020. "Effect of Antioxidants on High-Temperature Stability of Renewable Bio-Oils Revealed by an Innovative Method for the Determination of Kinetic Parameters of Oxidative Reactions" Antioxidants 9, no. 5: 399. https://doi.org/10.3390/antiox9050399
APA StyleMollica, F., Lucarini, M., Passerini, C., Carati, C., Pavoni, S., Bonoldi, L., & Amorati, R. (2020). Effect of Antioxidants on High-Temperature Stability of Renewable Bio-Oils Revealed by an Innovative Method for the Determination of Kinetic Parameters of Oxidative Reactions. Antioxidants, 9(5), 399. https://doi.org/10.3390/antiox9050399







