Correlating Volatile Lipid Oxidation Compounds with Consumer Sensory Data in Dairy Based Powders during Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Powder Samples
2.2. Compound Selection
2.3. Powder Composition
2.4. Microbial Analysis
2.5. Milk Powder Colour Measurements
2.6. Fatty Acid Analysis
2.7. Volatile Analysis
2.7.1. HS-SPME Conditions
2.7.2. GC Conditions
2.8. Sensory Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. General Powder Characteristics
3.2. Milk Powder Colour Analysis
3.3. Fatty Acid Analysis
3.4. HS-SPME-GCMS Volatile Analysis
3.4.1. FFWMP
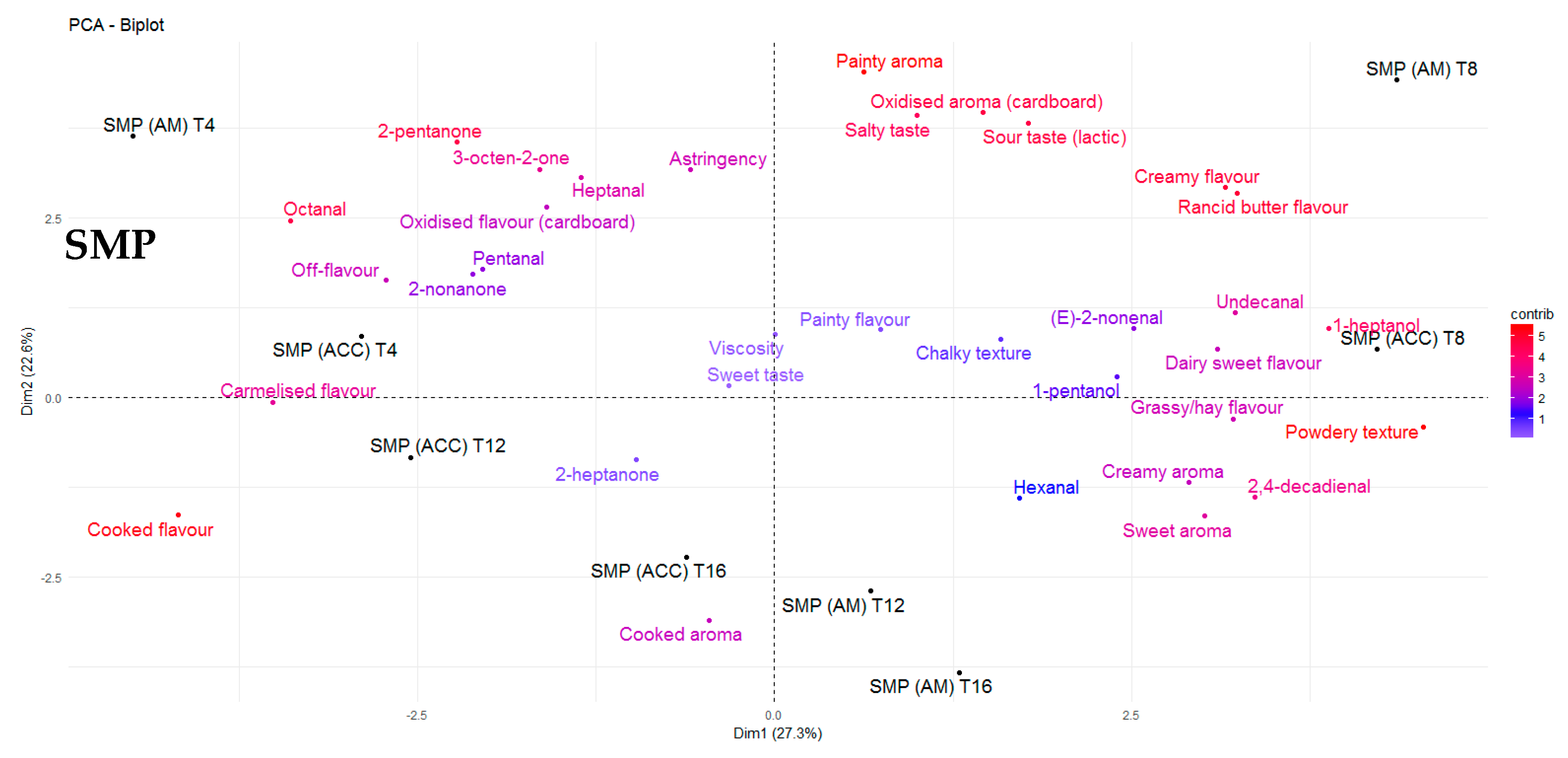
3.4.2. SMP
3.4.3. IMF
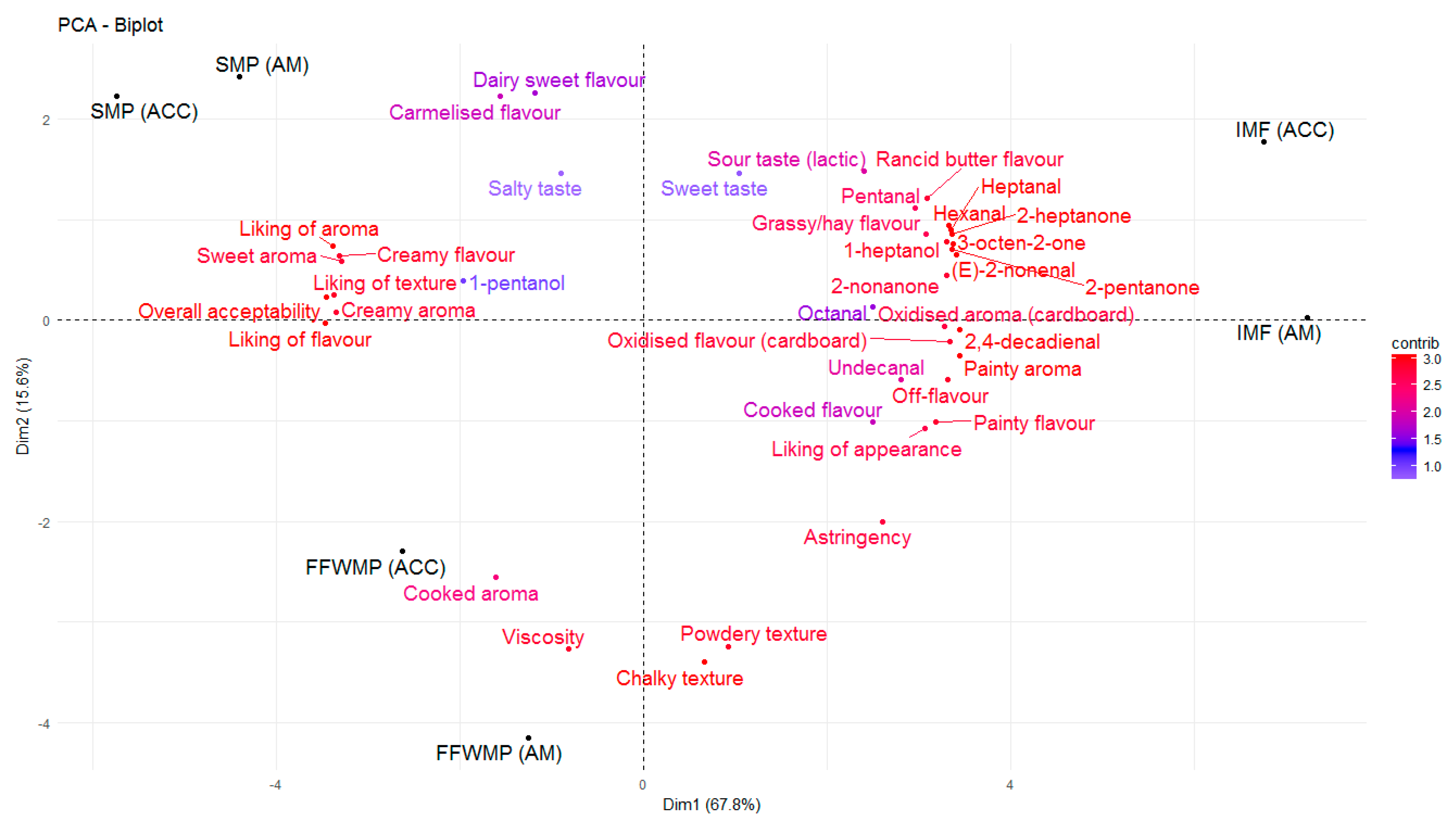
3.5. Sensory Evaluation
3.6. Range of Lipid Oxidation in Six Retail Infant Milk Formula Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Park, C.; Drake, M. The distribution of fat in dried dairy particles determines flavor release and flavor stability. J. Food Sci. 2014, 79, R452–R459. [Google Scholar] [CrossRef] [PubMed]
- Frankel, E.N. Lipid Oxidation; Woodhead Publishing: Cambridge, UK, 2014; p. 299. [Google Scholar]
- Aprea, E.; Romanzin, A.; Corazzin, M.; Favotto, S.; Betta, E.; Gasperi, F.; Bovolenta, S. Effects of grazing cow diet on volatile compounds as well as physicochemical and sensory characteristics of 12-month-ripened Montasio cheese. J. Dairy Sci. 2016, 99, 6180–6190. [Google Scholar] [CrossRef] [PubMed]
- Croissant, A.E.; Washburn, S.; Dean, L.; Drake, M. Chemical properties and consumer perception of fluid milk from conventional and pasture-based production systems. J. Dairy Sci. 2007, 90, 4942–4953. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Husny, J.; Rabe, S. Predicting fishiness off-flavour and identifying compounds of lipid oxidation in dairy powders by SPME-GC/MS and machine learning. Int. Dairy J. 2018, 77, 19–28. [Google Scholar] [CrossRef]
- Lloyd, M.; Drake, M.; Gerard, P. Flavor Variability and Flavor Stability of US-Produced Whole Milk Powder. J. Food Sci. 2009, 74, S334–S343. [Google Scholar] [CrossRef] [PubMed]
- Stapelfeldt, H.; Nielsen, B.R.; Skibsted, L.H. Effect of heat treatment, water activity and storage temperature on the oxidative stability of whole milk powder. Int. Dairy J. 1997, 7, 331–339. [Google Scholar] [CrossRef]
- Thomsen, M.K.; Lauridsen, L.; Skibsted, L.H.; Risbo, J. Temperature effect on lactose crystallization, Maillard reactions, and lipid oxidation in whole milk powder. J. Agric. Food Chem. 2005, 53, 7082–7090. [Google Scholar] [CrossRef]
- Fitzpatrick, J.; Iqbal, T.; Delaney, C.; Twomey, T.; Keogh, M. Effect of powder properties and storage conditions on the flowability of milk powders with different fat contents. J. Food Eng. 2004, 64, 435–444. [Google Scholar] [CrossRef]
- Ford, J.; Hurrell, R.; Finot, P. Storage of milk powders under adverse conditions: 2. Influence on the content of water-soluble vitamins. Br. J. Nutr. 1983, 49, 355–364. [Google Scholar] [CrossRef]
- Angulo, A.; Romera, J.; Ramirez, M.; Gil, A. Effects of storage conditions on lipid oxidation in infant formulas based on several protein sources. J. Am. Oil. Chem. Soc. 1998, 75, 1603–1607. [Google Scholar] [CrossRef]
- Cesa, S.; Casadei, M.; Cerreto, F.; Paolicelli, P. Infant milk formulas: Effect of storage conditions on the stability of powdered products towards autoxidation. Foods 2015, 4, 487–500. [Google Scholar] [CrossRef]
- Jia, H.-x.; Chen, W.-L.; Qi, X.-Y.; Su, M.-Y. The stability of milk-based infant formulas during accelerated storage. CyTA-J. Food 2019, 17, 96–104. [Google Scholar] [CrossRef]
- Lopez, C.; Cauty, C.; Guyomarc’h, F. Organization of lipids in milks, infant milk formulas and various dairy products: role of technological processes and potential impacts. Dairy Sci. Technol. 2015, 95, 863–893. [Google Scholar] [CrossRef]
- MacLean Jr, W.C.; Van Dael, P.; Clemens, R.; Davies, J.; Underwood, E.; O’Risky, L.; Rooney, D.; Schrijver, J. Upper levels of nutrients in infant formulas: comparison of analytical data with the revised Codex infant formula standard. J. Food Compos. Anal. 2010, 23, 44–53. [Google Scholar] [CrossRef]
- Tvrzicka, E.; Kremmyda, L.-S.; Stankova, B.; Zak, A. Fatty acids as biocompounds: their role in human metabolism, health and disease-a review. Part 1: classification, dietary sources and biological functions. Biomed. Pap. Med. Fac. Univ. 2011, 155, 117–130. [Google Scholar] [CrossRef]
- Kilcawley, K.N.; Faulkner, H.; Clarke, H.J.; O’Sullivan, M.G.; Kerry, J.P. Factors Influencing the Flavour of Bovine Milk and Cheese from Grass Based versus Non-Grass Based Milk Production Systems. Foods 2018, 7, 37. [Google Scholar] [CrossRef]
- Faulkner, H.; O’Callaghan, T.F.; McAuliffe, S.; Hennessy, D.; Stanton, C.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. Effect of different forage types on the volatile and sensory properties of bovine milk. J. Dairy Sci. 2018, 101, 1034–1047. [Google Scholar] [CrossRef]
- Chizoti, T.S.; Cruz, M.F.; Benassi, M.T.; Brugnaro, C.; de Medeiros, S.D.S. Sensory Analysis of Chocolate Milk for College Students. J. Obes. Overweig. 2018, 4. [Google Scholar] [CrossRef]
- Clarke, H.J.; Mannion, D.T.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. Development of a headspace solid-phase microextraction gas chromatography mass spectrometry method for the quantification of volatiles associated with lipid oxidation in whole milk powder using response surface methodology. Food Chem. 2019, 292, 75–80. [Google Scholar] [CrossRef]
- Van Aardt, M.; Duncan, S.; Marcy, J.; Long, T.; O’keefe, S.; Nielsen-Sims, S. Aroma analysis of light-exposed milk stored with and without natural and synthetic antioxidants. J. Dairy Sci. 2005, 88, 881–890. [Google Scholar] [CrossRef]
- De Jong, C.; Badings, H.T. Determination of free fatty acids in milk and cheese procedures for extraction, clean up, and capillary gas chromatographic analysis. J. High Resolut. Chromatogr. 1990, 13, 94–98. [Google Scholar] [CrossRef]
- O’Callaghan, T.F.; Mannion, D.; Apopei, D.; McCarthy, N.A.; Hogan, S.A.; Kilcawley, K.N.; Egan, M. Influence of Supplemental Feed Choice for Pasture-Based Cows on the Fatty Acid and Volatile Profile of Milk. Foods 2019, 8, 137. [Google Scholar] [CrossRef] [PubMed]
- International Dairy Federation (IDF). Standard 99C: Sensory Evaluation of Dairy Products by Scoring Reference Methodology; International Dairy Federation, Ed.; International Dairy Federation: Brussels, Belgium, 1997. [Google Scholar]
- R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.R-project.org/ (accessed on 20 April 2020).
- European Commission. Overview of Microbiological Criteria for Foodstuffs in Community Legislation in Force. 2001. Available online: http://europa.eu.int/comm/food/fs/sfp/mr/mr_crit_en.pdf (accessed on 20 April 2020).
- Pugliese, A.; Cabassi, G.; Chiavaro, E.; Paciulli, M.; Carini, E.; Mucchetti, G. Physical characterization of whole and skim dried milk powders. J. Food Sci. Technol. 2017, 54, 3433–3442. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.; Verdier-Metz, I.; Buchin, S.; Hurtaud, C.; Coulon, J.-B. How do the nature of forages and pasture diversity influence the sensory quality of dairy livestock products? Animal Sci. 2005, 81, 205–212. [Google Scholar] [CrossRef]
- Helmenstine, A.M. Why Milk is White. Available online: https://www.thoughtco.com/why-milk-is-white-606172 (accessed on 6 September 2019).
- Walstra, P.; Wouters, J.T.; Geurts, T.J. Dairy Science and Technology; CRC Press: Boca Raton, FL, USA, 2005; p. 169. [Google Scholar]
- Lock, A.L.; Bauman, D.E. Modifying milk fat composition of dairy cows to enhance fatty acids beneficial to human health. Lipids 2004, 39, 1197–1206. [Google Scholar] [CrossRef]
- Parodi, P.W. Milk fat in human nutrition. Aust. J. Dairy Technol. 2004, 59, 3. [Google Scholar]
- O‘Callaghan, T.; Hennessy, D.; McAuliffe, S.; Kilcawley, K.; O’Donovan, M.; Dillon, P.; Ross, R.; Stanton, C. Effect of pasture versus indoor feeding systems on raw milk composition and quality over an entire lactation. J. Dairy Sci. 2016, 99, 9424–9440. [Google Scholar] [CrossRef]
- Tripathi, M. Effect of nutrition on production, composition, fatty acids and nutraceutical properties of milk. J. Adv. Dairy Res. 2014, 2, 115–125. [Google Scholar]
- German, J.B.; Dillard, C.J. Composition, structure and absorption of milk lipids: a source of energy, fat-soluble nutrients and bioactive molecules. Crit Rev Food Sci Nutr. 2006, 46, 57–92. [Google Scholar] [CrossRef]
- Durmuş, M. Fish oil for human health: omega-3 fatty acid profiles of marine seafood species. J. Food Sci. Technol. 2018, 39, 1–8. [Google Scholar] [CrossRef]
- Aung, W.P.; Bjertness, E.; Htet, A.S.; Stigum, H.; Chongsuvivatwong, V.; Soe, P.P.; Kjøllesdal, M.K.R. Fatty Acid Profiles of Various Vegetable Oils and the Association between the Use of Palm Oil vs. Peanut Oil and Risk Factors for Non-Communicable Diseases in Yangon Region, Myanmar. Nutrients 2018, 10, 1193. [Google Scholar] [CrossRef]
- Castro, T.; Martinez, D.; Isabel, B.; Cabezas, A.; Jimeno, V. Vegetable Oils Rich in Polyunsaturated Fatty Acids Supplementation of Dairy Cows’ Diets: Effects on Productive and Reproductive Performance. Animals 2019, 9, 205. [Google Scholar] [CrossRef]
- Lee, J.H.; Min, D.B. Changes of headspace volatiles in milk with riboflavin photosensitization. J. Food Sci. 2009, 74, 563–568. [Google Scholar] [CrossRef]
- Decker, E.A.; Elias, R.J.; McClements, D.J. Oxidation in Foods and Beverages and Antioxidant Applications: Management in Different Industry Sectors; Woodhead Publishing: Cambridge, UK, 2010; pp. 190, 209. [Google Scholar]
- Charalambous, G. The Analysis and Control of Less Desirable Flavors in Foods and Beverages; Academic Press, Inc: New York, NY, USA, 2012; p. 62. [Google Scholar]
- Li, Y.; Zhang, L.; Wang, W. Formation of Aldehyde and Ketone Compounds during Production and Storage of Milk Powder. Molecules 2012, 17, 9900. [Google Scholar] [CrossRef]
- Park, C.W.; Drake, M. Condensed milk storage and evaporation affect the flavor of nonfat dry milk. J. Dairy Sci. 2016, 99, 9586–9597. [Google Scholar] [CrossRef]
- Chambers, E.; Koppel, K. Associations of volatile compounds with sensory aroma and flavor: The complex nature of flavor. Molecules 2013, 18, 4887–4905. [Google Scholar] [CrossRef]
- Karahadian, C.; Lindsay, R.C. Evaluation of compounds contributing characterizing fishy flavors in fish oils. J. Am. Oil Chem. Soc. 1989, 66, 953–960. [Google Scholar] [CrossRef]
- Chevance, F.F.; Farmer, L.J. Release of volatile odor compounds from full-fat and reduced-fat frankfurters. J. Agric. Food Chem. 1999, 47, 5161–5168. [Google Scholar] [CrossRef]
- Papastergiadis, A.; Mubiru, E.; Van Langenhove, H.; De Meulenaer, B. Malondialdehyde measurement in oxidized foods: evaluation of the spectrophotometric thiobarbituric acid reactive substances (TBARS) test in various foods. J. Agric. Food Chem. 2012, 60, 9589–9594. [Google Scholar] [CrossRef]
- Grotto, D.; Maria, L.S.; Valentini, J.; Paniz, C.; Schmitt, G.; Garcia, S.C.; Pomblum, V.J.; Rocha, J.B.T.; Farina, M. Importance of the lipid peroxidation biomarkers and methodological aspects for malondialdehyde quantification. Quim. Nova 2009, 32, 169–174. [Google Scholar] [CrossRef]
- Xu, L.; Yu, X.; Li, M.; Chen, J.; Wang, X. Monitoring oxidative stability and changes in key volatile compounds in edible oils during ambient storage through HS-SPME/GC–MS. Int. J. Food Prop. 2017, 20, S2926–S2938. [Google Scholar] [CrossRef]
- Ajmal, M.; Nadeem, M.; Imran, M.; Junaid, M. Lipid compositional changes and oxidation status of ultra-high temperature treated Milk. Lipids Health Dis. 2018, 17, 227. [Google Scholar] [CrossRef]
- Clare, D.; Bang, W.; Cartwright, G.; Drake, M.; Coronel, P.; Simunovic, J. Comparison of sensory, microbiological, and biochemical parameters of microwave versus indirect UHT fluid skim milk during storage. J. Dairy Sci. 2005, 88, 4172–4182. [Google Scholar] [CrossRef]
- Deeth, H. Improving UHT processing and UHT milk products. In Improving the Safety and Quality of Milk; Griffiths, M., Ed.; Woodhead Publishing: Cambridge, UK, 2010; pp. 302–329. [Google Scholar]
- Khan, I.T.; Nadeem, M.; Imran, M.; Ullah, R.; Ajmal, M.; Jaspal, M.H. Antioxidant properties of Milk and dairy products: a comprehensive review of the current knowledge. Lipids Health Dis. 2019, 18, 41. [Google Scholar] [CrossRef]
- Choe, E.; Lee, J.; Min, D. Chemistry for oxidative stability of edible oils. Healthful Lipids 2005, 558–590. [Google Scholar]
- McSweeney, S.L. Emulsifiers in infant nutritional products. In Food Emulsifiers and Their Applications; Springer: New York, NY, USA, 2008; pp. 233–261. [Google Scholar]
- Vignolles, M.; Lopez, C.; Madec, M.; Ehrhardt, J.; Méjean, S.; Schuck, P.; Jeantet, R. Fat properties during homogenization, spray-drying, and storage affect the physical properties of dairy powders. J. Dairy Sci. 2009, 92, 58–70. [Google Scholar] [CrossRef]
- Barrow, C.; Wang, B.; Adhikari, B.; Liu, H. Spray drying and encapsulation of omega-3 oils. In Food Enrichment with Omega-3 Fatty Acids; Jacobsen, C., Nielsen, N.S., Horn, A.F., Sorensen, M., Eds.; Woodhead Publishing: Cambridge, UK, 2013; pp. 194–225. [Google Scholar]
- Kaushik, P.; Dowling, K.; Barrow, C.J.; Adhikari, B. Microencapsulation of omega-3 fatty acids: A review of microencapsulation and characterization methods. J. Funct. Foods 2015, 19, 868–881. [Google Scholar] [CrossRef]



| Fatty Acids | FFWMP | SMP | IMF | p-Value |
|---|---|---|---|---|
| Butyric acid C4:0 | 0.09 ± 0.02 b | 21.03 ± 5.93 a | 1.03 ± 0.07 b | 0.014 |
| Caproic acid C6:0 | 0.04 ± 0.01 b | 6.64 ± 2.02 a | 0.41 ± 0.01 b | 0.018 |
| Octanoic acid C8:0 | 0.04 ± 0.01 | 1.32 ± 1.86 | 0.44 ± 0.01 | 0.549 |
| Decanoic acid C10:0 | 0.04 ± 0.01 b | 3.98 ± 0.28 a | 0.65 ± 0.03 b | <0.001 |
| Undecanoic acid C11:0 | ND | ND | 0.01 ± 0.01 | 0.465 |
| Lauric acid C12:0 | 0.28 ± 0.02 b | 4.40 ± 0.83 a | 3.03 ± 0.15 ac | 0.008 |
| Tridecanoic acid C13:0 | ND b | ND b | 0.02 ± 0.01 a | <0.001 |
| Myristic acid C14:0 | 0.49 ± 0.29 b | ND b | 2.43 ± 0.08 a | 0.002 |
| Myristoleic acid C14:1 c9 | 0.01 ± 0.01 b | ND b | 0.13 ± 0.01 a | 0.001 |
| Pentadecanoic acid C15:0 | 0.06 ± 0.01 b | ND c | 0.23 ± 0.01 a | <0.001 |
| Palmitic acid C16:0 | 32.9 ± 3.71 a | 17.18 ± 3.71 b,c | 20.06 ± 0.21 b | 0.027 |
| Palmitoleic acid C16:1 c9 | 0.13 ± 0.01 | 0.88 ±0.37 | 0.26 ± 0.01 | 0.075 |
| Heptadecanoic acid C17:0 | 0.08 ± 0.01 b | ND c | 0.14 ± 0.01 a | 0.001 |
| Stearic acid C18:0 | 3.36 ± 0.37 | 1.22 ± 1.72 | 4.31 ± 0.07 | 0.114 |
| Oleic acid C18:1 n9c | 29.22 ± 2.89 a | 10.59 ± 6.74 b | 26.77 ± 0.45 a,b | 0.040 |
| Elaidic acid C18:1 n9t | 2.59 ± 0.36 | 2.01 ± 2.85 | 2.92 ± 0.03 | 0.863 |
| Linoleic acid C18:2 n6c | 8.40 ± 3.19 b | 30.76 ± 2.99 a | 17.32 ± 0.24 b,c | 0.007 |
| trans-9,12-octadecadienoate C18:2 n6t | 21.60 ± 3.25 a | ND b | 15.91 ± 0.28 a | 0.003 |
| α-Linolenic acid C18:3 n3 | 0.17 ± 0.02 b | ND c | 1.79 ± 0.04 a | <0.001 |
| Gamma linolenic acid c18:3 n6 | 0.01 ± 0.01 | ND | 0.07 ± 0.01 | 0.151 |
| Eicosanoic acid C20:0 | 0.24 ± 0.03 a | ND b | 0.24 ± 0.01 a | 0.001 |
| cis-11-Eicosenoic acid C20:1 | 0.10 ± 0.01 b | ND c | 0.24 ± 0.01 a | <0.001 |
| Eicosenoic acid C20:2 | ND | ND | 0.01 ± 0.02 | 0.465 |
| Eicosadienoic acid C20:3 n6 | ND | ND | 0.01 ± 0.02 | 0.465 |
| Nervonic acid C24:1 n9 | ND b | ND b | 0.28 ± 0.05a | 0.004 |
| Eicosapentaenoic acid C20:5 | ND b | ND b | 0.05 ± 0.05 a | <0.001 |
| CLA C18:2 c9t11 | 0.14 ± 0.03 b | ND c | 1.28 ± 0.01 a | <0.001 |
| Class | Compound | Fatty Acid Source | Reference |
|---|---|---|---|
| Aldehyde | Hexanal | Oleic and linoleic acid | [17] |
| Pentanal | Arachidonic and linoleic acid | [17] | |
| Heptanal | Possibly linoleic and oleic acid | [39] | |
| Octanal | Oleic acid | [40] | |
| (E)-2-Nonenal | Linoleic and possibly palmitoleic acid | [40] | |
| 2,4-Decadienal | Linoleic acid | [40] | |
| Undecanal | Possibly oleic acid | - | |
| Ketone | 2-Nonanone | Decanoic acid | [41] |
| 2-Heptanone | Octanoic acid | [41] | |
| 2-Pentanone | Hexanoic acid | [41] | |
| 3-Octen-2-one | Arachidonic and linoleic acid | [17] | |
| Alcohol | 1-Heptanol | Possibly lipid oxidation of heptanal | - |
| 1-Pentanol | Lipid oxidation of pentanal | [17] |
| Compound | LRI | T0 FFWMP | T4 FFWMP (CON) | T8 FFWMP (CON) | T12 FFWMP (CON) | T16 FFWMP (CON) | p-Value | T4 FFWMP (AM) | T8 FFWMP (AM) | T12 FFWMP (AM) | T16 FFWMP (AM) | p-Value | T4 FFWMP (ACC) | T8 FFWMP (ACC) | T12 FFWMP (ACC) | T16 FFWMP (ACC) | p-Value | T4 FFWMP (HUM) | T8 FFWMP (HUM) | T12 FFWMP (HUM) | T16 FFWMP (HUM) | p-Value |
| Hexanal | 840 | 0 | 277 | 197 | 133 | 82 | * | 193 | 145 | 132 | 118 | *** | 259 | 189 | 324 | 382 | *** | 217 | 207 | 174 | 124 | * |
| Pentanal | 735 | 0 | 11 | 9 | 13 | 6 | * | 10 | 8 | 14 | 10 | *** | 13 | 13 | 56 | 41 | *** | 9 | 12 | 18 | 16 | * |
| Heptanal | 944 | 15 | 42 | 17 | 15 | 20 | * | 35 | 22 | 19 | 23 | NS | 31 | 20 | 40 | 50 | *** | 24 | 20 | 26 | 25 | * |
| Octanal | 1047 | 22 | 33 | 17 | 18 | 21 | * | 36 | 15 | 21 | 12 | NS | 28 | 12 | 30 | 37 | *** | 30 | 25 | 23 | 28 | * |
| (E)-2-Nonenal | 1151 | 14 | 5 | 9 | 10 | 9 | * | 6 | 11 | 16 | 12 | NS | 4 | 4 | 6 | 5 | *** | 12 | 9 | 9 | 11 | * |
| 2,4-Decadienal | 1399 | 37 | 2 | 15 | 20 | 17 | * | 6 | 33 | 61 | 54 | NS | 8 | 15 | 20 | 22 | *** | 6 | 53 | 31 | 29 | * |
| Undecanal | 1359 | 7 | 3 | 5 | 3 | 2 | * | 4 | 7 | 13 | 9 | *** | 4 | 4 | 32 | 17 | *** | 19 | 34 | 17 | 14 | * |
| 2-Nonanone | 1140 | 0 | 17 | 10 | 82 | 66 | * | 21 | 12 | 3 | 2 | *** | 5 | 1 | 30 | 109 | *** | 5 | 4 | 36 | 92 | NS |
| 2-Heptanone | 935 | 10 | 9 | 6 | 5 | 6 | NS | 28 | 7 | 7 | 6 | NS | 19 | 6 | 16 | 19 | *** | 22 | 6 | 8 | 8 | NS |
| 2-Pentanone | 730 | 2 | 1 | 3 | 3 | 1 | NS | 2 | 1 | 1 | 0 | *** | 1 | 2 | 2 | 10 | NS | 2 | 1 | 1 | 5 | * |
| 3-Octen-2-one | 1096 | 20 | 21 | 10 | 4 | 19 | NS | 24 | 9 | 5 | 7 | *** | 6 | 2 | 7 | 9 | *** | 6 | 6 | 3 | 3 | * |
| 1-Heptanol | 1016 | 0 | 0 | 41 | 19 | 31 | * | 0 | 36 | 50 | 32 | *** | 0 | 29 | 51 | 46 | NS | 0 | 55 | 48 | 38 | * |
| 1-Pentanol | 815 | 70 | 60 | 329 | 136 | 189 | NS | 54 | 274 | 311 | 17 | NS | 48 | 365 | 39 | 48 | NS | 74 | 35 | 124 | 1793 | NS |
| Compound | LRI | T0 SMP | T4 SMP (CON) | T8 SMP (CON) | T12 SMP (CON) | T16 SMP (CON) | p-Value | T4 SMP (AM) | T8 SMP (AM) | T12 SMP (AM) | T16 SMP (AM) | p-Value | T4 SMP (ACC) | T8 SMP (ACC) | T12 SMP (ACC) | T16 SMP (ACC) | p-Value | T4 SMP (HUM) | T8 SMP (HUM) | T12 SMP (HUM) | T16 SMP (HUM) | p-Value |
| Hexanal | 840 | 0 | 14 | 42 | 28 | 20 | NS | 2 | 4 | 29 | 11 | * | 14 | 48 | 29 | 10 | * | 8 | 42 | 19 | 8 | * |
| Pentanal | 735 | 3 | 2 | 1 | 2 | 1 | NS | 7 | 1 | 2 | 2 | NS | 2 | 4 | 1 | 1 | * | 3 | 3 | 1 | 1 | NS |
| Heptanal | 944 | 16 | 10 | 16 | 5 | 7 | NS | 16 | 13 | 6 | 8 | NS | 8 | 4 | 5 | 9 | * | 16 | 15 | 1 | 3 | * |
| Octanal | 1047 | 18 | 12 | 6 | 13 | 17 | * | 17 | 7 | 9 | 4 | NS | 10 | 5 | 7 | 7 | * | 10 | 9 | 10 | 12 | NS |
| (E)-2-Nonenal | 1151 | 20 | 2 | 4 | 2 | 2 | NS | 2 | 5 | 4 | 3 | NS | 2 | 2 | 1 | 2 | * | 3 | 2 | 2 | 3 | NS |
| 2,4-Decadienal | 1399 | 15 | 1 | 6 | 5 | 5 | NS | 1 | 8 | 8 | 7 | NS | 1 | 4 | 4 | 4 | * | 1 | 8 | 5 | 6 | NS |
| Undecanal | 1359 | 5 | 1 | 2 | 1 | 2 | NS | 1 | 4 | 3 | 2 | NS | 1 | 2 | 1 | 1 | * | 2 | 2 | 2 | 1 | NS |
| 2-Nonanone | 1140 | 7 | 6 | 5 | 3 | 3 | * | 7 | 5 | 4 | 3 | * | 4 | 4 | 6 | 8 | * | 4 | 3 | 4 | 4 | * |
| 2-Heptanone | 935 | 9 | 6 | 7 | 3 | 3 | NS | 7 | 5 | 6 | 4 | NS | 4 | 5 | 7 | 13 | * | 5 | 5 | 7 | 9 | NS |
| 2-Pentanone | 730 | 1 | 1 | 0 | 0 | 0 | NS | 1 | 0 | 0 | 0 | NS | 1 | 0 | 0 | 0 | NS | 1 | 0 | 0 | 0 | NS |
| 3-Octen-2-one | 1096 | 4 | 4 | 2 | 1 | 1 | NS | 5 | 3 | 0 | 2 | NS | 2 | 1 | 0 | 1 | * | 1 | 6 | 1 | 1 | * |
| 1-Heptanol | 1016 | 37 | 0 | 55 | 61 | 49 | NS | 0 | 61 | 16 | 22 | NS | 0 | 46 | 35 | 24 | NS | 0 | 41 | 43 | 53 | * |
| 1-Pentanol | 815 | 0 | 61 | 83 | 4710 | 82 | NS | 63 | 32 | 123 | 47 | * | 62 | 1014 | 82 | 50 | NS | 56 | 105 | 47 | 51 | NS |
| Compound | LRI | T0 IMF | T4 IMF (CON) | T8 IMF (CON) | T12 IMF (CON) | T16 IMF (CON) | p-Value | T4 IMF (AM) | T8 IMF (AM) | T12 IMF (AM) | T16 IMF (AM) | p-Value | T4 IMF (ACC) | T8 IMF (ACC) | T12 IMF (ACC) | T16 IMF (ACC) | p-Value | T4 IMF (HUM) | T8 IMF (HUM) | T12 IMF (HUM) | T16 IMF (HUM) | p-Value |
| Hexanal | 840 | 5986 | 10674 | 13408 | 11700 | 13408 | * | 14364 | 17047 | 14581 | 17047 | * | 15628 | 19874 | 20741 | 19874 | * | 12550 | 18947 | 14353 | 15659 | * |
| Pentanal | 735 | 1209 | 1200 | 1111 | 1520 | 1111 | * | 1367 | 1530 | 2006 | 1530 | * | 3934 | 3621 | 3999 | 3621 | * | 1348 | 1401 | 1628 | 1641 | * |
| Heptanal | 944 | 861 | 915 | 1080 | 915 | 1080 | * | 1310 | 1313 | 1143 | 1313 | * | 1322 | 1410 | 1588 | 1410 | * | 1095 | 1399 | 1055 | 1295 | * |
| Octanal | 1047 | 784 | 608 | 864 | 803 | 864 | * | 910 | 1095 | 1039 | 1095 | NS | 133 | 146 | 141 | 146 | * | 799 | 1370 | 841 | 1090 | NS |
| (E)-2-Nonenal | 1151 | 64 | 36 | 53 | 60 | 53 | * | 46 | 79 | 93 | 79 | * | 48 | 74 | 110 | 74 | * | 55 | 86 | 132 | 97 | * |
| 2,4-Decadienal | 1399 | 154 | 25 | 33 | 60 | 33 | * | 17 | 82 | 120 | 82 | NS | 39 | 72 | 136 | 72 | * | 37 | 82 | 148 | 122 | * |
| Undecanal | 1359 | 97 | 3 | 6 | 3 | 6 | NS | 6 | 18 | 11 | 18 | NS | 69 | 7 | 68 | 7 | * | 32 | 5 | 16 | 5 | * |
| 2-Nonanone | 1140 | 5170 | 31 | 30 | 23 | 30 | * | 53 | 44 | 29 | 44 | * | 29 | 32 | 30 | 32 | * | 7064 | 7048 | 3871 | 3066 | * |
| 2-Heptanone | 935 | 55 | 49 | 44 | 48 | 44 | * | 53 | 66 | 57 | 66 | NS | 51 | 69 | 81 | 69 | * | 49 | 49 | 48 | 57 | * |
| 2-Pentanone | 730 | 8 | 10 | 9 | 9 | 9 | * | 7 | 13 | 11 | 13 | * | 11 | 15 | 16 | 15 | * | 8 | 9 | 14 | 15 | * |
| 3-Octen-2-one | 1096 | 38 | 76 | 67 | 155 | 67 | * | 171 | 166 | 32 | 127 | NS | 174 | 147 | 218 | 147 | NS | 25 | 26 | 96 | 95 | * |
| 1-Heptanol | 1016 | 0 | 0 | 1485 | 1309 | 1485 | NS | 0 | 2341 | 888 | 2341 | NS | 0 | 1731 | 1419 | 1191 | NS | 0 | 2201 | 1656 | 1116 | NS |
| 1-Pentanol | 815 | 62 | 534 | 68 | 85 | 68 | * | 685 | 112 | 69 | 112 | * | 848 | 102 | 129 | 102 | * | 1250 | 107 | 85 | 85 | * |
| Volatile Organic Compound | Hexanal | Rancid Butter Flavour | Grassy/Hay Flavour | Painty Flavour | Painty Aroma | Oxidised Flavour | Oxidised Aroma | Creamy Flavour | Creamy Aroma |
|---|---|---|---|---|---|---|---|---|---|
| Hexanal | - | 0.87 | 0.82 | 0.89 | 0.92 | 0.92 | 0.88 | –0.80 | –0.81 |
| Pentanal | 0.91 | - | - | 0.81 | 0.80 | 0.83 | - | - | - |
| Heptanal | 0.99 | 0.87 | 0.81 | 0.89 | 0.93 | 0.92 | 0.89 | –0.80 | –0.83 |
| (E)-2-Nonenal | 0.93 | 0.83 | 0.85 | - | 0.91 | 0.90 | 0.84 | –0.84 | - |
| Octanal | - | - | - | - | 0.80 | - | - | - | - |
| 2,4-Decadienal | - | - | - | - | 0.80 | - | - | - | |
| 2-Heptanone | - | 0.82 | 0.80 | - | 0.90 | 0.88 | - | - | –0.81 |
| 2-Pentanone | - | 0.84 | 0.84 | - | 0.91 | 0.88 | - | - | - |
| 1-Heptanol | - | - | 0.81 | - | - | - | - | - | - |
| Typical Values (g) | Brand 1 | Brand 2 | Brand 3 | Brand 4 | Brand 5 | Brand 6 |
|---|---|---|---|---|---|---|
| Protein | 1.6 | 1.25 | 1.3 | 1.3 | 1.25 | 1.3 |
| Fat | 3.3 | 3.6 | 3.4 | 3.4 | 3.5 | 3.4 |
| saturated | 1.1 | 1.5 | 1.5 | 1.5 | 1.2 | 1.5 |
| unsaturated | 1.8 | 2.1 | 1.9 | 1.9 | 0.7 | 1.9 |
| LCPs (not specified) | 0.028 | - | 0.015 | 0.024 | 0.02 | 0.024 |
| Linoleic acid | - | 0.55 | - | - | 0.6 | - |
| α-linolenic acid | - | 0.067 | - | - | 0.07 | - |
| Arachidonic acid (AA) | 0.012 | 0.0084 | 0.006 | 0.011 | 0.012 | 0.011 |
| Docosahexaenoic acid (DHA) | 0.011 | 0.0084 | 0.006 | 0.01 | 0.007 | 0.01 |
| Vegetable oils | Y | Y | Y | Y | Y | Y |
| Fish oils | Y | Y | Y | Y | Y | Y |
| Class | Volatile Organic Compound | CAS No. | LRI | IMF Brand 1 | IMF Brand 2 | IMF Brand 3 | IMF Brand 4 | IMF Brand 5 | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Aldehyde | Hexanal | 66-25-1 | 840 | 17935 a | 488 b | 175 b | 285 b | 849 b | * |
| Pentanal | 110-62-3 | 735 | 2013 a | 10 b | 15 b | 28 b | 51 b | * | |
| Heptanal | 111-71-7 | 944 | 1401 a | 73 b | 53 b | 52 b | 109 b | * | |
| Octanal | 124-13-0 | 1047 | 1815 a | 99 b | 162 b | 142 b | 212 b | * | |
| (E)-2-Nonenal | 18829-56-6 | 1151 | 662 a,d | 100 b,c | 1304 c,d | 1017 c | 1119 d | * | |
| 2,4-Decadienal | 2363-88-4 | 1399 | 651 a | 1987 a | 14869 b | 13685 b | 16276 b | * | |
| Undecanal | 112-44-7 | 1359 | 1078 | 938 | 1229 | 984 | 405 | NS | |
| Ketone | 2-Nonanone | 821-55-6 | 1140 | 112 | 17 | 100 | 109 | 132 | NS |
| 2-Heptanone | 110-43-0 | 935 | 83 b | 2395 a | 22 b | 37 b | 41 b | * | |
| 2-Pentanone | 107-87-9 | 730 | 18 | 10 | 4 | 40 | 33 | NS | |
| 3-Octen-2-one | 1669-44-9 | 1096 | 1004 | 712 | 414 | 554 | 169 | NS | |
| Alcohol | 1-Heptanol | 111-70-6 | 1016 | 1177 a | 104 b | 176 b | 164 b | 271 b | * |
| 1-Pentanol | 71-41-0 | 815 | 2402 a | 538 b | 59 b | 93 b | 284 b | * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clarke, H.J.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. Correlating Volatile Lipid Oxidation Compounds with Consumer Sensory Data in Dairy Based Powders during Storage. Antioxidants 2020, 9, 338. https://doi.org/10.3390/antiox9040338
Clarke HJ, O’Sullivan MG, Kerry JP, Kilcawley KN. Correlating Volatile Lipid Oxidation Compounds with Consumer Sensory Data in Dairy Based Powders during Storage. Antioxidants. 2020; 9(4):338. https://doi.org/10.3390/antiox9040338
Chicago/Turabian StyleClarke, Holly J., Maurice G. O’Sullivan, Joseph P. Kerry, and Kieran N. Kilcawley. 2020. "Correlating Volatile Lipid Oxidation Compounds with Consumer Sensory Data in Dairy Based Powders during Storage" Antioxidants 9, no. 4: 338. https://doi.org/10.3390/antiox9040338
APA StyleClarke, H. J., O’Sullivan, M. G., Kerry, J. P., & Kilcawley, K. N. (2020). Correlating Volatile Lipid Oxidation Compounds with Consumer Sensory Data in Dairy Based Powders during Storage. Antioxidants, 9(4), 338. https://doi.org/10.3390/antiox9040338






