Variation in Macronutrient Content, Phytochemical Constitution and In Vitro Antioxidant Capacity of Green and Red Butterhead Lettuce Dictated by Different Developmental Stages of Harvest Maturity
Abstract
1. Introduction
2. Materials and Methods
2.1. Lettuce Cultivars and Climate-Chamber Conditions
2.2. Sampling and Fresh Yield of Microgreens and Lettuce Heads
2.3. Dry matter, Nitrate and Mineral Content
2.4. Chlorophylls and Total Ascorbic Acid
2.5. Carotenoids Separation and Quantification by HPLC-DAD
2.6. Polyphenols Extraction and Analysis by UHPLC-Q-Orbitrap HRMS
2.7. UHPLC-Q-Orbitrap HRMS Method Validation
2.8. Statistical Analysis and Principal Component Analysis
3. Results and Discussion
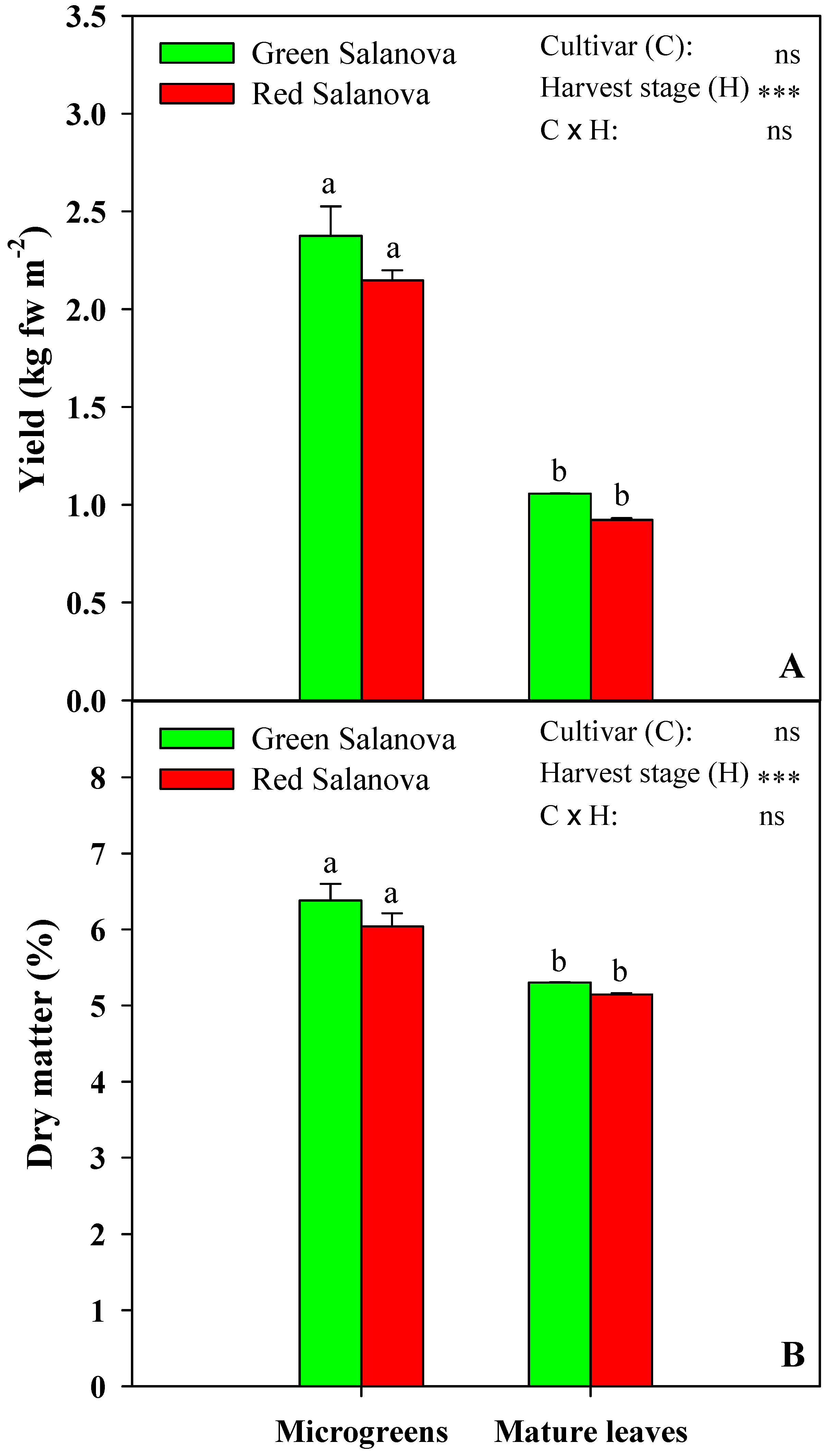
3.1. Yield and Dry Matter of Microgreens and Mature Salanova
3.2. Nitrate Concentration and Mineral Content of Microgreens and Mature Salanova
3.3. Pigments and Total Ascorbic Acid Content of Microgreens and Mature Salanova
3.4. Carotenoid Content of Microgreens and Mature Salanova
3.5. UHPLC-HRMS Orbitrap Validation
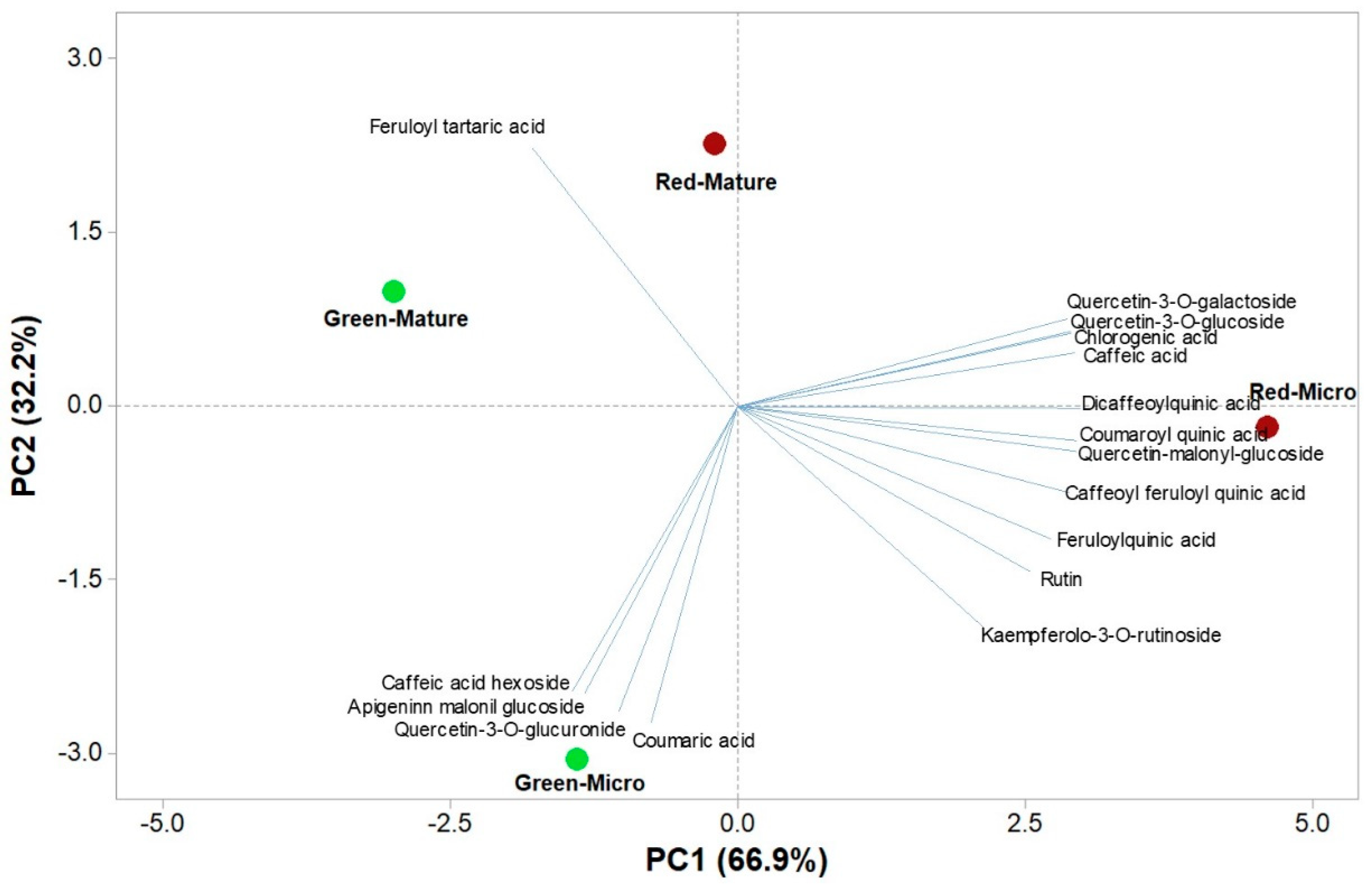
3.6. Polyphenols Profile of Microgreens and Mature Salanova
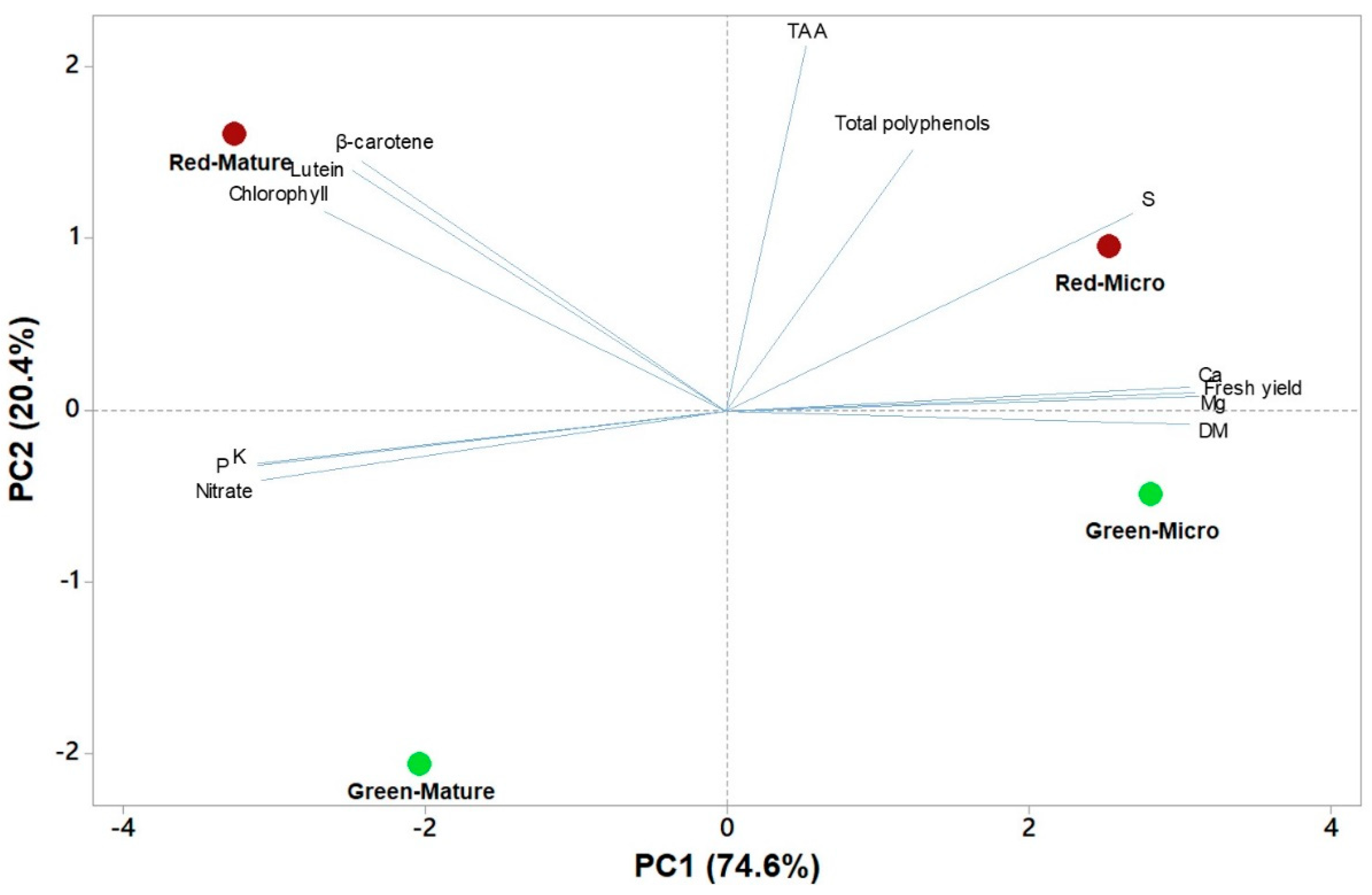
3.7. Principal Component Analysis of Functional and Nutritional Aspects of Green and Red Salanova at Microgreens and Mature Stages
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Raskin, I.; Ribnicky, D.M.; Komarnytsky, S.; Ilic, N.; Poulev, A.; Borisjuk, N.; O’Neal, J.M. Plants and human health in the twenty-first century. Trends Biotechnol. 2002, 20, 522–531. [Google Scholar] [CrossRef]
- Lewis, W.H.; Elvin-Lewis, M.P. Medical Botany: Plants Affecting Human Health; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Debnath, B.; Singh, W.S.; Das, M.; Goswami, S.; Singh, M.K.; Maiti, D.; Manna, K. Role of plant alkaloids on human health: A review of biological activities. Mater. Today Chem. 2018, 9, 56–72. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Rouphael, Y.; Di Gioia, F.; Kyratzis, A.; Serio, F.; Renna, M.; De Pascale, S.; Santamaria, P. Micro-scale vegetable production and the rise of microgreens. Trends Food Sci. Technol. 2016, 57, 103–115. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; El-Nakhel, C.; Graziani, G.; Pannico, A.; Soteriou, G.A.; Giordano, M.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Functional quality in novel food sources: Genotypic variation in the nutritive and phytochemical composition of thirteen microgreens species. Food Chem. 2019, 277, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Kyriacou, M.C.; El-Nakhel, C.; Pannico, A.; Graziani, G.; Soteriou, G.A.; Giordano, M.; Zarrelli, A.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Genotype-specific modulatory effects of select spectral bandwidths on the nutritive and phytochemical composition of microgreens. Front. Plant Sci. 2019, 10, 1501. [Google Scholar] [CrossRef] [PubMed]
- Oancea, S.; Oprean, L. Anthocyanins, from biosynthesis in plants to human health benefits. Acta Univ. Cinbinesis Ser. E Food Technol. 2011, 15. [Google Scholar]
- Boudet, A.M. Polyphenols: From plant adaptation to useful chemical resources. Recent Adv. Polyphenol. Res. 2012, 3, 41–70. [Google Scholar] [CrossRef]
- El-Nakhel, C.; Pannico, A.; Kyriacou, M.C.; Giordano, M.; De Pascale, S.; Rouphael, Y. Macronutrient deprivation eustress elicits differential secondary metabolites in red and green-pigmented butterhead lettuce grown in a closed soilless system. J. Sci. Food Agric. 2019, 99, 6962–6972. [Google Scholar] [CrossRef]
- Boudet, A.M. Evolution and current status of research in phenolic compounds. Phytochemistry 2007, 68, 2722–2735. [Google Scholar] [CrossRef]
- Imeh, U.; Khokhar, S. Distribution of conjugated and free phenols in fruits: Antioxidant activity and cultivar variations. J. Agric. Food Chem. 2002, 50, 6301–6306. [Google Scholar] [CrossRef]
- Di Gioia, F.; Tzortzakis, N.; Rouphael, Y.; Kyriacou, M.C.; Sampaio, S.L.; Ferreira, I.C.; Petropoulos, S.A. Grown to be Blue—Antioxidant properties and health effects of colored vegetables. Part II: Leafy, fruit, and other vegetables. Antioxidants 2020, 9, 97. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Jiang, X.; Xiao, Z.; Yu, L.; Pham, Q.; Sun, J.; Chen, P.; Yokoyama, W.; Yu, L.L.; Luo, Y.S.; et al. Red cabbage microgreens lower circulating low-density lipoprotein (LDL), liver cholesterol, and inflammatory cytokines in mice fed a high-fat diet. J. Agric. Food Chem. 2016, 64, 9161–9171. [Google Scholar] [CrossRef] [PubMed]
- Di Gioia, F.; Renna, M.; Santamaria, P. Sprouts, microgreens and baby leaf vegetables. In Minimally Processed Refrigerated Fruits and Vegetables; Springer: Boston, MA, USA, 2017; pp. 403–432. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; De Pascale, S.; Kyratzis, A.; Rouphael, Y. Microgreens as a component of space life support systems: A cornucopia of functional food. Front. Plant Sci. 2017, 8, 1587. [Google Scholar] [CrossRef] [PubMed]
- Choe, U.; Yu, L.L.; Wang, T.T. The science behind microgreens as an exciting new food for the 21st century. J. Agric. Food Chem. 2018, 66, 11519–11530. [Google Scholar] [CrossRef]
- Charlebois, S. Can greenbelt microgreens expand its model? A discussion on the future of microgreens. J. Agric. Stud. 2018, 6, 17. [Google Scholar] [CrossRef]
- Alrifai, O.; Hao, X.; Marcone, M.F.; Tsao, R. Current review of the modulatory effects of LED lights on photosynthesis of secondary metabolites and future perspectives of microgreens vegetables. J. Agric. Food Chem. 2019, 67, 6075–6090. [Google Scholar] [CrossRef]
- Pinto, E.; Almeida, A.A.; Aguiar, A.A.; Ferreira, I.M. Comparison between the mineral profile and nitrate content of microgreens and mature lettuces. J. Food Comp. Anal. 2015, 37, 38–43. [Google Scholar] [CrossRef]
- Weber, C.F. Nutrient content of cabbage and lettuce microgreens grown on vermicompost and hydroponic growing pads. J. Hortic. 2016, 3, 1–5. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G.; Giordano, M.; El-Nakhel, C.; Kyriacou, M.C.; De Pascale, S. Foliar applications of a legume-derived protein hydrolysate elicit dose-dependent increases of growth, leaf mineral composition, yield and fruit quality in two greenhouse tomato cultivars. Sci. Hortic. 2017, 226, 353–360. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Kampfenkel, K.; Vanmontagu, M.; Inze, D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Fonseca, J.M.; Choi, J.H.; Kubota, C.; Kwon, D.Y. Salt in irrigation water affects the nutritional and visual properties of romaine lettuce (Lactuca sativa L.). J. Agric. Food Chem. 2008, 56, 3772–3776. [Google Scholar] [CrossRef]
- Waterland, N.L.; Moon, Y.; Tou, J.C.; Kim, M.J.; Pena-Yewtukhiw, E.M.; Park, S. Mineral content differs among microgreen, baby leaf, and adult stages in three cultivars of kale. HortScience 2017, 52, 566–571. [Google Scholar] [CrossRef]
- European Commission (EC). European Commission Regulation (EC) No 1882/2006 of 19 December 2006 laying down methods of sampling and analysis for the official control of the levels of nitrates in certain foodstuffs. Off. J. Eur. Union 2006, 364, 25–31. [Google Scholar]
- Broadley, M.R.; Seginer, I.; Burns, A.; Escobar-Gutiérrez, A.J.; Burns, I.G.; White, P.J. The nitrogen and nitrate economy of butterhead lettuce (Lactuca sativa var. capitata L.). J. Exp. Bot. 2003, 54, 2081–2090. [Google Scholar] [CrossRef]
- Mengel, K.; Arneke, W.W. Effect of potassium on the water potential, the pressure potential, the osmotic potential and cell elongation in leaves of Phaseolus vulgaris. Physiol. Plant 1982, 54, 402–408. [Google Scholar] [CrossRef]
- Albornoz, F.; Lieth, J.H. N, P, K and S uptake response to various levels of CO2 assimilation and growth rate in lettuce. J. Plant Nutr. 2017, 40, 773–783. [Google Scholar] [CrossRef]
- Sim, C.C.; Zaharah, A.R.; Tan, M.S.; Goh, K.J. Rapid determination of leaf chlorophyll concentration, photosynthetic activity and NK concentration of Elaies guineensis via correlated SPAD-502 chlorophyll index. Asian J. Agric. Res. 2015, 9, 132–138. [Google Scholar] [CrossRef]
- El-Nakhel, C.; Giordano, M.; Pannico, A.; Carillo, P.; Fusco, G.M.; De Pascale, S.; Rouphael, Y. Cultivar-specific performance and qualitative descriptors for butterhead Salanova lettuce produced in closed soilless cultivation as a candidate salad crop for human life support in space. Life 2019, 9, 61. [Google Scholar] [CrossRef]
- Kizhedath, A.; Suneetha, V. Estimation of chlorophyll content in common household medicinal leaves and their utilization to avail health benefits of chlorophyll. J. Pharm. Res. 2011, 4, 1412–1413. [Google Scholar]
- Mou, B. Genetic variation of beta-carotene and lutein contents in lettuce. J. Am. Soc. Hortic. Sci. 2005, 130, 870–876. [Google Scholar] [CrossRef]
- Ebert, A.W.; Wu, T.H.; Yang, R.Y. Amaranth sprouts and microgreens–a homestead vegetable production option to enhance food and nutrition security in the rural-urban continuum. In Proceedings of the Regional Symposium on Sustaining Small-Scale Vegetable Production and Marketing Systems for Food and Nutrition Security (SEAVEG 2014), Bangkok, Thailand, 25–27 February 2014; pp. 25–27. [Google Scholar]
- Carillo, P.; Raimondi, G.; Kyriacou, M.C.; Pannico, A.; El-Nakhel, C.; Cirillo, V.; Colla, G.; De Pascale, S.; Rouphael, Y. Morpho-physiological and homeostatic adaptive responses triggered by omeprazole enhance lettuce tolerance to salt stress. Sci. Hortic. 2019, 49, 22–30. [Google Scholar] [CrossRef]
- Naidu, K.A. Vitamin C in human health and disease is still a mystery? An overview. Nutr. J. 2003, 2, 7. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, K.; Khan, A.; Khattak, M.M.A.K. Biological significance of ascorbic acid (vitamin C) in human health-a review. Pak. J. Nutr. 2004, 3, 5–13. [Google Scholar] [CrossRef]
- Khanam, U.K.S.; Oba, S.; Yanase, E.; Murakami, Y. Phenolic acids, flavonoids and total antioxidant capacity of selected leafy vegetables. J. Funct. Foods 2012, 4, 979–987. [Google Scholar] [CrossRef]
- Kijlstra, A.; Tian, Y.; Kelly, E.R.; Berendschot, T.T. Lutein: More than just a filter for blue light. Prog. Retin. Eye Res. 2012, 31, 303–315. [Google Scholar] [CrossRef]
- Johnson, E.J. Role of lutein and zeaxanthin in visual and cognitive function throughout the lifespan. Nutr. Rev. 2014, 72, 605–612. [Google Scholar] [CrossRef]
- Kim, D.E.; Shang, X.; Assefa, A.D.; Keum, Y.S.; Saini, R.K. Metabolite profiling of green, green/red, and red lettuce cultivars: Variation in health beneficial compounds and antioxidant potential. Food Res. Intern. 2018, 105, 361–370. [Google Scholar] [CrossRef]
- Moura, L.D.O.; Carlos, L.D.A.; Oliveira, K.G.D.; Martins, L.M.; Silva, E.C.D. Physicochemical characteristics of purple lettuce harvested at different ages. Rev. Caatinga 2016, 29, 489–495. [Google Scholar] [CrossRef]
- Mou, B. Nutritional quality of lettuce. Curr. Nutr. Food Sci. 2012, 8, 177–187. [Google Scholar] [CrossRef]
- Mou, B. Nutrient content of lettuce and its improvement. Curr. Nutr. Food Sci. 2009, 5, 242–248. [Google Scholar] [CrossRef]
- Oruna-Concha, M.J.; Lignou, S.; Feeney, E.L.; Beegan, K.; Kenny, O.; Harbourne, N. Investigating the Phytochemical, Flavour and Sensory Attributes of Mature and Microgreen Coriander (Coriandrum sativum); Siegmund, B., Leitner, E., Eds.; Flavour Sci., Verlag der tecnischen Universitat Graz: Graz, Austria, 2018. [Google Scholar] [CrossRef]
- Sharma, P.; Ghimeray, A.K.; Gurung, A.; Jin, C.W.; Rho, H.S.; Cho, D.H. Phenolic contents, antioxidant and α-glucosidase inhibition properties of Nepalese strain buckwheat vegetables. Afr. J. Biotechnol. 2012, 11, 184–190. [Google Scholar] [CrossRef]
| Harvest Stage | Cultivar | Nitrate | P | K | Ca | Mg | S | Na |
|---|---|---|---|---|---|---|---|---|
| (mg kg−1 fw) | (mg 100 g−1 fw) | (mg 100 g−1 fw) | (mg 100 g−1 fw) | (mg 100 g−1 fw) | (mg 100 g−1 fw) | (mg 100 g−1 fw) | ||
| Microgreens | Green | 69 ± 3.3 | 12.33 ± 0.58 | 117.3 ± 2.33 | 52.48 ± 0.85 b | 23.62 ± 0.61 | 6.51 ± 0.20 a | 33.42 ± 1.39 |
| Red | 117 ± 3.9 | 12.25 ± 0.44 | 127.4 ± 5.03 | 59.71 ± 0.88 a | 22.95 ± 0.60 | 6.85 ± 0.20 a | 32.56 ± 0.91 | |
| Mature leaves | Mean | 93 ± 11.0 B | 12.29 ± 0.33 B | 122.4 ± 3.36 B | 56.10 ± 1.71 A | 23.28 ± 0.41 A | 6.68 ± 0.15 A | 32.99 ± 0.77 A |
| Green | 1557 ± 40.8 | 18.94 ± 0.93 | 419.8 ± 8.18 | 29.17 ± 0.34 c | 12.49 ± 0.37 | 3.03 ± 0.03 c | 4.86 ± 0.18 | |
| Red | 1520 ± 31.3 | 19.28 ± 0.89 | 430.4 ± 3.76 | 21.85 ± 0.73 d | 10.50 ± 0.21 | 4.56 ± 0.26 b | 4.02 ± 0.12 | |
| Mean | 1538 ± 24.4 A | 19.11 ± 0.58 A | 425.1 ± 4.67 A | 25.51 ± 1.68 B | 11.49 ± 0.48 B | 3.80 ± 0.36 B | 4.44 ± 0.21 B | |
| Significance | ||||||||
| Cultivar (C) | ns | ns | ns | ns | ns | ns | ns | |
| Harvest stage (H) | *** | *** | *** | *** | *** | *** | *** | |
| C × H | ns | ns | ns | *** | ns | * | ns |
| Harvest Stage | Cultivar | TAA | Chlorophyll a | Chlorophyll b | Total Chlorophyll | Lutein | β-carotene |
|---|---|---|---|---|---|---|---|
| (mg AA 100 g−1 fw) | (mg 100 g−1 fw) | (mg 100 g−1 fw) | (mg 100 g−1 fw) | (μg 100 g−1 fw) | (μg 100 g−1 fw) | ||
| Microgreens | Green | 43.58 ± 2.70 b | 5.15 ± 0.32 c | 1.01 ± 0.03 d | 6.16 ± 0.28 d | 300 ± 20 c | 1058 ± 97 c |
| Red | 44.36 ± 2.69 b | 6.02 ± 0.57 c | 1.58 ± 0.10 c | 7.60 ± 0.60 c | 528 ± 50 b | 1567 ± 136 b | |
| Mature leaves | Mean | 43.97 ± 1.71 | 5.58 ± 0.35 B | 1.30 ± 0.14 B | 6.88 ± 0.44 B | 414 ± 56 B | 1313 ± 136 B |
| Green | 11.46 ± 0.18 c | 8.78 ± 0.12 b | 2.06 ± 0.11 b | 10.57 ± 0.09 b | 558 ± 48 b | 1516 ± 61 b | |
| Red | 55.12 ± 0.82 a | 17.80 ± 0.09 a | 4.64 ± 0.21 a | 22.48 ± 0.27 a | 1338 ± 36 a | 2656 ± 58 a | |
| Mean | 33.29 ± 9.77 | 13.29 ± 2.02 A | 3.35 ± 0.59 A | 16.52 ± 2.66 A | 948 ± 176 A | 2086 ± 258 A | |
| Significance | |||||||
| Cultivar (C) | * | ns | ns | ns | * | * | |
| Harvest stage (H) | ns | ** | ** | ** | * | * | |
| C × H | *** | *** | *** | *** | *** | * |
| Compound | LOD | LOQ | Linearity (R2) | Recovery % (n = 3) | Intra-Day Precision (RSD,%; (n = 3) | Intra-Day Precision (RSD,%; (n = 3) | ||
|---|---|---|---|---|---|---|---|---|
| ng g−1 | 1 mg kg−1 | 10 mg kg−1 | 50 mg kg−1 | 1 mg kg−1 | ||||
| Apigenin malonyl glucoside | 0.04 | 0.12 | 0.991 | 91.3 | 99.5 | 105.3 | 5 | 6 |
| Caffeic acid | 0.05 | 0.14 | 0.992 | 99.3 | 91.6 | 102.3 | 3 | 8 |
| Caffeic acid hexoside isomers | 0.05 | 0.16 | 0.995 | 99.1 | 98.1 | 102.5 | 6 | 9 |
| Caffeoyl feruloyl quinic acid | 0.04 | 0.12 | 0.995 | 100.1 | 100.0 | 102.3 | 7 | 10 |
| Chlorogenic acid | 0.04 | 0.12 | 0.991 | 96.3 | 98.4 | 103.6 | 5 | 5 |
| Coumaric acid | 0.05 | 0.14 | 0.994 | 98.5 | 99.5 | 103.6 | 4 | 7 |
| Coumaroyl quinic acid | 0.04 | 0.13 | 0.994 | 99.1 | 100.6 | 102.2 | 2 | 8 |
| Dicaffeoylquinic acid | 0.04 | 0.12 | 0.995 | 100.2 | 99.5 | 104.6 | 7 | 9 |
| Feruloyl quinic acid | 0.03 | 0.10 | 0.991 | 100.6 | 99.9 | 104.5 | 5 | 7 |
| Feruloyl tartaric acid | 0.05 | 0.14 | 0.997 | 98.5 | 99.2 | 105.9 | 6 | 6 |
| Quercetin malonyl glucoside | 0.03 | 0.10 | 0.987 | 93.0 | 95.0 | 100.1 | 3 | 8 |
| Quercetin-3-O-galactoside | 0.05 | 0.14 | 0.992 | 99.9 | 100.1 | 102.5 | 5 | 9 |
| Quercetin-3-O-glucoside | 0.05 | 0.14 | 0.989 | 99.8 | 100.2 | 105.5 | 5 | 6 |
| Quercetin-3-O-glucuronide | 0.04 | 0.13 | 0.992 | 100.3 | 102.6 | 103.1 | 8 | 9 |
| Rutin | 0.04 | 0.12 | 0.991 | 102.9 | 99.0 | 101.8 | 6 | 9 |
| Kaempferol-3-O-rutinoside | 0.05 | 0.16 | 0.995 | 99.2 | 98.2 | 100.2 | 6 | 7 |
| Polyphenols (µg g−1 fw) | Microgreens | Mature Leaves | Significance | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Green | Red | Mean | Green | Red | Mean | Cultivar (C) | Harvest stage (H) | C × H | |
| Apigenin malonil glucoside | 17.14 ± 0.41 a | 5.19 ± 0.23 c | 11.16 ± 2.68 | 9.70 ± 0.4 b | 1.42 ± 0.12 d | 5.56 ± 1.86 | *** | ns | *** |
| Caffeic acid | 0.56 ± 0.01 c | 8.21 ± 0.05 a | 4.39 ± 1.71 | 0.32 ± 0 c | 3.14 ± 0.3 b | 1.73 ± 0.64 | *** | ns | *** |
| Caffeic acid hexoside isomers | 1.58 ± 0.03 a | 0.59 ± 0.01 c | 1.09 ± 0.22 | 0.97 ± 0.03 b | 0.39 ± 0 d | 0.68 ± 0.13 | *** | ns | *** |
| Caffeoyl feruloyl quinic acid | 37.69 ± 0.02 b | 48.87 ± 0.37 a | 43.28 ± 2.5 A | 30.62 ± 0.02 d | 35.59 ± 0.17 c | 33.11 ± 1.11 B | * | ** | *** |
| Chlorogenic acid | 15.0 ± 0.34 c | 541 ± 29.5 a | 278 ± 118 | 6.92 ± 0.23 c | 234 ± 20.1 b | 121 ± 51.7 | *** | ns | *** |
| Coumaric acid | 0.93 ± 0.04 a | 0.49 ± 0 b | 0.71 ± 0.1 A | 0.51 ± 0 b | 0.34 ± 0.01 c | 0.42 ± 0.04 B | * | * | *** |
| Coumaroyl quinic acid | 33.46 ± 0.55 b | 86.45 ± 0.65 a | 59.96 ± 11.9 A | 20.37 ± 0.51 c | 34.89 ± 0.34 b | 27.63 ± 3.26 B | * | * | *** |
| Dicaffeoylquinic acid | 4.40 ± 0.04 | 18.14 ± 0.43 | 11.27 ± 3.08 | nd | 7.43 ± 0.35 | na | * | na | na |
| Feruloyl quinic acid | 1.97 ± 0.02 b | 3.15 ± 0.02 a | 2.56 ± 0.26 A | 0.84 ± 0.04 d | 1.35 ± 0.06 c | 1.09 ± 0.12 B | ns | *** | *** |
| Feruloyl tartaric acid | nd | nd | na | 58.62 ± 0.56 | 50.41 ± 0.04 | 54.51 ± 1.85 | *** | na | na |
| Kaempferol-3-O-rutinoside | 4.91 ± 0.01 | 5.16 ± 0.09 | 5.03 ± 0.07 A | 3.23 ± 0.07 | 3.81 ± 0.06 | 3.52 ± 0.14 B | ns | *** | ns |
| Quercetin-3-O-galactoside | 0.46 ± 0.01 c | 3.72 ± 0.02 a | 2.09 ± 0.73 | 0.33 ± 0 c | 2.09 ± 0.11 b | 1.21 ± 0.4 | *** | ns | *** |
| Quercetin-3-O-glucoside | 0.62 ± 0.01 c | 4.22 ± 0.02 a | 2.42 ± 0.8 | 0.30 ± 0.01 d | 2.33 ± 0.02 b | 1.31 ± 0.45 | *** | ns | *** |
| Quercetin-3-O-glucuronide | 13.66 ± 0.29 a | 4.19 ± 0.1 c | 8.92 ± 2.12 | 5.43 ± 0.37 b | 2.64 ± 0.04 d | 4.04 ± 0.65 | ** | ns | *** |
| Quercetin malonyl glucoside | 94.4 ± 1.63 b | 228 ± 4.31 a | 161 ± 29.9 A | 45.1 ± 3.36 c | 94.8 ± 4.35 b | 70.0 ± 11.38 B | * | * | *** |
| Rutin | 87.4 ± 0.59 b | 128 ± 1.36 a | 108 ± 9.15 A | 46.5 ± 1.84 d | 51.2 ± 1.19 c | 48.9 ± 1.44 B | ns | *** | *** |
| Total polyphenols | 314 ± 0.68 c | 1086 ± 25.8 a | 700 ± 173 | 230 ± 5.73 d | 526 ± 22.8 b | 378 ± 67.1 | ** | ns | *** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Nakhel, C.; Pannico, A.; Graziani, G.; Kyriacou, M.C.; Giordano, M.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Variation in Macronutrient Content, Phytochemical Constitution and In Vitro Antioxidant Capacity of Green and Red Butterhead Lettuce Dictated by Different Developmental Stages of Harvest Maturity. Antioxidants 2020, 9, 300. https://doi.org/10.3390/antiox9040300
El-Nakhel C, Pannico A, Graziani G, Kyriacou MC, Giordano M, Ritieni A, De Pascale S, Rouphael Y. Variation in Macronutrient Content, Phytochemical Constitution and In Vitro Antioxidant Capacity of Green and Red Butterhead Lettuce Dictated by Different Developmental Stages of Harvest Maturity. Antioxidants. 2020; 9(4):300. https://doi.org/10.3390/antiox9040300
Chicago/Turabian StyleEl-Nakhel, Christophe, Antonio Pannico, Giulia Graziani, Marios C. Kyriacou, Maria Giordano, Alberto Ritieni, Stefania De Pascale, and Youssef Rouphael. 2020. "Variation in Macronutrient Content, Phytochemical Constitution and In Vitro Antioxidant Capacity of Green and Red Butterhead Lettuce Dictated by Different Developmental Stages of Harvest Maturity" Antioxidants 9, no. 4: 300. https://doi.org/10.3390/antiox9040300
APA StyleEl-Nakhel, C., Pannico, A., Graziani, G., Kyriacou, M. C., Giordano, M., Ritieni, A., De Pascale, S., & Rouphael, Y. (2020). Variation in Macronutrient Content, Phytochemical Constitution and In Vitro Antioxidant Capacity of Green and Red Butterhead Lettuce Dictated by Different Developmental Stages of Harvest Maturity. Antioxidants, 9(4), 300. https://doi.org/10.3390/antiox9040300












