Effects of Trichoderma Biostimulation on the Phenolic Profile of Extra-Virgin Olive Oil and Olive Oil By-Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Fungal Material
2.3. Isolation and Characterization of Harzianic Acid and 6-Pentyl-α-Pyrone
2.4. Chemicals
2.5. Analytical Methods
2.5.1. Ultra High-Performance Liquid Chromatograph
2.5.2. Mass Spectrometry Analysis
2.5.3. Q Exactive Orbitrap UHPLC-MS/MS Method Validation
2.5.4. Extraction of Phenolics from the Olive Oil
2.5.5. Extraction of Phenolics from the Olive Leaves
2.5.6. Estimation of the Antioxidant Activity
2.5.7. Statistical Analysis
3. Results
3.1. Validation of the MS Method
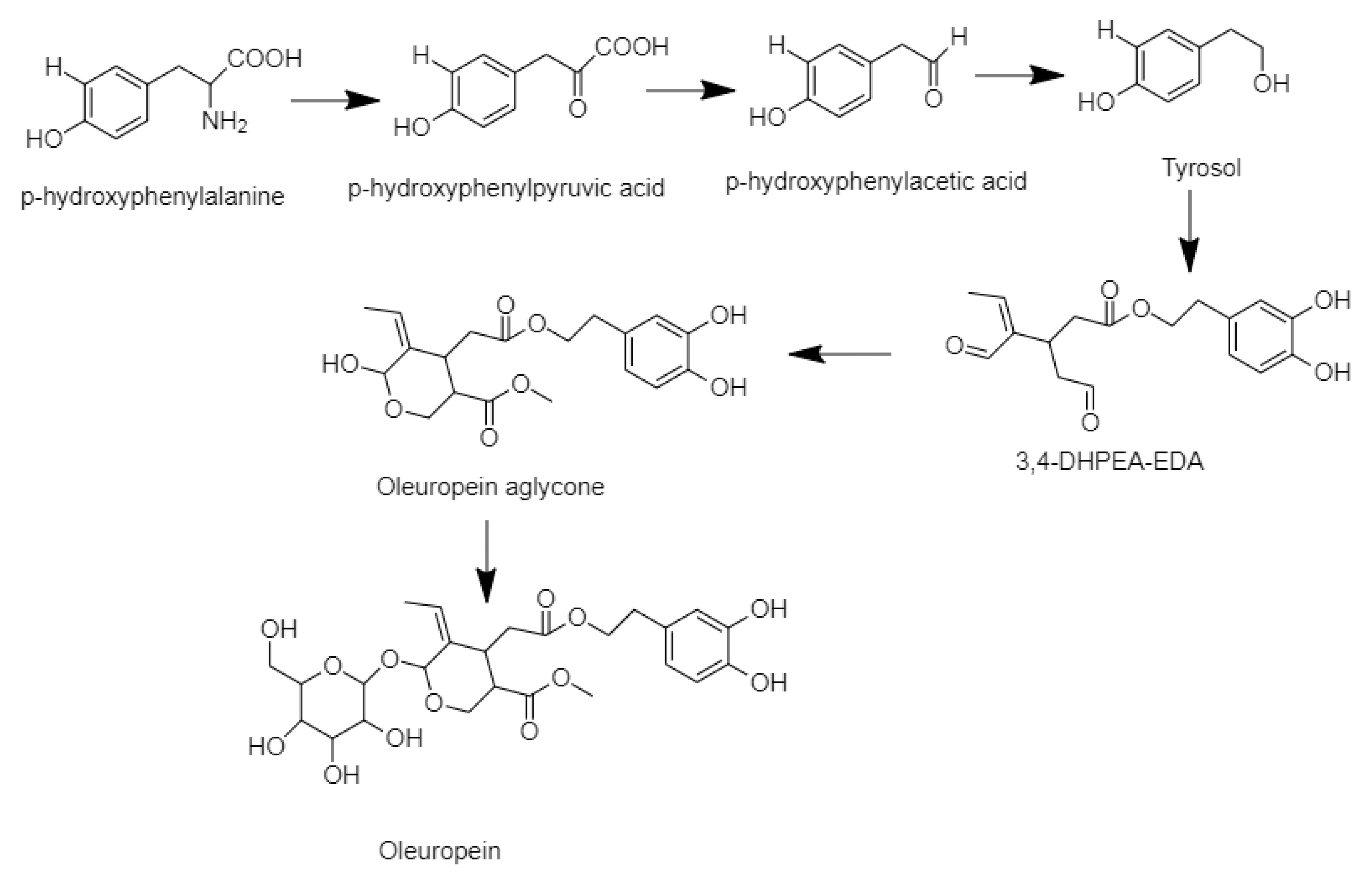
3.2. Identification of the Phenolic Compounds
3.3. Quantification of the Phenolic Compound
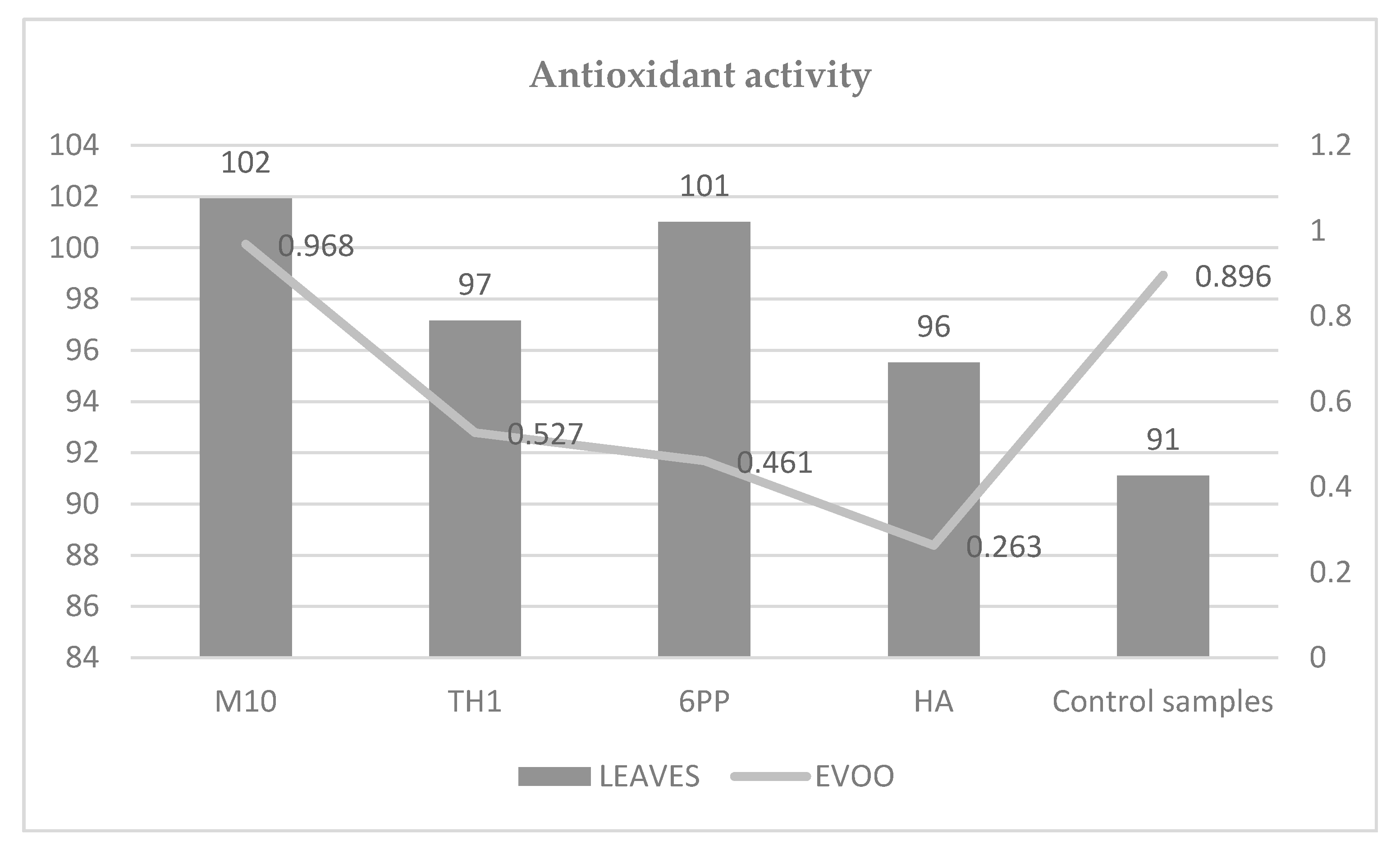
3.4. Antioxidant Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- De Luca, A.I.; Stillitano, T.; Falcone, G.; Squeo, G.; Caponio, F.; Strano, A.; Gulisano, G. Economic and environmental assessment of extra virgin olive oil processing innovations. Chem. Eng. Trans. 2018, 67, 133–138. [Google Scholar]
- Xu, L.; Geelen, D. Developing biostimulants from agro-food and industrial by-products. Front. Plant Sci. 2018, 9, 1567. [Google Scholar] [CrossRef] [PubMed]
- Abou Chehade, L.; Al Chami, Z.; De Pascali, S.A.; Cavoski, I.; Fanizzi, F.P. Biostimulants from food processing by-products: Agronomic, quality and metabolic impacts on organic tomato (Solanum lycopersicum L.). J. Sci. Food Agric. 2018, 98, 1426–1436. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.L.; Pepe, O. Microbial consortia: Promising probiotics as plant biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1801. [Google Scholar] [CrossRef] [PubMed]
- Ertani, A.; Schiavon, M.; Altissimo, A.; Franceschi, C.; Nardi, S. Phenol-containing organic substances stimulate phenylpropanoid metabolism in Zea mays. J. Plant Nutr. Soil Sci. 2011, 174, 496–503. [Google Scholar] [CrossRef]
- Ertani, A.; Schiavon, M.; Muscolo, A.; Nardi, S. Alfalfa plant-derived biostimulant stimulate short-term growth of salt stressed Zea mays L. plants. Plant Soil 2013, 364, 145–158. [Google Scholar] [CrossRef]
- Herrero, M.; Temirzoda, T.N.; Segura-Carretero, A.; Quirantes, R.; Plaza, M.; Ibañez, E. New possibilities for the valorization of olive oil by-products. J. Chromatogr. 2011, 1218, 7511–7520. [Google Scholar] [CrossRef]
- Rahmanian, N.; Jafari, S.M.; Galanakis, C.M. Recovery and removal of phenolic compounds from olive mill wastewater. J. Am. Oil Chem. Soc. 2014, 91, 1–18. [Google Scholar] [CrossRef]
- Dini, I.; Laneri, S. Nutricosmetic: A brief overview. Phytother. Res. 2019, 33, 3054–3063. [Google Scholar] [CrossRef]
- Pascale, A.; Vinale, F.; Manganiello, G.; Nigro, M.; Lanzuise, S.; Ruocco, M.; Marra, R.; Lombardi, N.; Woo, S.L.; Lorito, M. Trichoderma and its secondary metabolites improve yield and quality of grapes. Crop Prot. 2017, 92, 176–181. [Google Scholar] [CrossRef]
- Vázquez Roncero, A. Les polyphenols de l’huile d’olive et leur influence sur les caracteristiques de l’huile. Rev. Fr. Corps Gras 1978, 25, 21–26. [Google Scholar]
- Talhaoui, N.; Gómez-Caravaca, A.M.; Roldán, C.; León, L.; De la Rosa, R.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Chemometric analysis for the evaluation of phenolic patterns in olive leaves from six cultivars at different growth stages. J. Agric. Food Chem. 2015, 63, 1722–1729. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Servili, M.; Selvaggini, R.; Esposto, S.; Taticchi, A.; Montedoro, G.; Morozzi, G. Health and sensory properties of virgin olive oil hydrophilic phenols: Agronomic and technological aspects of production that affect their occurrence in oil. J. Chromatogr. 2004, 1054, 113–127. [Google Scholar] [CrossRef]
- Jerman, K.T.; Mozetič Vodopivec, B. The fate of olive fruit phenols during commercial olive oil processing: Traditional press versus continuous two- and three-phase centrifuge. LWT Food Sci. Technol. 2012, 49, 267–274. [Google Scholar] [CrossRef]
- Hatamnia, A.A.; Abbaspour, N.; Darvishzadeh, R. Antioxidant activity and phenolic profile of different parts of Bene (Pistacia atlantica subsp. kurdica) fruits. Food Chem. 2013, 62, 155–161. [Google Scholar] [CrossRef]
- Valentão, P.; Fernandes, E.; Carvalho, F.; Andrade, P.B.; Seabra, R.M.; Bastos, M.L. Hydroxyl radical and hypochlorous acid scavenging activity of small centaury (Centaurium erythraea) infusion. A comparative study with green tea (Camellia sinensis). Phytomedicine 2003, 10, 517–522. [Google Scholar] [CrossRef]
- Oksana, S.; Marian, B.; Mahendraand, R.; Bo, S.H. Plant phenolic compounds for food, pharmaceutical, and cosmetics production. J. Med. Plants Res. 2012, 6, 2526–2539. [Google Scholar]
- Klen, T.J.; Wondra, A.G.; Vrhovšek, U.; Mozetič, V.B. Phenolic profiling of olives and olive oil process-derived matrices using UPLC-DAD-ESI-QTOF-HRMS analysis. J. Agric. Food Chem. 2015, 63, 3859–3872. [Google Scholar] [CrossRef]
- Fernández-Escobar, R.; Beltrán, G.; Sánchez-Zamora, M.A.; García-Novelo, J.; Aguilera, M.P.; Uceda, M. Olive oil quality decreases with nitrogen over-fertilization. Hortscience 2006, 41, 215–219. [Google Scholar] [CrossRef]
- Dean, J.F.D.; La Fayette, P.R.; Rugh, C.; Tristram, A.H.; Hoopes, J.T.; Eriksson, K.-E.L.; Merkle, S.A. Laccases Associated with Lignifying Vascular Tissues. In ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2009; Volume 78, pp. 96–108. [Google Scholar]
- Ghasemzadeh, A.; Ghasemzadeh, N. Flavonoids and phenolic acids: Role and biochemical activity in plants and human. J. Med. Plants Res. 2011, 5, 6697–6703. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Barbetti, M.J.; Li, H.; Woo, S.L.; Lorito, M. A novel role for Trichoderma secondary metabolites in the interactions with plants. Physiol. Mol. Plant Pathol. 2008, 72, 80–86. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species, opportunistic avirulent plant symbionts. Nature 2004, 2, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Shoresh, M.; Harman, G.E.; Mastouri, F. Induced systemic resistance and plant responses to fungal biocontrol agents. Annu. Rev. Phytopathol. 2010, 48, 21–43. [Google Scholar] [CrossRef] [PubMed]
- Buer, C.S.; Muday, G.K.; Djordjevic, M.A. Flavonoids are differentially taken up and transported long distances in Arabidopsis. Plant Physiol. 2007, 145, 478–490. [Google Scholar] [CrossRef]
- García-Villalba, R.; Carrasco-Pancorbo, A.; Oliveras-Ferraros, C.; Vázquez-Martín, A.; Menéndez, J.A.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Characterization and quantification of phenolic compounds of extra-virgin olive oils with anticancer properties by a rapid and resolutive LC-ESI-TOF MS method. J. Pharm. Biomed. Anal. 2010, 51, 416–429. [Google Scholar] [CrossRef]
- Murkovic, M.; Lechner, S.; Pietzka, A.; Bratacos, M.; Katzogiannos, E. Analysis of minor components in olive oil. J. Biochem. Biophys. Methods 2004, 61, 155–160. [Google Scholar] [CrossRef]
- Ye, J.H.; Wijesundera, C.; Shi, M. Effects of agronomic and oil processing conditions on natural antioxidative phenolics in olive (Olea europea L.). Austin. J. Nutr. Food Sci. 2014, 2, 1050–1058. [Google Scholar]
- Fraser, C.M.; Chapple, C. The phenylpropanoid pathway in Arabidopsis. Am. Soc. Plant Biol. 2011, 9, e0152. [Google Scholar] [CrossRef]
- Jiménez, B.; Sánchez-Ortiz, A.; Lorenzo, M.L.; Rivas, A. Influence of fruit ripening on agronomic parameters, quality indices, sensory attributes, and phenolic compounds of Picudo olive oils. Food Res. Int. 2013, 54, 1860–1867. [Google Scholar] [CrossRef]
- Cardoso, S.M.; Falcao, S.I.; Peres, A.M.; Domingues, M.R.M. Oleuropein/ligstroside isomers and their derivatives in Portuguese olive mil wastewaters. Food Chem. 2011, 129, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Obied, H.K.; Prenzler, P.D.; Ryan, D.; Servili, M.; Taticchi, A.; Esposto, S.; Robards, K. Biosynthesis and biotransformations of phenol-conjugated oleosidic secoiridoids from Olea europaea L. Nat. Prod. Rep. 2008, 25, 1167–1179. [Google Scholar] [CrossRef] [PubMed]
- Hassen, I.; Casabianca, H.; Hosni, K. Biological activities of the natural antioxidant oleuropein: Exceeding the expectation a mini-review. J. Funct. Foods 2015, 18, 926–940. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J. Antioxidant activities of flavonoids as bioactive components of food. Biochem. Soc. Trans. 1996, 24, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Benavente-Garcia, O.; Castillo, J.; Lorente, J.; Ortuno, A.; Del Rio, J.A. Antioxidant activity of phenolics extracted from Olea europaea L. leaves. Food Chem. 2000, 68, 457–462. [Google Scholar] [CrossRef]
| Phenolic Compounds | Linearity (mg/L) | R2 | LOD (mg/L) | LOQ (mg/L) | Intraday RSD % (n = 3), 50 mg/L |
|---|---|---|---|---|---|
| Phenolic Acids | |||||
| 4-Hydroxybenzoic acid | 1–50 | 0.998 | 0.207 | 0.622 | 0.9 |
| 3-Hydroxybenzoic acid | 1–50 | 0.995 | 0.205 | 0.622 | 1.1 |
| Vanillic acid | 1–50 | 0.887 | 0.200 | 0.600 | 1.1 |
| Cinnamic acid | 1–50 | 0.991 | 0.200 | 0.600 | 0.9 |
| p-Coumaric acid | 1–50 | 1.000 | 0.100 | 0.300 | 1.8 |
| Ferulic acid | 1–50 | 0.912 | 0.100 | 0.300 | 1.7 |
| Flavonoids | |||||
| Luteolin | 0.5–50 | 0.991 | 0.066 | 0.200 | 1.4 |
| Apigenin | 0.5–50 | 0.899 | 0.066 | 0.200 | 2.1 |
| Lignans | |||||
| (+)Pinoresinol | 1–50 | 0.999 | 0.02 | 0.060 | 0.5 |
| (+)1-Acetoxypinoresinol | 1–50 | 0.899 | 0.233 | 0.700 | 1.5 |
| Phenolic Alcohols | |||||
| p-HPEA (Tyrosol) | 1–50 | 0.991 | 0.133 | 0.040 | 1.6 |
| 3.4 DHPEA (Hydroxytyrosol) | 1–50 | 0.992 | 0.666 | 2.000 | 3.0 |
| Secoiridoids | |||||
| Oleuropein | 1–50 | 0.991 | 0.166 | 0.500 | 5.0 |
| Ligstroside | 1–50 | 0.991 | 0.166 | 0.500 | 4.0 |
| Secologanoside | 1–50 | 0.967 | 0.333 | 1.000 | 2.1 |
| Elenaic acid | 1–50 | 0.991 | 0.333 | 1.000 | 0.7 |
| 3.4 DHPEA-EDA (Oleuropein aglycone dialdehyde) 3.4-DHPEA-EA (Oleuropein-aglycone monoaldehyde) | 1–50 | 0.998 | 1.000 | 3.000 | 2.1 |
| p-HPEA-EDA (Ligstroside-aglycone dialdehyde) | 1–50 | 0.899 | 0.416 | 1.250 | 3.0 |
| Phenolic Compounds | RT (min) | Formula | Theoretical m/z of deprotonated molecular ions [M − H]− | Experimental m/z of deprotonated molecular ions [M − H]− | Calculated Errors ∆ppm | Fragments | Collision Energy (eV) |
|---|---|---|---|---|---|---|---|
| Phenolic Acids | |||||||
| 4-Hydroxybenzoic acid | 2.57 | C7H6O3 | 137.02442 | 137.02456 | 1.02 | 93.03431 | 12 |
| 3-Hydroxybenzoic acid | 2.88 | C7H6O3 | 137.02442 | 137.02458 | 1.17 | 93.03431 | 12 |
| Vanillic acid | 4.30 | C8H8O4 | 167.03498 | 167.03522 | 1.44 | 152.01143 | 20 |
| Cinnamic acid | 11.54 | C9H8O2 | 147.04515 | 147.04536 | 1.43 | 103.04501 | 20 |
| p-Coumaric acid | 9.71 | C9H10O5 | 163.04007 | 163.04028 | 1.29 | 119.05023 | 20 |
| Ferulic acid | 11.81 | C10H10O4 | 193.05063 | 193.05084 | 1.09 | 178.02685 | 20 |
| Flavonoids | |||||||
| Luteolin | 19.07 | C15H10O6 | 285.04046 | 285.04106 | 2.10 | 133.02940 | 30 |
| Apigenin | 19.12 | C15H10O5 | 269.04555 | 269.04597 | 1.56 | 225.05592 | 35 |
| Lignans | |||||||
| (+) Pinoresinol | 17.00 | C20H22O6 | 357.13436 | 357.13487 | 1.43 | 151.03961 | 40 |
| (+) 1-Acetoxypinoresinol | 19.10 | C22H24O8 | 415.13984 | 415.14007 | 0.55 | 415.13821 | 40 |
| Phenolic Alcohols | |||||||
| Tyrosol (p-HPEA) | 2.75 | C8H10O2 | 137.06080 | 137.06096 | 1.17 | 119.05022 | 12 |
| Hydroxytyrosol (3,4 DHPEA) | 1.60 | C8H10O3 | 153.05572 | 153.05580 | 0.52 | 123.04561 | 12 |
| Secoiridoids | |||||||
| Oleuropein | 16.69 | C25H32O13 | 539.17701 | 539.17767 | 1.22 | 377.12393 | 20 |
| Ligstroside | 18.25 | C25H32O12 | 523.18210 | 523.18279 | 1.32 | 361.12914 | 12 |
| Secologanoside | 19.49 | C16H21O11 | 389.1092 | 389.109258 | 0.59 | 345.1195 | 12 |
| Elenaic acid | 13.14 | C11H14O6 | 241.07176 | 241.07212 | 1.49 | 209.04573 | 10 |
| Oleacein (3.4 DHPEA-EDA) | 16.14 | C17H20O6 | 319.11871 | 319.11898 | 0.85 | 301.1082 | 15 |
| Oleuropein-aglycone mono-aldehyde (3.4 DHPEA-EA) | 21.25 | C19H22O8 | 377.12419 | 377.12442 | 0.61 | 345.09790 | 12 |
| Ligstroside-aglycone dialdehyde (p-HPEA-EDA) | 18.59 | C17H20O5 | 303.12380 | 303.12441 | 2.01 | 301.1082 | 12 |
| Flavonoids (mg/kg) | Lignans (mg/kg) | |||
|---|---|---|---|---|
| Luteolin | Apigenin | Pinoresinol | Acetoxypinoresinol | |
| LEAF Samples | LEAF Samples | |||
| M10 | 5376.796 ± 19.561 (+23%) | 386.225 ± 2.12 (+1%) | 29.932 ± 0.038 (+56%) | 151.353 ± 3.269 (+58%) |
| TH1 | 5609.046 ± 56.141 (+26%) | 349.017 ± 8.094 (−10%) | 26.813 ± 2.045 (+50%) | 176.758 ± 6.716 (+64%) |
| 6PP | 10163.237 ± 50.790 (+59%) | 540.855 ± 3.289 (+29%) | 33.476 ± 2.813 (+60%) | 161.364 ± 11.729 (+61%) |
| HA | 8728.101±185.859 (+53%) | 627.880 ± 0.879 (+ 39%) | 35.846 ± 1.352 (+63%) | 126.704 ± 3.764 (+50%) |
| Control | 4134.259 ± 47.604 | 382.677 ± 0.560 | 13.278 ± 0.157 | 63.529 ± 0.290 |
| EVOO Samples | EVOO Samples | |||
| M10 | 7.317 ± 0.054 (+57%) | 0.251 ± 0.005 (+10%) | 0.203 ± 0.013 (+53%) | 9.829 ± 0.035 (+56%) |
| TH1 | 7.648 ± 0.072 (+58%) | 0.182 ± 0.002 (−26%) | 0.22 ± 0.009 (+57%) | 7.743 ± 0.062 (+44%) |
| 6PP | 6.145 ± 0.009 (+48%) | 0.201 ± 0.001 (−13%) | 0.172 ± 0.004 (+45%) | 11.52 ± 0.308 (+62%) |
| HA | 10.218 ± 0.014 (+70%) | 0.221 ± 0.001 (−3%) | 0.152 ± 0.005 (+37%) | 8.355 ± 0.14 (+48%) |
| Control | 3.178 ± 0.046 | 0.228 ± 0.001 | 0.095 ± 0.007 | 4.344 ± 0.097 |
| Phenolic Alcohols (mg/kg) | ||
|---|---|---|
| Tyrosol | Hydroxytyrosol | |
| LEAF Samples | ||
| M10 | 12.897 ± 1.325 (−390%) | 0.928 ± 0.008 (+31%) |
| TH1 | 11.524 ± 0.034 (−448%) | 0.498 ± 0.016 (−28%) |
| 6PP | 12.428 ± 0.269 (−408%) | 0.535 ± 0.01 (−19%) |
| HA | 12.198 ± 1.342 (−418%) | 0.683 ± 0.005 (+7%) |
| Control | 63.149 ± 2.143 | 0.636 ± 0.007 |
| EVOO Samples | ||
| M10 | 105.917 ± 1.698 (−325%) | 595.136 ± 17.946 (+74%) |
| TH1 | 153.018 ± 0.253 (−194%) | 236.603 ± 6.405 (+35%) |
| 6PP | 156.413 ± 1.237 (−188%) | 504.858 ± 3.7 (+70%) |
| HA | 118.196 ± 0.443 (−281%) | 180.862 ± 3.789 (+15%) |
| Control | 450.646 ± 6.736 | 152.706 ± 0.424 |
| Phenolic Acid (mg/kg) | ||||||
|---|---|---|---|---|---|---|
| 4-Hydroxybenzoic Acid | 3-Hydroxybenzoic Acid | Vanillic Acid | p-Coumaric Acid | Cinnamic Acid | Ferulic Acid | |
| LEAF Samples | ||||||
| M10 | 27.29 ± 1.787 (+42%) | 229.718 ± 2.840 (−7%) | 159.895 ± 0.498 (+41%) | 27.859 ± 0.092 (−116%) | 3.954 ± 0.06 (+67%) | 21.646 ± 0.023 (−70%) |
| TH1 | 20.41 ± 0.677 (+22%) | 235.590 ± 8.660 (−4%) | 168.819 ± 6.591 (+44%) | 23.787 ± 1.112 (−152%) | 2.986 ± 0.008 (+57%) | 22.456 ± 0.916 (−64%) |
| 6PP | 19.908 ± 0.227 (+20%) | 256.863 ± 0.801 (−4%) | 182.615 ± 3.141 (+48%) | 43.651± 0.342 (−38%) | 1.150 ± 0.061 (−12%) | 30.275 ± 0.107 (−22%) |
| HA | 20.190 ± 0.398 (+21%) | 249.687 ± 2.176 (+1%) | 178.680 ± 4.492 (+47%) | 26.627 ± 0.434 (−125%) | 2.651±0.33 (+51%) | 23.313 ± 0.279 (−58%) |
| Control | 15.833 ± 0.102 | 245.931±1.295 | 94.195 ± 0.497 | 60.067 + 0.865 | 1.287 + 0.031 | 36.836 ± 0.093 |
| EVOO Samples | ||||||
| M10 | 0.883 ± 0.007 (+31%) | 0.796 ± 0.004 (+66%) | 7.05 ± 0.059 (+62%) | 3.274 ± 0.024 (+56%) | 0.482 ± 0.009 (+9%) | 0.131 ± 0.001 (+51%) |
| TH1 | 0.473 ± 0.015 (−28%) | 0.845 ± 0.005 (+68%) | 8.35 ± 40.006 (+68%) | 3.422 ± 0.032 (+58%) | 0.348 ± 0.003 (−26%) | 0.219 ± 0.005 (+71%) |
| 6PP | 0.509 ± 0.009 (−27%) | 0.75 ± 0.005 (+64%) | 7.814 ± 0.067 (+66%) | 2.749 ± 0.004 (+48%) | 0.386 ± 0.002 (−13%) | 0.125 ± 0.003 (+49%) |
| HA | 0.649 ± 0.005 (+7%) | 1.172 ± 0.012 (+77%) | 13.17 ± 0.116 (+80%) | 4.572 ± 0.006 (+69%) | 0.423 ± 0.002 (−3%) | 0.238 ± 0.000 (+73%) |
| Control | 0.605 ± 0.007 | 0.27 ± 0.003 | 2.663 ± 0.012 | 1.422 ± 0.021 | 0.438 ± 0.002 | 0.064 ± 0.000 |
| Secoiridoid (mg/kg) | |||||||
|---|---|---|---|---|---|---|---|
| Ligstroside | Oleuropein | Secologanoside | Elenaic Acid | Oleacein | Oleuropein-Aglycone Mono-Aldehyde | Ligstroside-Aglycone di-Aldehyde | |
| LEAF Samples | |||||||
| M10 | 907.225 ± 25.038 (+69%) | 15225.842 ± 261.018 (+41%) | 307.740 ± 9.109 (+3%) | 370.346 ± 6.552 (−105%) | 6442.141 ± 43.543 (+87%) | 344.531 ± 5.578 (−71%) | 117.220 ± 2.866 (−34%) |
| TH1 | 754.591 ± 12.805 (+62%) | 14230.720 ± 297.140 (+36%) | 252.887 ± 9.855 (−18%) | 568.815 ± 6.404 (−33%) | 273.507 ± 2.253 (−209%) | 178.034 ± 4.785 (−230%) | 24.547 ± 0.547 (−547%) |
| 6PP | 238.574 ± 4.964 (−19%) | 11784.606 ± 108.787 (+23%) | 274.257 ± 3.357 (−8%) | 561.516 ± 2.589 (−35%) | 2650.549 ± 2.611 (+68%) | 247.182 ± 0.604 (−138%) | 152.650 ± 0.571 (−3%) |
| HA | 217.621 ± 7.405 (−31%) | 10272.050 ± 223.885 (+12%) | 274.860 ± 7.500 (−8%) | 498.886 ± 7.152 (−52%) | 364.933 ± 6.272 (−131%) | 124.221 ± 2.778 (−373%) | 55.490 ± 0.108 (−194%) |
| Control | 284.823 ± 2.205 | 9043.638 ± 189.420 | 297.833 ± 1.635 | 758.908 ± 22.919 | 844.611 ± 5.676 | 587.819 ± 5.041 | 157.254 ± 1.435 |
| EVOO Samples | |||||||
| M10 | 0.009 ± 0.001 (−433%) | 0.145 ± 0.029 (+4%) | 0.391 ± 0.002 (+52%) | 15.494 ± 0.13 (+62%) | 1031.14 ± 9.208 (−8%) | 344.531 ± 5.578 (−71%) | 712.316 ± 0.683 (−42%) |
| TH1 | 0.069 ± 0.01 (+30%) | 0.152 ± 0.01 (+8%) | 0.205 ± 0.003 (+10%) | 18.36 ± 0.014 (+68%) | 871.855 ± 0.506 (−27%) | 178.034 ± 4.785 (−230%) | 519.144 ± 3.714 (−95%) |
| 6PP | 0.007 ± 0.002 (−586%) | 0.15 ± 0.00 (+7%) | 0.207 ± 0.006 (+10%) | 17.174 ± 0.11 (+66%) | 736.801 ± 8.596 (−491%) | 247.182 ± 0.604 (−138%) | 505.106 ± 4.879 (−100%) |
| HA | 0.008 ± 0.002 (−500%) | 0.155 ± 0.013 (+10%) | 0.205 ± 0.00 (+10%) | 28.945 ± 0.255 (+80%) | 481.884 ± 0.819 (−128%) | 124.221 ± 2.778 (−373%) | 379.34 ± 2.044 (−166%) |
| Control | 0.048 ± 0.007 | 0.139 ± 0.007 | 0.186 ± 0.01 | 5.854 ± 0.026 | 1100.931 ± 5.181 | 587.819 ± 5.041 | 1011.245 ± 7.233 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dini, I.; Graziani, G.; Fedele, F.L.; Sicari, A.; Vinale, F.; Castaldo, L.; Ritieni, A. Effects of Trichoderma Biostimulation on the Phenolic Profile of Extra-Virgin Olive Oil and Olive Oil By-Products. Antioxidants 2020, 9, 284. https://doi.org/10.3390/antiox9040284
Dini I, Graziani G, Fedele FL, Sicari A, Vinale F, Castaldo L, Ritieni A. Effects of Trichoderma Biostimulation on the Phenolic Profile of Extra-Virgin Olive Oil and Olive Oil By-Products. Antioxidants. 2020; 9(4):284. https://doi.org/10.3390/antiox9040284
Chicago/Turabian StyleDini, Irene, Giulia Graziani, Francesca Luisa Fedele, Andrea Sicari, Francesco Vinale, Luigi Castaldo, and Alberto Ritieni. 2020. "Effects of Trichoderma Biostimulation on the Phenolic Profile of Extra-Virgin Olive Oil and Olive Oil By-Products" Antioxidants 9, no. 4: 284. https://doi.org/10.3390/antiox9040284
APA StyleDini, I., Graziani, G., Fedele, F. L., Sicari, A., Vinale, F., Castaldo, L., & Ritieni, A. (2020). Effects of Trichoderma Biostimulation on the Phenolic Profile of Extra-Virgin Olive Oil and Olive Oil By-Products. Antioxidants, 9(4), 284. https://doi.org/10.3390/antiox9040284










