Ginkgo biloba Prevents Oxidative Stress-Induced Apoptosis Blocking p53 Activation in Neuroblastoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Chemicals
2.2. Cell Proliferation Assay
2.3. Propidium Iodide and DAPI Staining Assay
2.4. Mitochondria Membrane Potential Measurement
2.5. RNA Extraction and q-PCR
2.6. Western Blot
2.7. Statistical Analysis
3. Results
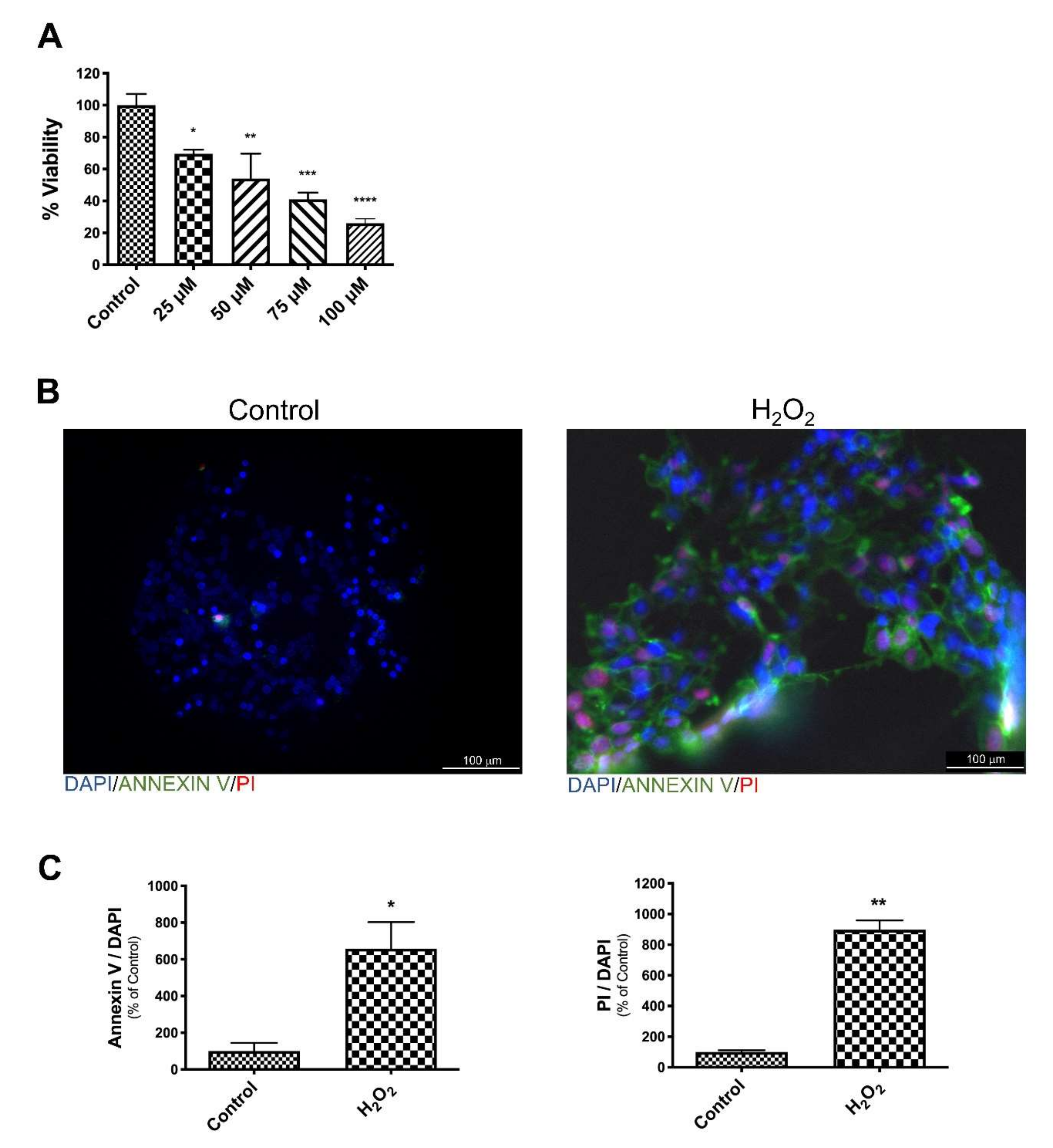
3.1. EGb Protects SK-N-BE Cells Against Oxidative Stress Induced Apoptotic Cell Death
3.2. EGb Protects SK-N-BE Cells Against Oxidative Stress Induced Apoptosis
3.3. EGb Mitigated the H2O2 Induced Decrease in Mitochondrial Membrane Potential
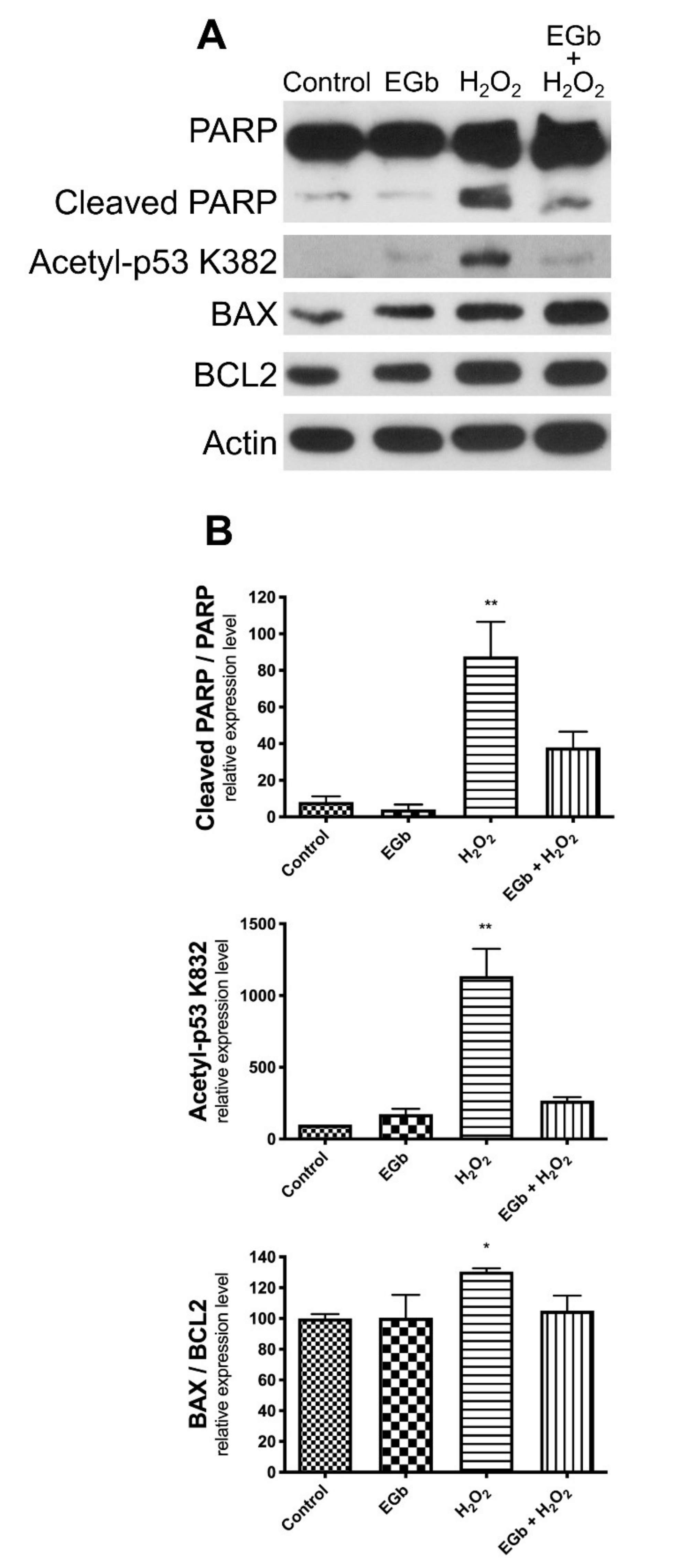
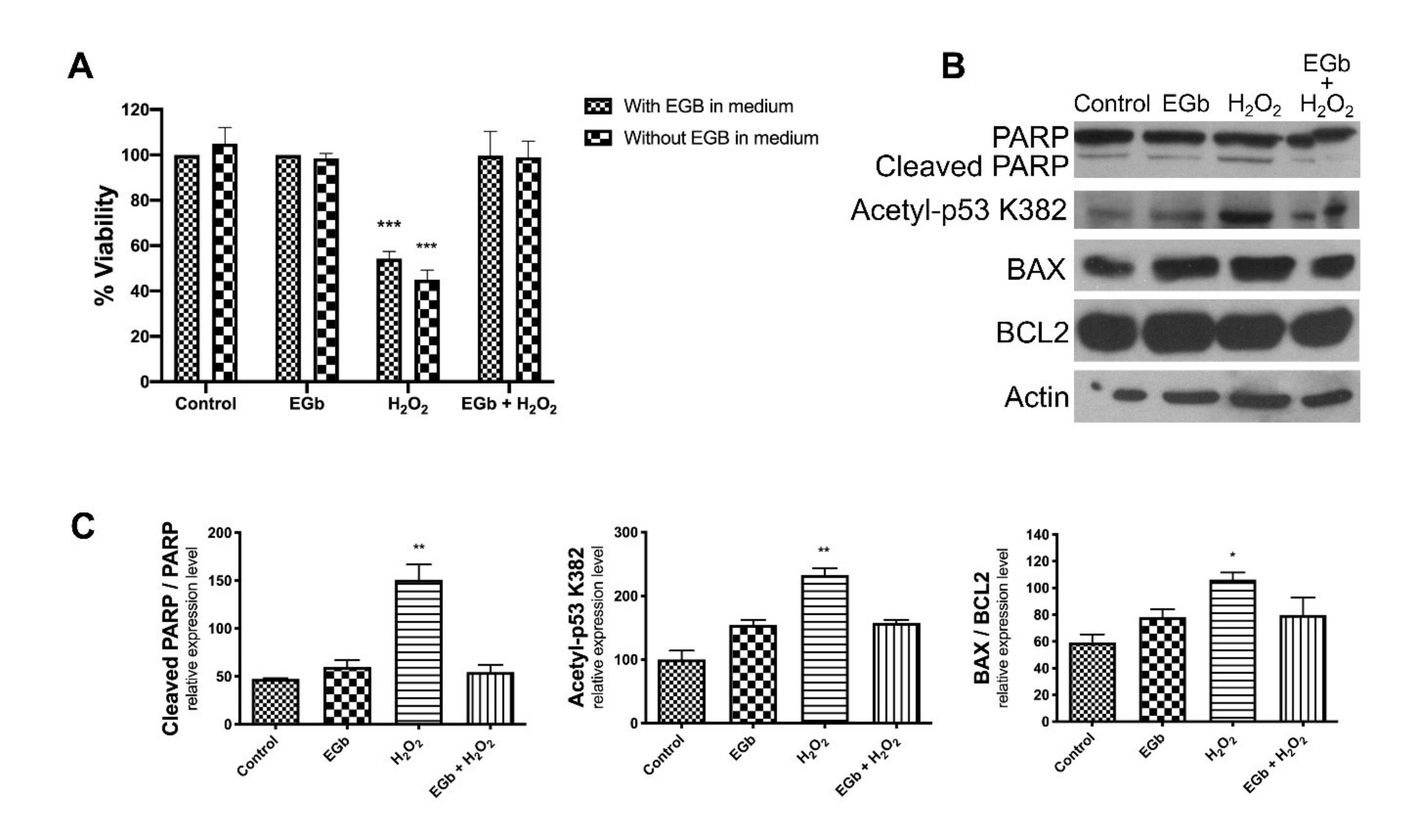
3.4. EGb Exhibits Intracellular Anti-Apoptotic Effect
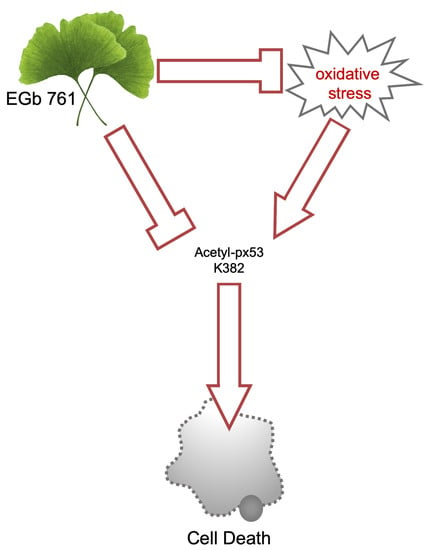
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bergamini, C.M.; Gambetti, S.; Dondi, A.; Cervellati, C. Oxygen, reactive oxygen species and tissue damage. Curr. Pharm. Des. 2004, 10, 1611–1626. [Google Scholar] [CrossRef] [PubMed]
- Behar, T.N.; Colton, C.A. Redox regulation of neuronal migration in a down syndrome model. Free Radic. Biol. Med. 2003, 35, 566–575. [Google Scholar] [CrossRef]
- Finkel, T. Redox-dependent signal transduction. FEBS Lett. 2000, 476, 52–54. [Google Scholar] [CrossRef]
- Martindale, J.L.; Holbrook, N.J. Cellular response to oxidative stress: Signaling for suicide and survival. J. Cell Physiol. 2002, 192, 1–15. [Google Scholar] [CrossRef]
- Filosa, S.; Pecorelli, A.; D’Esposito, M.; Valacchi, G.; Hajek, J. Exploring the possible link between MeCP2 and oxidative stress in rett syndrome. Free Radic. Biol. Med. 2015, 88, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Dias, V.; Junn, E.; Mouradian, M.M. The role of oxidative stress in Parkinson’s disease. J. Parkinsons Dis. 2013, 3, 461–491. [Google Scholar] [CrossRef]
- Lovell, M.A.; Markesbery, W.R. Oxidative DNA damage in mild cognitive impairment and late-stage Alzheimer’s disease. Nucleic Acids Res. 2007, 35, 7497–7504. [Google Scholar] [CrossRef] [PubMed]
- Dugger, B.N.; Dickson, D.W. Pathology of neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef]
- Yu, T.; Fan, Y.; Xu, Y.; Xu, L.; Xu, G.; Cao, F.; Jiang, H. Standardized Ginkgo biloba extract EGb 761(R) attenuates early brain injury following subarachnoid hemorrhage via suppressing neuronal apoptosis through the activation of Akt signaling. Biomed. Pharmacother. 2018, 107, 329–337. [Google Scholar] [CrossRef]
- Chávez-Morales, R.M.; Jaramillo-Juárez, F.; Rodríguez-Vázquez, M.L.; Martínez-Saldaña, M.C.; Del Río, F.A.P.; Garfias-López, J.A. The Ginkgo biloba extract (GbE) protects the kidney from damage produced by a single and low dose of carbon tetrachloride in adult male rats. Exp. Toxicol. Pathol. 2017, 69, 430–434. [Google Scholar] [CrossRef]
- Eckert, A.; Keil, U.; Scherping, I.; Hauptmann, S.; Muller, W.E. Stabilization of mitochondrial membrane potential and improvement of neuronal energy metabolism by Ginkgo biloba extract EGb 761. Ann. N. Y. Acad. Sci. 2005, 1056, 474–485. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wei, T.; Gao, Z.; Zhao, B.; Hou, J.; Xu, H.; Xin, W.; Packer, L. Different effects of the constituents of EGb761 on apoptosis in rat cerebellar granule cells induced by hydroxyl radicals. Biochem. Mol. Biol. Int. 1999, 47, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Gohil, K.; Packer, L. Bioflavonoid-rich botanical extracts show antioxidant and gene regulatory activity. Ann. N. Y. Acad. Sci. 2002, 957, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Qi, Y.; Chen, T. Long-term pre-treatment of antioxidant Ginkgo biloba extract EGb-761 attenuates cerebral-ischemia-induced neuronal damage in aged mice. Biomed. Pharmacother. 2017, 85, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Rapp, M.; Burkart, M.; Kohlmann, T.; Bohlken, J. Similar treatment outcomes with Ginkgo biloba extract EGb 761 and donepezil in Alzheimer’s dementia in very old age: A retrospective observational study. Int. J. Clin. Pharmacol. Ther. 2018, 56, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Dhiman, M.; Perez-Polo, J.R.; Mantha, A.K. Ginkgolide B revamps neuroprotective role of apurinic/apyrimidinic endonuclease 1 and mitochondrial oxidative phosphorylation against Abeta25-35 -induced neurotoxicity in human neuroblastoma cells. J. Neurosci. Res. 2015, 93, 938–947. [Google Scholar] [CrossRef]
- Vijayakumaran, S.; Nakamura, Y.; Henley, J.M.; Pountney, D.L. Ginkgolic acid promotes autophagy-dependent clearance of intracellular alpha-synuclein aggregates. Mol. Cell. Neurosci. 2019, 101, 103416. [Google Scholar] [CrossRef]
- Rojas, P.; Montes, P.; Rojas, C.; Serrano-Garcia, N.; Rojas-Castaneda, J.C. Effect of a phytopharmaceutical medicine, Ginko biloba extract 761, in an animal model of Parkinson’s disease: Therapeutic perspectives. Nutrition 2012, 28, 1081–1088. [Google Scholar] [CrossRef]
- Di Meo, F.; Filosa, S.; Madonna, M.; Giello, G.; Di Pardo, A.; Maglione, V.; Baldi, A.; Crispi, S. Curcumin C3 complex(R)/Bioperine(R) has antineoplastic activity in mesothelioma: An in vitro and in vivo analysis. J. Exp. Clin. Cancer Res. 2019, 38, 360. [Google Scholar] [CrossRef]
- Crispi, S.; Calogero, R.A.; Santini, M.; Mellone, P.; Vincenzi, B.; Citro, G.; Vicidomini, G.; Fasano, S.; Meccariello, R.; Cobellis, G.; et al. Global gene expression profiling of human pleural mesotheliomas: Identification of matrix metalloproteinase 14 (MMP-14) as potential tumour target. PLoS ONE 2009, 4, e7016. [Google Scholar] [CrossRef]
- Beyfuss, K.; Hood, D.A. A systematic review of p53 regulation of oxidative stress in skeletal muscle. Redox Rep. 2018, 23, 100–117. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.M.; Quelle, D.E. p53 Acetylation: Regulation and consequences. Cancers 2015, 7, 30–69. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Vaseva, A.V.; Moll, U.M. The mitochondrial p53 pathway. Biochim. Biophys. Acta 2009, 1787, 414–420. [Google Scholar] [CrossRef]
- Wyss-Coray, T. Ageing, neurodegeneration and brain rejuvenation. Nature 2016, 539, 180–186. [Google Scholar] [CrossRef]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef]
- Kandiah, N.; Ong, P.A.; Yuda, T.; Ng, L.L.; Mamun, K.; Merchant, R.A.; Chen, C.; Dominguez, J.; Marasigan, S.; Ampil, E.; et al. Treatment of dementia and mild cognitive impairment with or without cerebrovascular disease: Expert consensus on the use of Ginkgo biloba extract, EGb 761((R)). CNS Neurosci. Ther. 2019, 25, 288–298. [Google Scholar] [CrossRef]
- Spiegel, R.; Kalla, R.; Mantokoudis, G.; Maire, R.; Mueller, H.; Hoerr, R.; Ihl, R. Ginkgo biloba extract EGb 761((R)) alleviates neurosensory symptoms in patients with dementia: A meta-analysis of treatment effects on tinnitus and dizziness in randomized, placebo-controlled trials. Clin. Interv. Aging 2018, 13, 1121–1127. [Google Scholar] [CrossRef]
- Madabhushi, R.; Pan, L.; Tsai, L.H. DNA damage and its links to neurodegeneration. Neuron 2014, 83, 266–282. [Google Scholar] [CrossRef]
- Chow, H.M.; Herrup, K. Genomic integrity and the ageing brain. Nat. Rev. Neurosci. 2015, 16, 672–684. [Google Scholar] [CrossRef]
- Smith, J.V.; Luo, Y. Studies on molecular mechanisms of Ginkgo biloba extract. Appl. Microbiol. Biotechnol. 2004, 64, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Bridi, R.; Crossetti, F.P.; Steffen, V.M.; Henriques, A.T. The antioxidant activity of standardized extract of Ginkgo biloba (EGb 761) in rats. Phytother. Res. 2001, 15, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.; Colciaghi, F.; Cattabeni, F.; Di Luca, M. Ginkgo biloba extract: From molecular mechanisms to the treatment of Alzhelmer’s disease. Cell Mol. Biol. 2002, 48, 613–623. [Google Scholar] [PubMed]
- Smith, J.V.; Burdick, A.J.; Golik, P.; Khan, I.; Wallace, D.; Luo, Y. Anti-apoptotic properties of Ginkgo biloba extract EGb 761 in differentiated PC12 cells. Cell Mol. Biol. 2002, 48, 699–707. [Google Scholar] [PubMed]
- Shi, C.; Zhao, L.; Zhu, B.; Li, Q.; Yew, D.T.; Yao, Z.; Xu, J. Dosage effects of EGb761 on hydrogen peroxide-induced cell death in SH-SY5Y cells. Chem. Biol. Interact. 2009, 180, 389–397. [Google Scholar] [CrossRef]
- Jiang, X.; Nie, B.; Fu, S.; Hu, J.; Yin, L.; Lin, L.; Wang, X.; Lu, P.; Xu, X.M. EGb761 protects hydrogen peroxide-induced death of spinal cord neurons through inhibition of intracellular ROS production and modulation of apoptotic regulating genes. J. Mol. Neurosci. 2009, 38, 103–113. [Google Scholar] [CrossRef]
- Ni, Y.; Zhao, B.; Hou, J.; Xin, W. Preventive effect of Ginkgo biloba extract on apoptosis in rat cerebellar neuronal cells induced by hydroxyl radicals. Neurosci. Lett. 1996, 214, 115–118. [Google Scholar] [CrossRef]
- Savaskan, E.; Mueller, H.; Hoerr, R.; von Gunten, A.; Gauthier, S. Treatment effects of Ginkgo biloba extract EGb 761® on the spectrum of behavioral and psychological symptoms of dementia: Meta-analysis of randomized controlled trials. Int. Psychogeriatr. 2018, 30, 285–293. [Google Scholar] [CrossRef]
- Gauthier, S.; Schlaefke, S. Efficacy and tolerability of Ginkgo biloba extract EGb 761® in dementia: A systematic review and meta-analysis of randomized placebo-controlled trials. Clin. Interv. Aging 2014, 9, 2065–2077. [Google Scholar] [CrossRef]
- Corey, J.M.; Gertz, C.C.; Sutton, T.J.; Chen, Q.; Mycek, K.B.; Wang, B.S.; Martin, A.A.; Johnson, S.L.; Feldman, E.L. Patterning N-type and S-type neuroblastoma cells with Pluronic F108 and ECM proteins. J. Biomed. Mater. Res. A 2010, 93, 673–686. [Google Scholar] [CrossRef]
- Krishna, A.; Biryukov, M.; Trefois, C.; Antony, P.M.; Hussong, R.; Lin, J.; Heinaniemi, M.; Glusman, G.; Koglsberger, S.; Boyd, O.; et al. Systems genomics evaluation of the SH-SY5Y neuroblastoma cell line as a model for Parkinson’s disease. BMC Genomics 2014, 15, 1154. [Google Scholar] [CrossRef] [PubMed]
- Maraldi, T.; Riccio, M.; Zambonin, L.; Vinceti, M.; De Pol, A.; Hakim, G. Low levels of selenium compounds are selectively toxic for a human neuron cell line through ROS/RNS increase and apoptotic process activation. Neurotoxicology 2011, 32, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Mata, I.F.; Jang, Y.; Kim, C.H.; Hanna, D.S.; Dorschner, M.O.; Samii, A.; Agarwal, P.; Roberts, J.W.; Klepitskaya, O.; Shprecher, D.R.; et al. The RAB39B p.G192R mutation causes X-linked dominant Parkinson’s disease. Mol. Neurodegener. 2015, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Testa, G.; Staurenghi, E.; Giannelli, S.; Gargiulo, S.; Guglielmotto, M.; Tabaton, M.; Tamagno, E.; Gamba, P.; Leonarduzzi, G. A silver lining for 24-hydroxycholesterol in Alzheimer’s disease: The involvement of the neuroprotective enzyme sirtuin 1. Redox Biol. 2018, 17, 423–431. [Google Scholar] [CrossRef]
- Knights, C.D.; Catania, J.; Di Giovanni, S.; Muratoglu, S.; Perez, R.; Swartzbeck, A.; Quong, A.A.; Zhang, X.; Beerman, T.; Pestell, R.G.; et al. Distinct p53 acetylation cassettes differentially influence gene-expression patterns and cell fate. J. Cell Biol. 2006, 173, 533–544. [Google Scholar] [CrossRef]
- Olsson, A.; Manzl, C.; Strasser, A.; Villunger, A. How important are post-translational modifications in p53 for selectivity in target-gene transcription and tumour suppression? Cell Death Differ. 2007, 14, 1561–1575. [Google Scholar] [CrossRef]
- Brochier, C.; Dennis, G.; Rivieccio, M.A.; McLaughlin, K.; Coppola, G.; Ratan, R.R.; Langley, B. Specific acetylation of p53 by HDAC inhibition prevents DNA damage-induced apoptosis in neurons. J. Neurosci. 2013, 33, 8621–8632. [Google Scholar] [CrossRef]
- Ming, L.; Wang, P.; Bank, A.; Yu, J.; Zhang, L. PUMA Dissociates Bax and Bcl-X(L) to induce apoptosis in colon cancer cells. J. Biol. Chem. 2006, 281, 16034–16042. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, M.M. Flavonoids in Ginkgo biloba fallen leaves induce apoptosis through modulation of p53 activation in melanoma cells. Oncol. Rep. 2015, 33, 433–438. [Google Scholar] [CrossRef]
- Mattson, M.P. Apoptosis in neurodegenerative disorders. Nat. Rev. Mol. Cell Biol. 2000, 1, 120–129. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Meo, F.; Cuciniello, R.; Margarucci, S.; Bergamo, P.; Petillo, O.; Peluso, G.; Filosa, S.; Crispi, S. Ginkgo biloba Prevents Oxidative Stress-Induced Apoptosis Blocking p53 Activation in Neuroblastoma Cells. Antioxidants 2020, 9, 279. https://doi.org/10.3390/antiox9040279
Di Meo F, Cuciniello R, Margarucci S, Bergamo P, Petillo O, Peluso G, Filosa S, Crispi S. Ginkgo biloba Prevents Oxidative Stress-Induced Apoptosis Blocking p53 Activation in Neuroblastoma Cells. Antioxidants. 2020; 9(4):279. https://doi.org/10.3390/antiox9040279
Chicago/Turabian StyleDi Meo, Francesco, Rossana Cuciniello, Sabrina Margarucci, Paolo Bergamo, Orsolina Petillo, Gianfranco Peluso, Stefania Filosa, and Stefania Crispi. 2020. "Ginkgo biloba Prevents Oxidative Stress-Induced Apoptosis Blocking p53 Activation in Neuroblastoma Cells" Antioxidants 9, no. 4: 279. https://doi.org/10.3390/antiox9040279
APA StyleDi Meo, F., Cuciniello, R., Margarucci, S., Bergamo, P., Petillo, O., Peluso, G., Filosa, S., & Crispi, S. (2020). Ginkgo biloba Prevents Oxidative Stress-Induced Apoptosis Blocking p53 Activation in Neuroblastoma Cells. Antioxidants, 9(4), 279. https://doi.org/10.3390/antiox9040279