Development of Multifunctional Cosmetic Cream Using Bioactive Materials from Streptomyces sp. T65 with Synthesized Mesoporous Silica Particles SBA-15
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents, Cell Lines, and Equipment
2.2. Pathogenic Bacterial Strains
2.3. Isolation and Preservation
2.4. Screening, Identification, and Phylogenetic Position of Isolated Strains
2.5. Culture of Bacteria
2.6. Fermentation
2.7. Extraction
2.8. Collection of Active Fractions by Preparative HPLC (Prep-HPLC)
2.9. Cell Viability of HaCaT Cell
2.10. Evaluation of Antioxidant Activities
2.10.1. Evaluation of Toxicity in RAW264.7 Cells
2.10.2. Nitric Oxide Determination
2.10.3. DPPH Free Radical Scavenging Assay
2.11. Evaluation of Anti-Aging Activities
2.11.1. Evaluation of Cytotoxicity in CCD-986Sk Cells
2.11.2. Human Fibroblast Proliferation Using CCD-986Sk Cells
2.11.3. Collagen Type I Synthesis Assay
2.11.4. Elastase Inhibition Assay
2.12. Evaluation of Whitening Activities
2.12.1. Evaluation of Cytotoxicity in B16F1 Cells
2.12.2. Melanin Synthesis Inhibition in B16F10 Cells
2.12.3. Mushroom Tyrosinase Inhibition Assay
2.13. Analysis of Amino Acids
2.14. Fatty Acid
2.15. Antimicrobial Activities
2.16. Synthesis of Mesoporous Silica Particle
2.17. BET Surface Area Analysis
2.18. Sustainability Assessment
2.19. Cosmetic Formulation and Application
2.19.1. Formulation and Stability Test
2.19.2. Clinical Trials, Volunteers, and Application Methods
2.19.3. Image Capturing and Processing Methods
2.20. Statistical Analysis
3. Results and Discussions
3.1. Isolation, Selection, and Phylogeny of Selected Active Strains
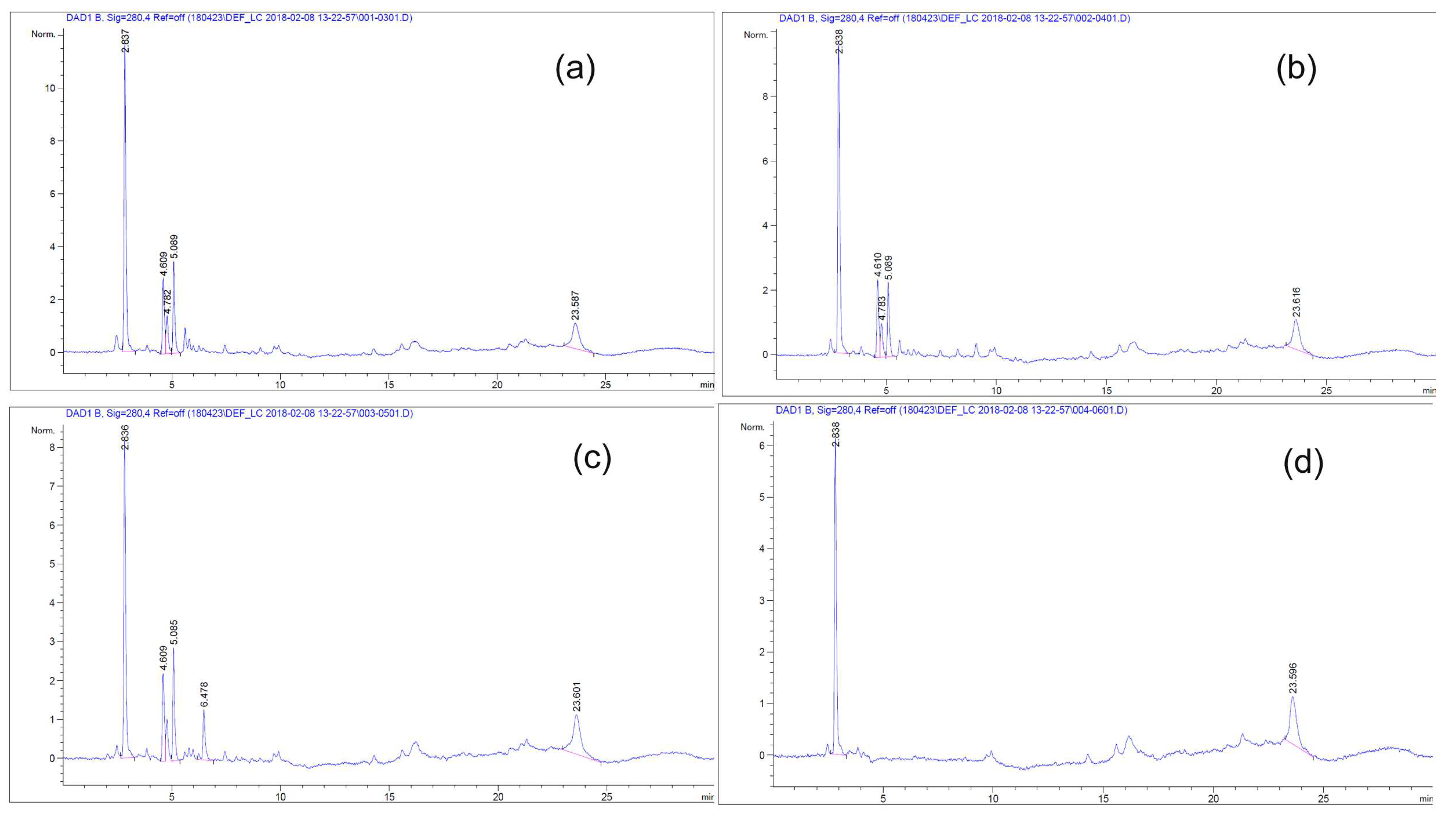
3.2. Detection and Collection of Bioactive Material
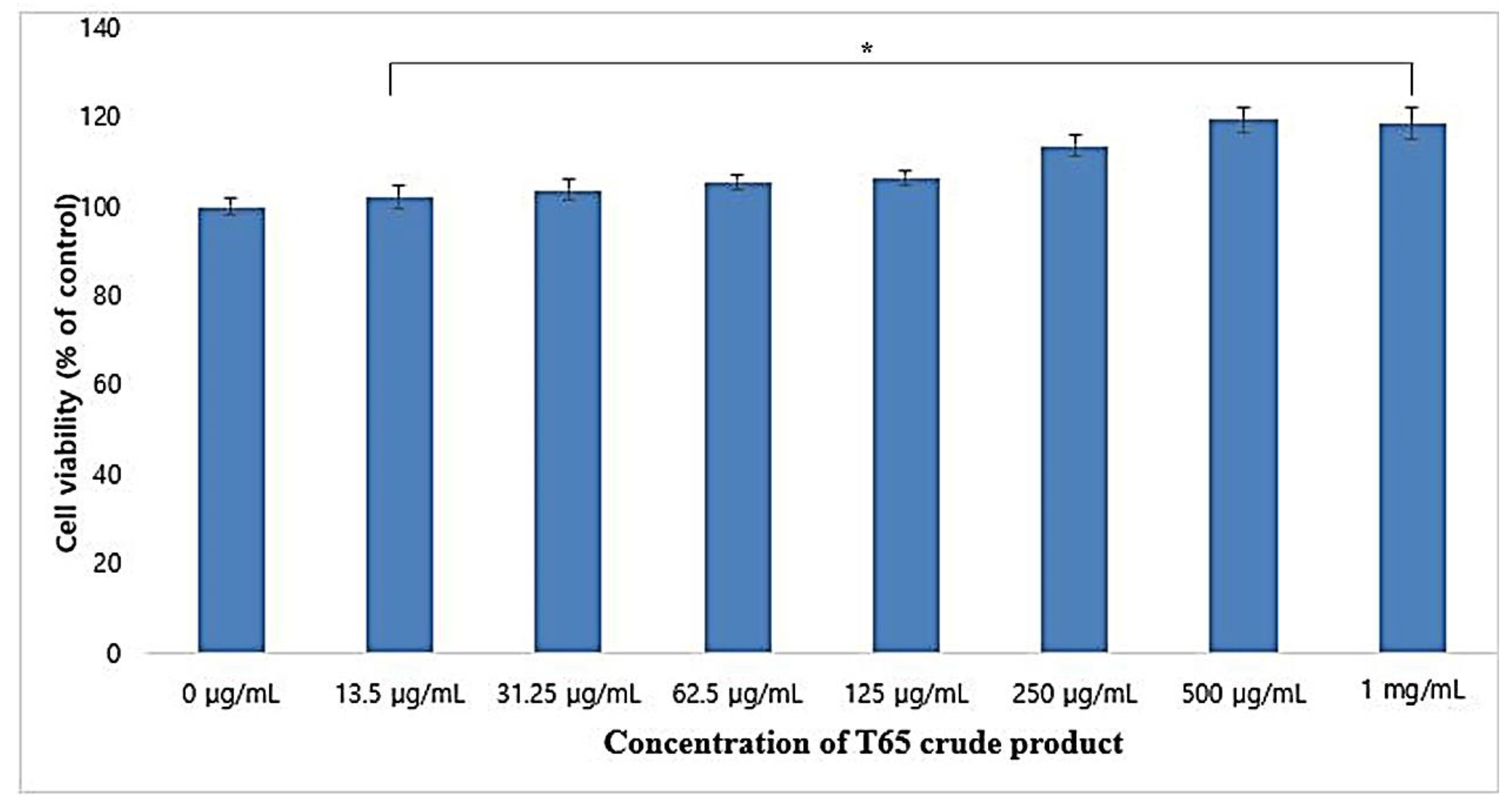
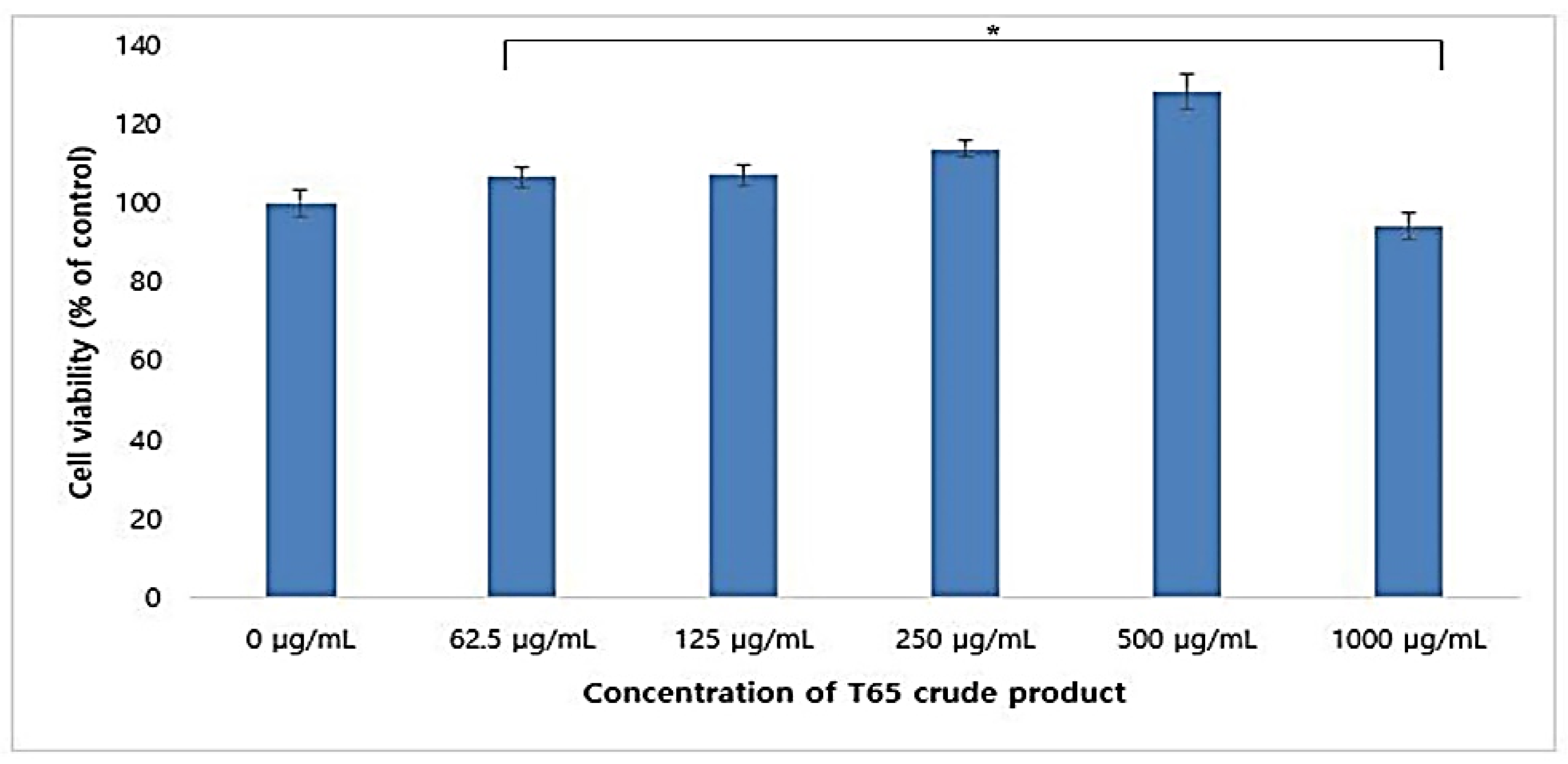
3.3. Cytotoxicity in HaCaT Cell
3.4. Antioxidant Activities
3.4.1. Cytotoxicity in RAW264.7 Cell
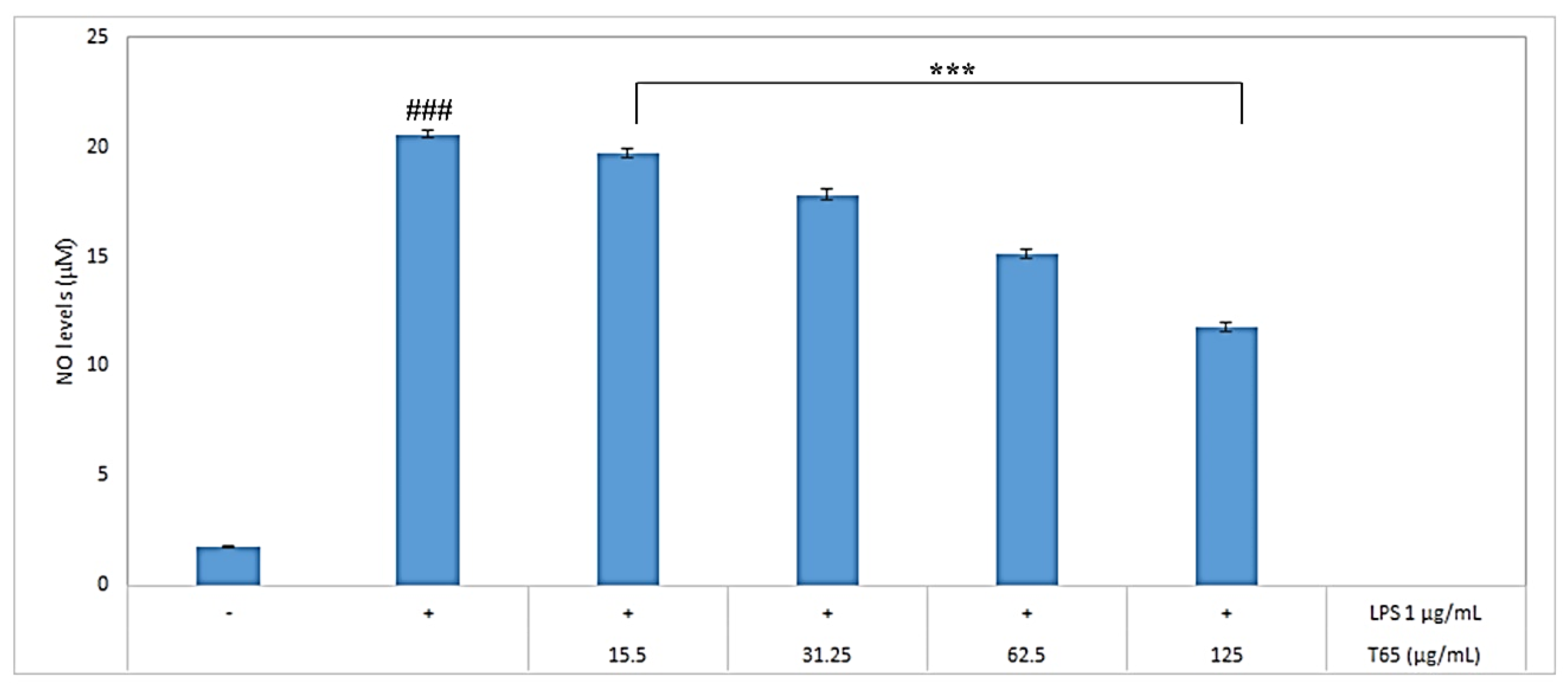
3.4.2. Inhibition of Nitric Oxide (NO) Production
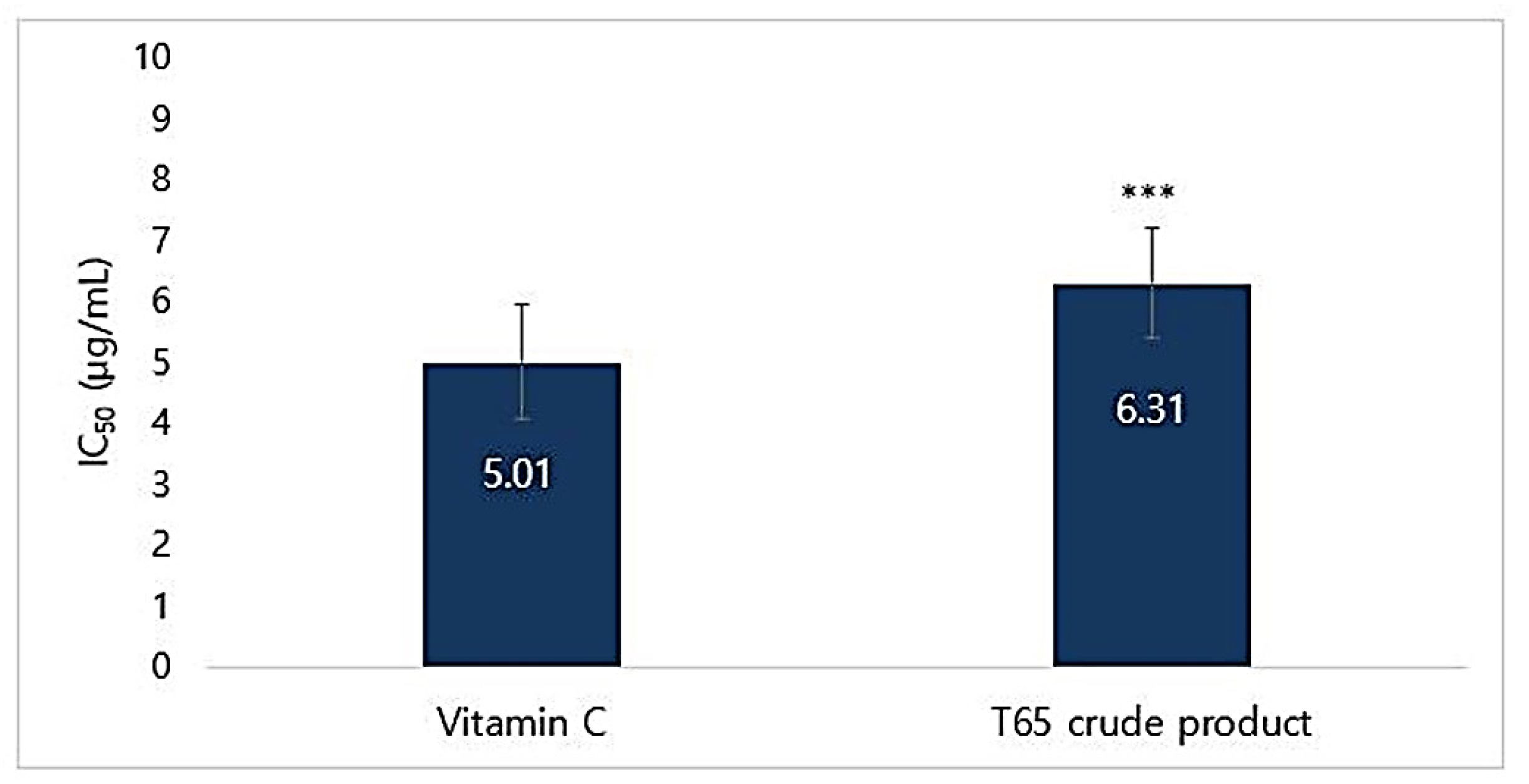
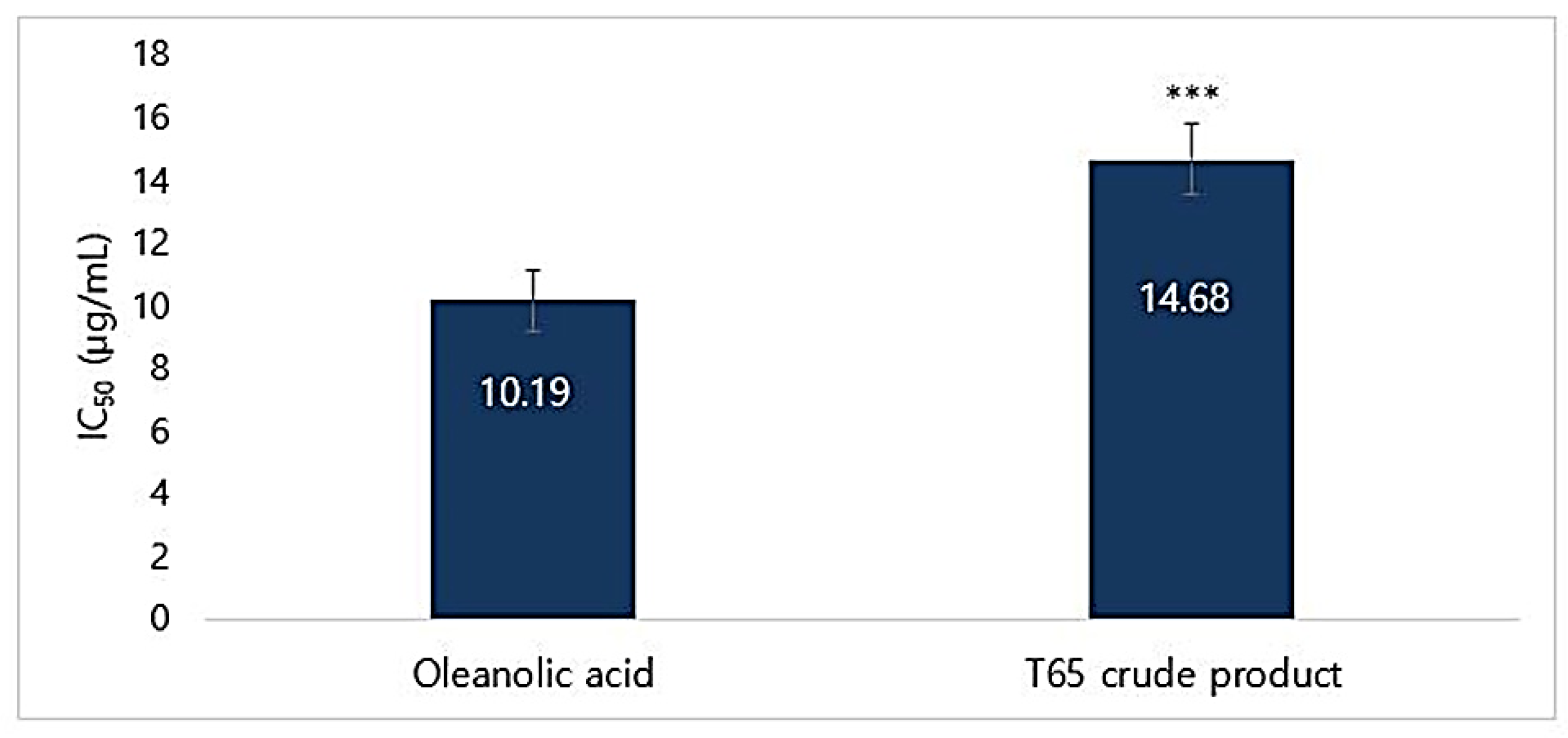
3.4.3. DPPH Radical Scavenging Activity
3.5. Antiaging Activities
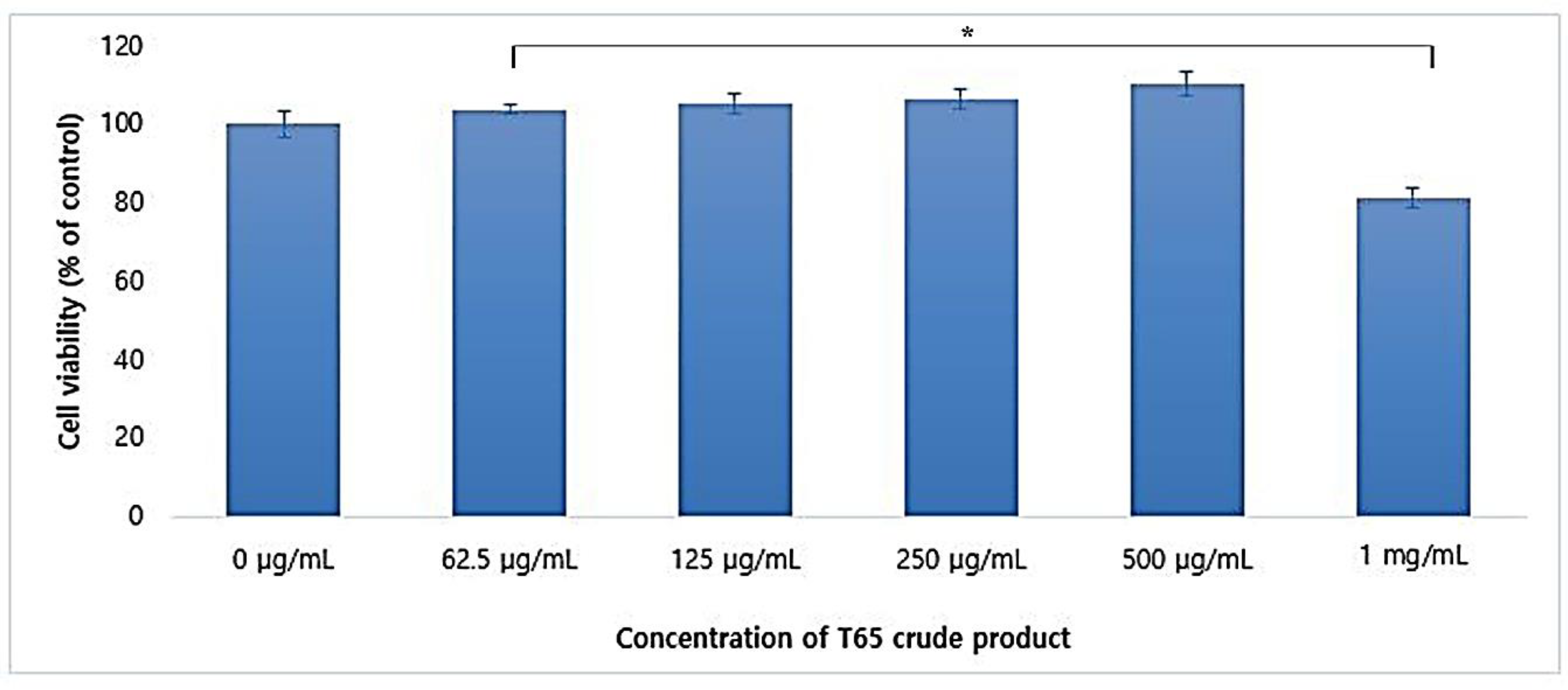
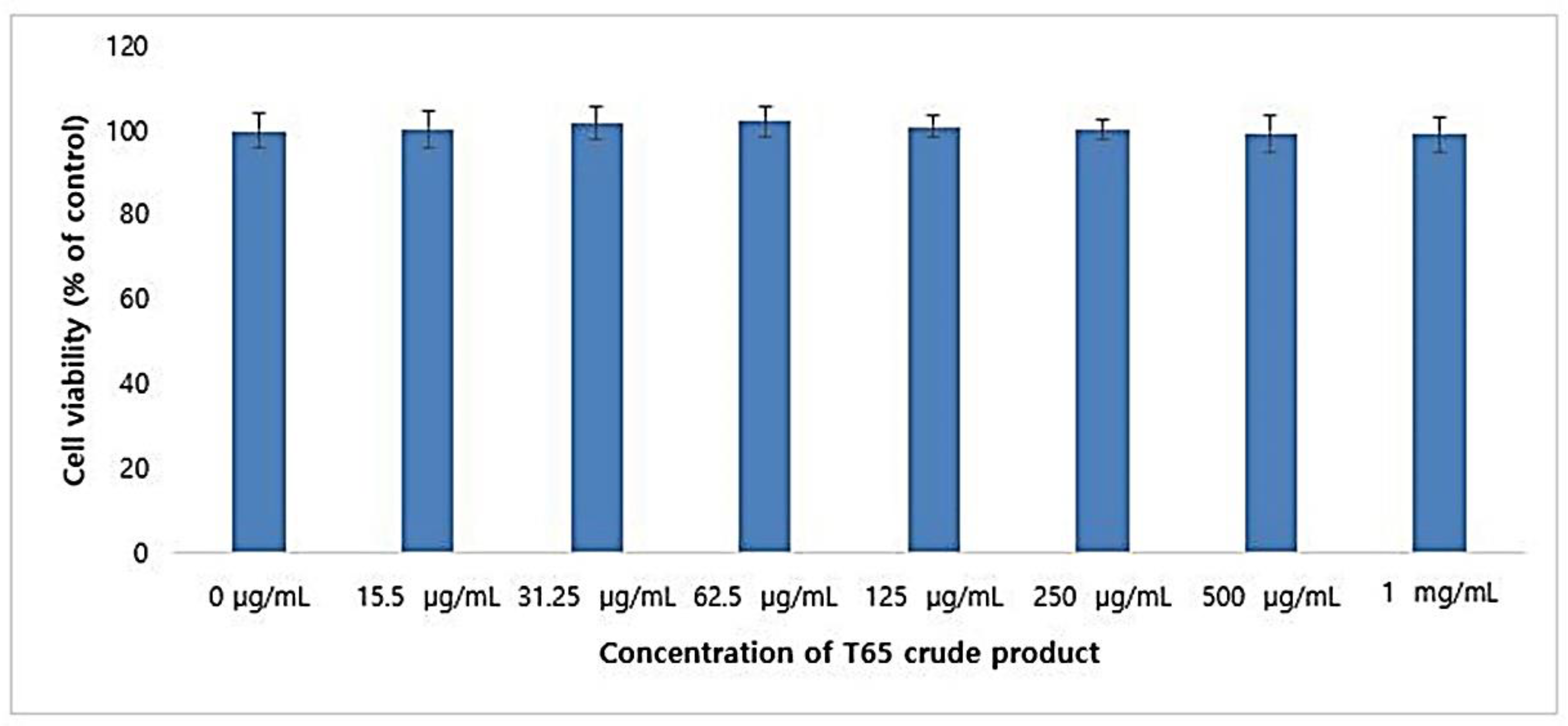
3.5.1. Cytotoxicity in CCD-986Sk Cells
3.5.2. Proliferation of HDF
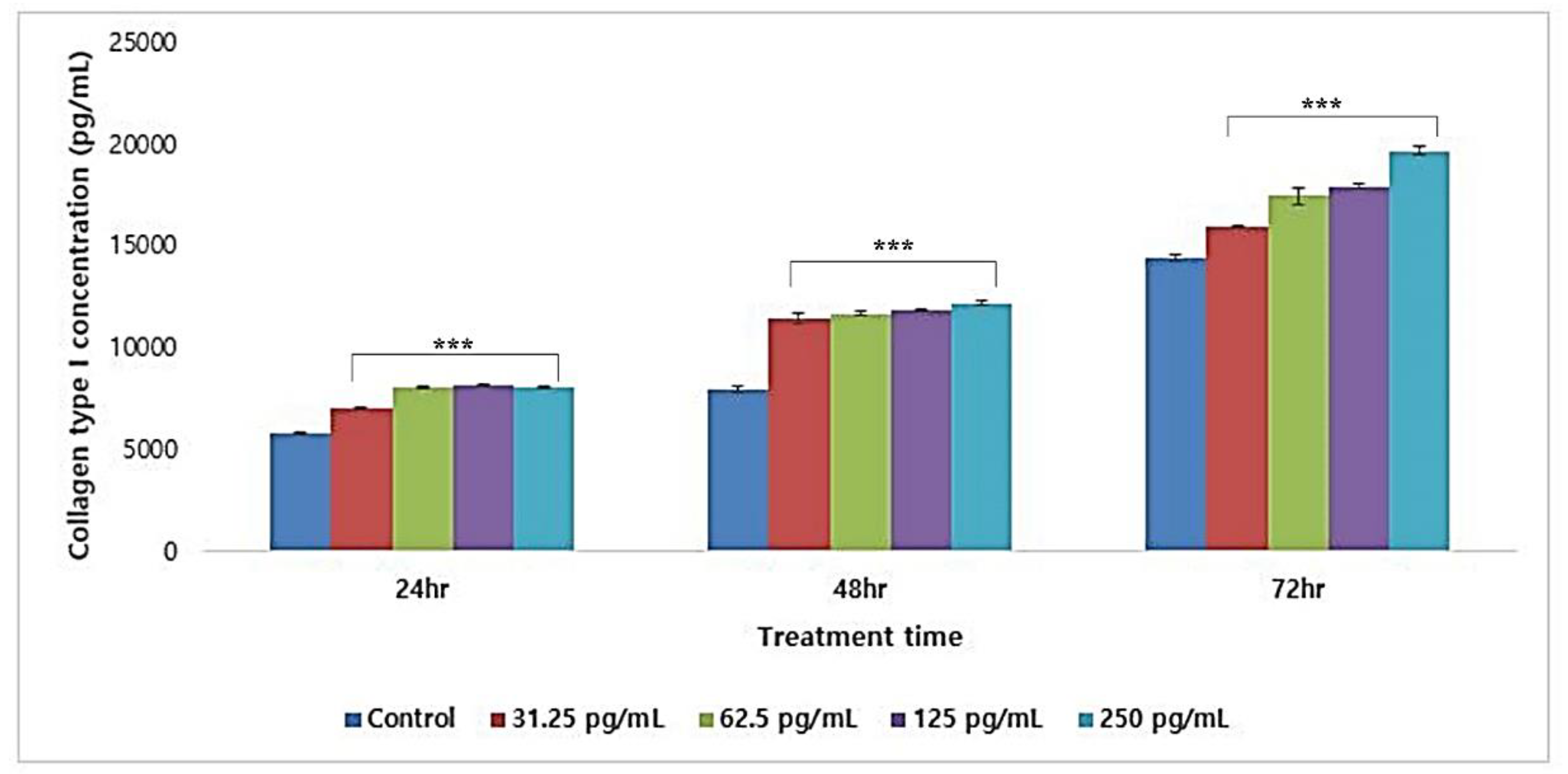
3.5.3. Collagen Type I Synthesis
3.5.4. Elastase Inhibition Assay
3.6. Whitening Activities
3.6.1. Cytotoxicity in B16F1 Cells
3.6.2. Melanin Synthesis Inhibition in B16F10 Cells
3.6.3. Mushroom Tyrosinase Inhibition Assay
3.7. Analysis of Amino Acids
3.8. Fatty Acids
3.9. Antimicrobial Activities
3.10. Mesoporous Silica Materials
3.11. Synthesis of Mesoporous Silica Particles
3.12. BET Surface Analyses
3.13. Sustainable Performance Evaluation
3.14. Cosmetic Formulation and Topical Application
3.14.1. Formulation Stability
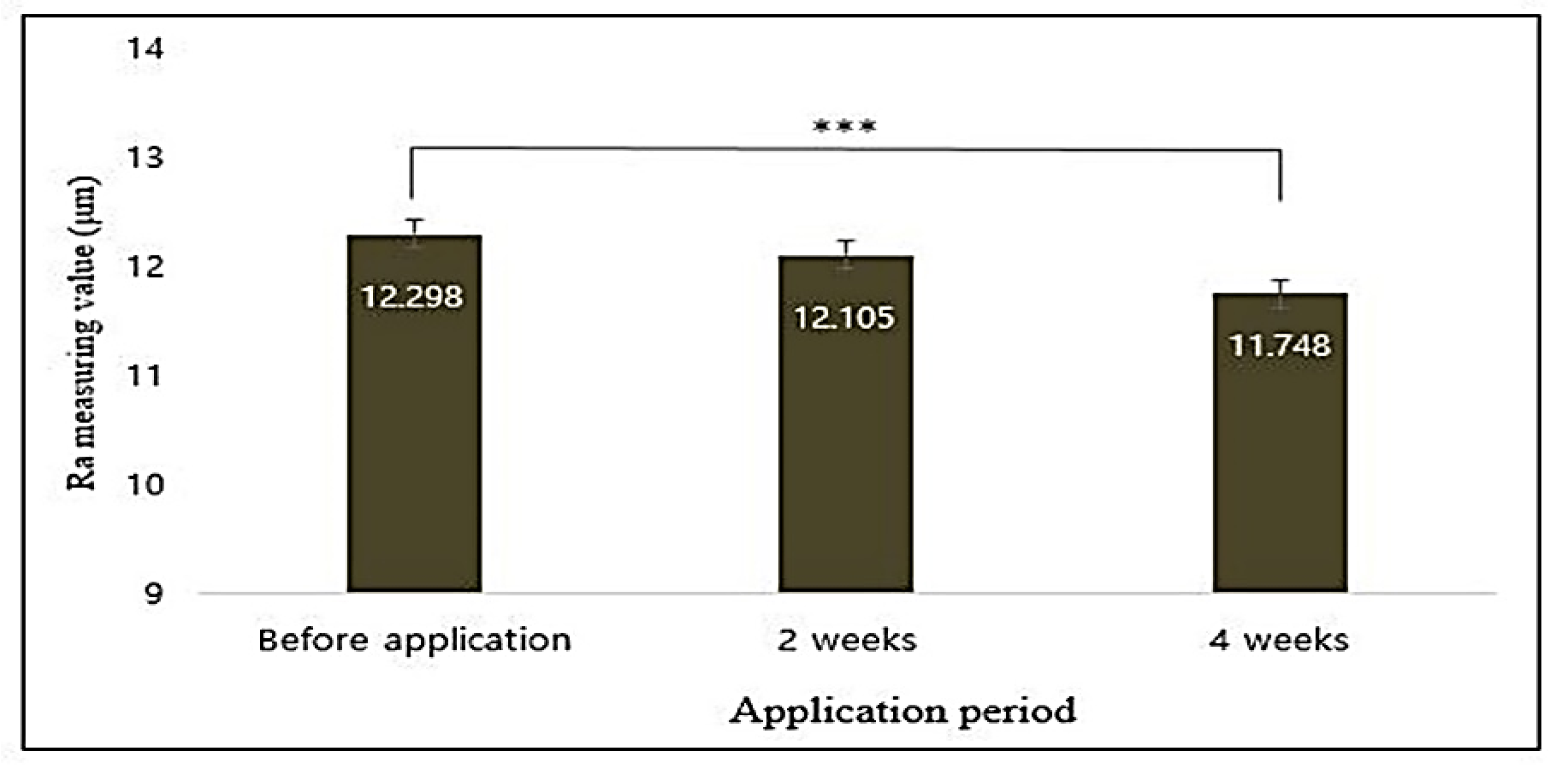
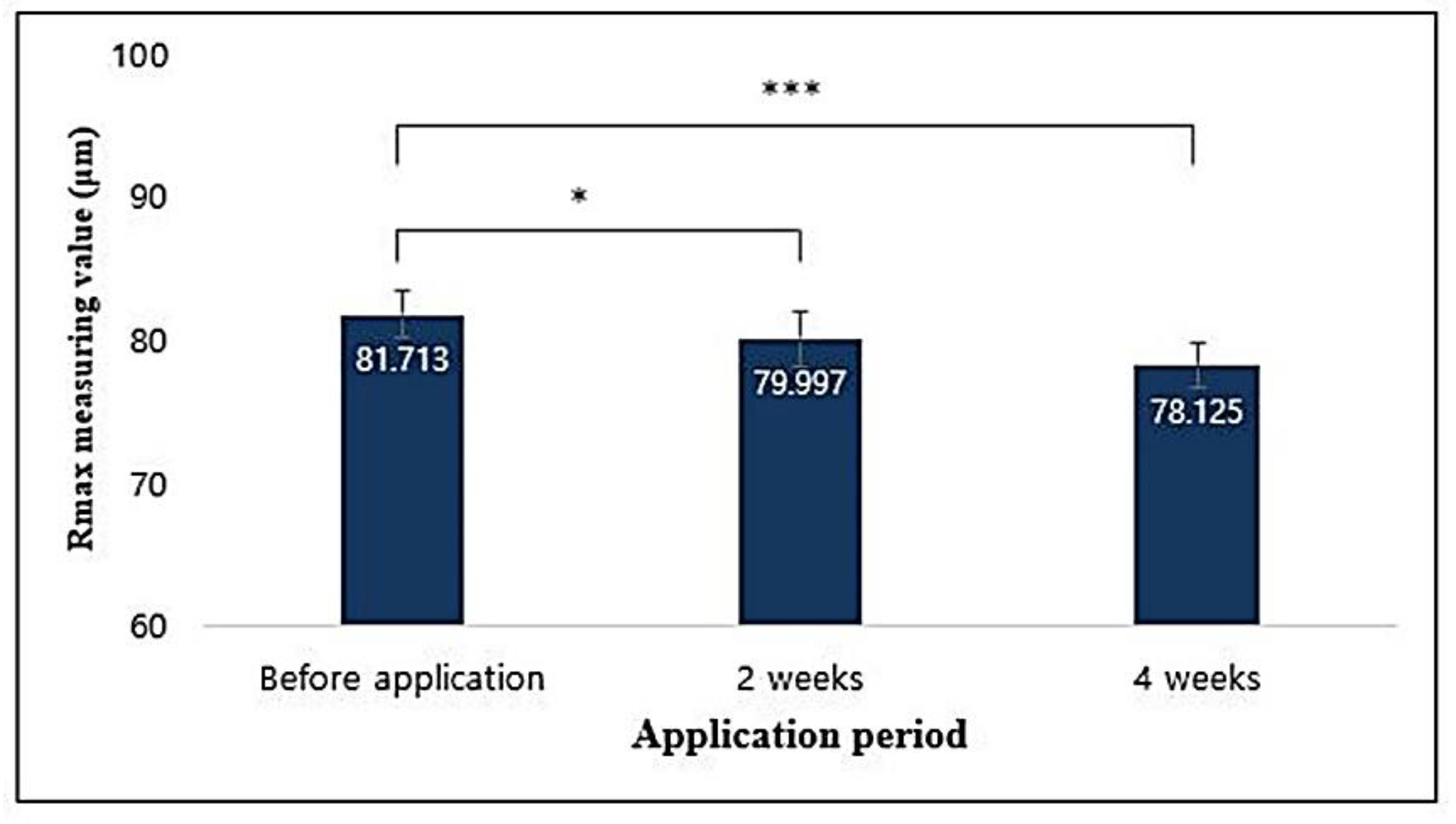
3.14.2. Clinical Trails
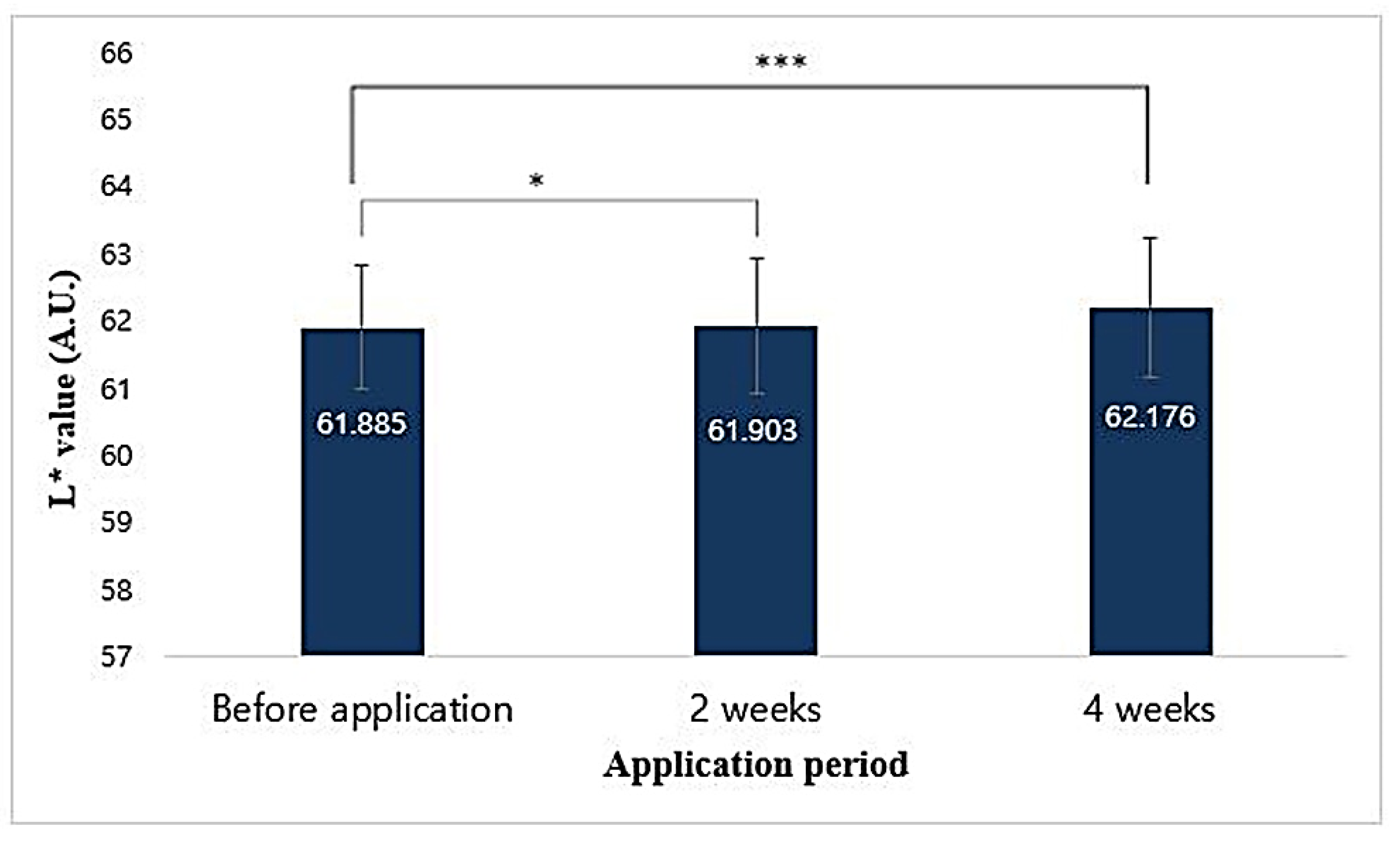
3.14.3. Image Capturing and Processing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Matts, P.J. New insights into skin appearance and measurement. J. Investig. Dermatology Symp. Proc. 2008, 13, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Fink, B.; Grammer, K.; Matts, P.J. Visible skin color distribution plays a role in the perception of age, attractiveness, and health in female faces. Evol. Hum. Behav. 2006, 27, 433–442. [Google Scholar] [CrossRef]
- Jones, A.L.; Russell, R.; Ward, R. Cosmetics alter biologically-based factors of beauty: Evidence from facial contrast. Evol. Psychol. 2015, 13, 210–229. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.L.; Kramer, R.S.S. Facial cosmetics and attractiveness: Comparing the effect sizes of professionally-applied cosmetics and identity. PLoS ONE 2016, 11, 1–17. [Google Scholar] [CrossRef]
- Taofiq, O.; Heleno, S.A.; Calhelha, R.C.; Alves, M.J.; Barros, L.; Barreiro, M.F.; González-Paramás, A.M.; Ferreira, I.C.F.R. Development of mushroom-based cosmeceutical formulations with anti-inflammatory, anti-tyrosinase, antioxidant, and antibacterial properties. Molecules 2016, 21, 1372. [Google Scholar] [CrossRef]
- Ribeiro, A.; Estanqueiro, M.; Oliveira, M.; Sousa Lobo, J. Main benefits and applicability of plant extracts in skin care products. Cosmetics 2015, 2, 48–65. [Google Scholar] [CrossRef]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Intrinsic and extrinsic factors in skin ageing: A review. Int. J. Cosmet. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef]
- Krutmann, J.; Bouloc, A.; Sore, G.; Bernard, B.A.; Passeron, T. The skin aging exposome. J. Dermatol. Sci. 2017, 85, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Dahal, R.H.; Shim, D.S.; Kim, J. Development of actinobacterial resources for functional cosmetics. J. Cosmet. Dermatol. 2017, 16, 243–252. [Google Scholar] [CrossRef]
- Long, A.; Crowther, J.; Beach, K.; Neil, J. Skin topometry changes demonstrate anti-aging efficacy of a topically applied cosmetic product via in vivo testing. J. Am. Acad. Dermatol. 2007, 56, 224. [Google Scholar]
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin anti-aging strategies. Dermatoendocrinology 2012, 4, 308–319. [Google Scholar] [CrossRef]
- Baxter, R.A. Anti-aging properties of resveratrol: Review and report of a potent new antioxidant skin care formulation. J. Cosmet. Dermatol. 2008, 7, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Date, A.; Patravale, V.; Korting, H.C.; Roeder, A.; Weindl, G. Retinoids in the treatment of skin aging: An overview of clinical efficacy and safety. Clin. Interv. Aging 2006, 1, 327–348. [Google Scholar] [CrossRef] [PubMed]
- Fubini, B.; Hubbard, A. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) generation by silica in inflammation and fibrosis. Free Radic. Biol. Med. 2003, 34, 1507–1516. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Pham-Huy, L.A.; Hua, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar] [PubMed]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef]
- Fawole, O.A.; Makunga, N.P.; Opara, U.L. Antibacterial, antioxidant and tyrosinase-inhibition activities of pomegranate fruit peel methanolic extract. BMC Complement. Altern. Med. 2012, 12, 1–11. [Google Scholar] [CrossRef]
- Kim, M.; Shibata, T.; Kwon, S.; Park, T.J.; Kang, H.Y. Ultraviolet-irradiated endothelial cells secrete stem cell factor and induce epidermal pigmentation. Sci. Rep. 2018, 8, 4235. [Google Scholar] [CrossRef]
- Hsu, K.D.; Chen, H.J.; Wang, C.S.; Lum, C.C.; Wu, S.P.; Lin, S.P.; Cheng, K.C. Extract of Ganoderma formosanum mycelium as a highly potent tyrosinase inhibitor. Sci. Rep. 2016, 6, 32854. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Thampi, A.; Puranik, M. Kinetics of melanin polymerization during enzymatic and nonenzymatic oxidation. J. Phys. Chem. B 2018, 122, 2047–2063. [Google Scholar] [CrossRef] [PubMed]
- Corinaldesi, C.; Barone, G.; Marcellini, F.; Dell’Anno, A.; Danovaro, R. Marine microbial-derived molecules and their potential use in cosmeceutical and cosmetic products. Mar. Drugs 2017, 15, 118. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-M. Fifty years of research and development of cosmeceuticals: A contemporary review. J. Cosmet. Dermatol. 2016, 15, 527–539. [Google Scholar] [CrossRef]
- Lee, J.; Ji, J.; Park, S.-H. Antiwrinkle and antimelanogenesis activity of the ethanol extracts of Lespedeza cuneata G. Don for development of the cosmeceutical ingredients. Food Sci. Nutr. 2018, 6, 1307–1316. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P.; Domagalska, B.W.; Młynarczyk, A. Essential oils and herbal extracts as antimicrobial agents in cosmetic emulsion. Indian J. Microbiol. 2013, 53, 232–237. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Di Lorenzo, A.; Izadi, M.; Sobarzo-Sánchez, E.; Daglia, M.; Nabavi, S.M. Antibacterial effects of cinnamon: From farm to food, cosmetic and pharmaceutical industries. Nutrients 2015, 7, 7729–7748. [Google Scholar] [CrossRef]
- Klaschka, U. Natural personal care products-analysis of ingredient lists and legal situation. Environ. Sci. Eur. 2016, 28, 8. [Google Scholar] [CrossRef]
- Alvarez-Rivera, G.; Llompart, M.; Lores, M.; Garcia–Jares, C. Preservatives in cosmetics: Regulatory aspects and analytical methods. Anal. Cosmet. Prod. 2018, 175–224. [Google Scholar]
- Lee, L.H.; Chan, K.G.; Stach, J.; Wellington, E.M.H.; Goh, B.H. Editorial: The search for biological active agent(s) from actinobacteria. Front. Microbiol. 2018, 9, 824. [Google Scholar] [CrossRef]
- Xu, R.; Shang, N.; Li, P. In vitro and in vivo antioxidant activity of exopolysaccharide fractions from Bifidobacterium animalis RH. Anaerobe 2011, 17, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Frank, J.A.; Reich, C.I.; Sharma, S.; Weisbaum, J.S.; Wilson, B.A.; Olsen, G.J. Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA genes. Appl. Environ. Microbiol. 2008, 74, 2461–2470. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Ha, S.M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/ (accessed on 25 March 2020).
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.-M.; Byung, H.K.; Eun, Y.C.; Jung, S.-H.; Yeong, S.K.; Kyung, R.M.; Kim, Y. Suppressive effect of novel aromatic diamine compound on nuclear factor-κB-dependent expression of inducible nitric oxide synthase in macrophages. Eur. J. Pharmacol. 2005, 521, 1–8. [Google Scholar] [CrossRef]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic triblock and star diblock copolymer and oligomeric sufactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Brightbill, H.D.; Modlin, R.L. Toll-like receptors: Molecular mechanisms of the mammalian immune response. Immunology 2000, 101, 1–10. [Google Scholar] [CrossRef]
- Emre, Y.; Hurtaud, C.; Nübel, T.; Criscuolo, F.; Ricquier, D.; Cassard-Doulcier, A.-M. Mitochondria contribute to LPS-induced MAPK activation via uncoupling protein UCP2 in macrophages. Biochem. J. 2007, 402, 271–278. [Google Scholar] [CrossRef]
- Sosroseno, W.; Barid, I.; Herminajeng, E.; Susilowati, H. Nitric oxide production by a murine macrophage cell line (RAW264.7) stimulated with lipopolysaccharide from Actinobacillus actinomycetemcomitans. Oral Microbiol. Immunol. 2002, 17, 72–78. [Google Scholar] [CrossRef]
- Ahmed, S.; Sulaiman, S.A.; Baig, A.A.; Ibrahim, M.; Liaqat, S.; Fatima, S.; Jabeen, S.; Shamim, N.; Othman, N.H. Honey as a potential natural antioxidant medicine: An insight into its molecular mechanisms of action. Oxid. Med. Cell. Longev. 2018, 2018, 8367846. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.-H.; Chen, S.; Corpe, C.; Dutta, A.; Dutta, S.K.; et al. Vitamin C as an antioxidant: Evaluation of its role in disease prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar] [CrossRef]
- Pratt, D. Natural antioxidants from plant material. In ACS Symphosium Series, Vol. 507; American Chemical Society: Washington, DC, USA, 1992; pp. 54–71. [Google Scholar]
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int. J. Biol. Sci. 2015, 11, 982–991. [Google Scholar] [CrossRef]
- Szymanska, R.; Pospisil, P.; Kruk, J. Plant-derived antioxidants in disease prevention. Oxid. Med. Cell. Longev. 2016, 2016, 1–2. [Google Scholar] [CrossRef]
- Narsing Rao, M.P.; Xiao, M.; Li, W.-J. Fungal and bacterial pigments: Secondary metabolites with wide applications. Front. Microbiol. 2017, 8, 1113. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant properties of probiotic bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef]
- Ko, R.K.; Kim, G.-O.; Hyun, C.-G.; Jung, D.S.; Lee, N.H. Compounds with tyrosinase inhibition, elastase inhibition and DPPH radical scavenging activities from the branches of Distylium racemosum Sieb. et Zucc. Phyther. Res. 2011, 25, 1451–1456. [Google Scholar] [CrossRef]
- Manivasagan, P.; Venkatesan, J.; Sivakumar, K.; Kim, S.-K. Pharmaceutically active secondary metabolites of marine actinobacteria. Microbiol. Res. 2014, 169, 262–278. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Roh, K.-B.; Li, Z.; Liu, G.; Tang, J.; Shin, S.; Park, D.; Jung, E.; You, J.; Roh, K.-B.; et al. The antiaging properties of andrographis paniculata by activation epidermal cell stemness. Molecules 2015, 20, 17557–17569. [Google Scholar] [CrossRef] [PubMed]
- Oliver, N.; Sternlicht, M.; Gerritsen, K.; Goldschmeding, R. Could aging human skin use a connective tissue growth factor boost to increase collagen content? J. Investig. Dermatol. 2010, 130, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Murakami, H.; Shimbo, K.; Inoue, Y.; Takino, Y.; Kobayashi, H. Importance of amino acid composition to improve skin collagen protein synthesis rates in UV-irradiated mice. Amino Acids 2012, 42, 2481–2489. [Google Scholar] [CrossRef]
- Burnett, C.L.; Heldreth, B.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; et al. Safety assessment of α-amino acids as used in cosmetics. Int. J. Toxicol. 2013, 32, 41S–64S. [Google Scholar] [CrossRef] [PubMed]
- Aramwit, P.; Luplertlop, N.; Kanjanapruthipong, T.; Ampawong, S. Effect of urea-extracted sericin on melanogenesis: Potential applications in post-inflammatory hyperpigmentation. Biol. Res. 2018, 51. [Google Scholar] [CrossRef] [PubMed]
- Marcuse, R. Antioxidative effect of amino-acids. Nature 1960, 186, 886–887. [Google Scholar] [CrossRef]
- Canfield, C.-A.; Bradshaw, P.C. Amino acids in the regulation of aging and aging-related diseases. Transl. Med. Aging 2019, 3, 70–89. [Google Scholar] [CrossRef]
- Kubglomsong, S.; Theerakulkait, C.; Reed, R.L.; Yang, L.; Maier, C.S.; Stevens, J.F. Isolation and identification of tyrosinase-inhibitory and copper-chelating peptides from hydrolyzed rice-bran-derived albumin. J. Agric. Food Chem. 2018, 66, 8346–8354. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.Y.; Yang, H.J.; Moon, H.I.; Cho, Y.S. Branched-chain amino acids complex inhibits melanogenesis in B16F0 melanoma cells. Immunopharmacol. Immunotoxicol. 2012, 34, 256–264. [Google Scholar] [CrossRef]
- Liu, D.Z.; Lin, Y.S.; Hou, W.C. Monohydroxamates of aspartic acid and glutamic acid exhibit antioxidant and angiotensin converting enzyme inhibitory activities. J. Agric. Food Chem. 2004, 52, 2386–2390. [Google Scholar] [CrossRef]
- Tejpal, C.S.; Vijayagopal, P.; Elavarasan, K.; Linga Prabu, D.; Lekshmi, R.G.K.; Asha, K.K.; Anandan, R.; Chatterjee, N.S.; Mathew, S. Antioxidant, functional properties and amino acid composition of pepsin-derived protein hydrolysates from whole tilapia waste as influenced by pre-processing ice storage. J. Food Sci. Technol. 2017, 54, 4257–4267. [Google Scholar] [CrossRef]
- Ali, H.M.; El-Gizawy, A.M.; El-Bassiouny, R.E.I.; Saleh, M.A. The role of various amino acids in enzymatic browning process in potato tubers, and identifying the browning products. Food Chem. 2016, 192, 879–885. [Google Scholar] [CrossRef]
- Richard, D.; Kefi, K.; Barbe, U.; Bausero, P.; Visioli, F. Polyunsaturated fatty acids as antioxidants. Pharmacol. Res. 2008, 57, 451–455. [Google Scholar] [CrossRef]
- Kumar, A.P.; Chougala, M.; Nandini, C.D.; Salimath, P.V. Effect of butyric acid supplementation on serum and renal antioxidant enzyme activities in streptozotocin-induced diabetic rats. J. Food Biochem. 2010, 34, 15–30. [Google Scholar] [CrossRef]
- Gular, G.O. Studies on antioxidant properties of the different solvent extracts and fatty acid composition of Hyoscyamus reticulatus L. J. Food Biochem. 2012, 36, 532–538. [Google Scholar] [CrossRef]
- Elagbar, Z.A.; Naik, R.R.; Shakya, A.K.; Bardaweel, S.K. Fatty acids analysis, antioxidant and biological activity of fixed oil of Annona muricata L. seeds. J. Chem. 2016, 2016. [Google Scholar] [CrossRef]
- Aktumsek, A.; Zengin, G.; Guler, G.O.; Cakmak, Y.S.; Duran, A. Assessment of the antioxidant potential and fatty acid composition of four Centaurea L. taxa from Turkey. Food Chem. 2013, 141, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuji, T.; Kao, M.C.; Fang, J.Y.; Zouboulis, C.C.; Zhang, L.; Gallo, R.L.; Huang, C.M. Antimicrobial property of lauric acid against Propionibacterium acnes: Its therapeutic potential for inflammatory acne vulgaris. J. Invest. Dermatol. 2009, 129, 2480–2488. [Google Scholar] [CrossRef]
- Otto, M. Staphylococcus colonization of the skin and antimicrobial peptides. Expert Rev. Dermatol. 2010, 5, 183–195. [Google Scholar] [CrossRef]
- Yanagisawa, T.; Shimizu, T.; Kuroda, K.; Kato, C. The preparation of alkyltriinethylaininonium–kaneinite complexes and their conversion to microporous materials. Bull. Chem. Soc. Jpn. 1990, 63, 988–992. [Google Scholar] [CrossRef]
- Beck, J.S.; Vartuli, J.C.; Roth, W.J.; Leonowicz, M.E.; Kresge, C.T.; Schmitt, K.D.; Chu, C.T.W.; Olson, D.H.; Sheppard, E.W.; McCullen, S.B.; et al. A new family of mesoporous molecular sieves prepared with liquid crystal templates. J. Am. Chem. Soc. 1992, 114, 10834–10843. [Google Scholar] [CrossRef]
- Trewyn, B.G.; Slowing, I.I.; Giri, S.; Chen, H.T.; Lin, V.S.Y. Synthesis and functionalization of a mesoporous silica nanoparticle based on the sol-gel process and applications in controlled release. Acc. Chem. Res. 2007, 40, 846–853. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef]
- Da Silva, L.S.; Moretti, A.L.; Cher, G.G.; Arroyo, P.A. Influence of crystallization and ageing time on the reproducibility of mesoporous molecular sieve SBA-15. Rev. Mater. 2019, 24. [Google Scholar] [CrossRef]
- Knežević, N.; Durand, J.O. Large pore mesoporous silica nanomaterials for application in delivery of biomolecules. Nanoscale 2015, 7, 2199–2209. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, M.L.; Esparza, J.M.; Campero, A.; Cordero, S.; Kornhauser, I.; Rojas, F. On comparing BJH and NLDFT pore-size distributions determined from N2 sorption on SBA-15 substrata. Phys. Chem. Chem. Phys. 2003, 5, 1859–1866. [Google Scholar] [CrossRef]


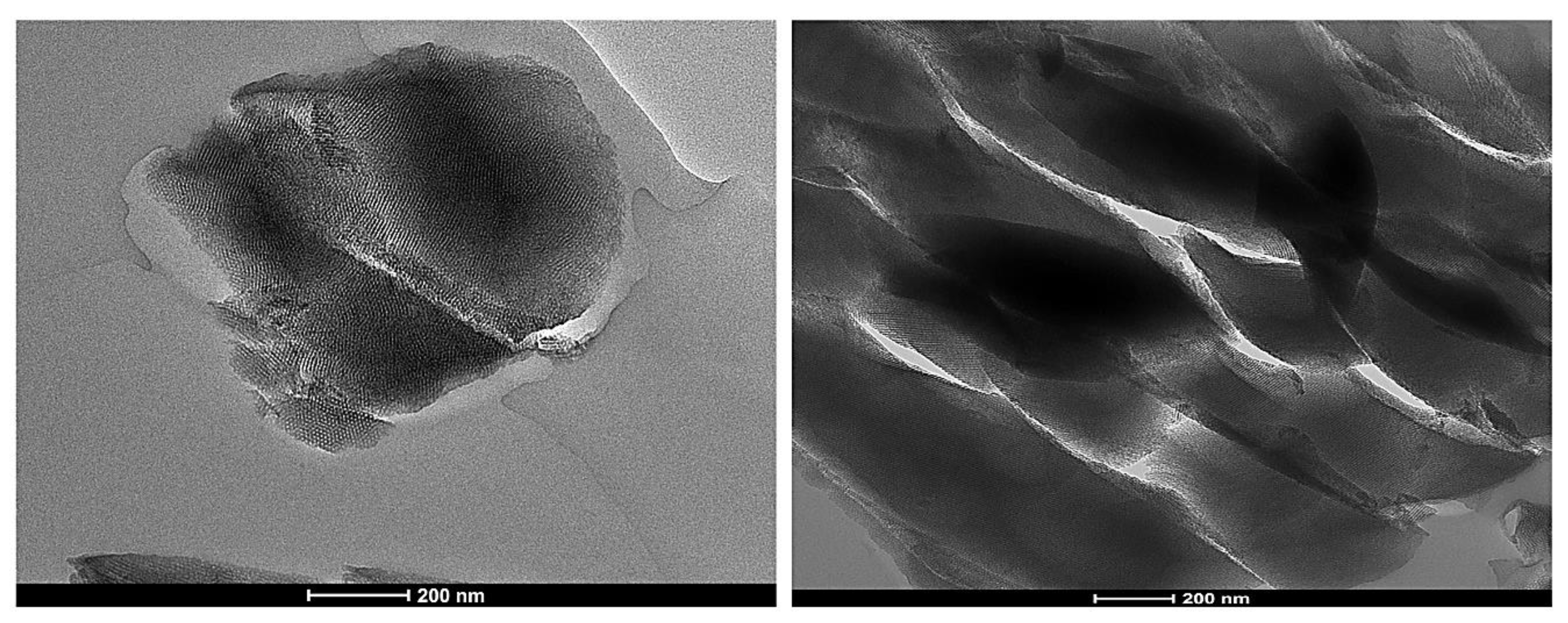
| SN | Sample | BSA | PV | PS |
|---|---|---|---|---|
| 1 | SB0053-18 | 708.8383 | 0.382060 | 21.5598 |
| 2 | SB0053-35 | 278.9649 | 0.367949 | 52.7593 |
| 3 | SB0053-36 | 345.9479 | 0.485290 | 56.1114 |
| 4 | SB0053-60 | 284.7321 | 0.372607 | 52.3449 |
| 5 | SB0053-3 | 348.2327 | 0.489289 | 56.2025 |
| 6 | SB0053-2 | 559.3040 | 0.423800 | 30.3096 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dahal, R.H.; Nguyen, T.M.; Shim, D.S.; Kim, J.Y.; Lee, J.; Kim, J. Development of Multifunctional Cosmetic Cream Using Bioactive Materials from Streptomyces sp. T65 with Synthesized Mesoporous Silica Particles SBA-15. Antioxidants 2020, 9, 278. https://doi.org/10.3390/antiox9040278
Dahal RH, Nguyen TM, Shim DS, Kim JY, Lee J, Kim J. Development of Multifunctional Cosmetic Cream Using Bioactive Materials from Streptomyces sp. T65 with Synthesized Mesoporous Silica Particles SBA-15. Antioxidants. 2020; 9(4):278. https://doi.org/10.3390/antiox9040278
Chicago/Turabian StyleDahal, Ram Hari, Tuan Manh Nguyen, Dong Seop Shim, Joon Young Kim, Jangyul Lee, and Jaisoo Kim. 2020. "Development of Multifunctional Cosmetic Cream Using Bioactive Materials from Streptomyces sp. T65 with Synthesized Mesoporous Silica Particles SBA-15" Antioxidants 9, no. 4: 278. https://doi.org/10.3390/antiox9040278
APA StyleDahal, R. H., Nguyen, T. M., Shim, D. S., Kim, J. Y., Lee, J., & Kim, J. (2020). Development of Multifunctional Cosmetic Cream Using Bioactive Materials from Streptomyces sp. T65 with Synthesized Mesoporous Silica Particles SBA-15. Antioxidants, 9(4), 278. https://doi.org/10.3390/antiox9040278






