Some Physiological and Biochemical Mechanisms during Seed-to-Seedling Transition in Tomato as Influenced by Garlic Allelochemicals
Abstract
1. Introduction
2. Materials and Methods
2.1. Garlic Extracts and Allelochemical Preparation
2.2. Experimental Conditions
2.2.1. Bioassays on Tomato Seed Priming with Aqueous Garlic Extracts (AGEs)
2.2.2. Bioassays on Comparative Effects of AGE, Diallyl Disulfide (DADS), and Allicin (AAS) on the Germination and Seedling Growth of Tomato
2.2.3. Germinating Tomato under the Volatile Influence of AGE
2.3. Germination Indices
2.4. Morphological Indices during Early Seedling Growth
2.5. Studying Physiology of Obtained Seedlings
2.5.1. Antioxidant Enzymes, MDA, and Soluble Protein Content
2.5.2. H2O2 Staining to Indicate Salinity Stress Conditions in Seedlings
2.5.3. Enzyme-Linked Immunosorbent Analysis (ELISA) for Hormonal Detection
2.5.4. Relative Expression of Genes
2.6. Data Analysis and Figure Construction
3. Results
3.1. Seed Priming with Aqueous Garlic Extract (AGE) Improves Seed Germination and Induces Post-Germination Defense System in Tomato
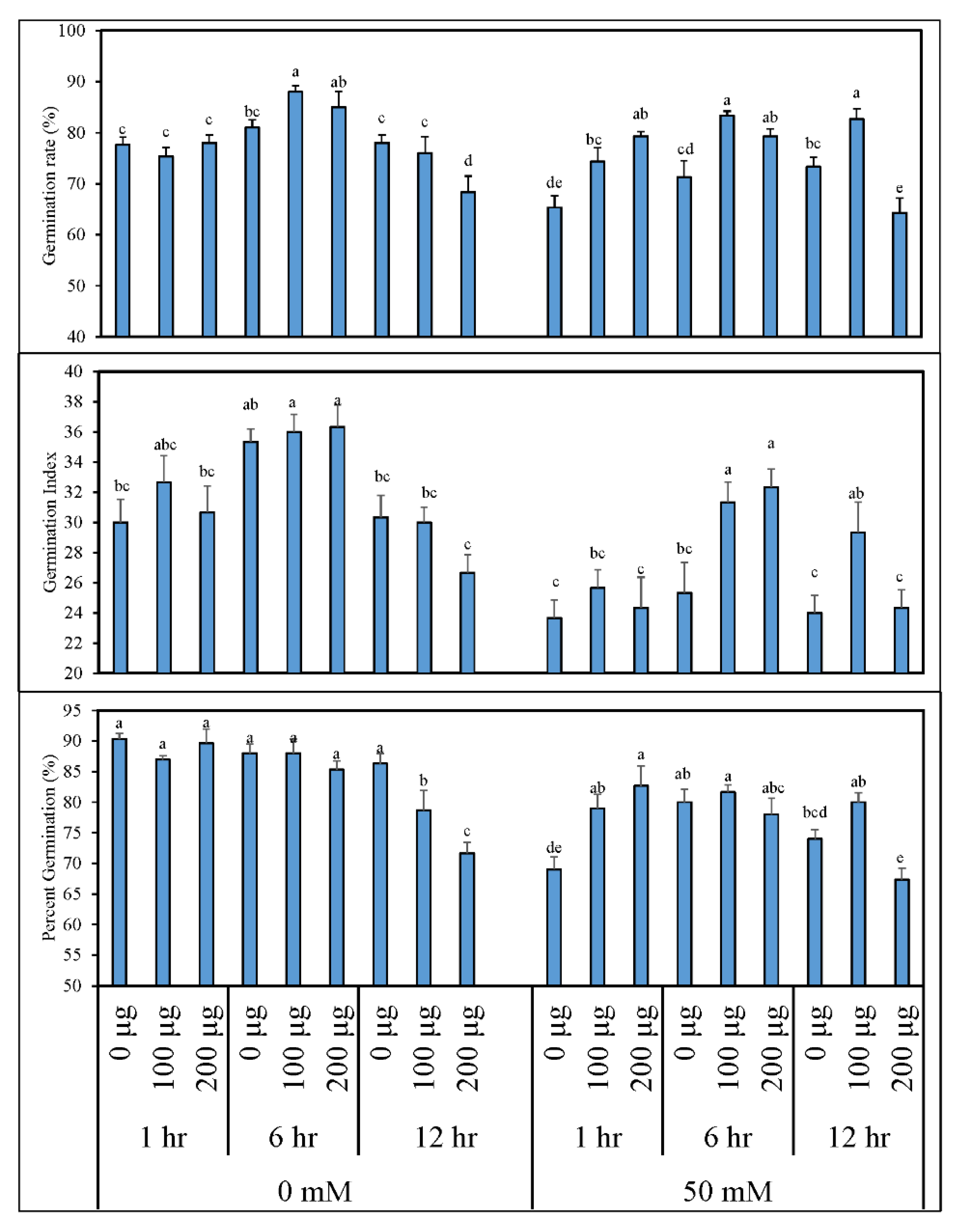
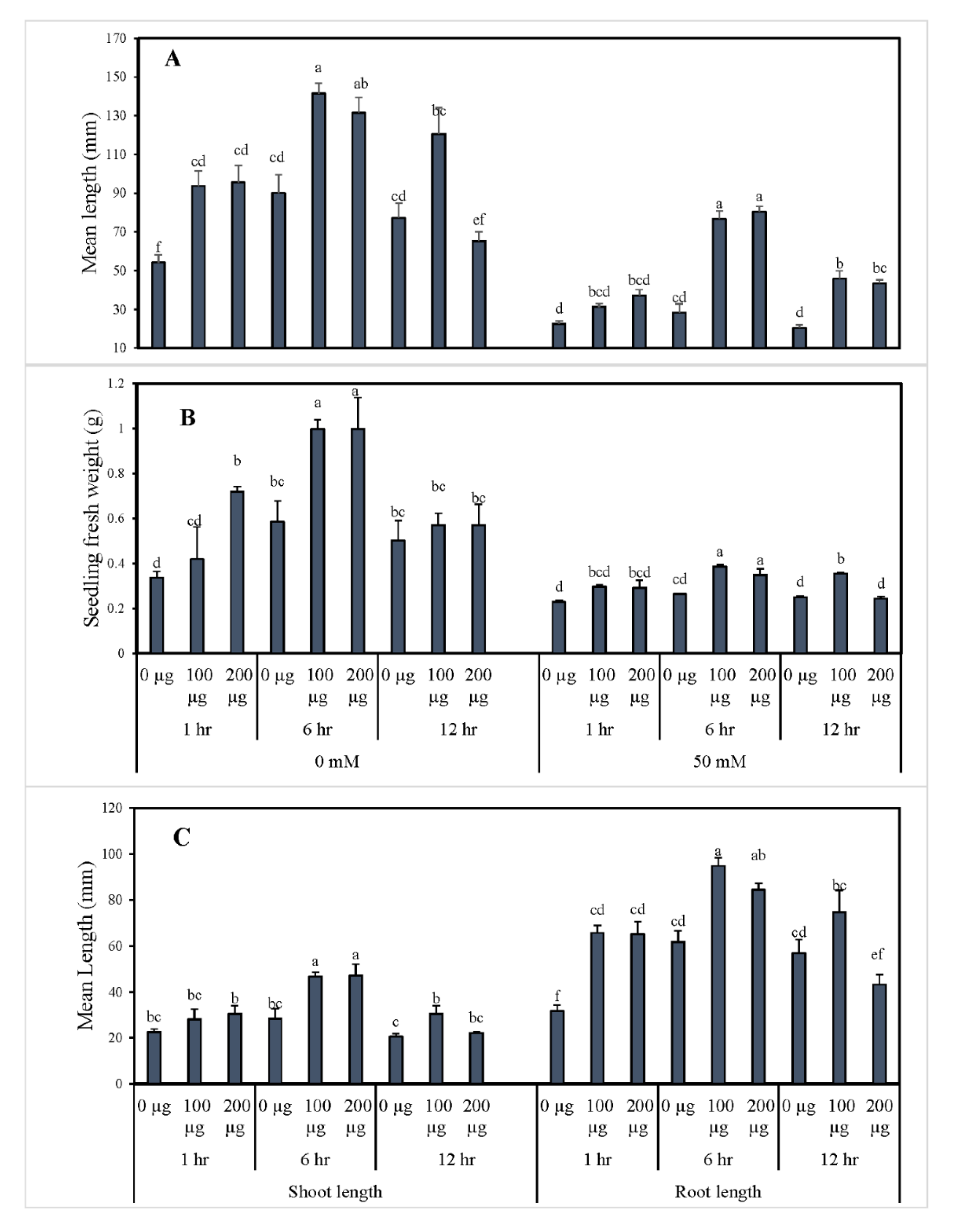
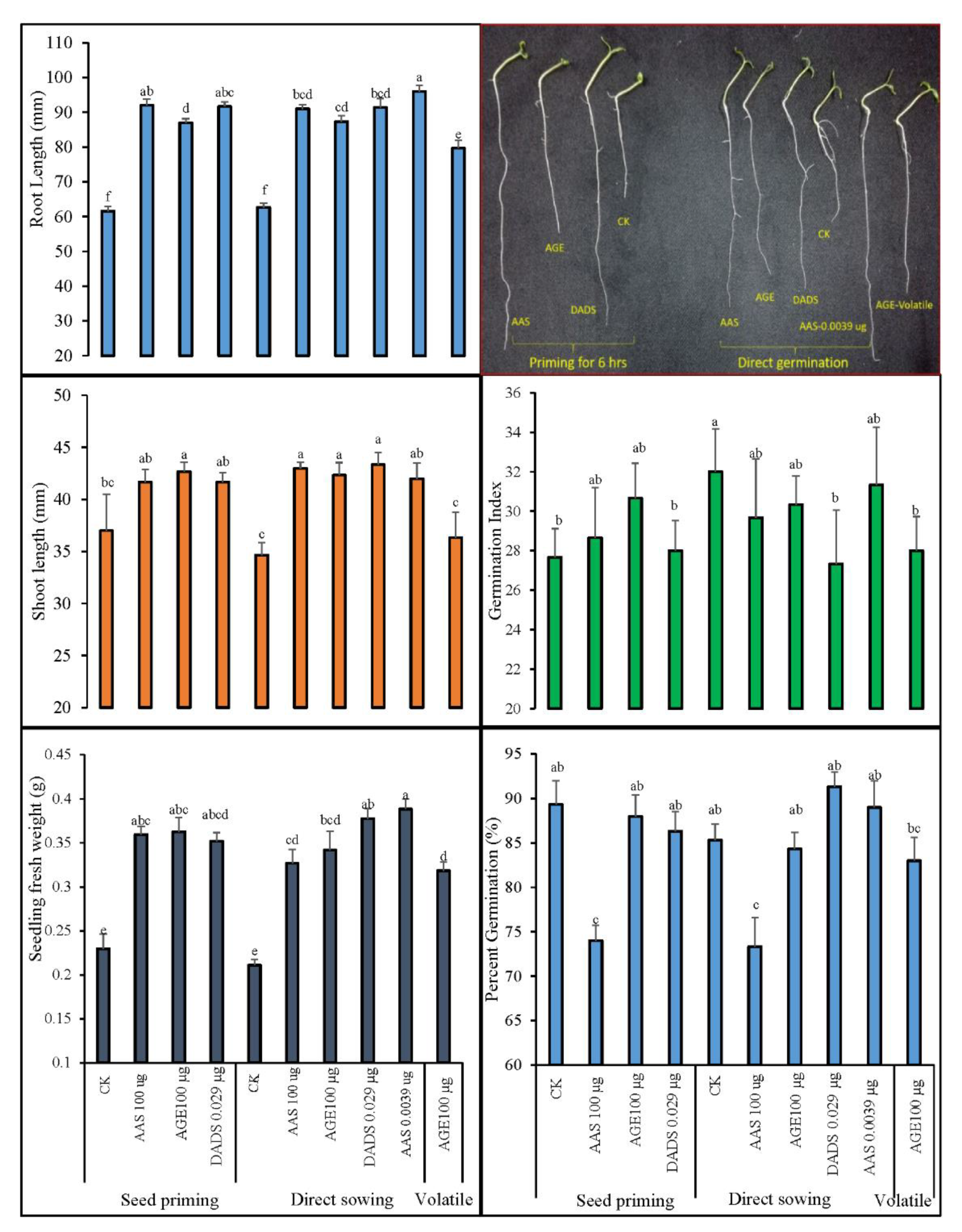
3.1.1. Germination Indices and Morphological Growth of Tomato after Seed Priming with AGE
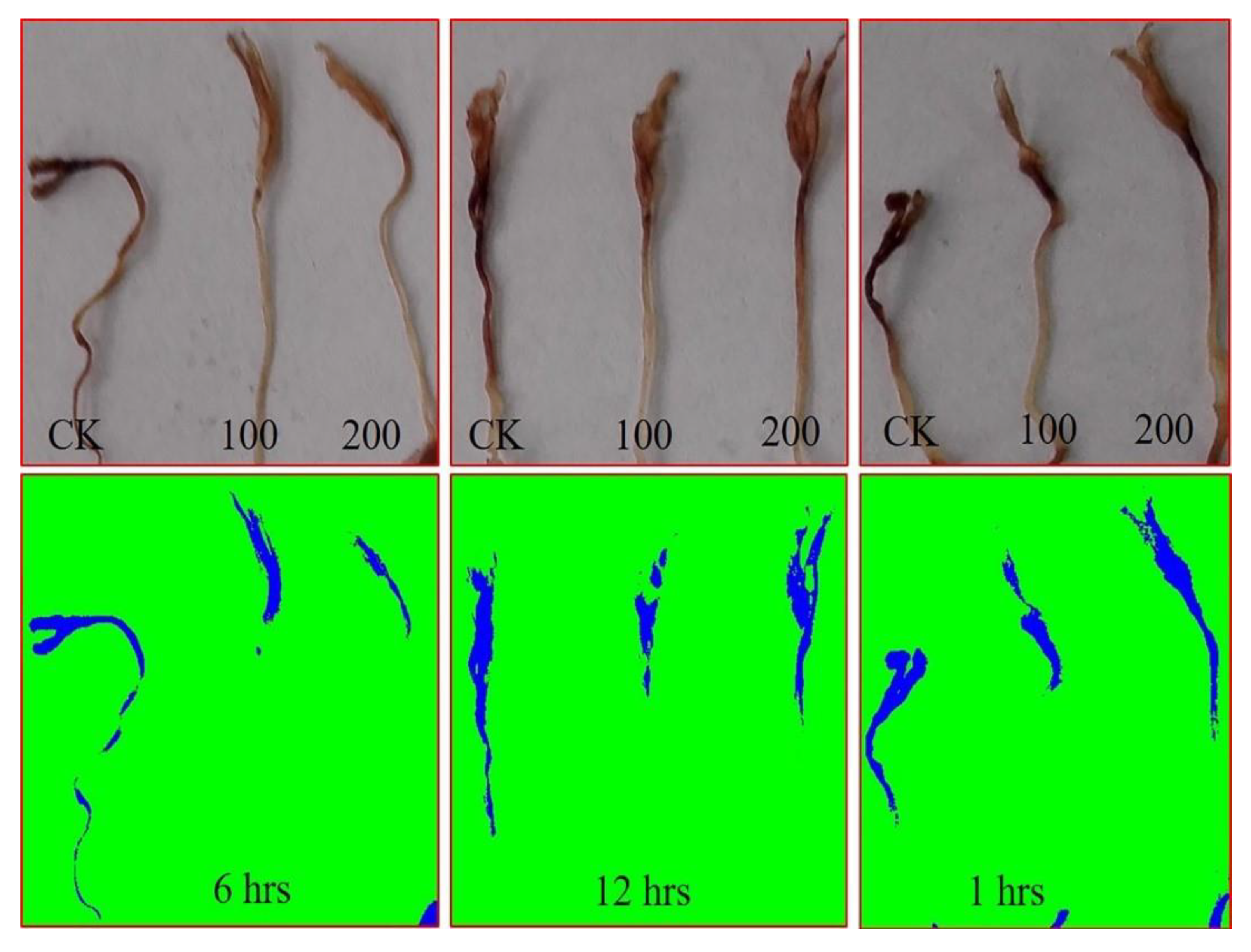
3.1.2. AGE Priming Alleviated Salt Stress during Germination by Affecting Reactive Oxygen Species (H2O2) Levels in Tomato Seedlings
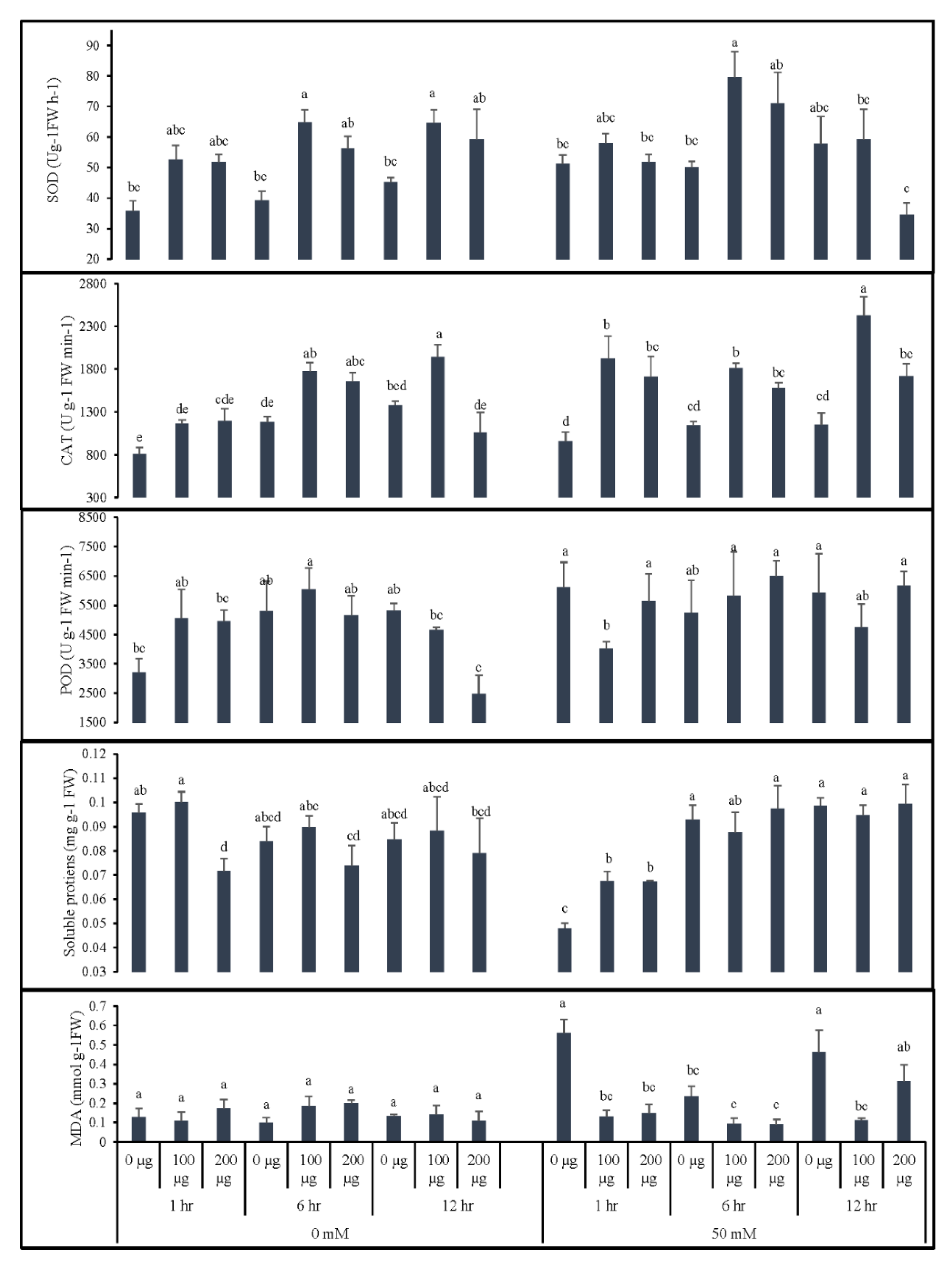
3.1.3. Seed Priming Improves Physiological Responses of Tomato Seedlings
3.2. Allelopathic Effects of AGE, Diallyl Disulfide, and Allicin on Tomato Seed Germination and Post-Germination Phytohormonal Regulation
3.2.1. Germination Indices and Post-Germination Morphological Observations
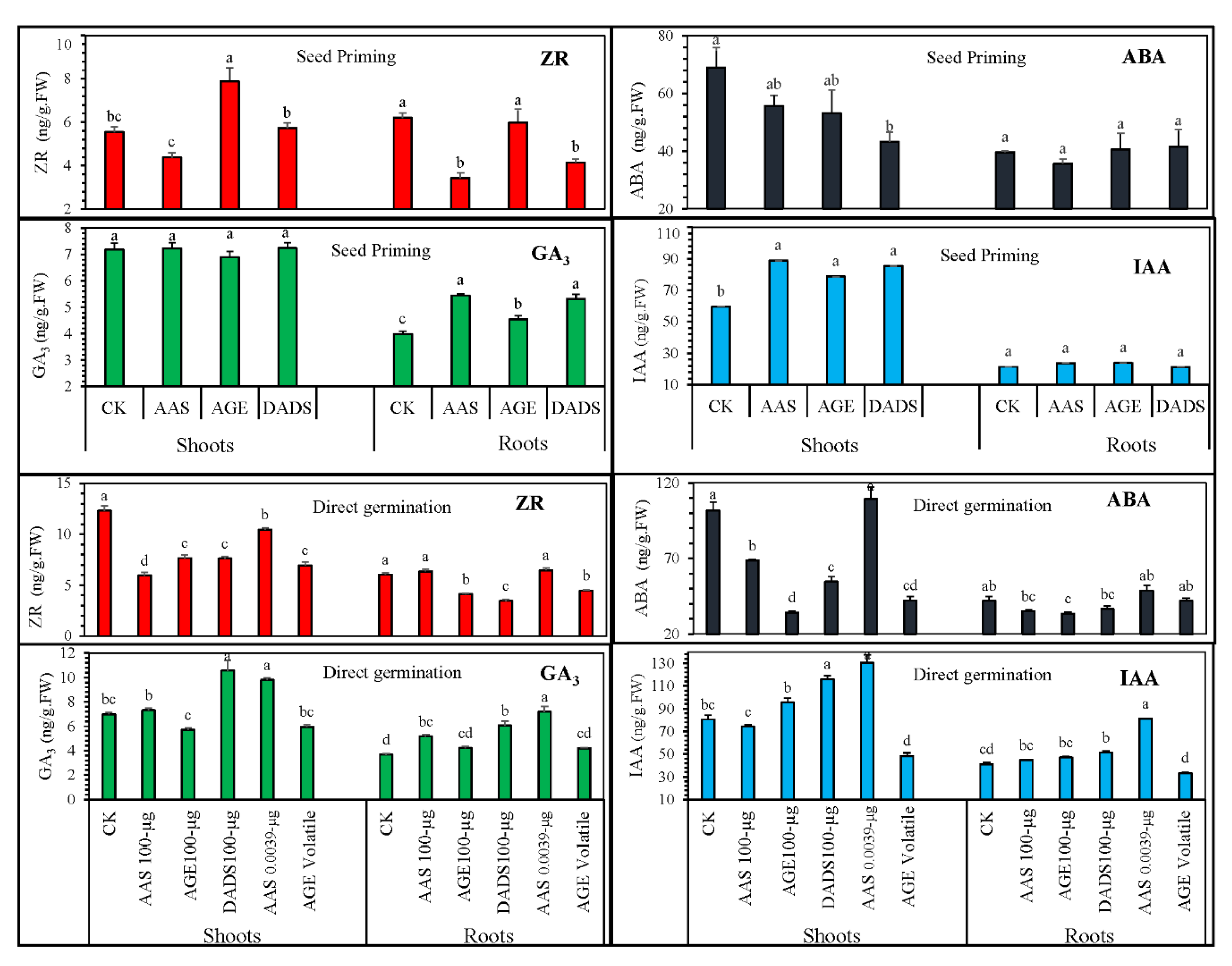
3.2.2. Influence of Various Treatments on the Endogenous Hormonal Levels
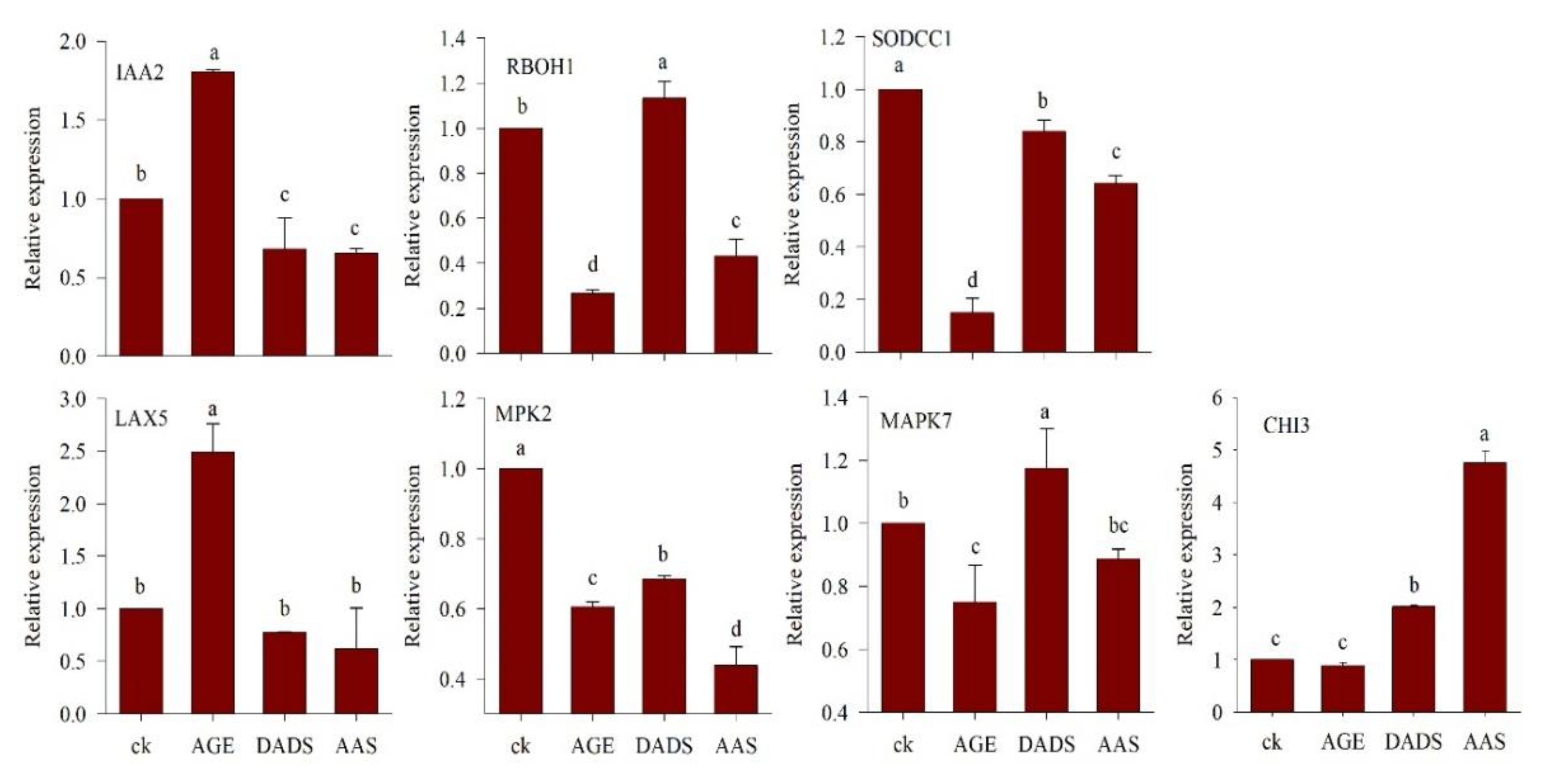
3.2.3. Relative Expression of Genes under AGE, DADS, and AAS Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sugeng, A.J.; Beamer, P.I.; Lutz, E.A.; Rosales, C.B. Hazard-ranking of agricultural pesticides for chronic health effects in Yuma County, Arizona. Sci. Total Environ. 2013, 463–464, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Gilden, R.C.; Huffling, K.; Sattler, B. Pesticides and health risks. JOGNN J. Obstet. Gynecol. Neonatal Nurs. 2010, 39, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.; Tisdell, C. Why farmers continue to use pesticides despite environmental, health and sustainability costs. Ecol. Econ. 2001, 39, 449–462. [Google Scholar] [CrossRef]
- Mostafalou, S.; Abdollahi, M. Pesticides and human chronic diseases: Evidences, mechanisms, and perspectives. Toxicol. Appl. Pharmacol. 2013, 268, 157–177. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Rouphael, Y.; Giordano, M.; Cardarelli, M.; Cozzolino, E.; Mori, M.; Kyriacou, M.; Bonini, P.; Colla, G. Plant- and seaweed-based extracts increase yield but differentially modulate nutritional quality of greenhouse spinach through biostimulant action. Agronomy 2018, 8, 126. [Google Scholar] [CrossRef]
- Hayat, S.; Ahmad, H.; Ali, M.; Hayat, K.; Khan, M.; Cheng, Z. Aqueous garlic extract as a plant biostimulant enhances physiology, improves crop quality and metabolite abundance, and primes the defense responses of receiver plants. Appl. Sci. 2018, 8, 1505. [Google Scholar] [CrossRef]
- Wilson, H.T.; Amirkhani, M.; Taylor, A.G. Evaluation of gelatin as a biostimulant seed treatment to improve plant performance. Front. Plant Sci. 2018, 9, 1006. [Google Scholar] [CrossRef]
- Marino, G.; Cellini, A.; Masia, A.; Simoni, A.; Francioso, O.; Gessa, C. In vitro treatment with a low molecular weight humic acid can improve growth and mineral uptake of pear plantlets during acclimatization. Acta Hortic. 2010, 884, 565–572. [Google Scholar] [CrossRef]
- Zhang, X.; Ervin, E.H.; Schmidt, R.E. Plant growth regulators can enhance the recovery of Kentucky Bluegrass sod from heat injury. Crop Sci. 2003, 43, 952–956. [Google Scholar] [CrossRef]
- Muñoz-Fambuena, N.; Mesejo, C.; Carmen González-Mas, M.; Primo-Millo, E.; Agustí, M.; Iglesias, D.J. Fruit regulates seasonal expression of flowering genes in alternate-bearing “Moncada” mandarin. Ann. Bot. 2011, 108, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Malo, I.; De Bastiani, M.; Arevalo, P.; Bernacchia, G. Natural extracts from pepper, wild rue and clove can activate defenses against pathogens in tomato plants. Eur. J. Plant Patol. 2017, 149, 89–101. [Google Scholar] [CrossRef]
- Hayat, S.; Cheng, Z.; Ahmad, H.; Ali, M.; Chen, X.; Wang, M.; Cheng, Z.; Ali, M. Garlic, from remedy to stimulant: Evaluation of antifungal potential reveals diversity in phytoalexin allicin content among garlic cultivars; allicin containing aqueous garlic extracts trigger antioxidants in cucumber. Front. Plant Sci. 2016, 7, 1235. [Google Scholar] [CrossRef] [PubMed]
- Shu, K.; Liu, X.D.; Xie, Q.; He, Z.H. Two Faces of One Seed: Hormonal regulation of dormancy and germination. Mol. Plant 2016, 9, 34–45. [Google Scholar] [CrossRef]
- Jeevan Kumar, S.P.; Rajendra Prasad, S.; Banerjee, R.; Thammineni, C. Seed birth to death: Dual functions of reactive oxygen species in seed physiology. Ann. Bot. 2015, 116, 4. [Google Scholar] [CrossRef]
- Bassel, G.W. To Grow or not to Grow? Trends Plant Sci. 2016, 21, 498–505. [Google Scholar] [CrossRef]
- Lin, F.; Ding, H.; Wang, J.; Zhang, H.; Zhang, A.; Zhang, Y.; Tan, M.; Dong, W.; Jiang, M. Positive feedback regulation of maize NADPH oxidase by mitogen-activated protein kinase cascade in abscisic acid signalling. J. Exp. Bot. 2009. [Google Scholar] [CrossRef]
- Baxter, A.; Mittler, R.; Suzuki, N. ROS as key players in plant stress signalling. J. Exp. Bot. 2014, 65, 1229–1240. [Google Scholar] [CrossRef]
- Hayat, S.; Ahmad, H.; Ren, K.; Ali, M.; Cheng, Z. Response of tomato growth to foliar spray and root drenching of aqueous garlic extract: A cocktail of antioxidative defenses, chlorophyll, carotenoid and soluble sugar contents. Int. J. Agric. Biol. Int. J. Agric. Biol. 2018, 20, 1251–1259. [Google Scholar]
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M.; De Pascale, S.; Bonini, P.; Colla, G. Arbuscular mycorrhizal fungi act as biostimulants in horticultural crops. Sci. Hortic. 2015, 196, 91–108. [Google Scholar] [CrossRef]
- Aremu, A.O.; Masondo, N.A.; Rengasamy, K.R.R.; Amoo, S.O.; Gruz, J.; Bíba, O.; Šubrtová, M.; Pěnčík, A.; Novák, O.; Doležal, K.; et al. Physiological role of phenolic biostimulants isolated from brown seaweed Ecklonia maxima on plant growth and development. Planta 2015, 241, 1313–1324. [Google Scholar] [CrossRef] [PubMed]
- Portz, D.; Koch, E.; Slusarenko, A.J. Effects of garlic (Allium sativum) juice containing allicin on Phytophthora infestans and downy mildew of cucumber caused by Pseudoperonospora cubensis. Eur. J. Plant Pathol. 2008, 122, 197–206. [Google Scholar] [CrossRef]
- Ayazi, M.; Ayazi, M.; Asadpour, L.; Kazemi, S.; Pourkhalili, S. Antibacterial activity of fresh juice of Allium sativum (garlic) against multi-drug resistant isolates of Staphylococcus aureus. Afr. J. Microbiol. Res. 2011, 5, 5776–5779. [Google Scholar] [CrossRef]
- Lee, J.; Gupta, S.; Huang, J.S.; Jayathilaka, L.P.; Lee, B.S. HPLC-MTT assay: Anticancer activity of aqueous garlic extract is from allicin. Anal. Biochem. 2013, 436, 187–189. [Google Scholar] [CrossRef]
- Wang, M.; Wu, C.; Cheng, Z.; Meng, H. Growth and physiological changes in continuously cropped eggplant (Solanum melongena L.) upon relay intercropping with garlic (Allium sativum L.). Front. Plant Sci. 2015, 6, 262. [Google Scholar] [CrossRef]
- Xiao, X.; Cheng, Z.; Meng, H.; Khan, M.A.; Li, H. Intercropping with garlic alleviated continuous cropping obstacle of cucumber in plastic tunnel. Acta Agric. Scand. Sect. B Soil Plant Sci. 2012, 62, 696–705. [Google Scholar] [CrossRef]
- Hayat, S.; Ahmad, H.; Ali, M.; Cheng, Z.; Ren, K.; Cheng, Z. Aqueous garlic extract stimulates growth and antioxidant enzymes activity of tomato (Solanum lycopersicum). Sci. Hortic. 2018, 240, 139–146. [Google Scholar] [CrossRef]
- Cheng, F.; Cheng, Z.; Meng, H.; Tang, X. The garlic allelochemical diallyl disulfide affects tomato root growth by influencing cell division, phytohormone balance and expansin gene expression. Front. Plant Sci. 2016, 7, 1199. [Google Scholar] [CrossRef]
- Huseynova, I.M.; Sultanova, N.F.; Aliyev, J.A. Histochemical visualization of ROS and antioxidant response to viral infections of vegetable crops grown in Azerbaijan. Plant Physiol. Biochem. 2014, 81, 26–35. [Google Scholar] [CrossRef]
- Abràmofff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with ImageJ Part II. Biophotonics Int. 2005, 11, 36–43. [Google Scholar]
- Ren, K.; Hayat, S.; Qi, X.; Liu, T.; Cheng, Z. The garlic allelochemical DADS influences cucumber root growth involved in regulating hormone levels and modulating cell cycling. J. Plant Physiol. 2018, 230, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Gruhlke, M.C.H.; Hemmis, B.; Noll, U.; Wagner, R.; Luhring, H.; Slusarenko, A.J. The defense substance allicin from garlic permeabilizes membranes of Beta vulgaris, Rhoeo discolor, Chara corallina and artificial lipid bilayers. Biochim. Biophys. Acta Gen. Subj. 2015, 1850, 602–611. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, L.; Ye, N.; Liu, R.; Jia, W.; Zhang, J. Nitric oxide-induced rapid decrease of abscisic acid concentration is required in breaking seed dormancy in Arabidopsis. New Phytol. 2009, 183, 1030–1042. [Google Scholar] [CrossRef] [PubMed]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative stress, prooxidants, and antioxidants: The interplay. Biomed Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Sekmen, A.H.; Turkan, I.; Tanyolac, Z.O.; Ozfidan, C.; Dinc, A. Different antioxidant defense responses to salt stress during germination and vegetative stages of endemic halophyte Gypsophila oblanceolata Bark. Environ. Exp. Bot. 2012, 77, 63–76. [Google Scholar] [CrossRef]
- Weitbrecht, K.; Müller, K.; Leubner-Metzger, G. First off the mark: Early seed germination. J. Exp. Bot. 2011, 62, 3289–3309. [Google Scholar] [CrossRef] [PubMed]
- McMahon, M.; Kofranek, A.; Rubatzky, V. Hartmann’s Plant Science: Growth, Development and Utilization of Cultivated Plants; Pearson Education, Inc., Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2007; ISBN 0131140752. [Google Scholar]
- Munné-bosch, S.; Müller, M. Hormonal cross-talk in plant development and stress responses. Front. Plant Sci. 2013, 4, 529. [Google Scholar] [CrossRef]
- Holdsworth, M.J.; Bentsink, L.; Soppe, W.J.J. Molecular networks regulating Arabidopsis seed maturation, after-ripening, dormancy and germination. New Phytol. 2008, 179, 33–54. [Google Scholar] [CrossRef]
- Bari, R.; Jones, J.D.G. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef]
- Jahan, M.S.; Shu, S.; Wang, Y.; Chen, Z.; He, M.; Tao, M.; Sun, J.; Guo, S. Melatonin alleviates heat-induced damage of tomato seedlings by balancing redox homeostasis and modulating polyamine and nitric oxide biosynthesis. BMC Plant Biol. 2019, 19, 414. [Google Scholar] [CrossRef]
- Yamauchi, T.; Yoshioka, M.; Fukazawa, A.; Mori, H.; Nishizawa, N.K.; Tsutsumi, N.; Yoshioka, H.; Nakazono, M. An NADPH oxidase RBOH functions in rice roots during lysigenous aerenchyma formation under oxygen-deficient conditions. Plant Cell 2017. [Google Scholar] [CrossRef] [PubMed]
- Orman-Ligeza, B.; Parizot, B.; de Rycke, R.; Fernandez, A.; Himschoot, E.; van Breusegem, F.; Bennett, M.J.; Périlleux, C.; Beeckman, T.; Draye, X. RBOH-mediated ROS production facilitates lateral root emergence in Arabidopsis. Development 2016, 143, 3328–3339. [Google Scholar] [CrossRef] [PubMed]
- Nie, W.F.; Wang, M.M.; Xia, X.J.; Zhou, Y.H.; Shi, K.; Chen, Z.; Yu, J.Q. Silencing of tomato RBOH1 and MPK2 abolishes brassinosteroid-induced H2O2 generation and stress tolerance. Plant Cell Environ. 2013, 36, 789–803. [Google Scholar] [CrossRef] [PubMed]
- Chao, W.S.; Doğramaci, M.; Horvath, D.P.; Anderson, J.V.; Foley, M.E. Phytohormone balance and stress-related cellular responses are involved in the transition from bud to shoot growth in leafy spurge. BMC Plant Biol. 2016, 16, 47. [Google Scholar] [CrossRef]
- Schuhegger, R.; Ihring, A.; Gantner, S.; Bahnweg, G.; Knappe, C.; Vogg, G.; Hutzler, P.; Schmid, M.; Van Breusegem, F.; Eberl, L.; et al. Induction of systemic resistance in tomato by N-acyl-L-homoserine lactone-producing rhizosphere bacteria. Plant Cell Environ. 2006, 29, 909–918. [Google Scholar] [CrossRef]
- Heribert, H.; Kazuo, S. Plant Responses to Abiotic Stress. In Topics in Current Genetics; Hirt, H., Shinozaki, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; Volume 4, p. 320. ISBN 3540200371. [Google Scholar]
- Pieterse, C.M.J.; Poelman, E.H.; Van Wees, S.C.M.; Dicke, M. Induced plant responses to microbes and insects. Front. Plant Sci. 2013, 4, 475. [Google Scholar] [CrossRef]
- Mauch-mani, B.; Baccelli, I.; Luna, E.; Flors, V. Defense priming: An adaptive part of induced resistance. Annu. Rev. Plant Bio. 2017, 68, 485–512. [Google Scholar] [CrossRef]
- Mittler, R.; Blumwald, E. The roles of ROS and ABA in systemic acquired acclimation. Plant Cell 2015, 27, 64–70. [Google Scholar] [CrossRef]
- Yu, L.; Yan, J.; Yang, Y.; He, L.; Zhu, W. Enhanced Tolerance to chilling stress in tomato by overexpression of a Mitogen-Activated protein kinase, SlMPK7. Plant Mol. Biol. Rep. 2016, 34, 76–88. [Google Scholar] [CrossRef]
- Nonogaki, H.; Bassel, G.W.; Bewley, J.D. Germination-still a mystery. Plant Sci. 2010, 179, 574–581. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayat, S.; Ahmad, H.; Nasir, M.; Khan, M.N.; Ali, M.; Hayat, K.; Khan, M.A.; Khan, F.; Ma, Y.; Cheng, Z. Some Physiological and Biochemical Mechanisms during Seed-to-Seedling Transition in Tomato as Influenced by Garlic Allelochemicals. Antioxidants 2020, 9, 235. https://doi.org/10.3390/antiox9030235
Hayat S, Ahmad H, Nasir M, Khan MN, Ali M, Hayat K, Khan MA, Khan F, Ma Y, Cheng Z. Some Physiological and Biochemical Mechanisms during Seed-to-Seedling Transition in Tomato as Influenced by Garlic Allelochemicals. Antioxidants. 2020; 9(3):235. https://doi.org/10.3390/antiox9030235
Chicago/Turabian StyleHayat, Sikandar, Husain Ahmad, Mubasher Nasir, Muhammad Numan Khan, Muhammad Ali, Kashif Hayat, Muhammad Ali Khan, Farmanullah Khan, Yongqing Ma, and Zhihui Cheng. 2020. "Some Physiological and Biochemical Mechanisms during Seed-to-Seedling Transition in Tomato as Influenced by Garlic Allelochemicals" Antioxidants 9, no. 3: 235. https://doi.org/10.3390/antiox9030235
APA StyleHayat, S., Ahmad, H., Nasir, M., Khan, M. N., Ali, M., Hayat, K., Khan, M. A., Khan, F., Ma, Y., & Cheng, Z. (2020). Some Physiological and Biochemical Mechanisms during Seed-to-Seedling Transition in Tomato as Influenced by Garlic Allelochemicals. Antioxidants, 9(3), 235. https://doi.org/10.3390/antiox9030235





