Blueberry Juice Antioxidants Protect Osteogenic Activity against Oxidative Stress and Improve Long-Term Activation of the Mineralization Process in Human Osteoblast-Like SaOS-2 Cells: Involvement of SIRT1
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of Blueberry Juice and Determination of Total Soluble Polyphenols
2.3. HPLC-PDA-MS Analysis of Phenolic Compounds
2.4. Cell Cultures, Treatments, Osteogenic Differentiation, and Cellular Viability
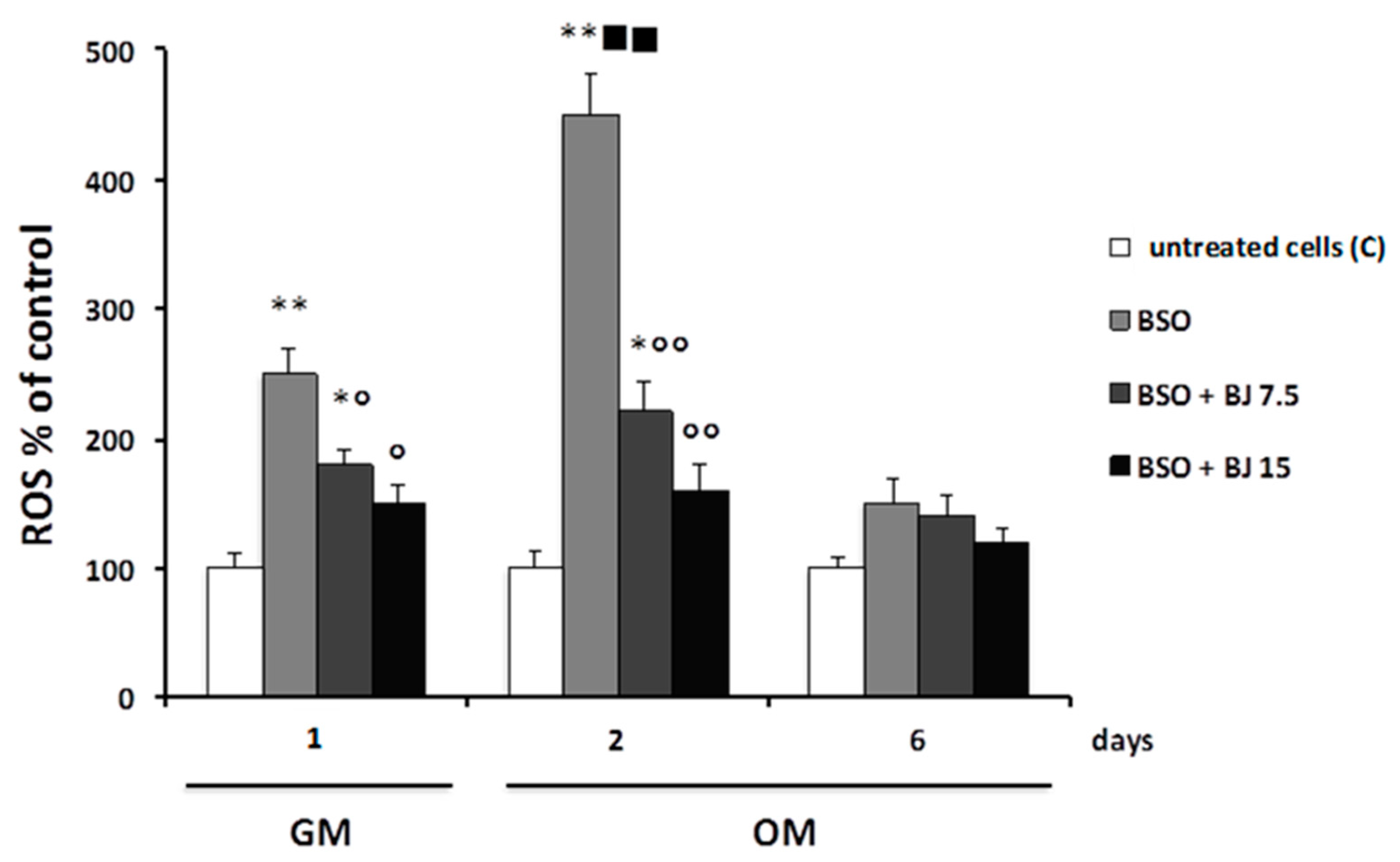
2.5. Determination of Intracellular ROS
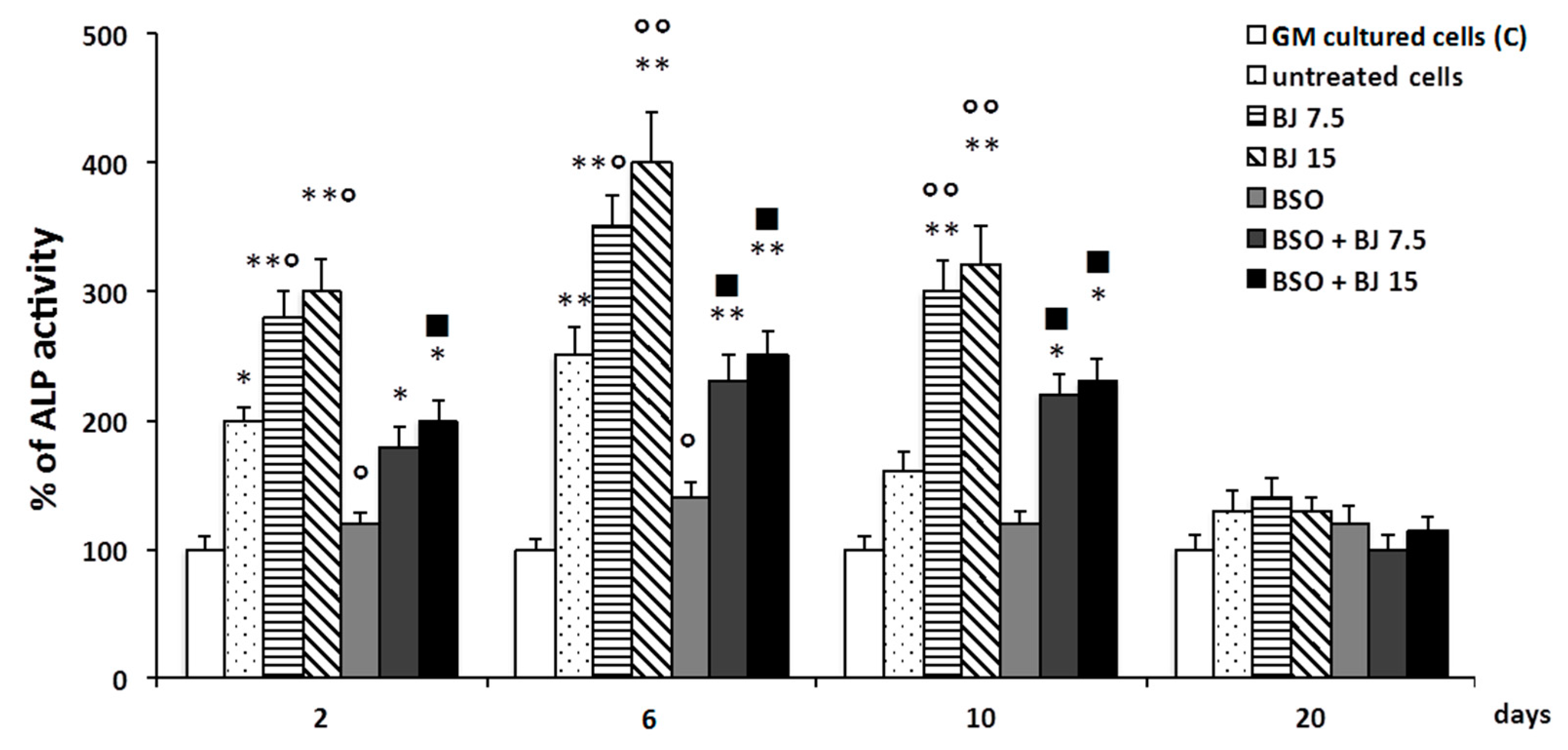
2.6. Alkaline Phosphatase Activity
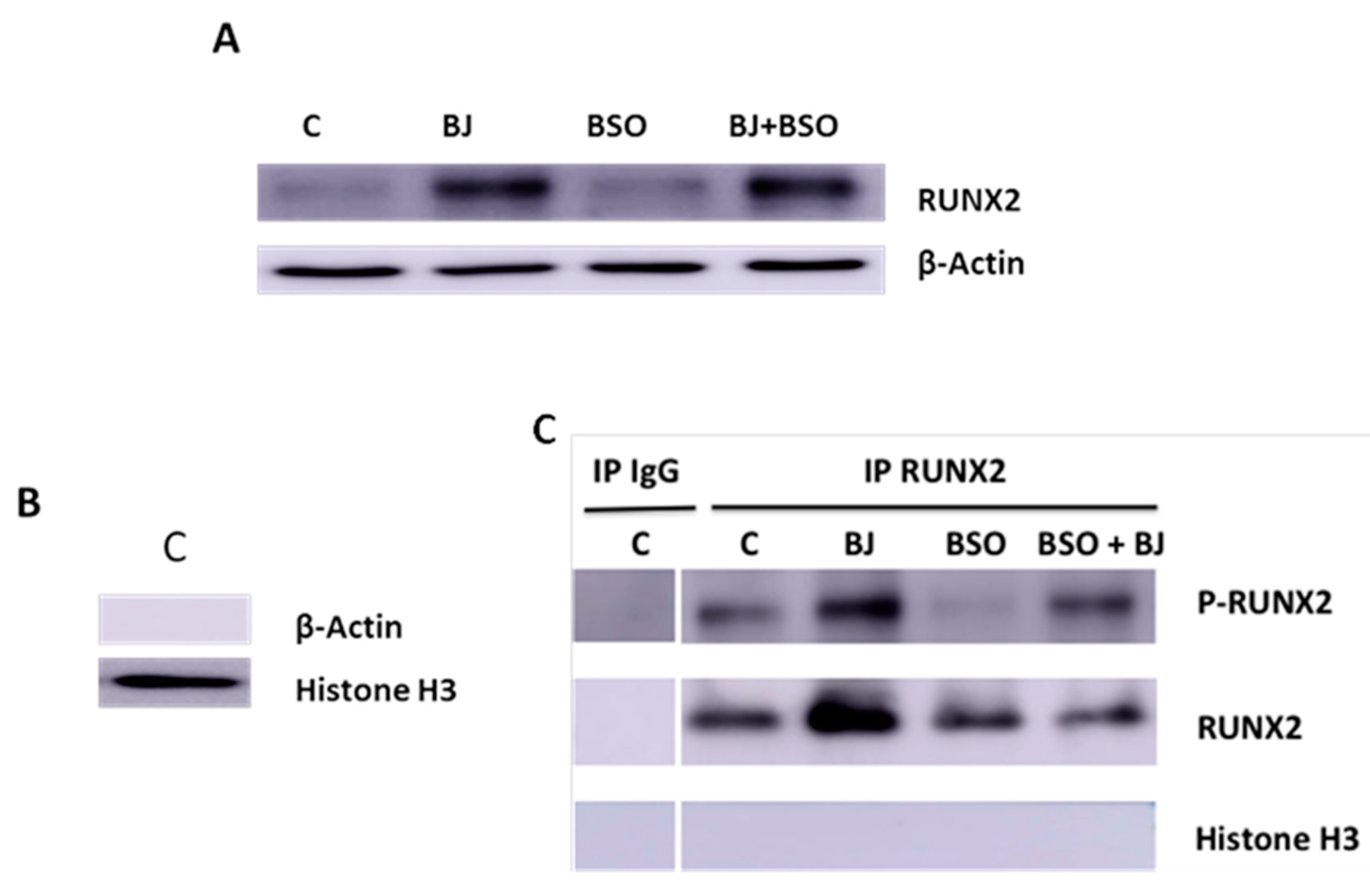
2.7. Western Blot Analysis of RUNX and RUNX-2 Phosphorylation
2.8. Alizarin Red S Assay
2.9. SIRT1 Expression Assay
2.10. Protein Assay
2.11. Statistical Analysis
3. Results
3.1. Polyphenolic Composition of BJ
3.2. Effect of BJ on Cell Viability and in Preventing Oxidative Stress Induced by BSO Treatment in SaOS-2 Cells
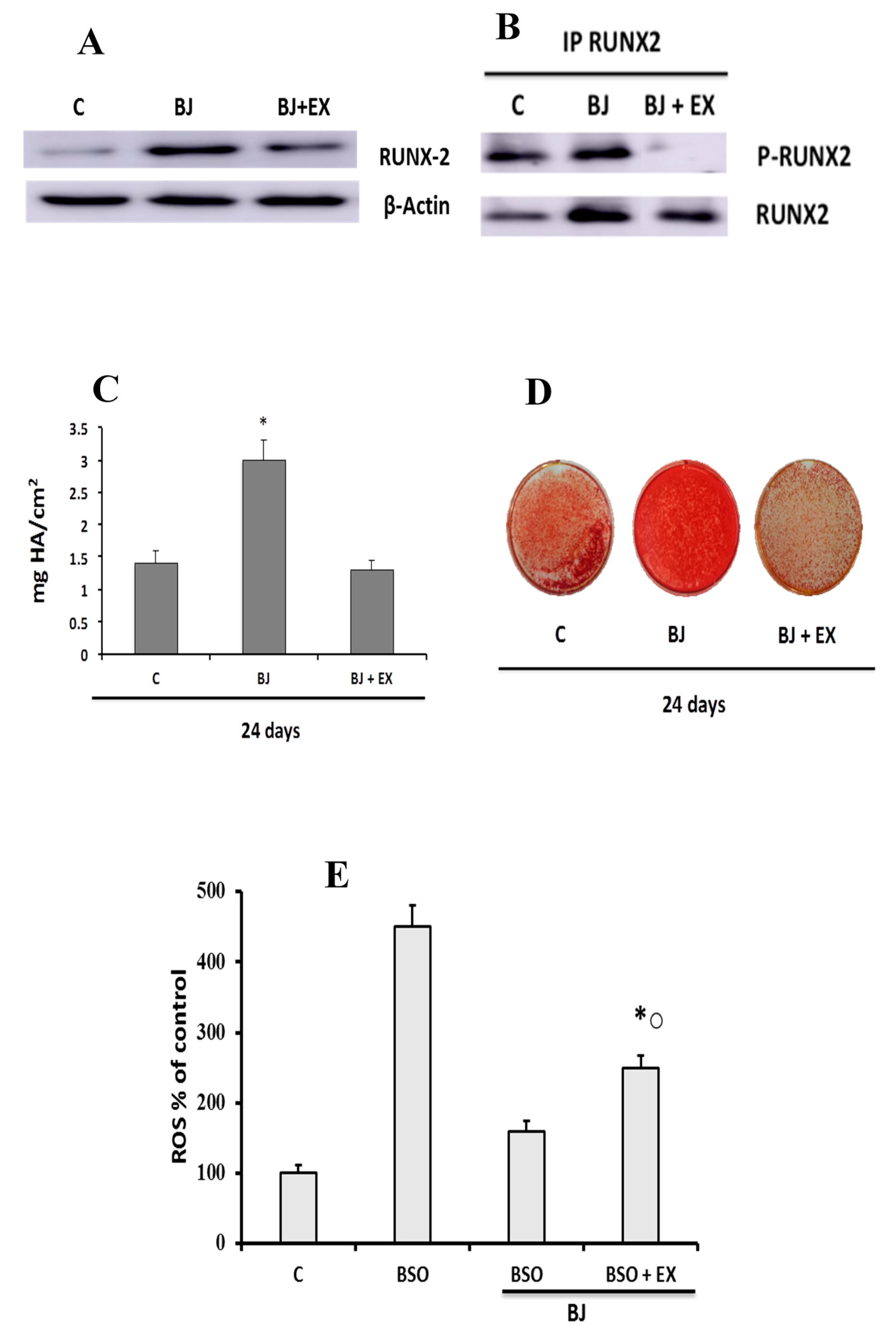
3.3. BJ Effect on the Markers of Differentiation and Osteogenic Process in SaOS-2 Cells in the Presence or Not of BSO-Induced Oxidative Stress
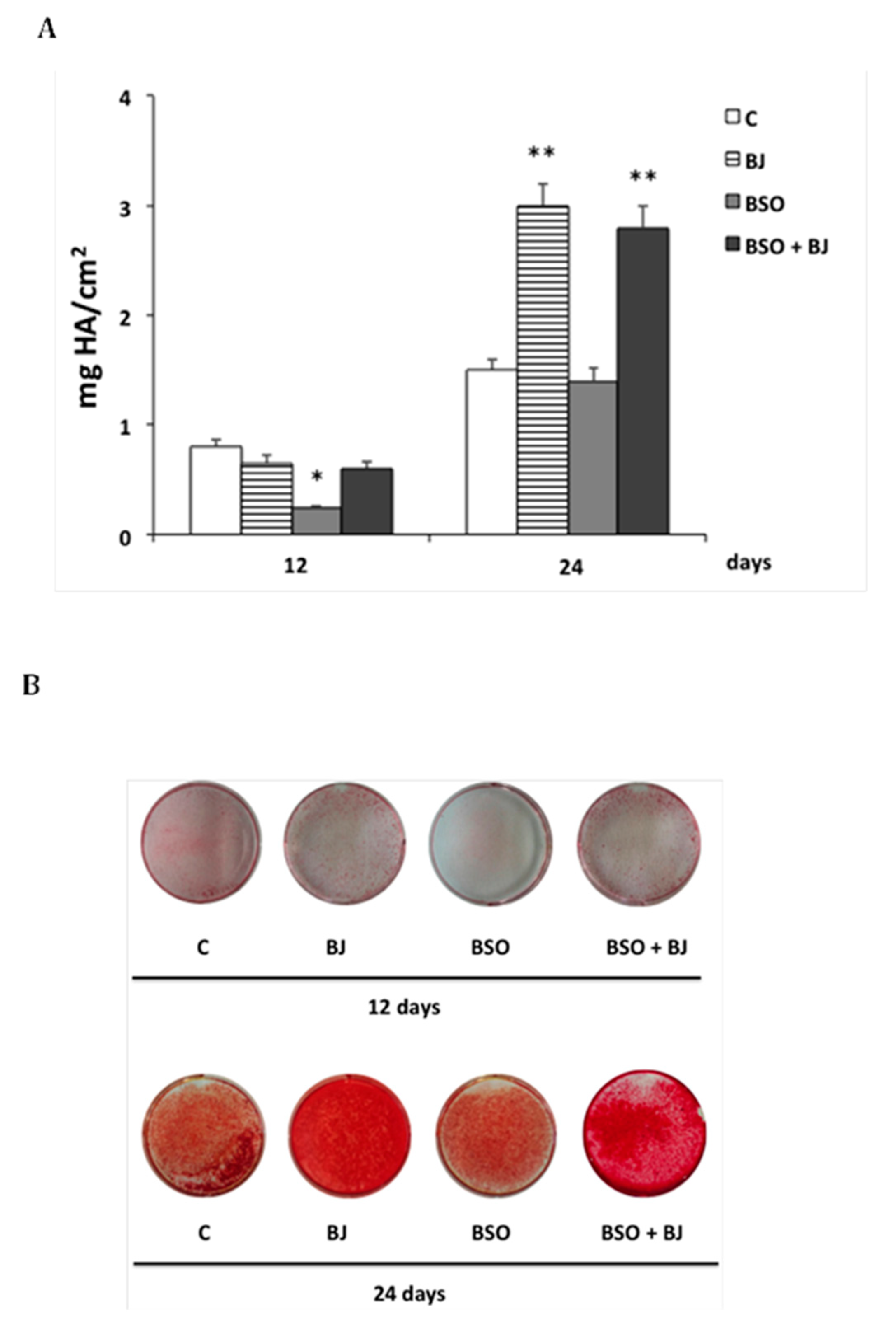
3.4. BJ Effect on the Mineralization Process in SaOS-2 Cells in the Presence or Not of BSO-Induced Oxidative Stress
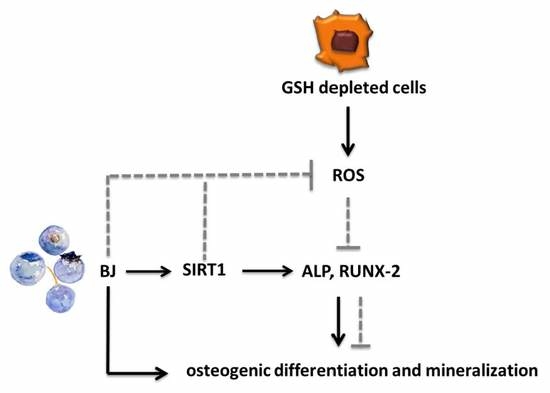
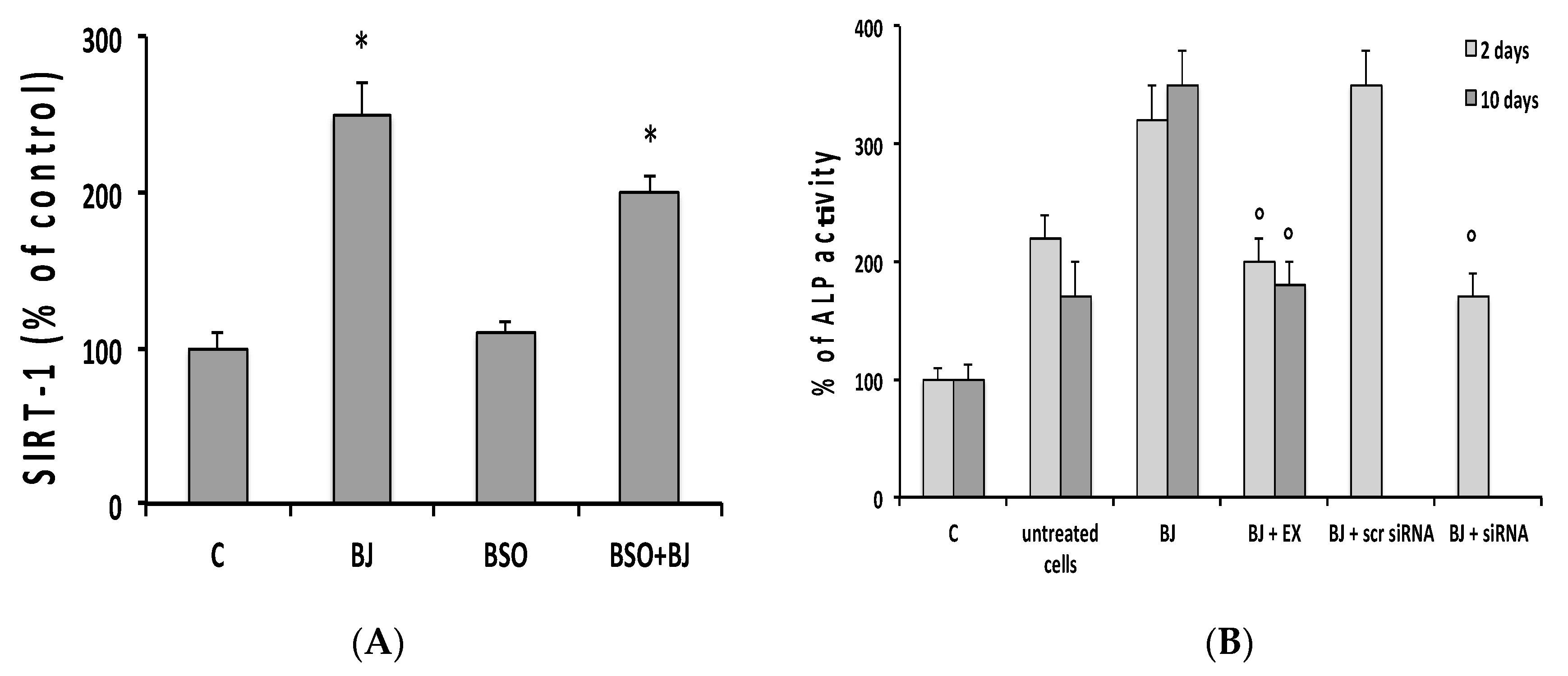
3.5. Involvement of SIRT1 on BJ-Induced Activation of Osteogenic Differentiation and Mineralization Process in SaOS-2 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Domazetovic, V.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Oxidative stress in bone remodeling: Role of antioxidants. Clin. Cases Miner. Bone. Metab. 2017, 14, 209–216. [Google Scholar] [CrossRef]
- Ohyama, Y.; Ito, J.; Kitano, V.J.; Shimada, J.; Hakeda, Y. The polymethoxy flavonoid sudachitin suppresses inflammatory bone destruction by directly inhibiting osteoclastogenesis due to reduced ROS production and MAPK activation in osteoclast precursors. PLoS ONE 2018, 13, e0191192. [Google Scholar] [CrossRef]
- Wauquier, F.; Leotoing, L.; Coxam, V.; Guicheux, J.; Wittrant, Y. Oxidative stress in bone remodelling and disease. Trends Mol. Med. 2009, 15, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Domazetovic, V.; Fontani, F.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Estrogen inhibits starvation-induced apoptosis in osteocytes by a redox-independent process involving association of JNK and glutathione S-transferase P1-1. FEBS Open Bio. 2017, 7, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Banfi, G.; Iorio, E.L.; Corsi, M.M. Oxidative stress, free radicals and bone remodeling. Clin. Chem. Lab. Med. 2008, 46, 1550–1555. [Google Scholar] [CrossRef] [PubMed]
- Fontani, F.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Glutathione, N-acetylcysteine and lipoic acid down-regulate starvation-induced apoptosis, RANKL/OPG ratio and sclerostin in osteocytes: Involvement of JNK and ERK1/2 signalling. Calcif. Tissue Int. 2015, 96, 335–346. [Google Scholar] [CrossRef]
- Almeida, M.; Martin-Millan, M.; Ambrogini, E.; Bradsher, R., 3rd; Han, L.; Chen, X.D.; Roberson, P.K.; Weinstein, R.S.; O’Brien, C.; Jilka, R.L.; et al. Estrogens attenuate oxidative stress and the differentiation and apoptosis of osteoblasts by DNA-binding-independent actions of the ERalpha. J. Bone Miner. Res. 2010, 25, 769–781. [Google Scholar]
- Doshi, S.B.; Agarwal, A. The role of oxidative stress in menopause. J. Midlife Health. 2013, 4, 140–146. [Google Scholar]
- Manolagas, S.C. From estrogen-centric to aging and oxidative stress: A revised perspective of the pathogenesis of osteoporosis. Endocr. Rev. 2010, 31, 266–300. [Google Scholar] [CrossRef]
- Baek, K.H.; Oh, K.W.; Lee, W.Y.; Lee, S.S.; Kim, M.K.; Kwon, H.S.; Rhee, E.J.; Han, J.H.; Song, K.H.; Cha, B.Y.; et al. Association of oxidative stress with postmenopausal osteoporosis and the effects of hydrogen peroxide on osteoclast formation in human bone marrow cell cultures. Calcif. Tissue Int. 2010, 87, 226–235. [Google Scholar] [CrossRef]
- Maggio, D.; Barabani, M.; Pierandrei, M.; Polidori, M.C.; Catani, M.; Mecocci, P.; Senin, U.; Pacifici, R.; Cherubini, A. Marked decrease in plasma antioxidants in aged osteoporotic women: Results of a cross-sectional study. J. Clin. Endocrinol. Metab. 2003, 88, 1523–1527. [Google Scholar] [CrossRef] [PubMed]
- Ostman, B.; Michaëlsson, K.; Helmersson, J.; Byberg, L.; Gedeborg, R.; Melhus, H.; Basu, S. Oxidative stress and bone mineral density in elderly men: Antioxidant activity of alpha-tocopherol. Free Radic. Biol. Med. 2009, 47, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, C.; Marcucci, G.; Favilli, F.; Zonefrati, R.; Mavilia, C.; Galli, G.; Tanini, A.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Role of GSH/GSSG redox couple in osteogenic activity and osteoclastogenic markers of human osteoblast-like SaOS-2 cells. FEBS J. 2013, 280, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Lean, J.M.; Jagger, C.J.; Kirstein, B.; Fuller, K.; Chambers, T.J. Hydrogen peroxide is essential for estrogen-deficiency bone loss and osteoclast formation. Endocrinology 2005, 146, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Oka, Y.; Iwai, S.; Amano, H.; Irie, Y.; Yatomi, K.; Ryu, K.; Yamada, S.; Inagaki, K.; Oguchi, K. Tea polyphenols inhibit rat osteoclast formation and differentiation. J. Pharmacol. Sci. 2012, 118, 55–64. [Google Scholar] [CrossRef]
- Yagi, H.; Tan, J.; Tuan, R.S. Polyphenols suppress hydrogen peroxide-induced oxidative stress in human bone-marrow derived mesenchymal stem cells. J. Cell. Biochem. 2013, 114, 1163–1173. [Google Scholar] [CrossRef]
- Tou, J.C. Resveratrol supplementation affects bone acquisition and osteoporosis: Pre-clinical evidence toward translational diet therapy. Biochim. Biophys. Acta 2015, 1852, 1186–1194. [Google Scholar] [CrossRef]
- Chen, J.R.; Lazarenko, O.P.; Wu, X.; Kang, J.; Blackburn, M.L.; Shankar, K.; Badger, T.M.; Ronis, M.J. Dietary-induced serum phenolic acids promote bone growth via p38 MAPK/β-catenin canonical Wnt signaling. J. Bone Miner. Res. 2010, 25, 2399–2411. [Google Scholar] [CrossRef]
- Li, T.; Wu, S.M.; Xu, Z.Y.; Ou-Yang, S. Rabbiteye blueberry prevents osteoporosis in ovariectomized rats. J. Orthop. Surg. Res. 2014, 9, 56. [Google Scholar]
- Weaver, C.M.; Alekel, D.L.; Ward, W.E.; Ronis, M.J. Flavonoid intake and bone health. J. Nutr. Gerontol. Geriatr. 2012, 31, 239–253. [Google Scholar] [CrossRef]
- Austermann, K.; Baecker, N.; Stehle, P.; Heer, M. Putative Effects of Nutritive Polyphenols on Bone Metabolism In Vivo—Evidence from Human Studies. Nutrients 2019, 11, 871. [Google Scholar] [CrossRef] [PubMed]
- Sendur, O.F.; Turan, Y.; Tastaban, E.; Serter, M. Antioxidant status in patients with osteoporosis: A controlled study. Jt. Bone Spine 2009, 76, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Domazetovic, V.; Fontani, F.; Tanini, D.; D’Esopo, V.; Marcucci, G.; Panzella, L.; Napolitano, A.; Brandi, M.L.; Capperucci, A.; Menichetti, S.; et al. Protective role of benzoselenophene derivatives of resveratrol on the induced oxidative stress in intestinal myofibroblasts and osteocytes. Chem. Biol. Interact. 2017, 275, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Hubert, P.A.; Lee, S.G.; Lee, S.-K.; Chun, O.K. Dietary Polyphenols, Berries, and Age-Related Bone Loss: A Review Based on Human, Animal, and Cell Studies. Antioxidants 2014, 3, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Alam, F.; Solayman, M.; Khalil, M.I.; Kamal, M.A.; Gan, S.H. Dietary Phytochemicals: Natural Swords Combating Inflammation and Oxidation-Mediated Degenerative Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 5137431. [Google Scholar] [CrossRef] [PubMed]
- Li, A.N.; Li, S.; Zhang, Y.J.; Xu, X.R.; Chen, Y.M.; Li, H.B. Resources and biological activities of natural polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef]
- Higgs, J.; Derbyshire, E.; Styles, K. Nutrition and osteoporosis prevention for the orthopaedic surgeon: A wholefoods approach. EFORT Open Rev. 2017, 2, 300–308. [Google Scholar] [CrossRef]
- Rachner, T.D.; Khosla, S.; Hofbauer, L.C. Osteoporosis: Now and the future. Lancet 2011, 377, 1276–1287. [Google Scholar] [CrossRef]
- Marcucci, G.; Brandi, M.L. Rare causes of osteoporosis. Clin. Cases Miner. Bone Metab. 2015, 12, 151–156. [Google Scholar] [CrossRef]
- Devareddy, L.; Hooshmand, S.; Collins, J.K.; Lucas, E.A.; Chai, S.C.; Arjmandi, B.H. Blueberry prevents bone loss in ovariectomized rat model of postmenopausal osteoporosis. J. Nutr. Biochem. 2008, 19, 694–699. [Google Scholar] [CrossRef]
- Ancillotti, C.; Ciofi, L.; Pucci, D.; Sagona, E.; Giordani, E.; Biricolti, S.; Gori, M.; Petrucci, W.A.; Giardi, F.; Bartoletti, R.; et al. Polyphenolic profiles and antioxidant and antiradical activity of Italian berries from Vaccinium myrtillus L. and Vaccinium uliginosum L. subsp. gaultherioides (Bigelow) S.B. Young. Food Chem. 2016, 204, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Prencipe, F.P.; Bruni, R.; Guerrini, A.; Rossi, D.; Benvenuti, S.; Pellati, F. Metabolite profiling of polyphenols in Vaccinium berries and determination of their chemopreventive properties. J Pharm. Biomed. Anal. 2014, 89, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Domazetovic, V.; Marcucci, G.; Pierucci, F.; Bruno, G.; Di Cesare Mannelli, L.; Ghelardini, C.; Brandi, M.L.; Iantomasi, T.; Meacci, E.; Vincenzini, M.T. Blueberry juice protects osteocytes and bone precursor cells against oxidative stress partly through SIRT1. FEBS Open Bio. 2019, 9, 1082–1096. [Google Scholar] [PubMed]
- Matsumoto, H.; Inaba, H.; Kishi, M.; Tominaga, S.; Hirayama, M.; Tsuda, T. Orally administered delphinidin 3-rutinoside and cyanidin 3-rutinoside are directly absorbed in rats and humans and appear in the blood as the intact forms. J. Agric. Food Chem. 2001, 49, 1546–1551. [Google Scholar] [CrossRef]
- Cao, G.; Muccitelli, H.U.; Sánchez-Moreno, C.; Prior, R.L. Anthocyanins are absorbed in glycated forms in elderly women: A pharmacokinetic study. Am. J. Clin. Nutr. 2001, 73, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Sandhu, A.; Edirisinghe, I.; Burton-Freeman, B. Characterization of Wild Blueberry Polyphenols Bioavailability and Kinetic Profile in Plasma over 24-h Period in Human Subjects. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Rodan, S.B.; Imai, Y.; Thiede, M.A.; Wesolowski, G.; Thompson, D.; Bar-Shavit, Z.; Shull, S.; Mann, K.; Rodan, G.A. Characterization of a human osteosarcoma cell line (Saos-2) with osteoblastic properties. Cancer Res. 1987, 47, 4961–4966. [Google Scholar]
- Boskey, A.L.; Roy, R. Cell culture systems for studies of bone and tooth mineralization. Chem. Rev. 2008, 108, 4716–4733. [Google Scholar] [CrossRef]
- McQuillan, D.J.; Richardson, M.D.; Bateman, J.F. Matrix deposition by a calcifying human osteogenic sarcoma cell line (SAOS-2). Bone 1995, 16, 415–426. [Google Scholar] [CrossRef]
- Gundle, R.; Beresford, J.N. The isolation and culture of cells from explants of human trabecular bone. Calcif. Tissue. Int. 1995, 56, S8–S10. [Google Scholar] [CrossRef]
- Hausser, H.J.; Brenner, R.E. Phenotypic instability of Saos-2 cells in long-term culture. Biochem. Biophys. Res. Commun. 2005, 333, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Díaz-García, M.C.; Obón, J.M.; Castellar, M.R.; Collado, J.; Alacid, M. Quantification by UHPLC of total individual polyphenols in fruit juices. Food Chem. 2013, 138, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Kotobuki, N.; Matsushima, A.; Kato, Y.; Kubo, Y.; Hirose, M.; Ohgushi, H. Small interfering RNA of alkaline phosphatase inhibits matrix mineralization. Cell Tissue Res. 2008, 332, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Regulation of osteoblast differentiation by transcription factors. J. Cell. Biochem. 2006, 99, 1233–1239. [Google Scholar] [CrossRef]
- Coffman, J.A. Runx transcription factors and the developmental balance between cell proliferation and differentiation. Cell. Biol. Int. 2003, 27, 315–324. [Google Scholar] [CrossRef]
- Cohen-Kfir, E.; Artsi, H.; Levin, A.; Abramowitz, E.; Bajayo, A.; Gurt, I.; Zhong, L.; D’Urso, A.; Toiber, D.; Mostoslavsky, R.; et al. Sirt1 Is a Regulator of Bone Mass and a Repressor of Sost Encoding for Sclerostin, a Bone Formation Inhibitor. Endocrinology 2011, 152, 4514–4524. [Google Scholar] [CrossRef]
- Liu, J.; Han, W.; Chen, L.; Tang, K. Mechanism of osteogenic and adipogenic differentiation of tendon stem cells induced by sirtuin 1. Mol. Med. Rep. 2016, 14, 1643–1648. [Google Scholar] [CrossRef]
- Zainabadi, K.; Liu, C.J.; Guarente, L. SIRT1 is a positive regulator of the master osteoblast transcription factor, RUNX2. PLoS ONE 2017, 12, e0178520. [Google Scholar] [CrossRef]
- Ren, T.; Huang, C.; Cheng, M. Dietary blueberry and bifidobacteria attenuate nonalcoholic fatty liver disease in rats by affecting SIRT1-mediated signaling pathway. Oxid. Med. Cell. Longev. 2014, 2014, 469059. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, S.I.; Moon, Y.J.; Kim, K.M.; Lee, K.B.; Park, B.H.; Jang, K.Y.; Kim, J.R. Overexpression of SIRT1 prevents hypoxia-induced apoptosis in osteoblast cells. Mol. Med. Rep. 2017, 16, 2969–2975. [Google Scholar] [CrossRef]
- Lean, J.M.; Davies, J.T.; Jagger, C.J.; Kirstein, B.; Partington, G.A.; Urry, Z.L.; Chambers, T.J. A crucial role for thiol antioxidants in estrogen-deficiency bone loss. J. Clin. Investig. 2003, 112, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Sanders, K.M.; Kotowicz, M.A.; Nicholson, G.C. Potential role of the antioxidant N-acetylcysteine in slowing bone resorption in early post-menopausal women: A pilot study. Transl. Res. 2007, 150, 215. [Google Scholar] [CrossRef] [PubMed]
- Lohachoompol, V.; Srzednicki, G.; Craske, J. The Change of Total Anthocyanins in Blueberries and Their Antioxidant Effect After Drying and Freezing. J. Biomed. Biotechnol. 2004, 2004, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Michalska, A.; Łysiak, G. Bioactive Compounds of Blueberries: Post-Harvest Factors Influencing the Nutritional Value of Products. Int. J. Mol. Sci. 2015, 16, 18642–18663. [Google Scholar] [CrossRef]
- Kuntz, S.; Rudloff, S.; Asseburg, H.; Borsch, C.; Fröhling, B.; Unger, F.; Dold, S.; Spengler, B.; Römpp, A.; Kunz, C. Uptake and bioavailability of anthocyanins and phenolic acids from grape/blueberry juice and smoothie in vitro and in vivo. Br. J. Nutr. 2015, 113, 1044–1055. [Google Scholar] [CrossRef]
- Bu, S.Y.; Hunt, T.S.; Smith, B.J. Dried plum polyphenols attenuate the detrimental effects of TNF-α on osteoblast function coincident with up-regulation of Runx2, Osterix and IGF-I. J. Nutr. Biochem. 2009, 20, 35–44. [Google Scholar] [CrossRef]
- Zhang, J.; Lazarenko, O.P.; Blackburn, M.L.; Shankar, K.; Badger, T.M.; Ronis, M.J.; Chen, J.R. Feeding blueberry diets in early life prevent senescence of osteoblasts and bone loss in ovariectomized adult female rats. PLoS ONE 2011, 6, e24486. [Google Scholar] [CrossRef]
- Artsi, H.; Cohen-Kfir, E.; Gurt, I.; Shahar, R.; Bajayo, A.; Kalish, N.; Bellido, T.M.; Gabet, Y.; Dresner-Pollak, R. The Sirtuin1 Activator SRT3025 Down-Regulates Sclerostin and Rescues Ovariectomy-Induced Bone Loss and Biomechanical Deterioration in Female Mice. Endocrinology 2014, 155, 3508–3515. [Google Scholar] [CrossRef]
- Gurt, I.; Artsi, H.; Cohen-Kfir, E.; Hamdani, G.; Ben-Shalom, G.; Feinstein, B.; El-HAj, M.; Dresner-Pollak, R. The Sirt1 Activators SRT2183 and SRT3025 Inhibit RANKL-Induced Osteoclastogenesis in Bone Marrow-Derived Macrophages and Down-Regulate Sirt3 in Sirt1 Null Cells. PLoS ONE 2015, 10, e0134391. [Google Scholar] [CrossRef]
- Novotny, R.; Daida, Y.G.; Grove, J.S.; Acharya, S.; Vogt, T.M.; Paperny, D. Adolescent dairy consumption and physical activity associated with bone mass. Prev. Med. 2004, 39, 355–360. [Google Scholar] [CrossRef]
- Tylavsky, F.A.; Holliday, K.; Danish, R.; Womack, C.; Norwood, J.; Carbone, L. Fruit and vegetable intakes are an independent predictor of bone size in early pubertal children. Am. J. Clin. Nutr. 2004, 79, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lazarenko, O.P.; Kang, J.; Blackburn, M.L.; Ronis, M.J.; Badger, T.M.; Chen, J.R. Feeding blueberry diets to young rats dose-dependently inhibits bone resorption through suppression of RANKL in stromal cells. PLoS ONE 2013, 8, e70438. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.R.; Lazarenko, O.P.; Zhang, J.; Blackburn, M.L.; Ronis, M.J.; Badger, T.M. Diet-derived phenolic acids regulate osteoblast and adipocyte lineage commitment and differentiation in young mice. J. Bone. Miner. Res. 2014, 29, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- Gilsanz, V.; Wren, T. Assessment of bone acquisition in childhood and adolescence. Pediatrics 2007, 119, S145–S149. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, U.; Iolascon, G.; Cianferotti, L.; Masi, L.; Marcucci, G.; Giusti, F.; Marini, F.; Parri, S.; Feola, M.; Rao, C.; et al. Clinical guidelines for the prevention and treatment of osteoporosis: Summary statements and recommendations from the Italian Society for Orthopaedics and Traumatology. J. Orthop. Traumatol. 2017, 18, 3–36. [Google Scholar] [CrossRef]
| Polyphenol Group | Identified Constituents | Total Polyphenols Expressed as Gallic Acid (mg ± SD/100 mL) |
|---|---|---|
| Anthocyanins | dephinidin 3-O-galactoside | |
| delphinidin 3-O-glucoside | ||
| cyanidin 3-O-galactoside | ||
| delphinidin 3-O-arabinoside | ||
| cyanidin 3-O-glucoside | ||
| petunidin 3-O-galactoside | ||
| petunidin 3-O-glucoside | ||
| cyanidin 3-O-arabinoside | ||
| peonidin 3-O-galactoside | ||
| petunidin 3-O-arabinoside | ||
| malvidin 3-O-galactoside | ||
| peonidin 3-O-glucoside | ||
| malvidin 3-O-glucoside | ||
| malvidin 3-O-arabinoside | ||
| Total anthocyanins | 107.0 ± 9.3 | |
| Flavonols | quercetin 3-O-arabinoside | |
| quercetin 3-O-galactoside | ||
| quercetin 3-O-glucoside | ||
| myricetin 3-O-glucoside | ||
| myricetin 3-O-galactoside | ||
| Total flavonols | 2.9 ± 0.9 | |
| Flavan-3-ols | catechin | |
| epicatechin | ||
| Total flavanols | 5.8 ± 1.4 | |
| Hydroxybenzoic acids | ellagic acid | 12.5 ± 2.4 |
| Hydroxycinnamic acids | chlorogenic acid | 30.6 ± 6.3 |
| Days | 1 | 2 |
|---|---|---|
| Control | 100 ± 8 | 100 ± 11 |
| BSO | 80 ± 10 | 83 ± 8 |
| BJ | 90 ± 8 | 85 ± 10 |
| BSO + BJ | 79 ± 9 | 80 ± 7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domazetovic, V.; Marcucci, G.; Falsetti, I.; Bilia, A.R.; Vincenzini, M.T.; Brandi, M.L.; Iantomasi, T. Blueberry Juice Antioxidants Protect Osteogenic Activity against Oxidative Stress and Improve Long-Term Activation of the Mineralization Process in Human Osteoblast-Like SaOS-2 Cells: Involvement of SIRT1. Antioxidants 2020, 9, 125. https://doi.org/10.3390/antiox9020125
Domazetovic V, Marcucci G, Falsetti I, Bilia AR, Vincenzini MT, Brandi ML, Iantomasi T. Blueberry Juice Antioxidants Protect Osteogenic Activity against Oxidative Stress and Improve Long-Term Activation of the Mineralization Process in Human Osteoblast-Like SaOS-2 Cells: Involvement of SIRT1. Antioxidants. 2020; 9(2):125. https://doi.org/10.3390/antiox9020125
Chicago/Turabian StyleDomazetovic, Vladana, Gemma Marcucci, Irene Falsetti, Anna Rita Bilia, Maria Teresa Vincenzini, Maria Luisa Brandi, and Teresa Iantomasi. 2020. "Blueberry Juice Antioxidants Protect Osteogenic Activity against Oxidative Stress and Improve Long-Term Activation of the Mineralization Process in Human Osteoblast-Like SaOS-2 Cells: Involvement of SIRT1" Antioxidants 9, no. 2: 125. https://doi.org/10.3390/antiox9020125
APA StyleDomazetovic, V., Marcucci, G., Falsetti, I., Bilia, A. R., Vincenzini, M. T., Brandi, M. L., & Iantomasi, T. (2020). Blueberry Juice Antioxidants Protect Osteogenic Activity against Oxidative Stress and Improve Long-Term Activation of the Mineralization Process in Human Osteoblast-Like SaOS-2 Cells: Involvement of SIRT1. Antioxidants, 9(2), 125. https://doi.org/10.3390/antiox9020125