Seasonality Modulates the Cellular Antioxidant Activity and Antiproliferative Effect of Sonoran Desert Propolis
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Propolis Collection and Methanolic Extraction
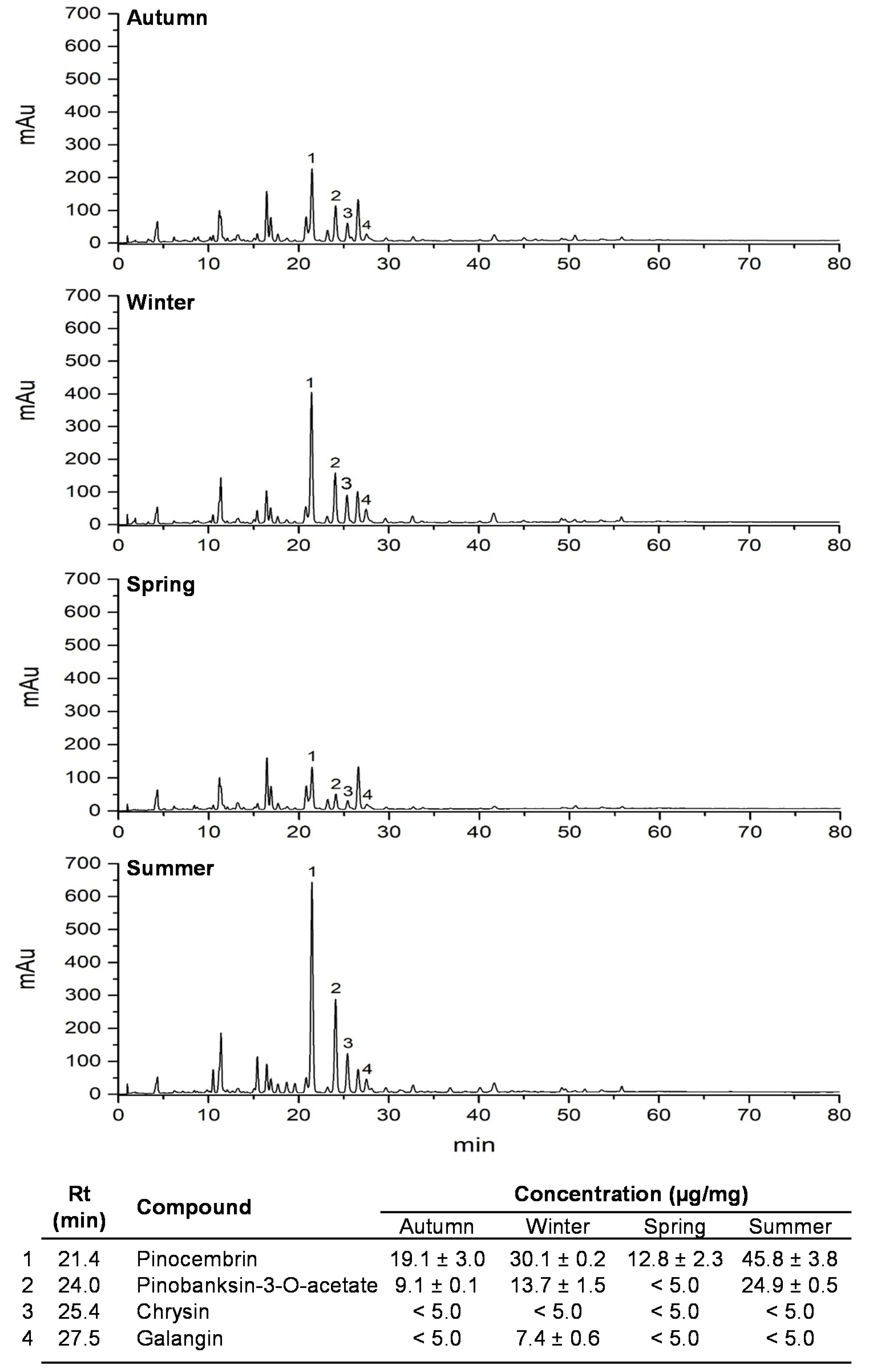
2.3. Polyphenolic Profile Analyses by HPLC–UV–DAD
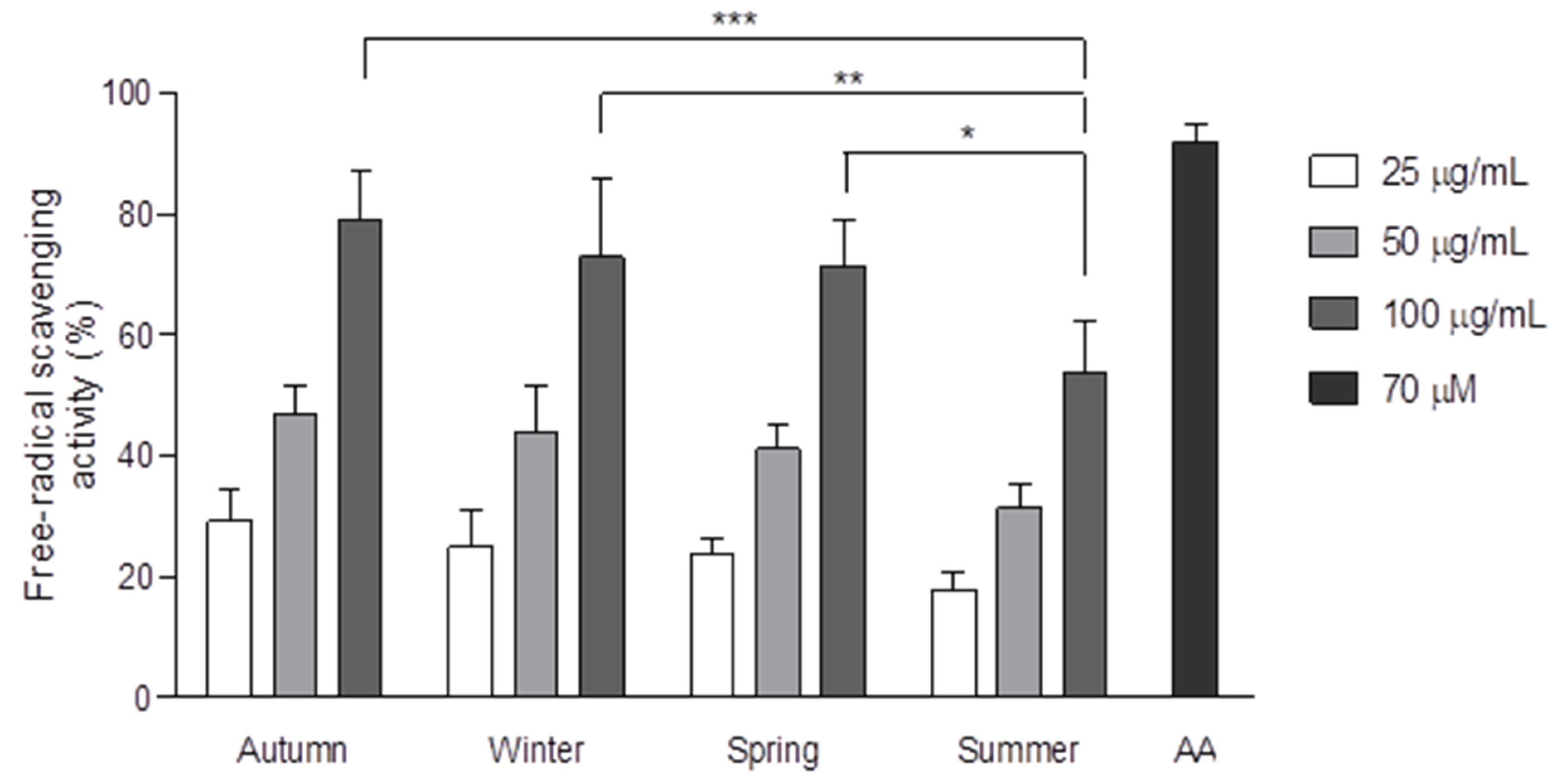
2.4. Free Radical Scavenging Activity (DPPH)
2.5. Cell Culture and Antiproliferative Activity Assay
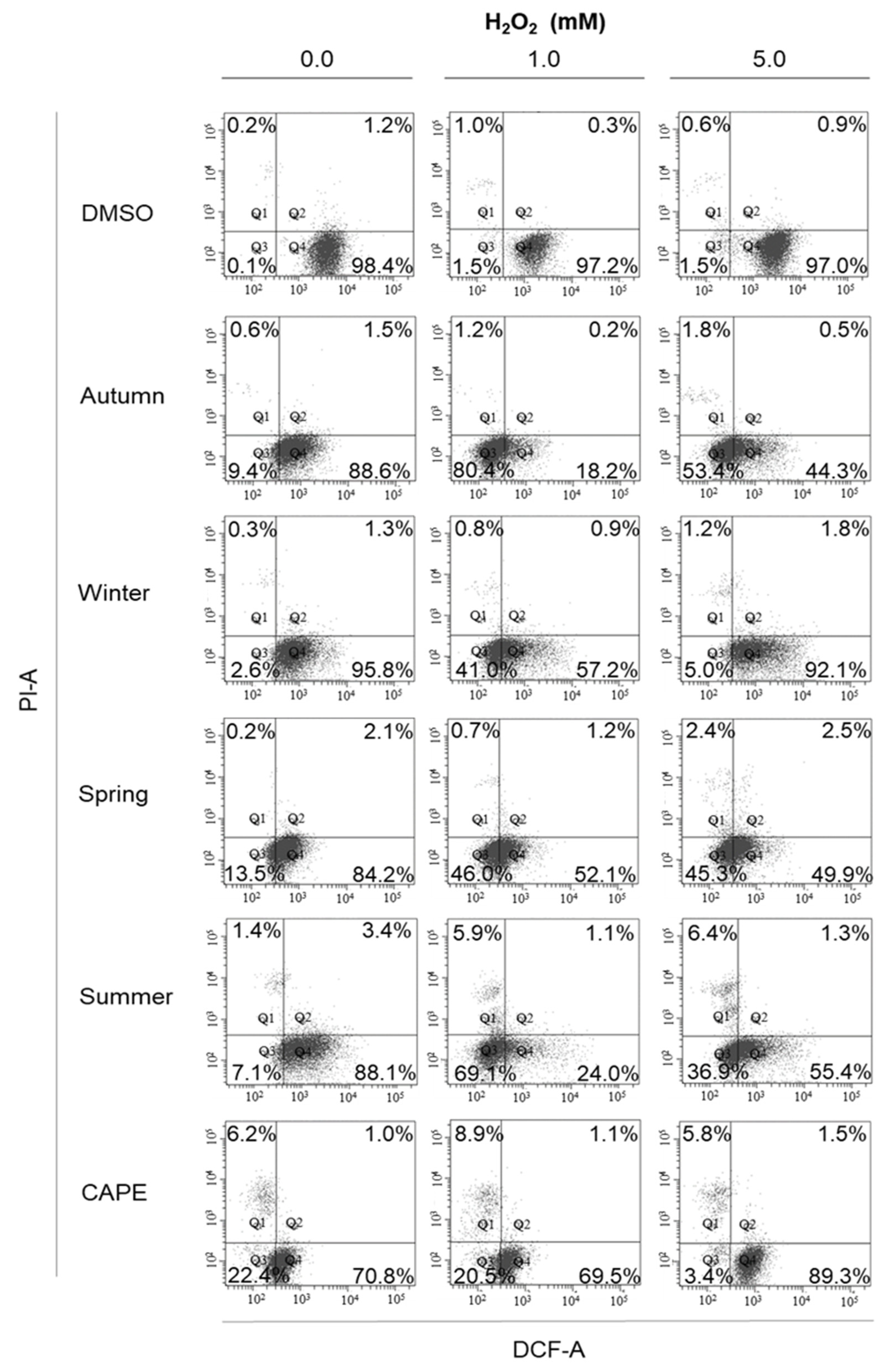
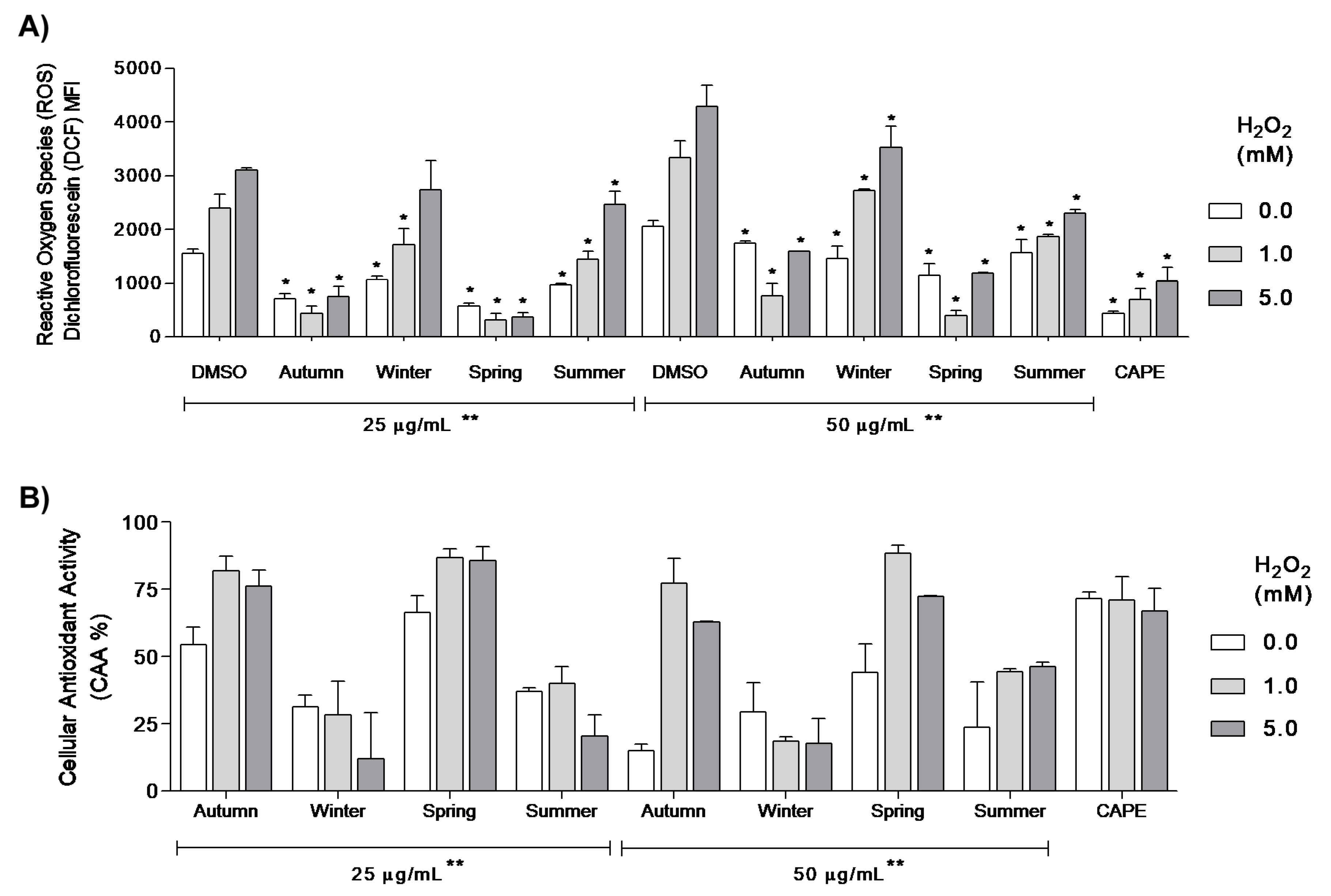
2.6. Cellular Anti-Oxidant Activity Assay (CAA)
2.7. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, E.-K.; Jang, M.; Song, M.-J.; Kim, D.; Kim, Y.; Jang, H.H. Redox-Mediated Mechanism of Chemoresistance in Cancer Cells. Antioxidants 2019, 8, 471. [Google Scholar] [CrossRef]
- Kaushal, G.P.; Chandrashekar, K.; Juncos, L.A. Molecular Interactions Between Reactive Oxygen Species and Autophagy in Kidney Disease. Int. J. Mol. Sci. 2019, 20, 3791. [Google Scholar] [CrossRef]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K. Reactive oxygen species (ROS) and cancer: Role of antioxidative nutraceuticals. Cancer Lett. 2017, 387, 95–105. [Google Scholar] [CrossRef]
- Carocho, M.; Ferreira, I.C.F.R. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef]
- Mittler, R. ROS Are Good. Trends Plant. Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef] [PubMed]
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef] [PubMed]
- Sabharwal, S.S.; Schumacker, P.T. Mitochondrial ROS in cancer: Initiators, amplifiers or an Achilles’ heel? Nat. Rev. Cancer 2014, 14, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Lokesh, K.N.; Channarayappa; Venkatarangana, M. Exemplified screening standardization of potent antioxidant nutraceuticals by principles of design of experiments. J. Funct. Foods 2015, 17, 260–270. [Google Scholar]
- Medić-Šarić, M.; Rastija, V.; Bojić, M.; Maleš, Ž.; Bradamante, V.; Lacković, Z.; Havsteen, B.; Smirnoff, N.; Castalado, S.; Capasso, F.; et al. From functional food to medicinal product: Systematic approach in analysis of polyphenolics from propolis and wine. Nutr. J. 2009, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Babich, H.; Schuck, A.G.; Weisburg, J.H.; Zuckerbraun, H.L. Research strategies in the study of the pro-oxidant nature of polyphenol nutraceuticals. J. Toxicol. 2011, 2011, 467305. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.B.; Pawlus, A.D.; Brinkman, D.; Gardner, G.; Hegeman, A.D.; Spivak, M.; Cohen, J.D. 3-Acyl dihydroflavonols from poplar resins collected by honey bees are active against the bee pathogens Paenibacillus larvae and Ascosphaera apis. Phytochemistry 2017, 138, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Bankova, V.; Bertelli, D.; Borba, R.; Conti, B.J.; da Silva Cunha, I.B.; Danert, C.; Eberlin, M.N.; Falcão, S.I.; Isla, M.I.; Moreno, M.I.N.; et al. Standard methods for Apis mellifera propolis research. J. Apic. Res. 2016, 8839, 1–49. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, C.P.; Wang, K.; Li, G.Q.; Hu, F.L. Recent advances in the chemical composition of propolis. Molecules 2014, 19, 19610–19632. [Google Scholar] [CrossRef]
- Cornara, L.; Biagi, M.; Xiao, J.; Burlando, B. Therapeutic properties of bioactive compounds from different honeybee products. Front. Pharmacol. 2017, 8, 412. [Google Scholar] [CrossRef]
- Sforcin, J. Biological Properties and Therapeutic Applications of Propolis. Phyther. Res. 2016, 30, 894–905. [Google Scholar] [CrossRef]
- Sforcin, J.M.; Bankova, V. Propolis: Is there a potential for the development of new drugs? J. Ethnopharmacol. 2011, 133, 253–260. [Google Scholar] [CrossRef]
- Do Nascimento, T.G.; dos Santos Arruda, R.E.; da Cruz Almeida, E.T.; dos Santos Oliveira, J.M.; Basílio-Júnior, I.D.; Celerino de Moraes Porto, I.C.; Rodrigues Sabino, A.; Tonholo, J.; Gray, A.; Ebel, R.E.; et al. Comprehensive multivariate correlations between climatic effect, metabolite-profile, antioxidant capacity and antibacterial activity of Brazilian red propolis metabolites during seasonal study. Sci. Rep. 2019, 9, 18293. [Google Scholar] [CrossRef]
- Alday, E.; Valencia, D.; Garibay-Escobar, A.; Domínguez-Esquivel, Z.; Piccinelli, A.L.; Rastrelli, L.; Monribot-Villanueva, J.; Guerrero-Analco, J.A.; Robles-Zepeda, R.E.; Hernandez, J.; et al. Plant origin authentication of Sonoran Desert propolis: An antiproliferative propolis from a semi-arid region. Sci. Nat. 2019, 106, 25. [Google Scholar] [CrossRef]
- Valencia, D.; Alday, E.; Robles-Zepeda, R.; Garibay-Escobar, A.; Galvez-Ruiz, J.C.; Salas-Reyes, M.; Jiménez-Estrada, M.; Velazquez-Contreras, E.; Hernandez, J.; Velazquez, C. Seasonal effect on chemical composition and biological activities of Sonoran propolis. Food Chem. 2012, 131, 645–651. [Google Scholar] [CrossRef]
- Sforcin, J.M.; Fernandes Júnior, A.; Lopes, C.A.M.; Funari, S.R.C.; Bankova, V. Seasonal effect of brazilian propolis on Candida albicans and Candida tropicalis. J. Venom. Anim. Toxins 2001, 7, 139–144. [Google Scholar] [CrossRef]
- Sforcin, J.M.; Fernandes, A.; Lopes, C.A.M.; Bankova, V.; Funari, S.R.C. Seasonal effect on Brazilian propolis antibacterial activity. J. Ethnopharmacol. 2000, 73, 243–249. [Google Scholar] [CrossRef]
- Alday-Provencio, S.; Diaz, G.; Rascon, L.; Quintero, J.; Alday, E.; Robles-Zepeda, R.; Garibay-Escobar, A.; Astiazaran, H.; Hernandez, J.; Velazquez, C. Sonoran Propolis and Some of its Chemical Constituents Inhibit in vitro Growth of Giardia lamblia Trophozoites. Planta Med. 2015, 81, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Bueno-Silva, B.; Marsola, A.; Ikegaki, M.; Alencar, S.M.; Rosalen, P.L. The effect of seasons on Brazilian red propolis and its botanical source: Chemical composition and antibacterial activity. Nat. Prod. Res. 2017, 31, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.B.; Spivak, M.; Hegeman, A.D.; Rendahl, A.; Cohen, J.D. Metabolomics Reveals the Origins of Antimicrobial Plant Resins Collected by Honey Bees. PLoS ONE 2013, 8, e77512. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Kumazawa, S.; Hamasaka, T.; Nakayama, T. Antioxidant activity of propolis of various geographic origins. Food Chem. 2004, 84, 329–339. [Google Scholar] [CrossRef]
- Nina, N.; Quispe, C.; Jiménez-Aspee, F.; Theoduloz, C.; Feresín, G.E.; Lima, B.; Leiva, E.; Schmeda-Hirschmann, G. Antibacterial activity, antioxidant effect and chemical composition of propolis from the Región del Maule, central Chile. Molecules 2015, 20, 18144–18167. [Google Scholar] [CrossRef]
- Daleprane, J.B.; Abdalla, D.S. Emerging roles of propolis: Antioxidant, cardioprotective, and antiangiogenic actions. Evid. Based Complement. Altern. Med. 2013, 2013, 175135. [Google Scholar] [CrossRef]
- Galeotti, F.; Maccari, F.; Fachini, A.; Volpi, N. Chemical Composition and Antioxidant Activity of Propolis Prepared in Different Forms and in Different Solvents Useful for Finished Products. Foods 2018, 7, 41. [Google Scholar] [CrossRef]
- Piccinelli, A.L.; Mencherini, T.; Celano, R.; Mouhoubi, Z.; Tamendjari, A.; Aquino, R.P.; Rastrelli, L. Chemical composition and antioxidant activity of Algerian propolis. J. Agric. Food Chem. 2013, 61, 5080–5088. [Google Scholar] [CrossRef] [PubMed]
- Eruslanov, E.; Kusmartsev, S. Identification of ROS Using Oxidized DCFDA and Flow-Cytometry. In Advanced Protocols in Oxidative Stress II, Methods in Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2010; Volume 594, pp. 57–72. ISBN 978-1-60761-410-4. [Google Scholar]
- Wang, H.; Joseph, J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic. Biol. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef] [PubMed]
- Carreño, A.L.; Alday, E.; Quintero, J.; Pérez, L.; Valencia, D.; Robles-Zepeda, R.; Valdez-Ortega, J.; Hernandez, J.; Velazquez, C. Protective effect of Caffeic Acid Phenethyl Ester (CAPE) against oxidative stress. J. Funct. Foods 2017, 29, 179–184. [Google Scholar] [CrossRef]
- Hernandez, J.; Goycoolea, F.M.; Quintero, J.; Acosta, A.; Castañeda, M.; Dominguez, Z.; Robles, R.; Vazquez-Moreno, L.; Velazquez, E.F.; Astiazaran, H.; et al. Sonoran propolis: Chemical composition and antiproliferative activity on cancer cell lines. Planta Med. 2007, 73, 1469–1474. [Google Scholar] [CrossRef]
- Wollenweber, E.; Buchmann, S.L. Feral honey bees in the Sonoran Desert: Propolis sources other than poplars (Populus spp.). Z. Nat. C 1997, 52, 530–535. [Google Scholar] [CrossRef]
- Wollenweber, E.; Hradetzky, D.; Mann, K.; Roitman, J.N.; Yatskievych, G.; Proksch, M.; Proksch, P. Exudate Flavonoids from Aerial Parts of Five Ambrosia Species. J. Plant. Physiol. 1987, 131, 37–43. [Google Scholar] [CrossRef]
- Velazquez, C.; Navarro, M.; Acosta, A.; Angulo, A.; Dominguez, Z.; Robles, R.; Robles-Zepeda, R.; Lugo, E.; Goycoolea, F.M.; Velazquez, E.F.; et al. Antibacterial and free-radical scavenging activities of Sonoran propolis. J. Appl. Microbiol. 2007, 103, 1747–1756. [Google Scholar] [CrossRef]
- Grunberger, D.; Banerjee, R.; Eisinger, K.; Oltz, E.M.; Efros, L.; Caldwell, M.; Estevez, V.; Nakanishi, K. Preferential cytotoxicity on tumor cells by caffeic acid phenethyl ester isolated from propolis. Experientia 1988, 44, 230–232. [Google Scholar] [CrossRef]
- Conti, B.J.; Bankova, V.; Sforcin, J.M. Chemical composition of the same brazilian propolis sample analyzed in 1997 and in 2012: No freezing effect. Nat. Prod. Commun. 2015, 10, 1279–1280. [Google Scholar] [CrossRef]
- Usia, T.; Banskota, A.H.; Tezuka, Y.; Midorikawa, K.; Matsushige, K.; Kadota, S. Constituents of Chinese Propolis and Their Antiproliferative Activities. J. Nat. Prod. 2002, 65, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Wade, W.F.; Chen, Z.Z.; Maki, R.; McKercher, S.; Palmer, E.; Cambier, J.C.; Freed, J.H. Altered I-A protein-mediated transmembrane signaling in B cells that express truncated I-Ak protein. Proc. Natl. Acad. Sci. USA 1989, 86, 6297–6301. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Fiaschi, T.; Chiarugi, P. Oxidative stress, tumor microenvironment, and metabolic reprogramming: A diabolic liaison. Int. J. Cell Biol. 2012, 2012, 762825. [Google Scholar] [CrossRef] [PubMed]
- Amar, Y.; Meddah, B.; Bonacorsi, I.; Costa, G.; Pezzino, G.; Saija, A.; Cristani, M.; Boussahel, S.; Ferlazzo, G.; Meddah, A.T. Phytochemicals, antioxidant and antiproliferative properties of rosmarinus officinalis L on U937 and CaCo-2 cells. Iran. J. Pharm. Res. 2017, 16, 315–327. [Google Scholar] [PubMed]
- Neto, M.R.; Tintino, S.R.; da Silva, A.R.; do Socorro Costa, M.; Boligon, A.A.; Matias, E.F.; de Queiroz Balbino, V.; Menezes, I.R.; Coutinho, H.D. Seasonal variation of Brazilian red propolis: Antibacterial activity, synergistic effect and phytochemical screening. Food Chem. Toxicol. 2017, 107, 572–580. [Google Scholar]
- Dudonné, S.; Poupard, P.; Coutiére, P.; Woillez, M.; Richard, T.; Mérillon, J.M.; Vitrac, X. Phenolic composition and antioxidant properties of poplar bud (Populus nigra) extract: Individual antioxidant contribution of phenolics and transcriptional effect on skin aging. J. Agric. Food Chem. 2011, 59, 4527–4536. [Google Scholar] [CrossRef]
- Shreve, F.; Wiggins, I.L. Vegetation and Flora of the Sonoran Desert; Stanford University Press: Stanford, CA, USA, 1964; Volume 1, ISBN 0804701636. [Google Scholar]
| Seasonal CP | IC50 (µg/mL or µM) |
|---|---|
| Autumn | 5.9 ± 0.6 a,b |
| Winter | 12.1 ± 0.5 b |
| Spring | 9.2 ± 1.3 a |
| Summer | 9.5 ± 0.4 a |
| CAPE | 1.4 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendez-Pfeiffer, P.; Alday, E.; Carreño, A.L.; Hernández-Tánori, J.; Montaño-Leyva, B.; Ortega-García, J.; Valdez, J.; Garibay-Escobar, A.; Hernandez, J.; Valencia, D.; et al. Seasonality Modulates the Cellular Antioxidant Activity and Antiproliferative Effect of Sonoran Desert Propolis. Antioxidants 2020, 9, 1294. https://doi.org/10.3390/antiox9121294
Mendez-Pfeiffer P, Alday E, Carreño AL, Hernández-Tánori J, Montaño-Leyva B, Ortega-García J, Valdez J, Garibay-Escobar A, Hernandez J, Valencia D, et al. Seasonality Modulates the Cellular Antioxidant Activity and Antiproliferative Effect of Sonoran Desert Propolis. Antioxidants. 2020; 9(12):1294. https://doi.org/10.3390/antiox9121294
Chicago/Turabian StyleMendez-Pfeiffer, Pablo, Efrain Alday, Ana Laura Carreño, Jorge Hernández-Tánori, Beatriz Montaño-Leyva, Jesús Ortega-García, Judith Valdez, Adriana Garibay-Escobar, Javier Hernandez, Dora Valencia, and et al. 2020. "Seasonality Modulates the Cellular Antioxidant Activity and Antiproliferative Effect of Sonoran Desert Propolis" Antioxidants 9, no. 12: 1294. https://doi.org/10.3390/antiox9121294
APA StyleMendez-Pfeiffer, P., Alday, E., Carreño, A. L., Hernández-Tánori, J., Montaño-Leyva, B., Ortega-García, J., Valdez, J., Garibay-Escobar, A., Hernandez, J., Valencia, D., & Velazquez, C. (2020). Seasonality Modulates the Cellular Antioxidant Activity and Antiproliferative Effect of Sonoran Desert Propolis. Antioxidants, 9(12), 1294. https://doi.org/10.3390/antiox9121294







