Influence of the Location of Ascorbic Acid in Walnut Oil Spray-Dried Microparticles with Outer Layer on the Physical Characteristics and Oxidative Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Walnut Oil Purification
2.2.2. Purified Walnut Oil (PWO) Characterization
2.2.3. PWO Emulsion Preparation
2.2.4. Encapsulation of PWO Emulsion by Spray-Drying
2.2.5. Formation and Confirmation of a Sodium Alginate Outer Layer Using a Three-Fluid Nozzle.
Confirmation of Sodium Alginate Outer-Layer Using a Three-Fluid Nozzle
2.2.6. Incorporation of Ascorbic Acid (AA) in PWO-C/SA Microparticles
2.2.7. Characterization of PWO-C/SA Microparticles with Ascorbic Acid
Water Activity, Moisture Content and Hygroscopicity
Ascorbic Acid Recovery
Morphology of the Microparticles
Induction Period (IP)
Thermal Analysis
2.2.8. Oxidative Stability Assays
Encapsulated Oil Extraction
Determination of Triacylglycerol Dimers and Polymers
2.2.9. Statistical Analysis
3. Results and Discussion
3.1. PWO Emulsion Preparation
3.2. PWO Encapsulation by Spray Drying
3.3. Study of the SA Outer Layer Formation in PWO-C Microparticles
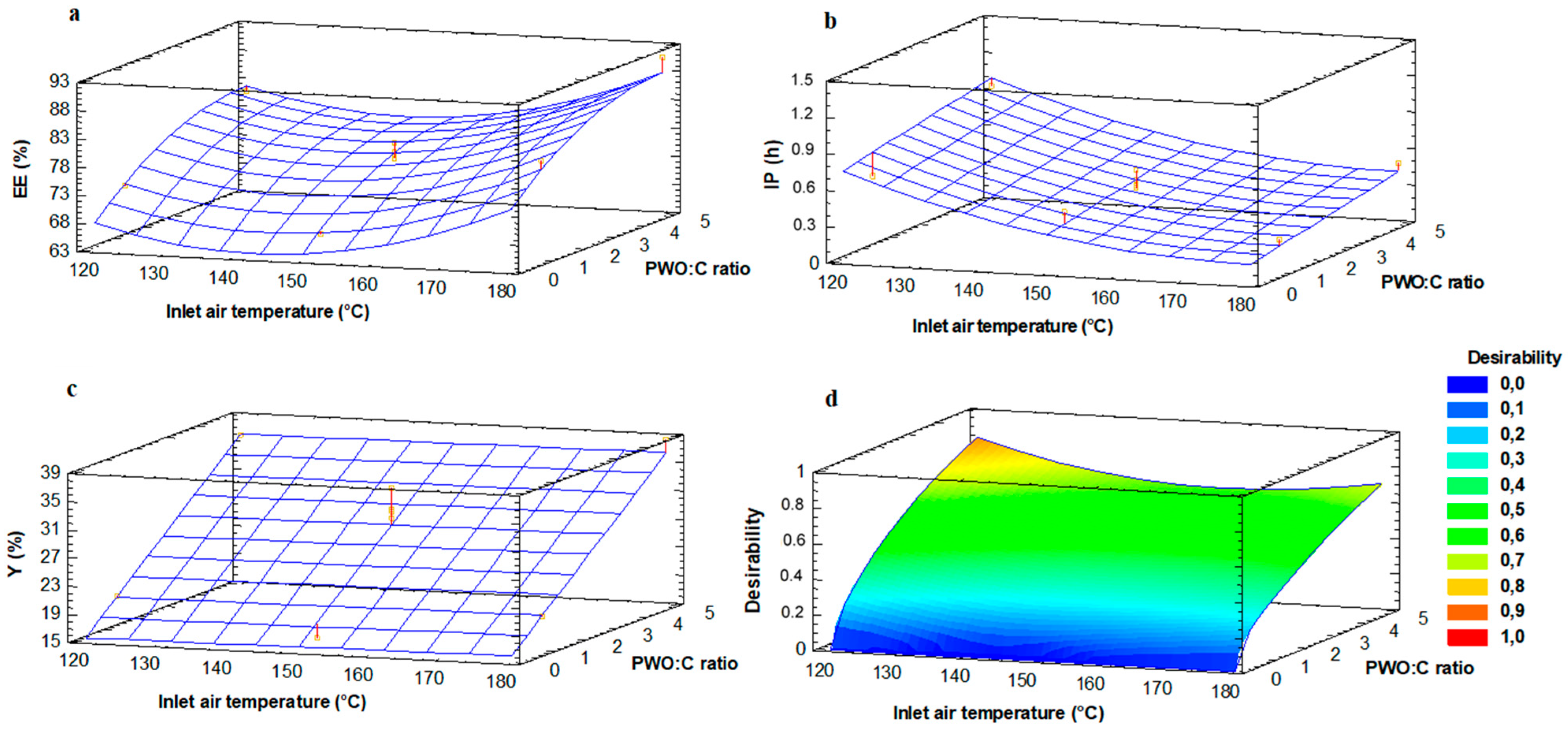
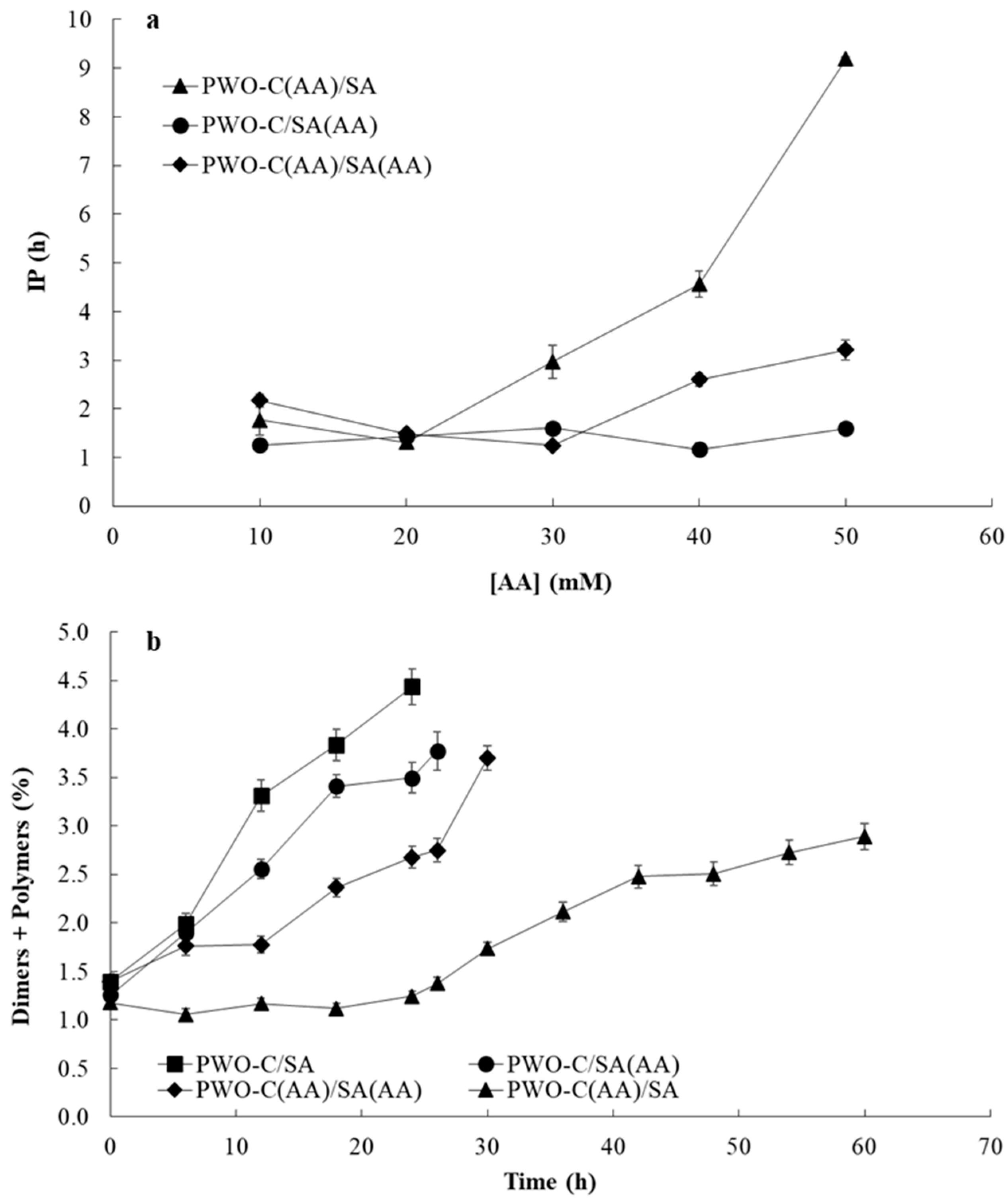
3.4. Effect of the Concentration and Localization of AA on the Oxidative Stability of Encapsulated PWO
3.5. Characterization of PWO Microparticles Obtained under Optimal Conditions
3.5.1. Encapsulation Efficiency (EE)
3.5.2. Induction Period (IP)
3.5.3. AA Content
3.5.4. Moisture Content, Water Activity, Hygroscopicity
3.5.5. Morphology
3.6. Thermal Analysis
3.7. Stability of Encapsulated PWO During Storage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bakry, A.M.; Abbas, S.; Ali, B.; Majeed, H.; Abouelwafa, M.Y.; Mousa, A.; Liang, L. Microencapsulation of oils: A comprehensive review of benefits, techniques, and applications. Compr. Rev. Food Sci. Food Saf. 2016, 15, 143–182. [Google Scholar] [CrossRef]
- Ruiz Ruiz, J.C.; Ortiz Vazquez, E.L.; Segura Campos, M.R. Encapsulation of vegetable oils as source of omega-3 fatty acids for enriched functional foods. Crit. Rev. Food Sci. Nutr. 2017, 57, 1423–1434. [Google Scholar] [CrossRef] [PubMed]
- Shamaei, S.; Seiiedlou, S.S.; Aghbashlo, M.; Tsotsas, E.; Kharaghani, A. Microencapsulation of walnut oil by spray drying: Effects of wall material and drying conditions on physicochemical properties of microcapsules. Innov. Food Sci. Emerg. Technol. 2017, 39, 101–112. [Google Scholar] [CrossRef]
- Cunnane, S.; Drevon, C.A.; Harris, W.; Sinclair, A.; Spwctor, A. Recommendations for intakes of polyunsaturated fatty acids in healthy adults. J. Pharm. Sci. 2004, 11, 12–25. [Google Scholar]
- Geranpour, M.; Assadpour, E.; Jafari, S.M. Recent advances in the spray drying encapsulation of essential fatty acids and functional oils. Trends Food Sci. Technol. 2020, 102, 71–90. [Google Scholar] [CrossRef]
- Calvo, P.; Lozano, M.; Espinosa-Mansilla, A.; González-Gómez, D. In-vitro evaluation of the availability of ω-3 and ω-6 fatty acids and tocopherols from microencapsulated walnut oil. Food Res. Int. 2012, 48, 316–321. [Google Scholar] [CrossRef]
- Luna-Guevara, J.J.; Ochoa-Velasco, C.E.; Hernández-Carranza, P.; Guerrero-Beltrán, J.A. Microencapsulation of walnut, peanut and pecan oils by spray drying. Food Struct. 2017, 12, 26–32. [Google Scholar] [CrossRef]
- Martínez, M.L.; Curti, M.I.; Roccia, P.; Llabot, J.M.; Penci, M.C.; Bodoira, R.M.; Ribotta, P.D. Oxidative stability of walnut (Juglans regia L.) and chia (Salvia hispanica L.) oils microencapsulated by spray drying. Powder Technol. 2015, 270, 271–277. [Google Scholar] [CrossRef]
- Linke, A.; Linke, T.; Kohlus, R. Contribution of the internal and external oxygen to the oxidation of microencapsulated fish oil. Eur. J. Lipid Sci. Technol. 2020, 1900381. [Google Scholar] [CrossRef]
- Rubilar, M.; Morales, E.; Sáez, R.; Acevedo, F.; Palma, B.; Villarroel, M.; Shene, C. Polyphenolic fractions improve the oxidative stability of microencapsulated linseed oil. Eur. J. Lipid Sci. Technol. 2012, 114, 760–771. [Google Scholar] [CrossRef]
- George, M.; Abraham, T.E. pH sensitive alginate–guar gum hydrogel for the controlled delivery of protein drugs. Int. J. Pharm. 2007, 335, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Legako, J.; Dunford, N.T. Effect of spray nozzle design on fish oil-whey protein microcapsule properties. J. Food Sci. 2010, 75, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Kašpar, O.; Jakubec, M.; Štěpánek, F. Characterization of spray dried chitosan–TPP microparticles formed by two-and three-fluid nozzles. Powder Technol. 2013, 240, 31–40. [Google Scholar] [CrossRef]
- Cichello, S. Oxygen absorbers in food preservation: A review. Int. J. Food Sci. Technol. 2015, 52, 1889–1895. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Kondo, I.; Kajimoto, G. Participation of free fatty acids in the oxidation of purified soybean oil during microwave heating. J. Am. Oil Chem. Soc. 1992, 69, 1136–1140. [Google Scholar] [CrossRef]
- AOCS Official Method Cd 8-89. Tocopherols and Tocotrienols in Vegetable Oils and Fats by HPLC. In Official Methods and Recommended Practices of the AOCS, 3rd ed.; AOCS Publishing: Champaing, IL, USA, 1993. [Google Scholar]
- Morelo, G.; Giménez, B.; Márquez-Ruiz, G.; Holgado, F.; Romero-Hasler, P.; Soto-Bustamante, E.; Robert, P. Influence of the Physical State of Spray-Dried Flavonoid-Inulin Microparticles on Oxidative Stability of Lipid Matrices. Antioxidants 2019, 8, 520. [Google Scholar] [CrossRef]
- AOCS Official Method Cd 8-53. Peroxide value—Acetic acid-chloroform method. In Official Methods and Recommended Practices of the AOCS; AOCS Publishing: Champaing, IL, USA, 1992. [Google Scholar]
- AOCS Official Method Cd 3d-63. Acid value. In Official Methods and Recommended Practices of the AOCS, 3rd ed.; AOCS Publishing: Champaing, IL, USA, 1993. [Google Scholar]
- Pu, J.; Bankston, J.D.; Sathivel, S. Developing microencapsulated flaxseed oil containing shrimp (Litopenaeus setiferus) astaxanthin using a pilot scale spray dryer. Biosyst. Eng. 2011, 108, 121–132. [Google Scholar] [CrossRef]
- Cai, Y.Z.; Corke, H. Production and properties of spray-dried amaranthus betacyanin pigments. J. Food Sci. 2000, 65, 1248–1252. [Google Scholar] [CrossRef]
- Odriozola-Serrano, I.; Hernández-Jover, T.; Martín-Belloso, O. Comparative evaluation of UV-HPLC methods and reducing agents to determine vitamin C in fruits. Food Chem. 2007, 105, 1151–1158. [Google Scholar] [CrossRef]
- Márquez-Ruiz, G.; Dobarganes, C. HPSEC in lipid analysis. In Lipid Analysis and Lipidomics: New Techniques and Applications, 1st ed.; Mossoba, M.M., Kramer, J.K.G., Brenna, J.T., McDonald, R.E., Eds.; AOCS Publishing: Champaign, IL, USA, 2006; pp. 205–238. [Google Scholar]
- Berton-Carabin, C.C.; Ropers, M.H.; Genot, C. Lipid oxidation in oil-in-water emulsions: Involvement of the interfacial layer. Compr. Rev. Food Sci. Food Saf. 2014, 13, 945–977. [Google Scholar] [CrossRef]
- Serfert, Y.; Drusch, S.; Schwarz, K. Chemical stabilisation of oils rich in long-chain polyunsaturated fatty acids during homogenisation, microencapsulation and storage. Food Chem. 2009, 113, 1106–1112. [Google Scholar] [CrossRef]
- Sánchez-Reinoso, Z.; Gutiérrez, L.F. Effects of the emulsion composition on the physical properties and oxidative stability of Sacha Inchi (Plukenetia volubilis L.) oil microcapsules produced by spray drying. Food Bioprocess Technol. 2017, 10, 1354–1366. [Google Scholar] [CrossRef]
- Gallardo, G.; Guida, L.; Martínez, V.; López, M.C.; Bernhardt, D.; Blasco, R.; Predrozas-Islas, R.; Hermida, L.G. Microencapsulation of linseed oil by spray drying for functional food application. Food Res. Int. 2013, 52, 473–482. [Google Scholar] [CrossRef]
- Can Karaca, A.; Low, N.; Nickerson, M. Encapsulation of flaxseed oil using a benchtop spray dryer for legume protein–maltodextrin microcapsule preparation. J. Agric. Food Chem. 2013, 61, 5148–5155. [Google Scholar] [CrossRef] [PubMed]
- Partanen, R.; Hakala, P.; Sjövall, O.; Kallio, H.; Forssell, P. Effect of relative humidity on the oxidative stability of microencapsulated sea buckthorn seed oil. J. Food Sci. 2005, 70, E37–E43. [Google Scholar] [CrossRef]
- Encina, C.; Vergara, C.; Giménez, B.; Oyarzún-Ampuero, F.; Robert, P. Conventional spray-drying and future trends for the microencapsulation of fish oil. Trends Food Sci. Technol. 2016, 56, 46–60. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Rouaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res. Int. 2007, 10, 1107–1121. [Google Scholar] [CrossRef]
- Pawar, S.N.; Edgar, K.J. Alginate derivatization: A review of chemistry, properties and applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef]
- Pierucci, A.P.T.; Andrade, L.R.; Baptista, E.B.; Volpato, N.M.; Rocha-Leão, M.H.M. New microencapsulation system for ascorbic acid using pea protein concentrate as coat protector. J. Microencapsul. 2016, 23, 654–662. [Google Scholar] [CrossRef]
- Botrel, D.A.; de Barros Fernandes, R.V.; Borges, S.V.; Yoshida, M.I. Influence of wall matrix systems on the properties of spray-dried microparticles containing fish oil. Food Res. Int. 2014, 62, 344–352. [Google Scholar] [CrossRef]
- Carvalho, A.G.S.; Silva, V.M.; Hubinger, M.D. Microencapsulation by spray drying of emulsified green coffee oil with two-layered membranes. Food Res. Int. 2014, 61, 236–245. [Google Scholar] [CrossRef]
- Silva, E.K.; Azevedo, V.M.; Cunha, R.L.; Hubinger, M.D.; Meireles, M.A.A. Ultrasound-assisted encapsulation of annatto seed oil: Whey protein isolate versus modified starch. Food Hydrocol. 2016, 56, 71–83. [Google Scholar] [CrossRef]
- Tian, X.L.; Tian, D.F.; Wang, Z.Y.; Mo, F.K. Synthesis and Evaluation of Chitosan-Vitamin C Complex. Indian J. Pharm. Sci. 2009, 71, 371–376. [Google Scholar] [PubMed]
- Çaykara, T.; Demirci, S. Preparation and Characterization of Blend Films of Poly(Vinyl Alcohol) and Sodium Alginate. J. Macromol. Sci. A 2006, 43, 1113–1121. [Google Scholar] [CrossRef]
- Márquez-Ruiz, G.; Martín-Polvillo, M.; Dobarganes, C. Effect of temperature and addition of alpha-tocopherol on the oxidation of trilinolein model systems. Lipids 2003, 38, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Martín-Polvillo, M.; Márquez-Ruiz, G.; Dobarganes, C. Oxidative stability of sunflower oils differing in unsaturation degree during long term storage at room temperature. J. Am. Oil Chem. Soc. 2004, 81, 577–583. [Google Scholar] [CrossRef]
- Young, S.; Basiana, E.; Nitin, N. Effects of interfacial composition on the stability of emulsion and encapsulated bioactives after thermal and high pressure processing. J. Food Eng. 2018, 231, 22–29. [Google Scholar] [CrossRef]
- Reineccius, G.A.; Yan, C. Factors controlling the deterioration of spray dried flavourings and unsaturated lipids. Flavour Frag. J. 2016, 31, 5–21. [Google Scholar] [CrossRef]


| Parameter | PWO-C | PWO-C/SA | PWO-C(AA)/SA | PWO-C/SA(AA) | PWO-C(AA)/SA(AA) |
|---|---|---|---|---|---|
| EE (%) | 80.2 ± 0.6 a | 94.0 ± 1.3 b | 93.8 ± 1.5 b | 93.9 ± 5.0 b | 92.9 ± 1.2 b |
| IP (h) | 0.99 ± 0.1 a | 1.84 ± 0.1 b | 4.56 ± 0.27d | 1.17 ± 0.13 a | 2.6 ± 0.14c |
| Recovery AA (%) | - | - | 86.3 ± 1.7 a | 85.2 ± 2.2 a | 83.8 ± 2.0 a |
| Humidity (%) | 2.3 ± 0.1 a | 2.0 ± 0.1 a | 2.50 ± 0.4 a | 2.3 ± 0.2 a | 3.1 ± 0.5 a |
| aw | 0.18 ± 0.0 a | 0.20 ± 0.01 a | 0.20 ± 0.01 a | 0.22 ± 0.02 a | 0.20 ± 0.02 a |
| Hygroscopicity (%) | 23.43 ± 1.3 a | 44.29 ± 1.6 b | 45.59 ± 1.5 b | 45.34 ± 1.3 b | 45.64 ± 1.6 b |
| Sample | Tg (°C) | M.P. (°C) | ΔH (J/g) |
|---|---|---|---|
| C | 60.49 | 163.96 | 120.6 |
| SA | n.d. | 148.9 | n.d. |
| PWO-C | 53.23 | 166.74/191.66 | 4.48/n.d. |
| PWO-C/SA | 67.13 | 159.60 | 94.29 |
| PWO-C(AA)/SA | 51.83 | 139.13 | 64.71 |
| PWO-C/SA(AA) | 51.67 | 147.67 | 75.29 |
| PWO-C(AA)/SA(AA) | 50.69 | 148.30 | 85.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cáceres, D.; Giménez, B.; Márquez-Ruiz, G.; Holgado, F.; Vergara, C.; Romero-Hasler, P.; Soto-Bustamante, E.; Robert, P. Influence of the Location of Ascorbic Acid in Walnut Oil Spray-Dried Microparticles with Outer Layer on the Physical Characteristics and Oxidative Stability. Antioxidants 2020, 9, 1272. https://doi.org/10.3390/antiox9121272
Cáceres D, Giménez B, Márquez-Ruiz G, Holgado F, Vergara C, Romero-Hasler P, Soto-Bustamante E, Robert P. Influence of the Location of Ascorbic Acid in Walnut Oil Spray-Dried Microparticles with Outer Layer on the Physical Characteristics and Oxidative Stability. Antioxidants. 2020; 9(12):1272. https://doi.org/10.3390/antiox9121272
Chicago/Turabian StyleCáceres, Denisse, Begoña Giménez, Gloria Márquez-Ruiz, Francisca Holgado, Cristina Vergara, Patricio Romero-Hasler, Eduardo Soto-Bustamante, and Paz Robert. 2020. "Influence of the Location of Ascorbic Acid in Walnut Oil Spray-Dried Microparticles with Outer Layer on the Physical Characteristics and Oxidative Stability" Antioxidants 9, no. 12: 1272. https://doi.org/10.3390/antiox9121272
APA StyleCáceres, D., Giménez, B., Márquez-Ruiz, G., Holgado, F., Vergara, C., Romero-Hasler, P., Soto-Bustamante, E., & Robert, P. (2020). Influence of the Location of Ascorbic Acid in Walnut Oil Spray-Dried Microparticles with Outer Layer on the Physical Characteristics and Oxidative Stability. Antioxidants, 9(12), 1272. https://doi.org/10.3390/antiox9121272





