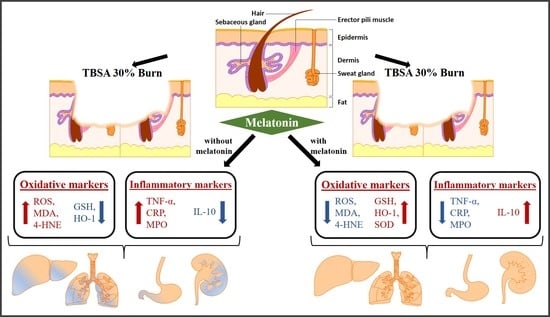
Protective Effects of Melatonin against Severe Burn-Induced Distant Organ Injury: A Systematic Review and Meta-Analysis of Experimental Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Study Selection
2.4. Data Extraction
2.5. Assessment of Methodological Quality
2.6. Data Analysis
2.7. Publication Bias
3. Results
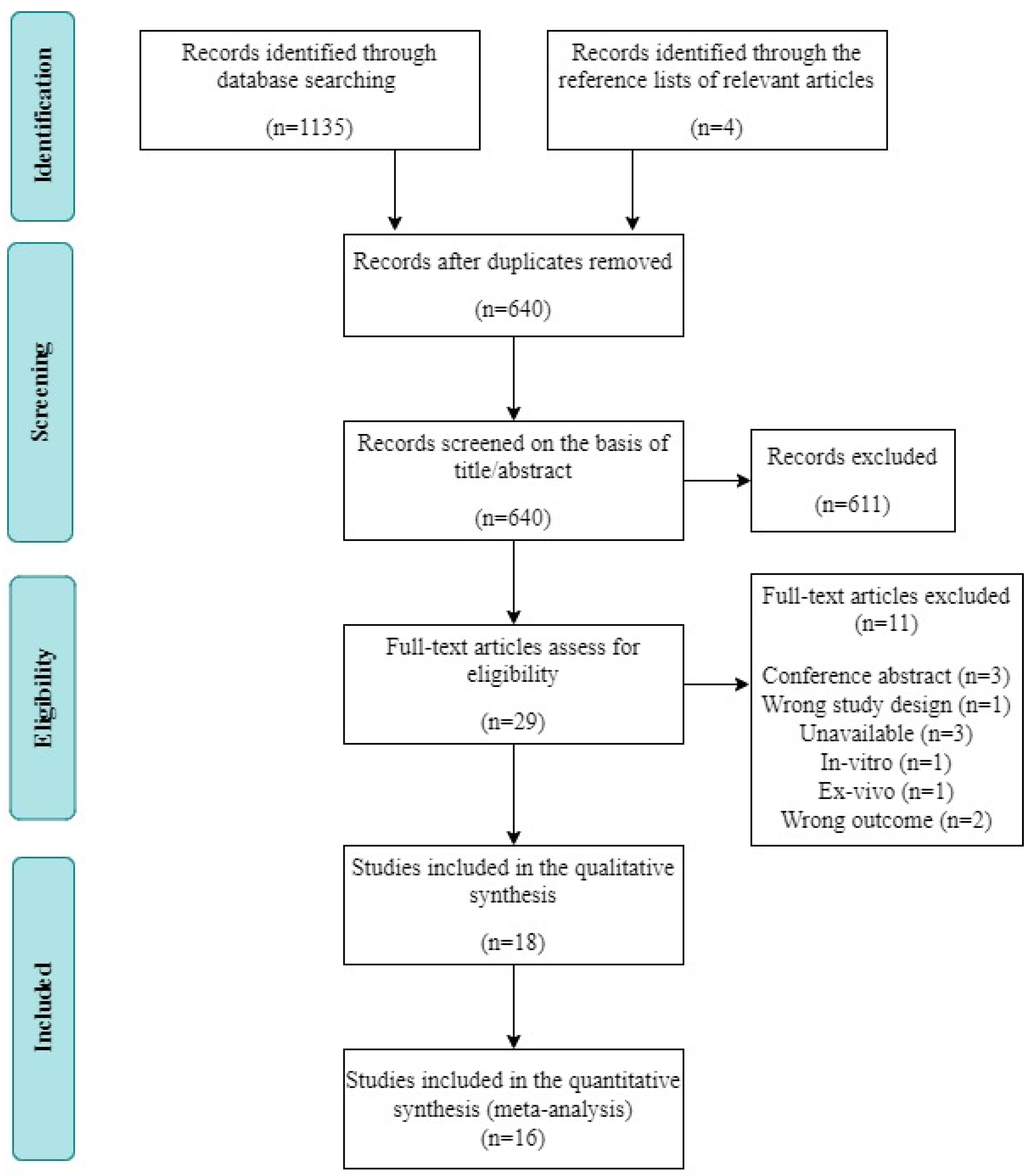
3.1. Study Selection
3.2. Study Characteristics
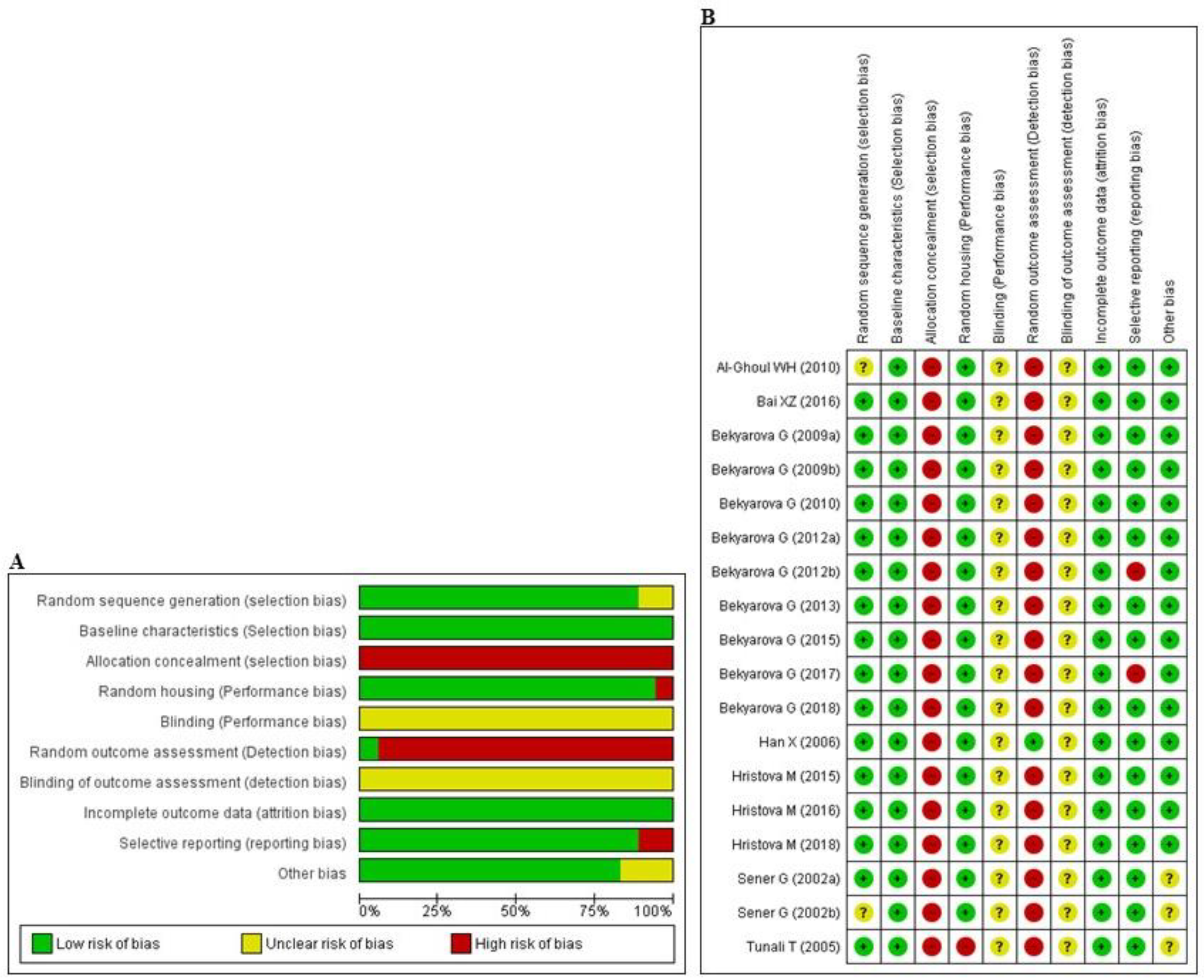
3.3. Risk of Bias and Quality of Reporting
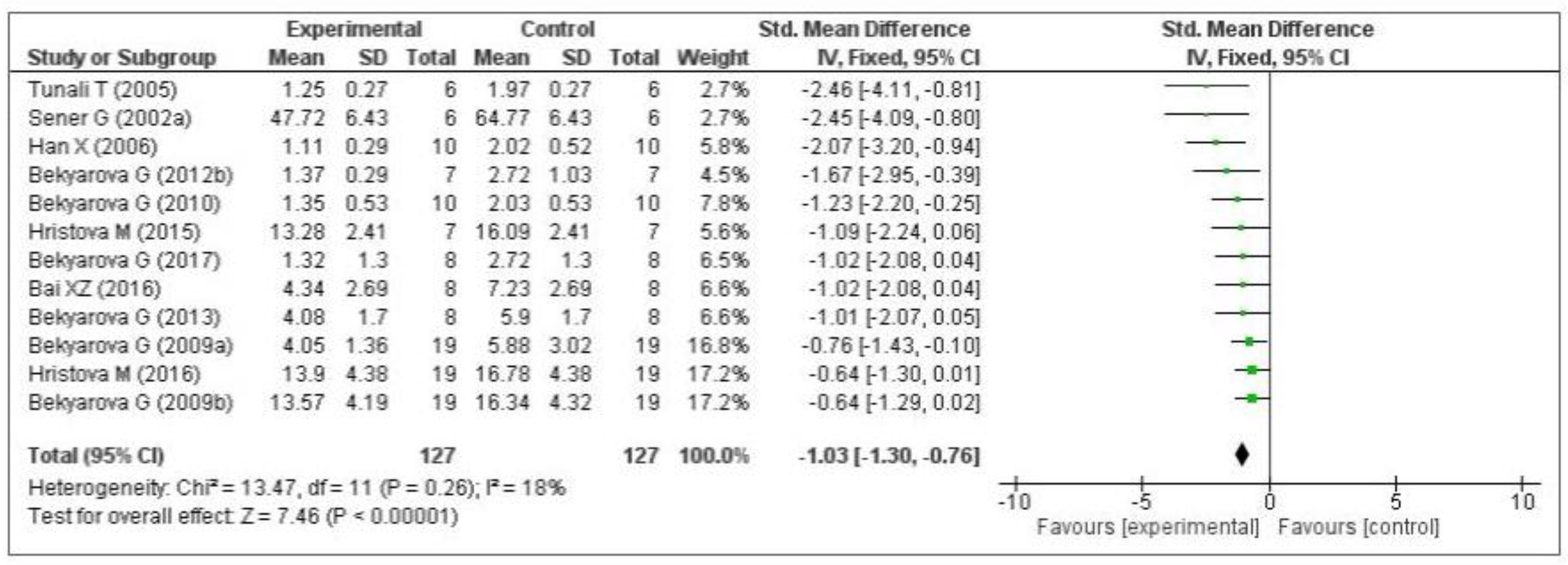
3.4. Data Analysis
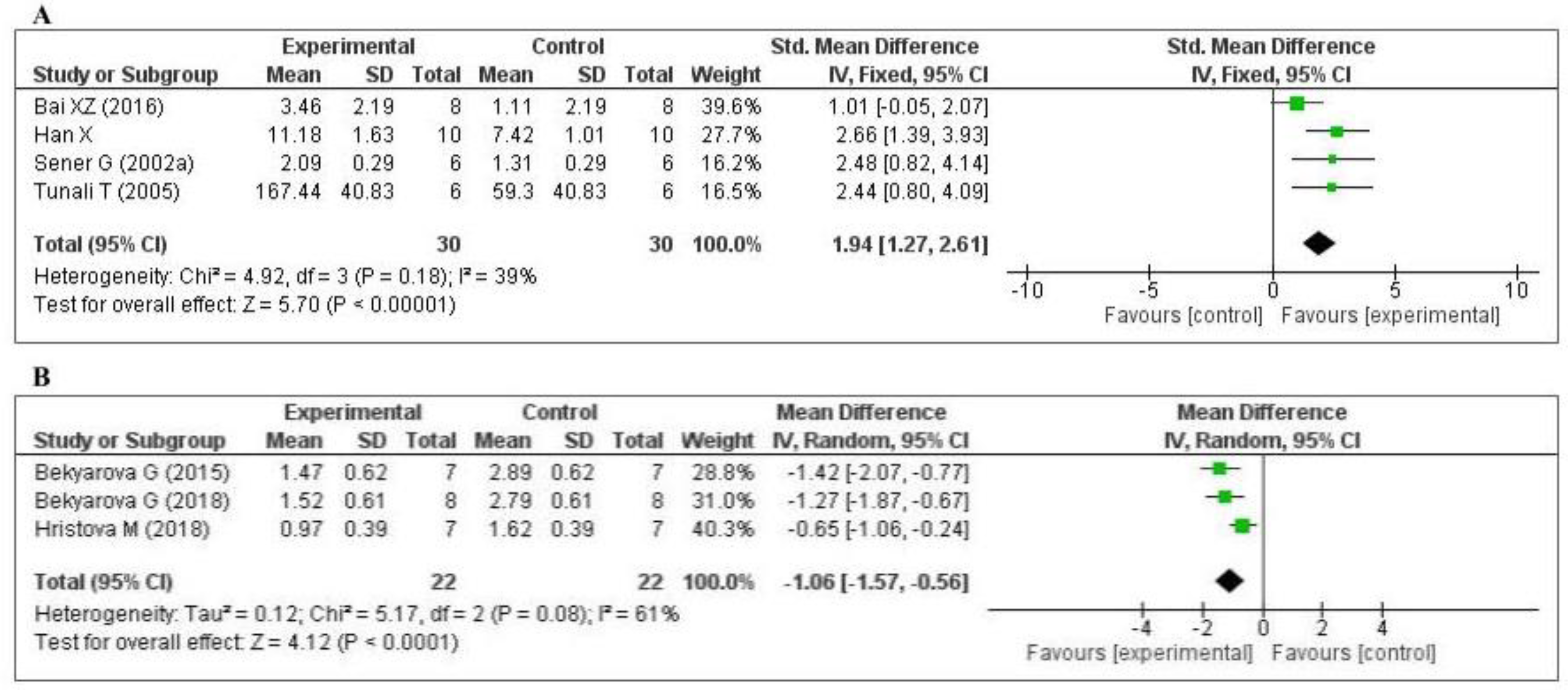
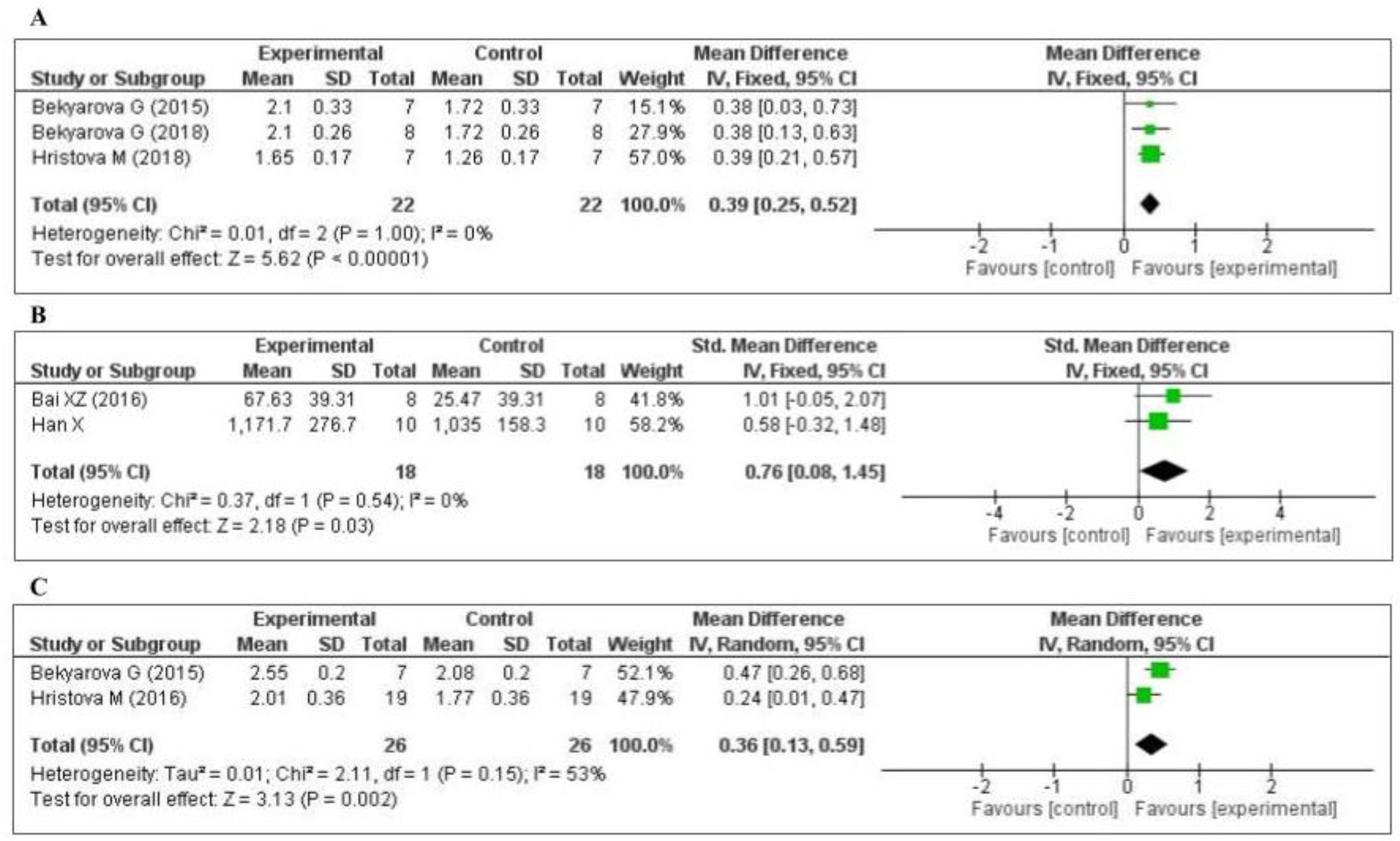
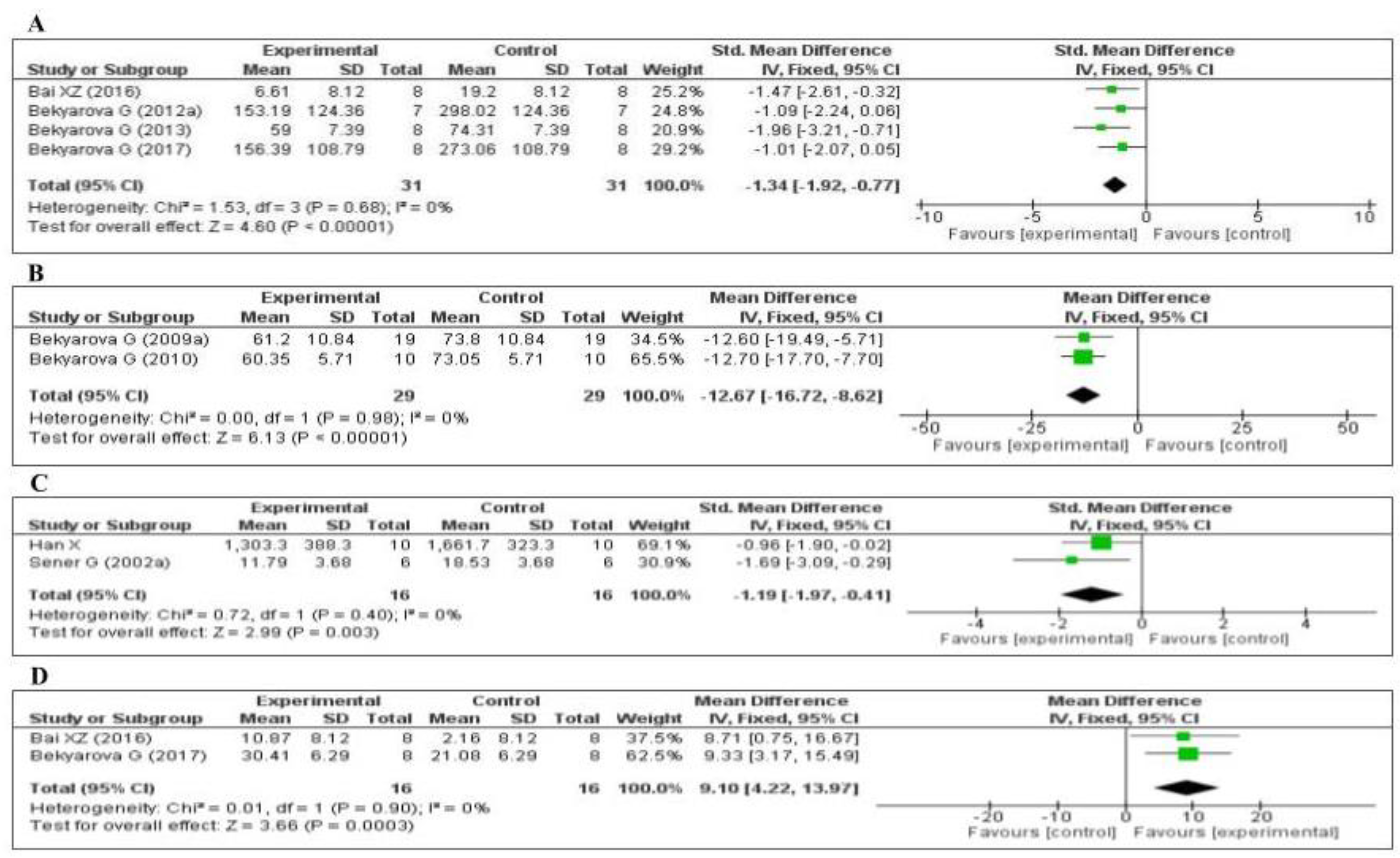
3.4.1. Effect of MT on Oxidative Stress Markers
3.4.2. Effect of MT on Inflammatory Markers
3.4.3. Effect of MT on Other Inflammatory Markers
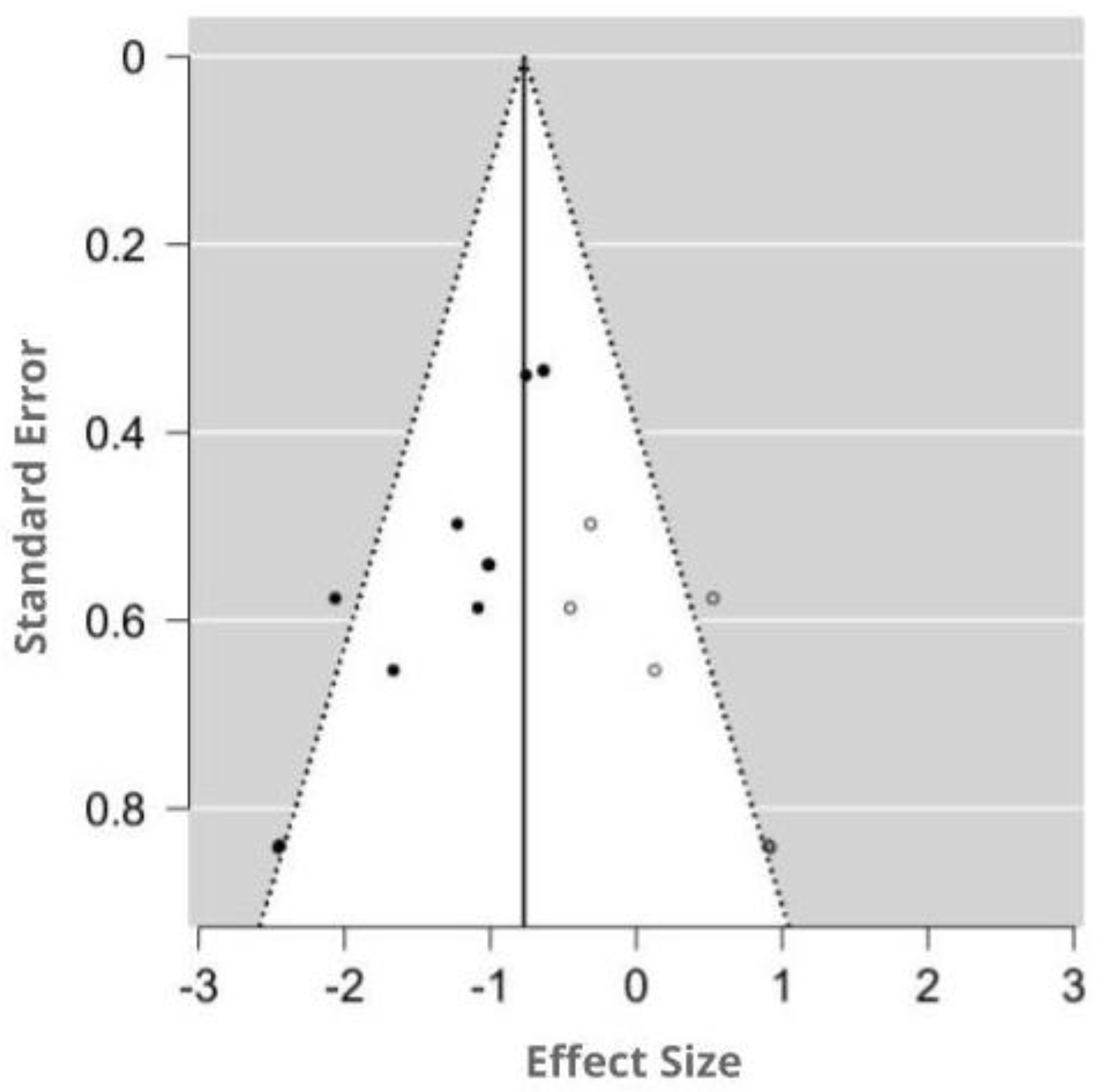
3.4.4. Assessment of Publication Bias
4. Discussion
4.1. Main Findings
4.2. Strengths and Limitations
4.3. Clinical Importance
4.4. Implications for Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- American Burn Association. National Burn Repository 2019 Update, Report of Data from 2009–2018; American Burn Association: Chicago, IL, USA, 2019. [Google Scholar]
- Peck, M.D. Epidemiology of burns throughout the world. Part I: Distribution and risk factors. Burns 2011, 37, 1087–1100. [Google Scholar] [CrossRef] [PubMed]
- Hop, M.J.; Polinder, S.; van der Vlies, C.H.; Middelkoop, E.; van Baar, M.E. Costs of burn care: A systematic review. Wound Repair Regen. 2014, 22, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Stokes, M.A.R.; Johnson, W.D. Burns in the Third World: An unmet need. Ann. Burns Fire Disasters 2017, 30, 243–246. [Google Scholar] [PubMed]
- Smolle, C.; Cambiaso-Daniel, J.; Forbes, A.A.; Wurzer, P.; Hundeshagen, G.; Branski, L.K.; Huss, F.; Kamolz, L.-P. Recent trends in burn epidemiology worldwide: A systematic review. Burns 2017, 43, 249–257. [Google Scholar] [CrossRef]
- Brusselaers, N.; Monstrey, S.; Vogelaers, D.; Hoste, E.; Blot, S. Severe burn injury in Europe: A systematic review of the incidence, etiology, morbidity, and mortality. Crit. Care 2010, 14, R188. [Google Scholar] [CrossRef]
- Seada, A.; Younis, G. Identification of predisposing factors to multiple organ dysfunctions syndrome among burned patients in intensive care unit at Mansoura University. Int. Acad. J. Health Med. Nurs. 2020, 2, 12–25. [Google Scholar]
- Jeschke, M.G.; van Baar, M.E.; Choudhry, M.A.; Chung, K.K.; Gibran, N.S.; Logsetty, S. Burn injury. Nat. Rev. Dis. Prim. 2020, 6, 11. [Google Scholar] [CrossRef]
- Kallinen, O.; Maisniemi, K.; Böhling, T.; Tukiainen, E.; Koljonen, V. Multiple organ failure as a cause of death in patients with severe burns. J. Burn Care Res. 2012, 33, 206–211. [Google Scholar] [CrossRef]
- AbuBakr, H.O.; Aljuaydi, S.H.; Abou-Zeid, S.M.; El-Bahrawy, A. Burn-Induced Multiple Organ Injury and Protective Effect of Lutein in Rats. Inflammation 2018, 41, 760–772. [Google Scholar] [CrossRef]
- Stanojcic, M.; Abdullahi, A.; Rehou, S.; Parousis, A.; Jeschke, M.G. Pathophysiological Response to Burn Injury in Adults. Ann. Surg. 2018, 267, 576–584. [Google Scholar] [CrossRef]
- Beiraghi-Toosi, A.; Askarian, R.; Sadrabadi Haghighi, F.; Safarian, M.; Kalantari, F.; Hashemy, S.I. Burn-induced Oxidative Stress and Serum Glutathione Depletion; a Cross Sectional Study. Emergency 2018, 6, e54. [Google Scholar] [PubMed]
- Nielson, C.B.; Duethman, N.C.; Howard, J.M.; Moncure, M.; Wood, J.G. Burns. J. Burn Care Res. 2017, 38, e469–e481. [Google Scholar] [CrossRef] [PubMed]
- Marsden, N.J.; Van, M.; Dean, S.; Azzopardi, E.A.; Hemington-Gorse, S.; Evans, P.A.; Whitaker, I.S. Measuring coagulation in burns: An evidence-based systematic review. Scars Burn. Health 2017, 3, 205951311772820. [Google Scholar] [CrossRef] [PubMed]
- Parihar, A.; Parihar, M.S.; Milner, S.; Bhat, S. Oxidative stress and anti-oxidative mobilization in burn injury. Burns 2008, 34, 6–17. [Google Scholar] [CrossRef]
- Bekyarova, G.; Yankova, T.; Galunska, B. Increased antioxidant capacity, suppression of free radical damage and erythrocyte aggrerability after combined application of alpha-tocopherol and FC-43 perfluorocarbon emulsion in early postburn period in rats. Artif. Cells Blood Substit. Immobil. Biotechnol. 1996, 24, 629–641. [Google Scholar] [CrossRef]
- Ding, H.Q.; Zhou, B.J.; Liu, L.; Cheng, S. Oxidative stress and metallothionein expression in the liver of rats with severe thermal injury. Burns 2002, 28, 215–221. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Naito, Y.; Ueda, S.; Oyamada, H.; Takemura, T.; Yoshida, N.; Sugino, S.; Kondo, M. Role of Oxygen-Derived Free Radicals in the Pathogenesis of Gastric Mucosal Lesions in Rats. J. Clin. Gastroenterol. 1990, 12, S65–S71. [Google Scholar] [CrossRef]
- Gong, Z.-Y.; Yuan, Z.-Q.; Dong, Z.-W.; Peng, Y.-Z. Glutamine with probiotics attenuates intestinal inflammation and oxidative stress in a rat burn injury model through altered iNOS gene aberrant methylation. Am. J. Transl. Res. 2017, 9, 2535–2547. [Google Scholar]
- Alican, İ.; Ünlüer, E.E.; Yeğen, C.; Yeğen, B.Ç. Bombesin improves burn-induced intestinal injury in the rat. Peptides 2000, 21, 1265–1269. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, W.; Deng, J.; Lan, L.; Xue, X.; Zhang, C.; Cai, G.; Luo, X.; Liu, J. Polydatin protects cardiac function against burn injury by inhibiting sarcoplasmic reticulum Ca2+ leak by reducing oxidative modification of ryanodine receptors. Free Radic. Biol. Med. 2013, 60, 292–299. [Google Scholar] [CrossRef]
- Deng, J.; Wang, G.; Huang, Q.; Yan, Y.; Li, K.; Tan, W.; Jin, C.; Wang, Y.; Liu, J. Oxidative stress-induced leaky sarcoplasmic reticulum underlying acute heart failure in severe burn trauma. Free Radic. Biol. Med. 2008, 44, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Li, T.; Sun, Q.; Xin, Q.; Xu, T.; Yu, J.; Wang, Y.; Wei, L. Protective effect of baicalin against severe burn-induced remote acute lung injury in rats. Mol. Med. Rep. 2017, 17, 2689–2694. [Google Scholar] [CrossRef] [PubMed]
- Till, G.O.; Beauchamp, C.H.A.R.L.E.S.; Menapace, D.A.V.I.D.; Tourtellotte, W., Jr.; Kunkel, R.; Johnson, K.J.; Ward, P.A. Oxygen Radical Dependent Lung Damage following Thermal Injury of Rat Skin. J. Trauma Inj. Infect. Crit. Care 1983, 23, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.W. Free radicals and lipid peroxidation mediated injury in burn trauma: The role of antioxidant therapy. Toxicology 2003, 189, 75–88. [Google Scholar] [CrossRef]
- Demling, R.H.; Lalonde, C. Systemic Lipid Peroxidation and Inflammation Induced by Thermal Injury Persists into the Post-resuscitation Period. J. Trauma Inj. Infect. Crit. Care 1990, 30, 69–74. [Google Scholar] [CrossRef]
- Youn, Y.-K.; Suh, G.-J.; Jung, S.-E.; Oh, S.-K.; Demling, R. Recombinant Human Growth Hormone Decreases Lung and Liver Tissue Lipid Peroxidation and Increases Antioxidant Activity after Thermal Injury in Rats. J. Burn Care Rehabil. 1998, 19, 542. [Google Scholar] [CrossRef]
- Hansbrough, J.F.; Wikström, T.; Braide, M.; Tenenhaus, M.; Rennekampff, O.H.; Kiessig, V.; Bjursten, L.M. Neutrophil Activation and Tissue Neutrophil Sequestration in a Rat Model of Thermal Injury. J. Surg. Res. 1996, 61, 17–22. [Google Scholar] [CrossRef][Green Version]
- Cetinkale, O.; Senel, O.; Bulan, R. The effect of antioxidant therapy on cell-mediated immunity following burn injury in an animal model. Burns 1999, 25, 113–118. [Google Scholar] [CrossRef]
- Rizzo, J.A.; Rowan, M.P.; Driscoll, I.R.; Chung, K.K.; Friedman, B.C. Vitamin C in Burn Resuscitation. Crit. Care Clin. 2016, 32, 539–546. [Google Scholar] [CrossRef]
- Omran AL-Watify, D.G.; Abdul-Khaleq Abd-Zaid, W. Effecieny of Antioxidant defenses System in Burned Patients of Both Sexes with Second and Third Degrees of Burn. J. Pure Sci. 2017, 21, 81–92. [Google Scholar]
- Youn, Y.K.; Lalonde, C.; Demling, R. Oxidants and the pathophysiology of burn and smoke inhalation injury. Free Radic. Biol. Med. 1992, 12, 409–415. [Google Scholar] [CrossRef]
- Klein, G.L. Why so little effort to study anti-oxidant therapy in burns? Burn. Trauma 2016, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Adjepong, M.; Agbenorku, P.; Brown, P.; Oduro, I. The role of antioxidant micronutrients in the rate of recovery of burn patients: A systematic review. Burn. Trauma 2016, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.L.; Shahrokhi, S.; Jeschke, M.G. Enteral nutrition support in burn care: A review of current recommendations as instituted in the Ross Tilley Burn Centre. Nutrients 2012, 4, 1554–1565. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.A.; Hong, Y.; Choi, J.; Lee, Y.; Jin, Y.; Hong, Y. Melatonin: A Potent Therapeutic Candidate in Degenerative Neural Damages. Chronobiol. Med. 2020, 2, 85–95. [Google Scholar] [CrossRef]
- Mistraletti, G.; Paroni, R.; Umbrello, M.; D’Amato, L.; Sabbatini, G.; Taverna, M.; Formenti, P.; Finati, E.; Favero, G.; Bonomini, F.; et al. Melatonin Pharmacological Blood Levels Increase Total Antioxidant Capacity in Critically Ill Patients. Int. J. Mol. Sci. 2017, 18, 759. [Google Scholar] [CrossRef]
- Agabiti-Rosei, C.; Favero, G.; De Ciuceis, C.; Rossini, C.; Porteri, E.; Rodella, L.F.; Franceschetti, L.; Maria Sarkar, A.; Agabiti-Rosei, E.; Rizzoni, D.; et al. Effect of long-term treatment with melatonin on vascular markers of oxidative stress/inflammation and on the anticontractile activity of perivascular fat in aging mice. Hypertens. Res. 2017, 40, 41–50. [Google Scholar] [CrossRef]
- Galano, A.; Tan, D.X.; Reiter, R.J. Melatonin as a natural ally against oxidative stress: A physicochemical examination. J. Pineal Res. 2011, 51, 1–16. [Google Scholar] [CrossRef]
- Manchester, L.C.; Coto-Montes, A.; Boga, J.A.; Andersen, L.P.H.; Zhou, Z.; Galano, A.; Vriend, J.; Tan, D.-X.; Reiter, R.J. Melatonin: An ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 2015, 59, 403–419. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.; Manchester, L.C.; Qi, W. Biochemical Reactivity of Melatonin with Reactive Oxygen and Nitrogen Species: A Review of the Evidence. Cell Biochem. Biophys. 2001, 34, 237–256. [Google Scholar] [CrossRef]
- Leon, J.; Acuna-Castroviejo, D.; Escames, G.; Tan, D.-X.; Reiter, R.J. Melatonin mitigates mitochondrial malfunction. J. Pineal Res. 2005, 38, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tiong, Y.L.; Ng, K.Y.; Koh, R.Y.; Ponnudurai, G.; Chye, S.M. Melatonin Prevents Oxidative Stress-Induced Mitochondrial Dysfunction and Apoptosis in High Glucose-Treated Schwann Cells via Upregulation of Bcl2, NF-κB, mTOR, Wnt Signalling Pathways. Antioxidants 2019, 8, 198. [Google Scholar] [CrossRef] [PubMed]
- Han, X.N.; Wen, G.Q.; Xu, L. Protective effect of melatonin against remal dysfunction following severe burn in rats. Chin. Crit. Care Med. 2007, 12, 721–723. [Google Scholar]
- Hristova, M.; Tzaneva, M.; Bekyarova, G.; Chivchibashi, D.; Stefanova, N.; Kiselova-Kaneva, Y. Molecular Mechanisms of Melatonin Protection from Gastric Mucosal Apoptotic Injury in Experimental Burns. Molecules 2018, 23, 749. [Google Scholar] [CrossRef]
- Wiggins-Dohlvik, K.; Han, M.S.; Stagg, H.W.; Alluri, H.; Shaji, C.A.; Oakley, R.P.; Davis, M.L.; Tharakan, B. Melatonin inhibits thermal injury–induced hyperpermeability in microvascular endothelial cells. J. Trauma Acute Care Surg. 2014, 77, 899–905. [Google Scholar] [CrossRef]
- Kayapinar, M. Saving the zone of stasis in burns with melatonin: An experimental study in rats. Turk. J. Trauma Emerg. Surg. 2015, 21, 419–424. [Google Scholar] [CrossRef][Green Version]
- Bekyarova, G.; Tzaneva, M.; Hristova, M.; Hristov, K. Melatonin protection against burn-induced liver injury. A review. Open Med. 2014, 9, 148–158. [Google Scholar] [CrossRef]
- Maldonado, M.-D.; Murillo-Cabezas, F.; Calvo, J.-R.; Lardone, P.-J.; Tan, D.-X.; Guerrero, J.-M.; Reiter, R.J. Melatonin as pharmacologic support in burn patients: A proposed solution to thermal injury-related lymphocytopenia and oxidative damage. Crit. Care Med. 2007, 35, 1177–1185. [Google Scholar] [CrossRef]
- Halpern, B.; Mancini, M.C.; Bueno, C.; Barcelos, I.P.; de Melo, M.E.; Lima, M.S.; Carneiro, C.G.; Sapienza, M.T.; Buchpiguel, C.A.; do Amaral, F.G.; et al. Melatonin Increases Brown Adipose Tissue Volume and Activity in Patients with Melatonin Deficiency: A Proof-of-Concept Study. Diabetes 2019, 68, 947–952. [Google Scholar] [CrossRef]
- Genario, R.; Cipolla-Neto, J.; Bueno, A.A.; Santos, H.O. Melatonin supplementation in the management of obesity and obesity-associated disorders: A review of physiological mechanisms and clinical applications. Pharmacol. Res. 2020. [Google Scholar] [CrossRef]
- Permuy, M.; López-Peña, M.; González-Cantalapiedra, A.; Muñoz, F. Melatonin: A Review of Its Potential Functions and Effects on Dental Diseases. Int. J. Mol. Sci. 2017, 18, 865. [Google Scholar] [CrossRef] [PubMed]
- Bazyar, H.; Gholinezhad, H.; Moradi, L.; Salehi, P.; Abadi, F.; Ravanbakhsh, M.; Zare Javid, A. The effects of melatonin supplementation in adjunct with non-surgical periodontal therapy on periodontal status, serum melatonin and inflammatory markers in type 2 diabetes mellitus patients with chronic periodontitis: A double-blind, placebo-controlled t. Inflammopharmacology 2019, 27, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Tabrizi, R.; Vakili, S.; Akbari, M.; Mirhosseini, N.; Lankarani, K.B.; Rahimi, M.; Mobini, M.; Jafarnejad, S.; Vahedpoor, Z.; Asemi, Z. The effects of curcumin-containing supplements on biomarkers of inflammation and oxidative stress: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2019, 33, 253–262. [Google Scholar] [CrossRef] [PubMed]
- 10.4.3.1 Recommendations on Testing for Funnel Plot Asymmetry. Available online: https://handbook-5-1.cochrane.org/chapter_10/10_4_3_1_recommendations_on_testing_for_funnel_plot_asymmetry.htm (accessed on 11 July 2020).
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Bekyarova, G.; Hristova, M.; Tzaneva, M.; Kotzev, A. Hepatoprotective effect of melatonin via activation of Nrf2 and anti-apoptotic proteins in burn rats. Oxid. Antioxid. Med. Sci. 2018, 8, 11. [Google Scholar] [CrossRef]
- Bekyarova, G. Melatonin modulates inflammatory response and suppresses burn-induced apoptotic injury. J. Mind Med. Sci. 2017, 4, 59–66. [Google Scholar] [CrossRef]
- Bekyarova, G.; Tzaneva, M.; Hristova, M. Melatonin protects against burn-induced hepatic oxidative injury by inducing HO-1 via the Nrf2 pathway. Vet. Med. 2016, 60, 621–628. [Google Scholar] [CrossRef]
- Bekyarova, G.; Tzaneva, M.; Hristova, M. Melatonin modulates the expression of Bcl-2 family proteins in liver after thermal injury in rats. Adv. Biosci. Biotechnol. 2013, 4, 41–47. [Google Scholar] [CrossRef]
- Bekyarova, G.; Apostolova, M.; Kotzev, I. Melatonin protection against burn-induced hepatic injury by down-regulation of nuclear factor kappa B activation. Int. J. Immunopathol. Pharmacol. 2012, 25, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Bekyarova, G.; Bratchkova, Y.; Tancheva, S.; Hristova, M. Effective melatonin protection of burn-induced hepatic disorders in rats. Open Med. 2012, 7, 533–538. [Google Scholar] [CrossRef]
- Bekyarova, G.; Tancheva, S.; Hristova, M. Protective effect of melatonin against oxidative hepatic injury after experimental thermal trauma. Methods Find. Exp. Clin. Pharmacol. 2009, 31, 11–14. [Google Scholar] [CrossRef]
- Sener, G.; Sehirli, A.O.; Satiroğlu, H.; Keyer-Uysal, M.; Yeğen, B.C. Melatonin improves oxidative organ damage in a rat model of thermal injury. Burns 2002, 28, 419–425. [Google Scholar] [CrossRef]
- Hristova, M.; Bekyarova, G.; Tzaneva, M. Heme oxygenase-1 expression and oxidative stress—Related markers in gastric mucosa in skin burns and protection with melatonin. Trakia J. Sci. 2016, 14, 307–313. [Google Scholar] [CrossRef]
- Hristova, M.; Bekyarova, G.; Tzaneva, M. Heme oxygenase-1 upregulated by melatonin: Potential protection against burn-induced oxidative gastric mucosal injury. J. IMAB Annu. Proc. Sci. Pap. 2015, 21, 779–783. [Google Scholar] [CrossRef][Green Version]
- Bekyarova, G.; Galunska, B.; Ivanova, D.; Yankova, T. Effect of melatonin on burn-induced gastric mucosal injury in rats. Burns 2009, 35, 863–868. [Google Scholar]
- Sener, G.; Sehirli, A.O.; Satiroğlu, H.; Keyer-Uysal, M.; Yeğen, B.C. Melatonin prevents oxidative kidney damage in a rat model of thermal injury. Life Sci. 2002, 70, 2977–2985. [Google Scholar] [CrossRef]
- Bai, X.-Z.; He, T.; Gao, J.-X.; Liu, Y.; Liu, J.-Q.; Han, S.-C.; Li, Y.; Shi, J.-H.; Han, J.-T.; Tao, K.; et al. Melatonin prevents acute kidney injury in severely burned rats via the activation of SIRT1. Sci. Rep. 2016, 6, 32199. [Google Scholar] [CrossRef] [PubMed]
- Bekyarova, G.; Tancheva, S.; Hristova, M. The effects of melatonin on burn-induced inflammatory responses and coagulation disorders in rats. Methods Find. Exp. Clin. Pharmacol. 2010, 32, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Tunali, T.; Sener, G.; Yarat, A.; Emekli, N. Melatonin reduces oxidative damage to skin and normalizes blood coagulation in a rat model of thermal injury. Life Sci. 2005, 76, 1259–1265. [Google Scholar] [CrossRef]
- Al-Ghoul, W.M.; Abu-Shaqra, S.; Park, B.G.; Fazal, N. Melatonin plays a protective role in postburn rodent gut pathophysiology. Int. J. Biol. Sci. 2010, 6, 282–293. [Google Scholar] [CrossRef]
- Han, X.; Xu, L. The protective effect of melatonin on acute lung injury in severely burned rats. Chin. J. Clin. Rehabil. 2006, 10, 70–72. [Google Scholar]
- Vaughn, L.; Beckel, N. Severe burn injury, burn shock, and smoke inhalation injury in small animals. Part 1: Burn classification and pathophysiology. J. Vet. Emerg. Crit. Care 2012, 22, 179–186. [Google Scholar] [CrossRef]
- Nishiura, T.; Nishimura, T.; DeSerres, S.; Godfrey, V.; Bradham, C.A.; Nakagawa, T.; Brenner, D.A.; Meyer, A.A. Gene expression and cytokine and enzyme activation in the liver after a burn injury. J. Burn Care Rehabil. 2000, 21, 135–141. [Google Scholar] [CrossRef]
- Rao, R.; Orman, M.A.; Berthiaume, F.; Androulakis, I.P. Dynamics of hepatic gene expression and serum cytokine profiles in single and double-hit burn and sepsis animal models. Data Br. 2015, 3, 229–233. [Google Scholar] [CrossRef][Green Version]
- Fang, W.-H.; Yao, Y.-M.; Shi, Z.-G.; Yu, Y.; Wu, Y.; Lu, L.-R.; Sheng, Z.-Y. The mRNA expression patterns of tumor necrosis factor-alpha and TNFR-I in some vital organs after thermal injury. World J. Gastroenterol. 2003, 9, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Rawlingson, A. Nitric oxide, inflammation and acute burn injury. Burns 2003, 29, 631–640. [Google Scholar] [CrossRef]
- Guo, Y.; You, Y.; Lv, D.; Yan, J.; Shang, F.-F.; Wang, X.; Zhang, C.; Fan, Q.; Luo, S. Inducible nitric oxide synthase contributes to insulin resistance and cardiac dysfunction after burn injury in mice. Life Sci. 2019, 239, 116912. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.-Y.; Wang, Q.-C.; Chen, X.-L.; Wang, Q.; Sun, C.-S.; Sun, Y.-X.; Wang, C.-H.; Su, M.-X.; Wang, H.-Y.; Wu, X.-S. Hypertonic saline resuscitation protects against kidney injury induced by severe burns in rats. Burns 2019, 45, 641–648. [Google Scholar] [CrossRef]
- He, W.; Wang, Y.; Wang, P.; Wang, F. Intestinal barrier dysfunction in severe burn injury. Burn. Trauma 2019, 7, 24. [Google Scholar] [CrossRef]
- Morris, N.L.; Li, X.; Earley, Z.M.; Choudhry, M.A. Regional variation in expression of pro-inflammatory mediators in the intestine following a combined insult of alcohol and burn injury. Alcohol 2015, 49, 507–511. [Google Scholar] [CrossRef]
- Song, Y.; Li, Y.; Xiao, Y.; Hu, W.; Wang, X.; Wang, P.; Zhang, X.; Yang, J.; Huang, Y.; He, W.; et al. Neutralization of interleukin-17A alleviates burn-induced intestinal barrier disruption via reducing pro-inflammatory cytokines in a mouse model. Burn. Trauma 2019, 7, 37. [Google Scholar] [CrossRef]
- Banerjee, S.; Shah, S.K.; Melnyk, S.B.; Pathak, R.; Hauer-Jensen, M.; Pawar, S.A. Cebpd Is Essential for Gamma-Tocotrienol Mediated Protection against Radiation-Induced Hematopoietic and Intestinal Injury. Antioxidants 2018, 7, 55. [Google Scholar] [CrossRef]
- Pepe, G.; Rapa, S.F.; Salviati, E.; Bertamino, A.; Auriemma, G.; Cascioferro, S.; Autore, G.; Quaroni, A.; Campiglia, P.; Marzocco, S. Bioactive Polyphenols from Pomegranate Juice Reduce 5-Fluorouracil-Induced Intestinal Mucositis in Intestinal Epithelial Cells. Antioxidants 2020, 9, 699. [Google Scholar] [CrossRef]
- Murata, M.; Kawanishi, S. Oxidative DNA damage induced by nitrotyrosine, a biomarker of inflammation. Biochem. Biophys. Res. Commun. 2004, 316, 123–128. [Google Scholar] [CrossRef]
- Kaneki, M.; Fukushima, Y.; Shinozaki, S.; Fukaya, M.; Habiro, M.; Shimizu, N.; Chang, K.; Yasuhara, S.; Martyn, J.A.J. iNOS inhibitor, L-NIL, reverses burn-induced glycogen synthase kinase-3β activation in skeletal muscle of rats. Metabolism 2013, 62, 341–346. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sheng, Z. Prevention of multiple organ dysfunction syndrome in patients with extensive deep burns. Chin. J. Traumatol. = Zhonghua Chuang Shang Za Zhi 2002, 5, 195–199. [Google Scholar] [PubMed]
- Nguyen, L.N.; Nguyen, T.G. Characteristics and outcomes of multiple organ dysfunction syndrome among severe-burn patients. Burns 2009, 35, 937–941. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.S.; Yang, Z.C.; Liu, X.S.; Chen, F.M.; He, B.B.; Li, A.; Crowther, R.S. Serial experimental and clinical studies on the pathogenesis of multiple organ dysfunction syndrome (MODS) in severe burns. Burns 1998, 24, 706–716. [Google Scholar] [CrossRef]
- Lv, Q.; Gu, Y.; Qi, Y.; Liu, Z.; Ma, G.-E. Effects of lentinan on NF-κB activity in the liver of burn rats with sepsis. Exp. Ther. Med. 2020, 20, 2279–2283. [Google Scholar]
- Bortolin, J.A.; Quintana, H.T.; de Tomé, T.C.; Ribeiro, F.A.P.; Ribeiro, D.A.; de Oliveira, F. Burn injury induces histopathological changes and cell proliferation in liver of rats. World J. Hepatol. 2016, 8, 322–330. [Google Scholar] [CrossRef]
- Luo, G.; Peng, D.; Zheng, J.; Chen, X.; Wu, J.; Elster, E.; Tadaki, D. The role of NO in macrophage dysfunction at early stage after burn injury. Burns 2005, 31, 138–144. [Google Scholar] [CrossRef]
- Saitoh, D.; Okada, Y.; Ookawara, T.; Yamashita, H.; Takahara, T.; Ishihara, S.; Ohno, H.; Mimura, K. Prevention of ongoing lipid peroxidation by wound excision and superoxide dismutase treatment in the burned rat. Am. J. Emerg. Med. 1994, 12, 142–146. [Google Scholar] [CrossRef]
- Thomson, P.D.; Till, G.O.; Woolliscroft, J.O.; Smith, D.J.; Prasad, J.K. Superoxide dismutase prevents lipid peroxidation in burned patients. Burns 1990, 16, 406–408. [Google Scholar] [CrossRef]
- Sussan, T.E.; Jun, J.; Thimmulappa, R.; Bedja, D.; Antero, M.; Gabrielson, K.L.; Polotsky, V.Y.; Biswal, S. Disruption of Nrf2, a key inducer of antioxidant defenses, attenuates ApoE-mediated atherosclerosis in mice. PLoS ONE 2008, 3, e3791. [Google Scholar] [CrossRef]
- Braun, S.; Hanselmann, C.; Gassmann, M.G.; auf dem Keller, U.; Born-Berclaz, C.; Chan, K.; Kan, Y.W.; Werner, S. Nrf2 transcription factor, a novel target of keratinocyte growth factor action which regulates gene expression and inflammation in the healing skin wound. Mol. Cell. Biol. 2002, 22, 5492–5505. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Wang, H.; Xu, J.; Li, T.; Zhang, L.; Ding, Y.; Zhu, L.; He, J.; Zhou, M. Melatonin stimulates antioxidant enzymes and reduces oxidative stress in experimental traumatic brain injury: The Nrf2-ARE signaling pathway as a potential mechanism. Free Radic. Biol. Med. 2014, 73, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, C.; Zhang, K.; Lan, X.; Chen, X.; Zang, W.; Wang, Z.; Guan, F.; Zhu, C.; Yang, X.; et al. Melatonin receptor activation provides cerebral protection after traumatic brain injury by mitigating oxidative stress and inflammation via the Nrf2 signaling pathway. Free Radic. Biol. Med. 2019, 131, 345–355. [Google Scholar] [CrossRef] [PubMed]
- LaLonde, C.; Nayak, U.; Hennigan, J.; Demling, R.H. Excessive liver oxidant stress causes mortality in response to burn injury combined with endotoxin and is prevented with antioxidants. J. Burn Care Rehabil. 1997, 18, 187–192. [Google Scholar] [CrossRef]
- Sahib, A.S.; Al-Jawad, F.H.; Alkaisy, A.A. Effect of antioxidants on the incidence of wound infection in burn patients. Ann. Burns Fire Disasters 2010, 23, 199–205. [Google Scholar] [PubMed]
- Ghanayem, H. Towards evidence based emergency medicine: Best BETs from the Manchester Royal Infirmary. BET 3: Vitamin C in severe burns. Emerg. Med. J. 2012, 29, 1017–1018. [Google Scholar]
- Kremer, T.; Harenberg, P.; Hernekamp, F.; Riedel, K.; Gebhardt, M.M.; Germann, G.; Heitmann, C.; Walther, A. High-dose vitamin C treatment reduces capillary leakage after burn plasma transfer in rats. J. Burn Care Res. 2010, 31, 470–479. [Google Scholar] [CrossRef]
- Tanaka, H.; Matsuda, T.; Miyagantani, Y.; Yukioka, T.; Matsuda, H.; Shimazaki, S. Reduction of resuscitation fluid volumes in severely burned patients using ascorbic acid administration: A randomized, prospective study. Arch. Surg. 2000, 135, 326–331. [Google Scholar] [CrossRef]
- De Grooth, H.-J.; Manubulu-Choo, W.-P.; Zandvliet, A.S.; Spoelstra-de Man, A.M.E.; Girbes, A.R.; Swart, E.L.; Oudemans-van Straaten, H.M. Vitamin C Pharmacokinetics in Critically Ill Patients: A Randomized Trial of Four IV Regimens. Chest 2018, 153, 1368–1377. [Google Scholar] [CrossRef]
- Ferraro, P.M.; Curhan, G.C.; Gambaro, G.; Taylor, E.N. Total, Dietary, and Supplemental Vitamin C Intake and Risk of Incident Kidney Stones. Am. J. Kidney Dis. 2016, 67, 400–407. [Google Scholar] [CrossRef]
- Andersen, L.P.H.; Werner, M.U.; Rosenkilde, M.M.; Fenger, A.Q.; Petersen, M.C.; Rosenberg, J.; Gögenur, I. Pharmacokinetics of high-dose intravenous melatonin in humans. J. Clin. Pharmacol. 2016, 56, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Maras, A.; Schroder, C.M.; Malow, B.A.; Findling, R.L.; Breddy, J.; Nir, T.; Shahmoon, S.; Zisapel, N.; Gringras, P. Long-term efficacy and safety of pediatric prolonged-release melatonin for insomnia in children with autism spectrum disorder. J. Child Adolesc. Psychopharmacol. 2018, 28, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Alagbonsi, I.A.; Olayaki, L.A. Role of oxidative stress in Cannabis sativa-associated spermatotoxicity: Evidence for ameliorative effect of combined but not separate melatonin and vitamin C. Middle East Fertil. Soc. J. 2017, 22, 136–144. [Google Scholar] [CrossRef]
- Gitto, E.; Tan, D.X.; Reiter, R.J.; Karbownik, M.; Manchester, L.C.; Cuzzocrea, S.; Fulia, F.; Barberi, I. Individual and synergistic antioxidative actions of melatonin: Studies with vitamin E, vitamin C, glutathione and desferrioxamine (desferoxamine) in rat liver homogenates. J. Pharm. Pharmacol. 2001, 53, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- Chitsazi, M.; Faramarzie, M.; Sadighi, M.; Shirmohammadi, A.; Hashemzadeh, A. Effects of adjective use of melatonin and vitamin C in the treatment of chronic periodontitis: A randomized clinical trial. J. Dent. Res. Dent. Clin. Dent. Prospect. 2017, 11, 236–240. [Google Scholar]
- Akinci, A.; Esrefoglu, M.; Cetin, A.; Ates, B. Melatonin is more effective than ascorbic acid and β-carotene in improvement of gastric mucosal damage induced by intensive stress. Arch. Med. Sci. 2015, 11, 1129–1136. [Google Scholar]
- Thalji, S.Z.; Kothari, A.N.; Kuo, P.C.; Mosier, M.J. Acute Kidney Injury in Burn Patients: Clinically Significant Over the Initial Hospitalization and 1 Year After Injury: An Original Retrospective Cohort Study. Ann. Surg. 2017, 266, 376–382. [Google Scholar] [CrossRef]
- Fear, V.S.; Boyd, J.H.; Rea, S.; Wood, F.M.; Duke, J.M.; Fear, M.W. Burn Injury Leads to Increased Long-Term Susceptibility to Respiratory Infection in both Mouse Models and Population Studies. PLoS ONE 2017, 12, e0169302. [Google Scholar] [CrossRef]
- Duke, J.M.; Randall, S.M.; Fear, M.W.; Boyd, J.H.; Rea, S.; Wood, F.M. Understanding the long-term impacts of burn on the cardiovascular system. Burns 2016, 42, 366–374. [Google Scholar] [CrossRef]
- Kraft, R.; Herndon, D.N.; Finnerty, C.C.; Shahrokhi, S.; Jeschke, M.G. Occurrence of multiorgan dysfunction in pediatric burn patients: Incidence and clinical outcome. Ann. Surg. 2014, 259, 381–387. [Google Scholar] [CrossRef]
- Barrett, L.W.; Fear, V.S.; Waithman, J.C.; Wood, F.M.; Fear, M.W. Understanding acute burn injury as a chronic disease. Burn. Trauma 2019, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.; Maestroni, G.J.M.; Cardinali, D.P.; Esquifino, A.I.; Perumal, S.R.P.; Miller, S.C. Melatonin, immune function and aging. Immun. Ageing 2005, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Vico, A.; Lardone, P.J.; Alvarez-Sánchez, N.; Rodríguez-Rodríguez, A.; Guerrero, J.M. Melatonin: Buffering the immune system. Int. J. Mol. Sci. 2013, 14, 8638–8683. [Google Scholar] [CrossRef]
- Calvo, J.R.; González-Yanes, C.; Maldonado, M.D. The role of melatonin in the cells of the innate immunity: A review. J. Pineal Res. 2013, 55, 103–120. [Google Scholar] [CrossRef] [PubMed]
| Author (year) | Animal Characteristics | Study Characteristics | Intervention Characteristics | Outcome Measures | |||||
|---|---|---|---|---|---|---|---|---|---|
| Species (Gender) | Weight (gm) | Model (Exposure Time) | Exp Group (n) | Con Group (n) | Study Area | MT Use Time | MT in Dose | ||
| Bekyarova G (2018) [63] | Rat (Male) | 220–250 | 30% of TBSA burns by 90 °C hot water (10 s) | B + MT (8) | B + MT (8) | Liver | Immediately after 30% of TBSA burns | 10 mg/kg | 4-HNE, Nrf2 |
| Bekyarova G (2017) [64] | Rat (Male) | 220–250 | 30% of TBSA burns by 98 °C hot water (10 s) | B + MT (8) | B + V (8) | Liver | Immediately after 30% of TBSA burns | 10 mg/kg | TNF-α, IL-10, MDA |
| Bekyarova G (2015) [72] | Rat (Male) | 220–250 | 30% of TBSA burns by 90 °C hot water (10 s) | B + MT (7) | B + V (7) | Liver | Immediately after 30% of TBSA burns | 10 mg/kg | 4-HNE, Nrf2, H0-1 |
| Bekyarova G (2013) [66] | Rat (Male) | 220–250 | 30% of TBSA burns by 90 °C hot water (10 s) | B + MT (8) | B + V (8) | Liver | Immediately after 30% of TBSA burns | 10 mg/kg | MDA, TNF-α |
| Bekyarova G (2012a) [67] | Rat (Male) | 220–250 | 30% of TBSA burns by 98 °C hot water (10 s) | B + MT (7) | B + V (7) | Liver | Immediately after 30% of TBSA burns | 10 mg/kg | NF-kB, TNF-α |
| Bekyarova G (2012b) [68] | Rat (Male) | 220–250 | 30% of TBSA burns by 90 °C hot water (10 s) | B + MT (7) | B + V (7) | Liver | Immediately after 30% of TBSA burns | 10 mg/kg | MDA |
| Bekyarova G (2009a) [69] | Rat (Male) | 220–250 | 20% of TBSA burns by 90 °C hot water (10 s) | B + MT (19) | B + V (19) | Liver | Immediately after 20% of TBSA burns | 10 mg/kg | MDA, CRP |
| Sener G (2002a) [70] | Rat (Both sex) | 200–250 | 30% of TBSA burns by 90 °C hot water (10 s) | B + MT (6) | B + V (6) | Liver, Lung, Intestine | Immediately after 30% of TBSA burns | 10 mg/kg | GSH, MDA, PO, MPO |
| Hristova M (2018) [45] | Rat (Male) | 220–250 | 30% of TBSA burns by 90 °C hot water (10 s) | B + MT (7) | B + V (7) | Gastric mucosa | Immediately after 30% of TBSA burns | 10 mg/kg | 4-HNE, Nrf2 |
| Hristova M (2016) [71] | Rat (Male) | 220–250 | 30% of TBSA burns by 98 °C hot water (10 s) | B + MT (19) | B + V (19) | Gastric mucosa | Immediately after 30% of TBSA burns | 10 mg/kg | MDA, iNOS, HO-1 |
| Hristova M (2015) [72] | Rat (Male) | 220–250 | 30% of TBSA burns by 98 °C hot water (10 s) | B + MT (7) | B + V (7) | Gastric mucosa | Immediately after 30% of TBSA burns | 10 mg/kg | MDA |
| Bekyarova G (2009b) [73] | Rat (Male) | 220–250 | 30% of TBSA burns by 98 °C hot water (10 s) | B + MT (19) | B + V (19) | Gastric mucosa | Immediately after 30% of TBSA burns | 10 mg/kg | MDA |
| Sener G (2002b) [74] | Rat (both sex) | 200–250 | 30% of TBSA burns by 90 °C hot water (10 s) | B + MT Not reported | B + V Not reported | Kidney | Immediately after 30% of TBSA burns | 10 mg/kg | MDA, GSH, PO, MPO |
| Bai XZ (2016) [75] | Rat (Male) | 200–250 | 40% of TBSA burns by 98 °C hot water (12 s) | B + MT (8) | B + V (8) | Kidney | Immediately after 40% of TBSA burns | 10 mg/kg | MDA, GSH, SOD, IL-1β, TNF-α, IL-10 |
| Bekyarova G (2010) [76] | Rat (Male) | 220–250 | 30% of TBSA burns by 90 °C hot water (10 s) | B + MT (10) | B + V (10) | Plasma | Immediately after 30% of TBSA burns | 10 mg/kg | MDA, CRP |
| Tunali T (2005) [77] | Rat (Both sex) | 200–250 | 30% of TBSA burns by 90 °C hot water (10 s) | B + MT (6) | B + V (6) | Plasma | Immediately after 30% of TBSA burns | 10 mg/kg | MDA, GSH |
| Al-Ghoul WM (2010) [78] | Rat (Male) | 250–300 | 30% of TBSA burns by (95–97) °C hot water (10 s) | B + MT (3) | B + V (3) | Intestine | Daily for 3 days | 1.86 mg/kg | Nitrotyrosine |
| Han Xiaohua (2006) [79] | Rat (Male) | 200–250 | 30% of TBSA burns by 100 °C hot water (15 s) | B + MT (10) | B + V (10) | Lung | Immediately after 30% of TBSA burns | 10 mg/kg | GSH, MDA, GPx, SOD, MPO |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sumsuzzman, D.M.; Choi, J.; Khan, Z.A.; Hong, Y. Protective Effects of Melatonin against Severe Burn-Induced Distant Organ Injury: A Systematic Review and Meta-Analysis of Experimental Studies. Antioxidants 2020, 9, 1196. https://doi.org/10.3390/antiox9121196
Sumsuzzman DM, Choi J, Khan ZA, Hong Y. Protective Effects of Melatonin against Severe Burn-Induced Distant Organ Injury: A Systematic Review and Meta-Analysis of Experimental Studies. Antioxidants. 2020; 9(12):1196. https://doi.org/10.3390/antiox9121196
Chicago/Turabian StyleSumsuzzman, Dewan Md., Jeonghyun Choi, Zeeshan Ahmad Khan, and Yonggeun Hong. 2020. "Protective Effects of Melatonin against Severe Burn-Induced Distant Organ Injury: A Systematic Review and Meta-Analysis of Experimental Studies" Antioxidants 9, no. 12: 1196. https://doi.org/10.3390/antiox9121196
APA StyleSumsuzzman, D. M., Choi, J., Khan, Z. A., & Hong, Y. (2020). Protective Effects of Melatonin against Severe Burn-Induced Distant Organ Injury: A Systematic Review and Meta-Analysis of Experimental Studies. Antioxidants, 9(12), 1196. https://doi.org/10.3390/antiox9121196