Oral Antioxidant Vitamins and Magnesium Limit Noise-Induced Hearing Loss by Promoting Sensory Hair Cell Survival: Role of Antioxidant Enzymes and Apoptosis Genes
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Antioxidant (ACEMg) Supplementation
2.3. Noise Exposure Protocol
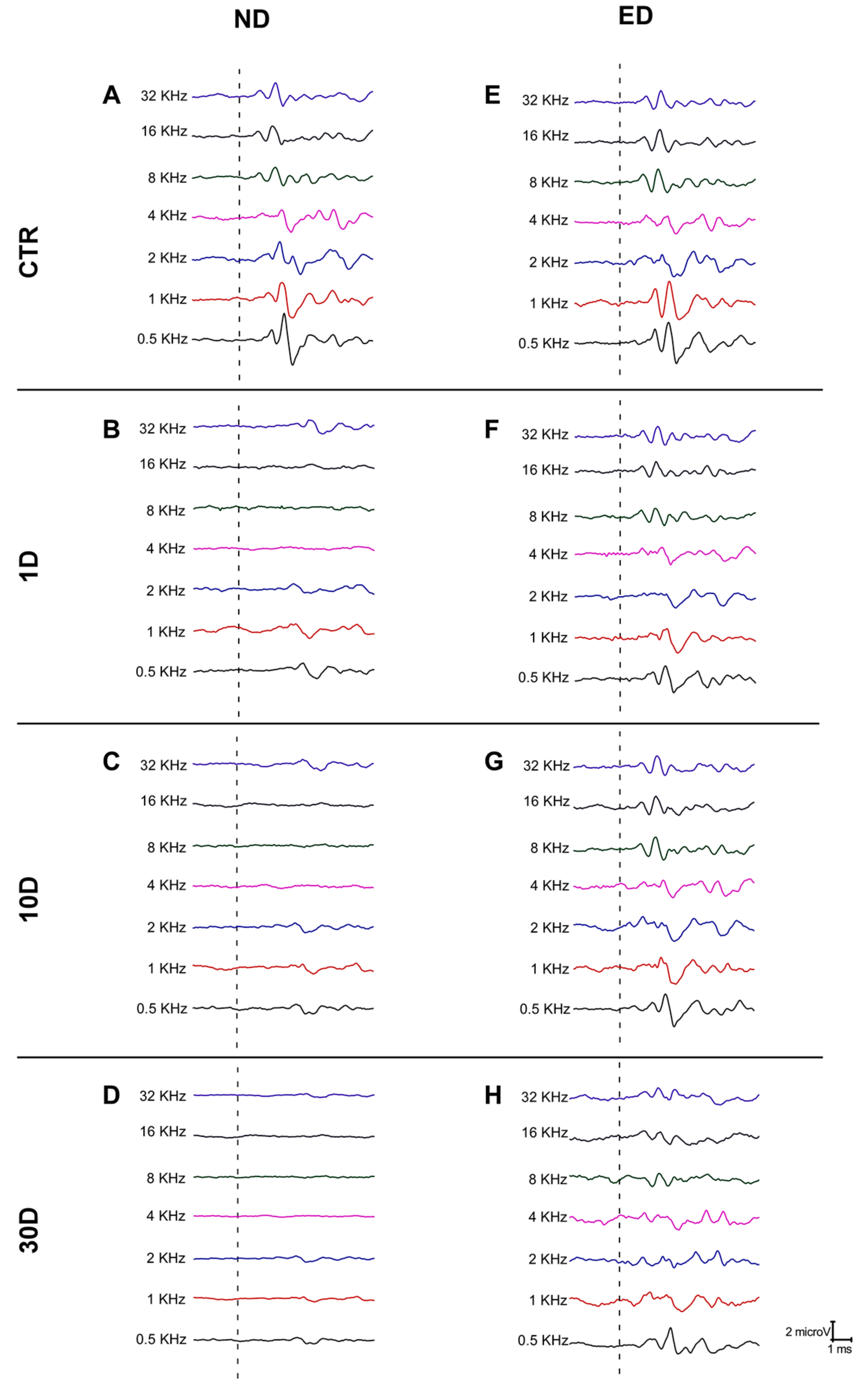
2.4. Auditory Brainstem Response (ABR) Recordings
ABR Data Analysis
2.5. Real Time-Quantitative Polymerase Chain Reaction (qPCR)
2.5.1. Cochlear Dissection and RNA Extraction
2.5.2. cDNA Synthesis and qPCR
2.6. Cochlear Whole-Mount Preparations
2.7. Cochlear Immunohistochemistry
2.8. Statistical Analysis
2.9. Preparation of Figures
3. Results
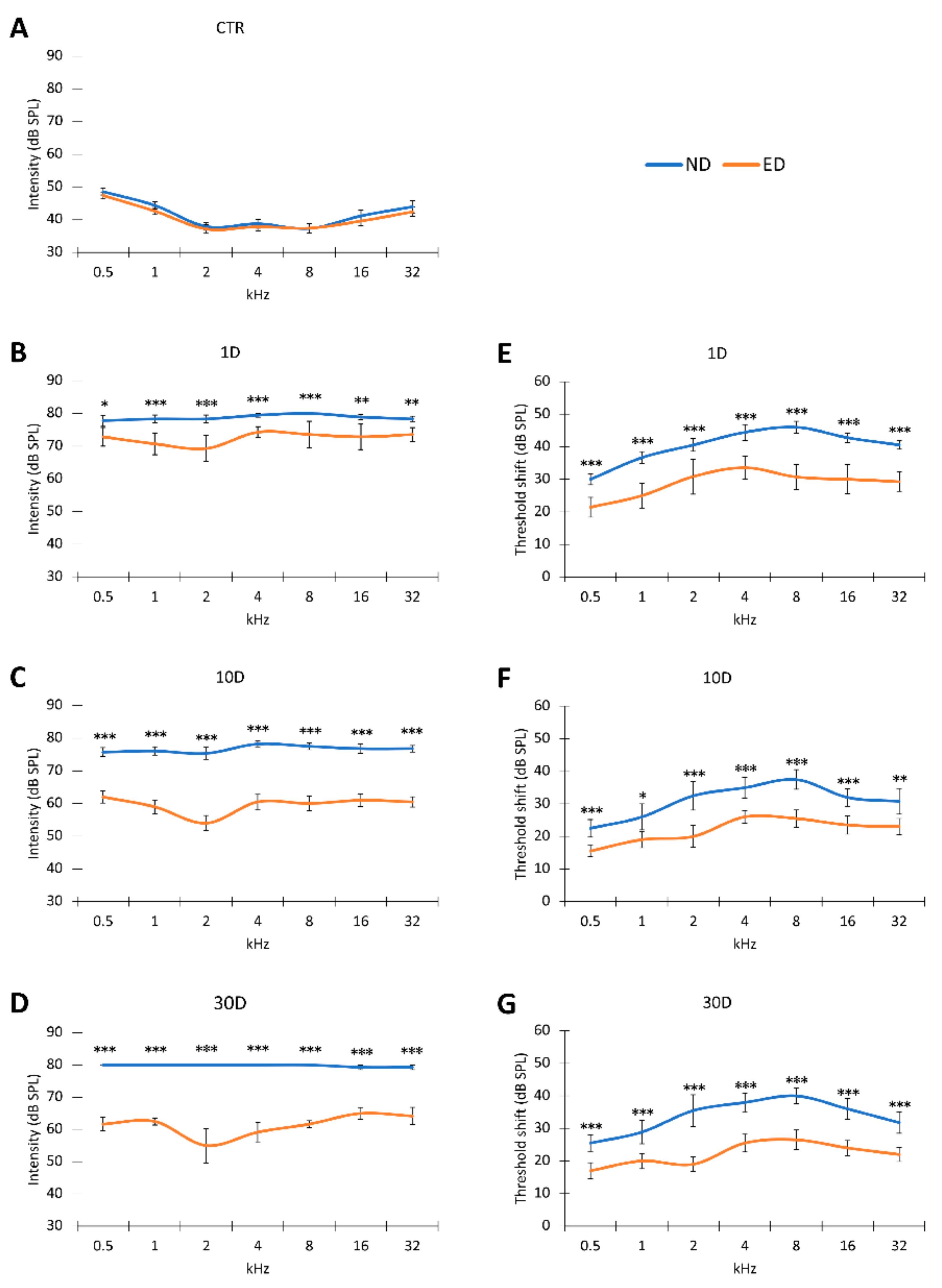
3.1. ACEMg Otoprotection Against NIHL: ABR Recordings
3.1.1. Auditory Thresholds and Threshold Shift after NIHL
3.1.2. Recovery of Auditory Thresholds and Threshold Shifts after Oral Administration of ACEMg
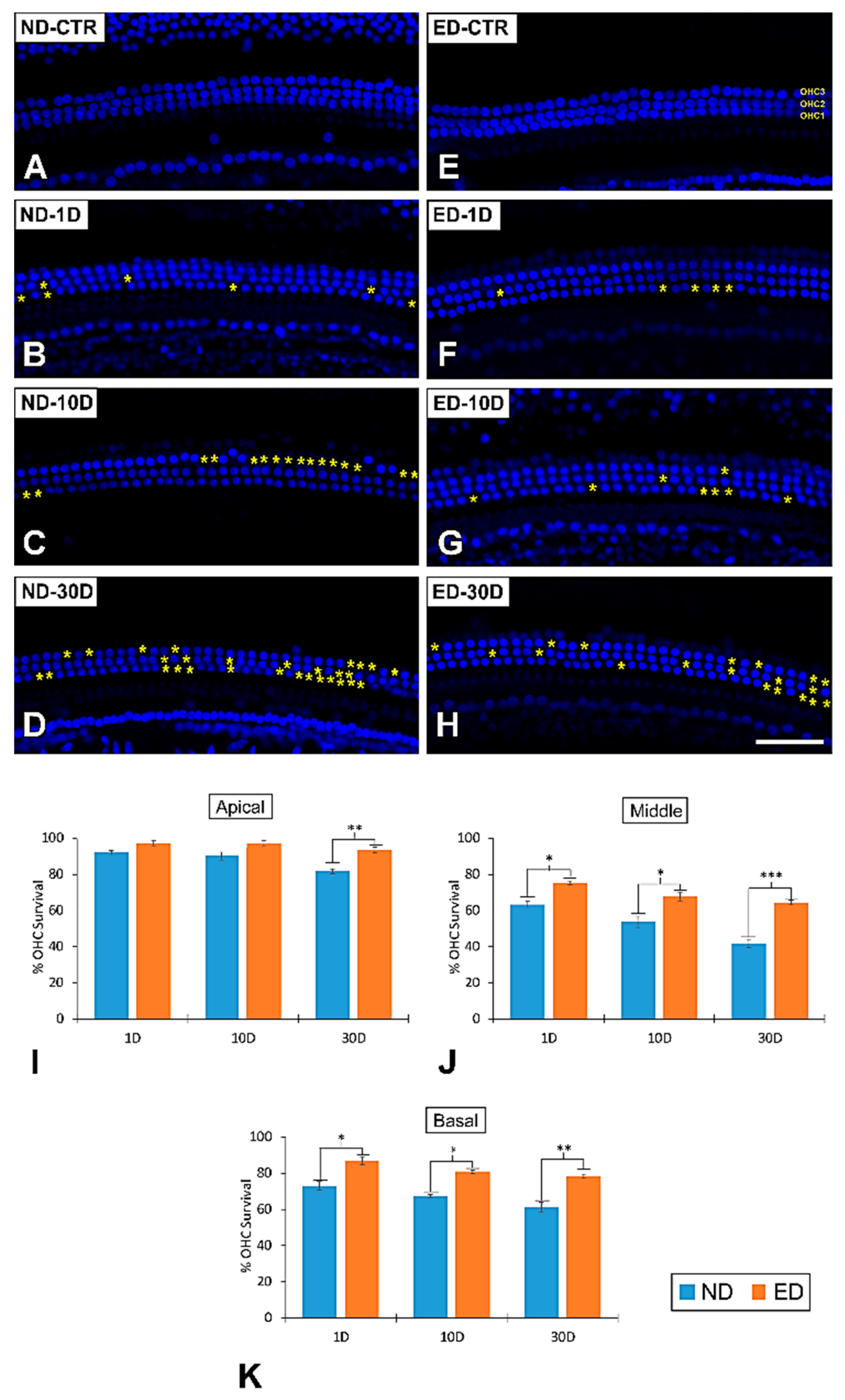
3.2. Loss of OHCs in the Cochlea after NIHL and ACEMg Otoprotection
3.2.1. Noise-Exposed, Untreated Rats
3.2.2. Oral Administration of ACEMg
3.3. Time Expression of Antioxidant Enzymes and Apoptosis Genes in the Cochlea after NIHL and ACEMg Otoprotection
3.3.1. Noise-Exposed, Untreated Rats
3.3.2. Oral Administration of ACEMg
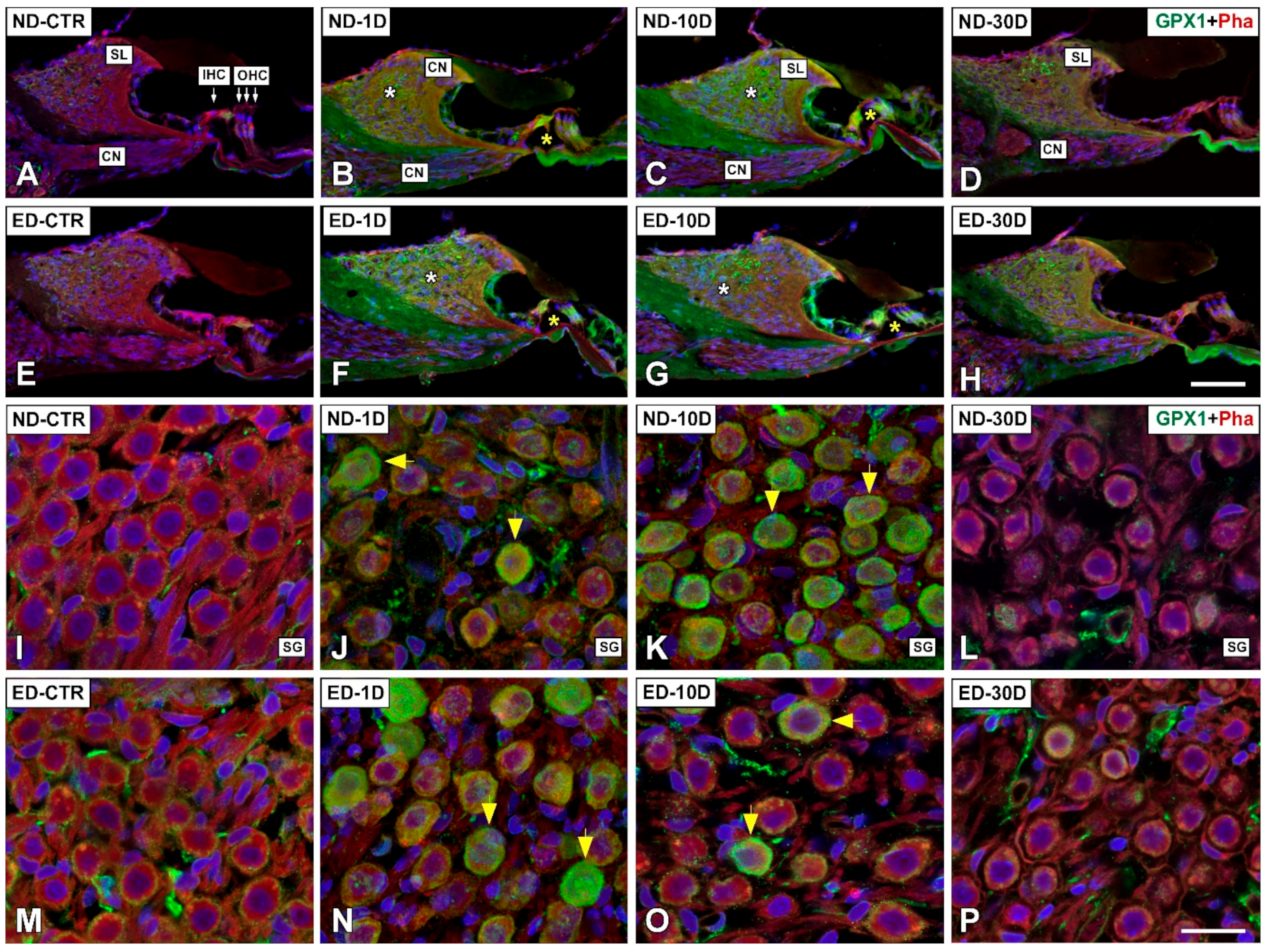
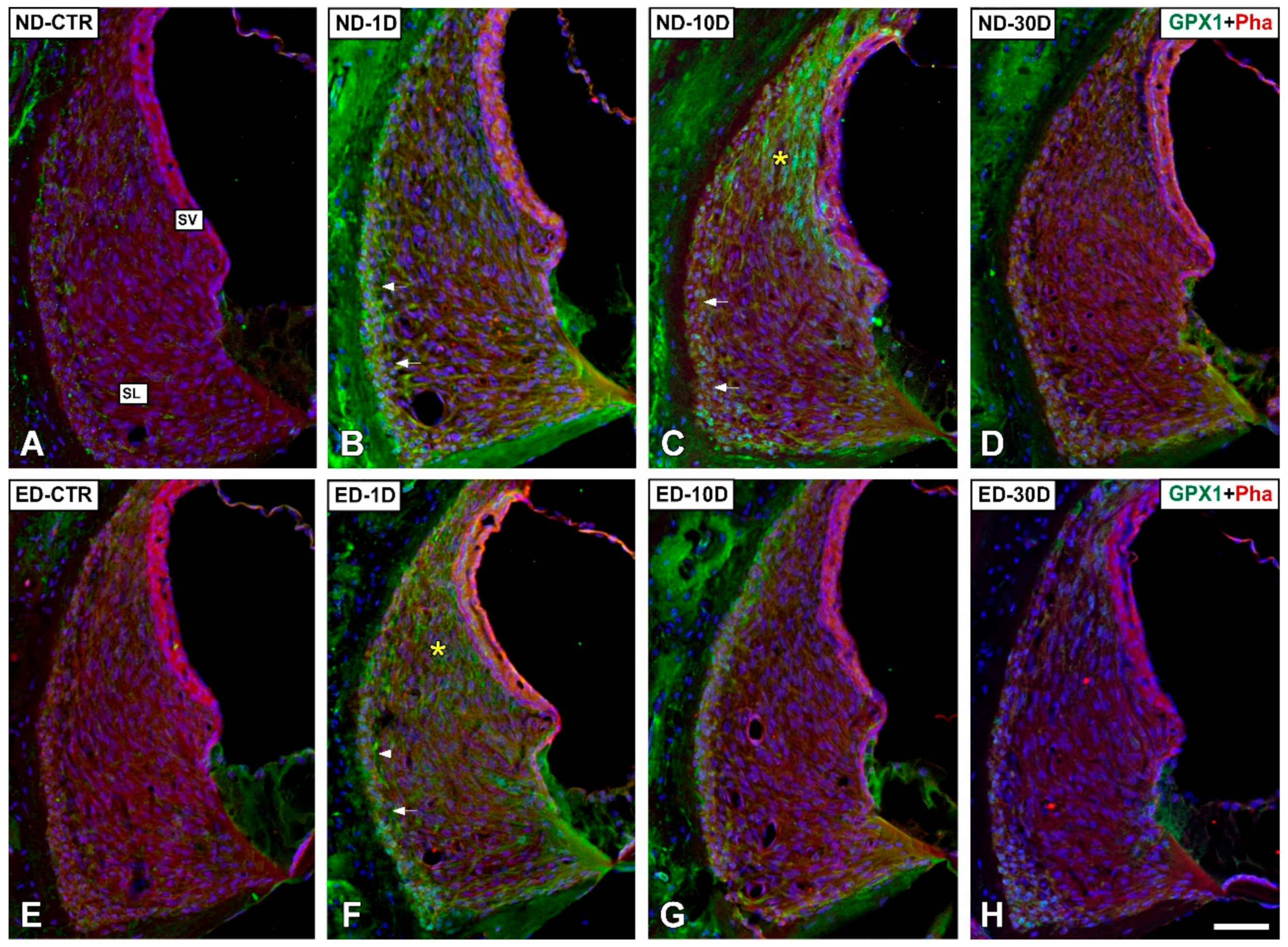
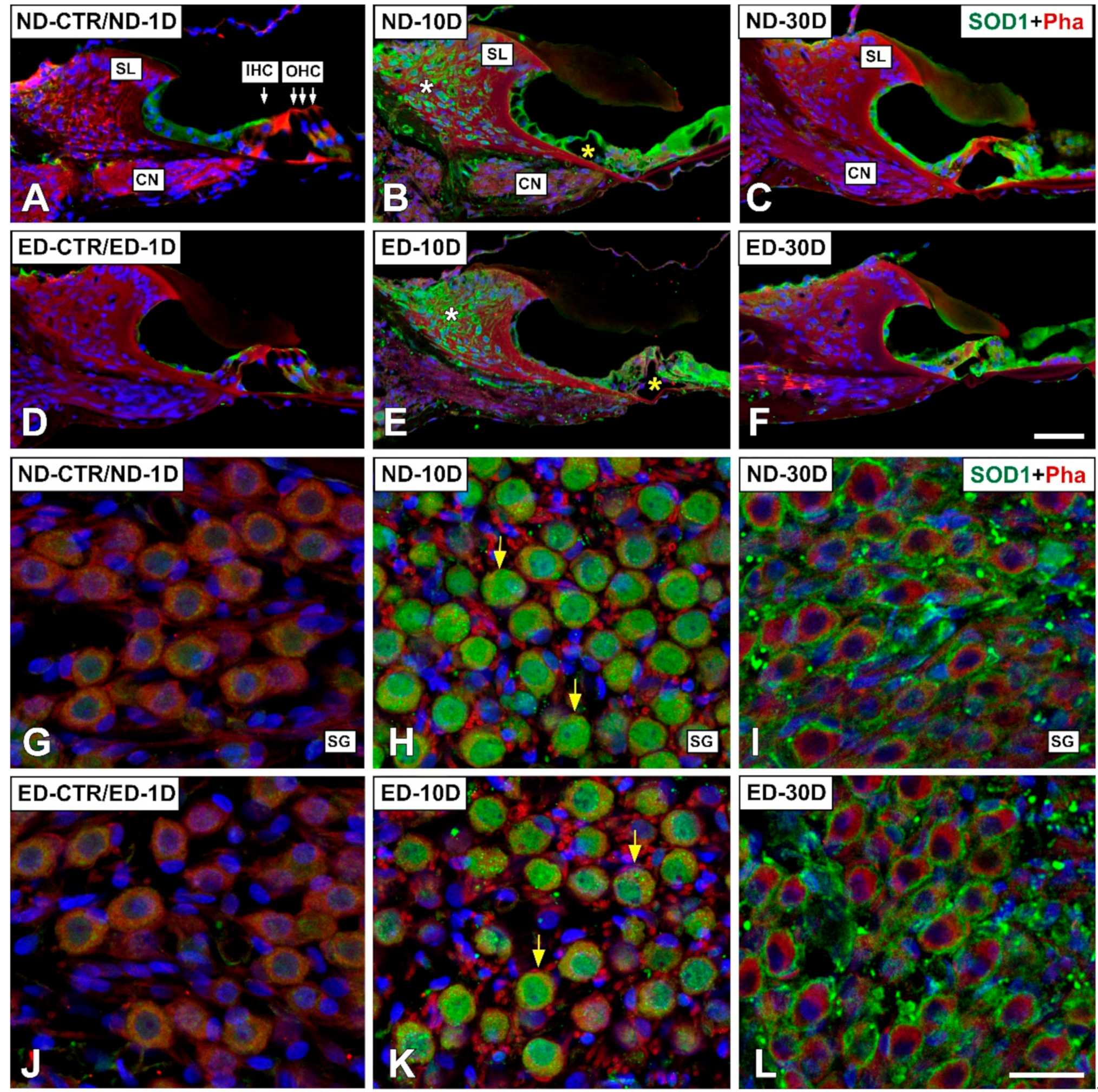
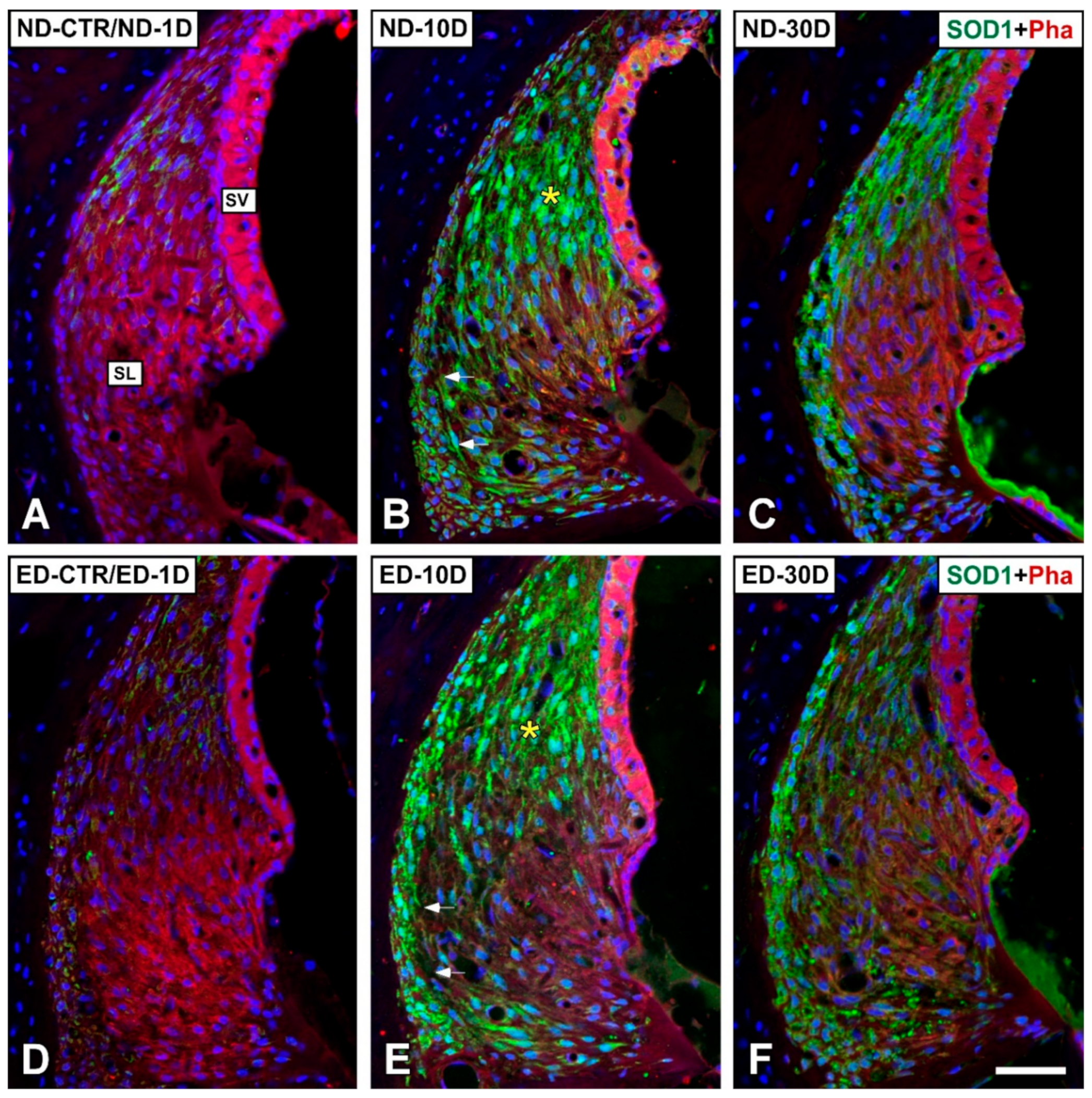
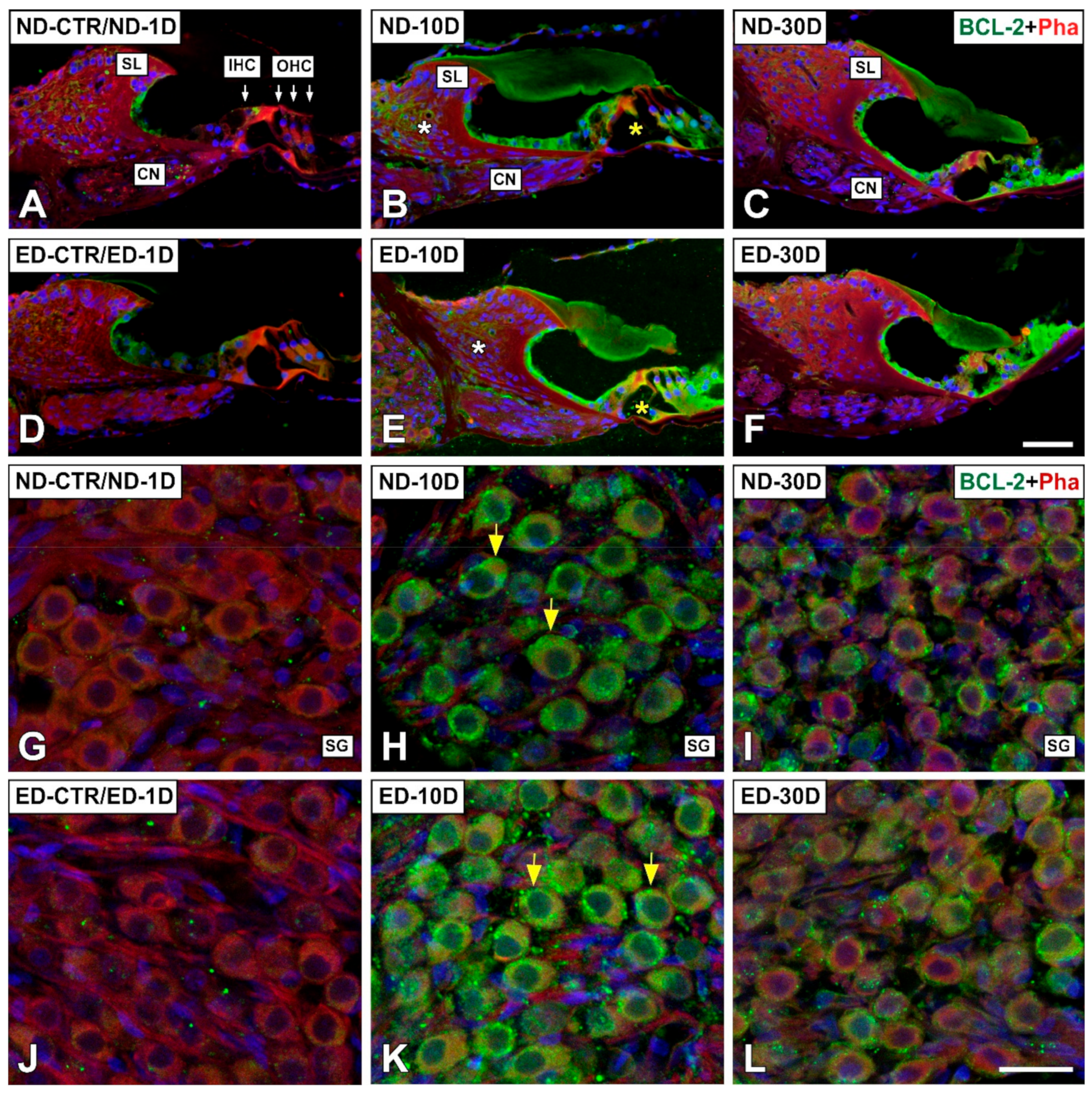
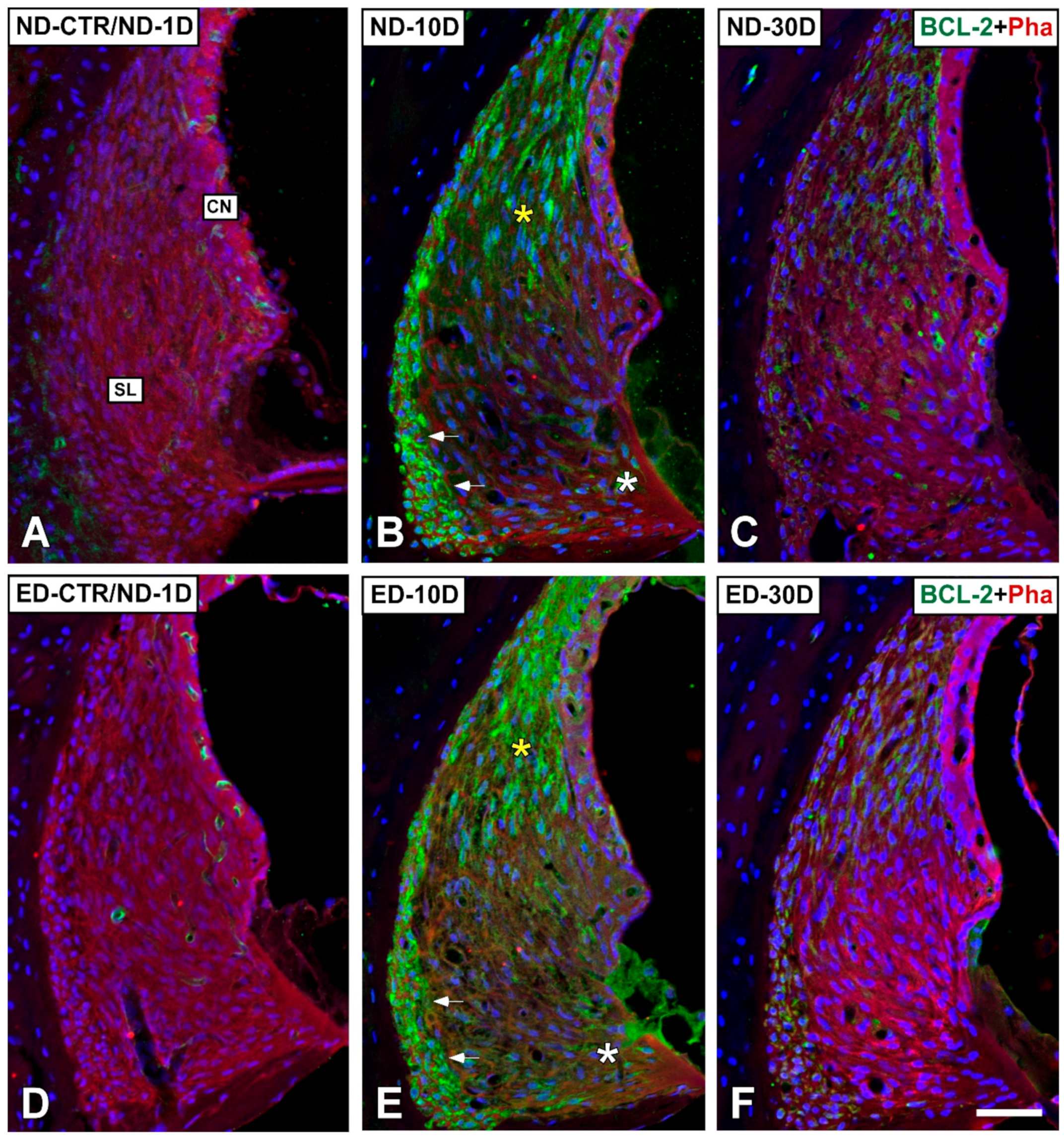
3.4. Distribution of Immunostaining for Antioxidant Enzymes and Apoptosis-Related Proteins in the Cochlea after NIHL and ACEMg Otoprotection
3.4.1. Noise-Exposed, Untreated Rats
3.4.2. Oral Administration of ACEMg
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Altschuler, R.A.; Dolan, D. Basic Mechanisms Underlying Noise-Induced Hearing Loss. In Free Radicals in ENT Pathology; Miller, J.M., Le Prell, C.G., Rybak, L., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 129–146. ISBN 978-3-319-13472-7. [Google Scholar]
- Le, T.N.; Straatman, L.V.; Lea, J.; Westerberg, B. Current insights in noise-induced hearing loss: A literature review of the underlying mechanism, pathophysiology, asymmetry, and management options. J. Otolaryngol.-Head Neck Surg. 2017, 46, 41. [Google Scholar] [CrossRef] [PubMed]
- Carroll, Y.I.; Eichwald, J.; Scinicariello, F.; Hoffman, H.J.; Deitchman, S.; Radke, M.S.; Themann, C.L.; Breysse, P. Vital Signs: Noise-Induced Hearing Loss Among Adults—United States 2011–2012. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 139–144. [Google Scholar] [CrossRef]
- Sakat, M.S.; Kilic, K.; Bercin, S. Pharmacological agents used for treatment and prevention in noise-induced hearing loss. Eur. Arch. Oto-Rhino-Laryngol. 2016, 273, 4089–4101. [Google Scholar] [CrossRef] [PubMed]
- Kurabi, A.; Keithley, E.M.; Housley, G.D.; Ryan, A.F.; Wong, A.C.-Y. Cellular mechanisms of noise-induced hearing loss. Hear. Res. 2017, 349, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Dinh, C.T.; Goncalves, S.; Bas, E.; Van De Water, T.R.; Zine, A. Molecular regulation of auditory hair cell death and approaches to protect sensory receptor cells and/or stimulate repair following acoustic trauma. Front. Cell. Neurosci. 2015, 9, 96. [Google Scholar] [CrossRef] [PubMed]
- Fetoni, A.R.; Troiani, D.; Petrosini, L.; Paludetti, G. Cochlear Injury and adaptive plasticity of the auditory cortex. Front. Aging Neurosci. 2015, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Henderson, D.; Bielefeld, E.C.; Harris, K.C.; Hu, B.H. The role of oxidative stress in noise-induced hearing loss. Ear Hear 2006, 27, 1–19. [Google Scholar] [CrossRef]
- Wong, A.C.Y.; Ryan, A.F. Mechanisms of sensorineural cell damage, death and survival in the cochlea. Front. Aging Neurosci. 2015, 7, 58. [Google Scholar] [CrossRef]
- Poirrier, A.L.; Pincemail, J.; Van Den Ackerveken, P.; Lefebvre, P.P.; Malgrange, B. Oxidative stress in the cochlea: An update. Curr. Med. Chem. 2010, 17, 3591–3604. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive Species and Antioxidants: Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef]
- Yamasoba, T.; Nuttall, A.L.; Harris, C.; Raphael, Y.; Miller, J.M. Role of glutathione in protection against noise-induced hearing loss. Brain Res. 1998, 784, 82–90. [Google Scholar] [CrossRef]
- Ohinata, Y.; Yamasoba, T.; Schacht, J.; Miller, J.M. Glutathione limits noise-induced hearing loss. Hear. Res. 2000, 146, 28–34. [Google Scholar] [CrossRef]
- Pourbakht, A.; Yamasoba, T. Ebselen attenuates cochlear damage caused by acoustic trauma. Hear. Res. 2003, 181, 100–108. [Google Scholar] [CrossRef]
- Kil, J.; Pierce, C.; Tran, H.; Gu, R.; Lynch, E.D. Ebselen treatment reduces noise induced hearing loss via the mimicry and induction of glutathione peroxidase. Hear. Res. 2007, 226, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Kil, J.; Lobarinas, E.; Spankovich, C.; Griffiths, S.K.; Antonelli, P.J.; Lynch, E.D.; Le Prell, C.G. Safety and efficacy of ebselen for the prevention of noise-induced hearing loss: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2017, 390, 969–979. [Google Scholar] [CrossRef]
- Campbell, K.C.M.; Meech, R.P.; Klemens, J.J.; Gerberi, M.T.; Dyrstad, S.S.W.; Larsen, D.L.; Mitchell, D.L.; El-Azizi, M.; Verhulst, S.J.; Hughes, L.F. Prevention of noise- and drug-induced hearing loss with d-methionine. Hear. Res. 2007, 226, 92–103. [Google Scholar] [CrossRef]
- Campbell, K.; Claussen, A.; Meech, R.; Verhulst, S.; Fox, D.; Hughes, L. d-methionine (d-met) significantly rescues noise-induced hearing loss: Timing studies. Hear. Res. 2011, 282, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Azzi, A.; Gysin, R.; Kempná, P.; Munteanu, A.; Negis, Y.; Villacorta, L.; Visarius, T.; Zingg, J.-M. Vitamin E mediates cell signaling and regulation of gene expression. Ann. N. Y. Acad. Sci. 2004, 1031, 86–95. [Google Scholar] [CrossRef]
- Le Prell, C.G.; Hughes, L.; Miller, J.M. Free radical scavengers vitamins A, C, and E plus magnesium reduce noise trauma. Free Radic. Biol. Med. 2007, 42, 1454–1463. [Google Scholar] [CrossRef]
- Prasad, K.N.; Bondy, S.C. Increased oxidative stress, inflammation, and glutamate: Potential preventive and therapeutic targets for hearing disorders. Mech. Ageing Dev. 2020, 185, 111191. [Google Scholar] [CrossRef]
- Green, K.L.; Swiderski, D.L.; Prieskorn, D.M.; DeRemer, S.J.; Beyer, L.A.; Miller, J.M.; Green, G.E.; Raphael, Y. ACEMg Diet supplement modifies progression of hereditary deafness. Sci. Rep. 2016, 6, 22690. [Google Scholar] [CrossRef] [PubMed]
- Boston University. Lamorte’s Power Calculations. Available online: https://www.bu.edu/researchsupport/compliance/animal-care/working-with-animals/research/sample-size-calculations-iacuc/ (accessed on 7 February 2020).
- Fuentes-Santamaría, V.; Alvarado, J.C.; Melgar-Rojas, P.; Gabaldón-Ull, M.C.; Miller, J.M.; Juiz, J.M. The role of glia in the peripheral and central auditory system following noise overexposure: Contribution of TNF-α and IL-1β to the pathogenesis of hearing loss. Front. Neuroanat. 2017, 11, 9. [Google Scholar] [CrossRef]
- Alvarado, J.C.; Fuentes-Santamaría, V.; Jareño-Flores, T.; Blanco, J.L.; Juiz, J.M. Normal variations in the morphology of auditory brainstem response (ABR) waveforms: A study in wistar rats. Neurosci. Res. 2012, 73, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, J.C.; Fuentes-Santamaría, V.; Gabaldón-Ull, M.C.; Jareño-Flores, T.; Miller, J.M.; Juiz, J.M. Noise-Induced “toughening” effect in wistar rats: Enhanced auditory brainstem responses are related to calretinin and nitric oxide synthase upregulation. Front. Neuroanat. 2016, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, J.C.; Fuentes-Santamaría, V.; Gabaldón-Ull, M.C.; Juiz, J.M. Age-related hearing loss is accelerated by repeated short-duration loud sound stimulation. Front. Neurosci. 2019, 13, 77. [Google Scholar] [CrossRef]
- Alvarado, J.C.; Fuentes-Santamaría, V.; Gabaldón-Ull, M.C.; Blanco, J.L.; Juiz, J.M. Wistar rats: A forgotten model of age-related hearing loss. Front. Aging Neurosci. 2014, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Santamaría, V.; Alvarado, J.C.; López-Muñoz, D.F.; Melgar-Rojas, P.; Gabaldón-Ull, M.C.; Juiz, J.M. Glia-related mechanisms in the anteroventral cochlear nucleus of the adult rat in response to unilateral conductive hearing loss. Front. Neurosci. 2014, 8, 319. [Google Scholar] [CrossRef]
- Gourévitch, B.; Doisy, T.; Avillac, M.; Edeline, J.-M. Follow-up of latency and threshold shifts of auditory brainstem responses after single and interrupted acoustic trauma in guinea pig. Brain Res. 2009, 1304, 66–79. [Google Scholar] [CrossRef]
- Melgar-Rojas, P.; Alvarado, J.C.; Fuentes-Santamaría, V.; Gabaldón-Ull, M.C.; Juiz, J.M. Validation of reference genes for RT–qPCR analysis in noise–induced hearing loss: A study in Wistar rat. PLoS ONE 2015, 10, e0138027. [Google Scholar] [CrossRef]
- Subramaniam, M.; Henderson, D.; Campo, P.; Spongr, V. The effect of “conditioning” on hearing loss from a high frequency traumatic exposure. Hear. Res. 1992, 58, 57–62. [Google Scholar] [CrossRef]
- Trowe, M.-O.; Maier, H.; Schweizer, M.; Kispert, A. Deafness in mice lacking the T-box transcription factor Tbx18 in otic fibrocytes. Development 2008, 135, 1725–1734. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, J.C.; Fuentes-Santamaría, V.; Gabaldón-Ull, M.C.; Juiz, J.M. An oral combination of vitamins A, C, E, and Mg++ improves auditory thresholds in age-related hearing loss. Front. Neurosci. 2018, 12, 527. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef]
- Fetoni, A.R.; De Bartolo, P.; Eramo, S.L.M.; Rolesi, R.; Paciello, F.; Bergamini, C.; Fato, R.; Paludetti, G.; Petrosini, L.; Troiani, D. Noise-induced hearing loss (NIHL) as a target of oxidative stress-mediated damage: Cochlear and cortical responses after an increase in antioxidant defense. J. Neurosci. 2013, 33, 4011–4023. [Google Scholar] [CrossRef]
- Fuentes-Santamaría, V.; Alvarado, J.C.; la Rosa, L.R.-D.; Juiz, J.M.; Varela-Nieto, I. Neuroglial involvement in abnormal glutamate transport in the cochlear nuclei of the Igf1−/− mouse. Front. Cell. Neurosci. 2019, 13, 67. [Google Scholar] [CrossRef]
- Viberg, A.; Canlon, B. The guide to plotting a cochleogram. Hear. Res. 2004, 197, 1–10. [Google Scholar] [CrossRef]
- Yoshida, T.; Sawamura, S.; Ota, T.; Higuchi, T.; Ogata, G.; Hori, K.; Nakagawa, T.; Doi, K.; Sato, M.; Nonomura, Y.; et al. Fibrocytes in the cochlea of the mammalian inner ear: Their molecular architecture, physiological properties, and pathological relevance. Med. Res. Arch. 2017, 5. [Google Scholar] [CrossRef]
- Furness, D.N. Forgotten Fibrocytes: A neglected, supporting cell type of the cochlea with the potential to be an alternative therapeutic target in hearing loss. Front. Cell. Neurosci. 2019, 13, 532. [Google Scholar] [CrossRef]
- Le Prell, C.G.; Gagnon, P.M.; Bennett, D.C.; Ohlemiller, K.K. Nutrient-enhanced diet reduces noise-induced damage to the inner ear and hearing loss. Transl. Res. 2011, 158, 38–53. [Google Scholar] [CrossRef]
- Chen, G.D.; Daszynski, D.M.; Ding, D.; Jiang, H.; Woolman, T.; Blessing, K.; Kador, P.F.; Salvi, R. Novel oral multifunctional antioxidant prevents noise-induced hearing loss and hair cell loss. Hear. Res. 2020, 388, 107880. [Google Scholar] [CrossRef] [PubMed]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Gossweiler, A.G.; Martinez-Mier, E.A. Chapter 6: Vitamins and oral health. In Monographs in Oral Science; Zohoori, F.V., Duckworth, R.M., Eds.; Karger: Basel, Switzerland, 2020; Volume 28, pp. 59–67. ISBN 978-3-318-06516-9. [Google Scholar]
- Jacono, A.A.; Hu, B.; Kopke, R.D.; Henderson, D.; Van De Water, T.R.; Steinman, H.M. Changes in cochlear antioxidant enzyme activity after sound conditioning and noise exposure in the chinchilla. Hear. Res. 1998, 117, 31–38. [Google Scholar] [CrossRef]
- Ohlemiller, K.K.; McFadden, S.L.; Ding, D.L.; Lear, P.M.; Ho, Y.S. Targeted mutation of the gene for cellular glutathione peroxidase (Gpx1) increases noise-induced hearing loss in mice. J. Assoc. Res. Otolaryngol. 2000, 1, 243–254. [Google Scholar] [CrossRef]
- Ohlemiller, K.K.; McFadden, S.L.; Ding, D.-L.; Flood, D.G.; Reaume, A.G.; Hoffman, E.K.; Scott, R.W.; Wright, J.S.; Putcha, G.V.; Salvi, R.J. targeted deletion of the cytosolic Cu/Zn-superoxide dismutase gene (sod1) increases susceptibility to noise-induced hearing loss. Audiol. Neurotol. 1999, 4, 237–246. [Google Scholar] [CrossRef]
- McFadden, S.L.; Ohlemiller, K.K.; Ding, D.; Shero, M.; Salvi, R.J. The influence of superoxide dismutase and glutathione peroxidase deficiencies on noise-induced hearing loss in mice. Noise Health 2001, 3, 49–64. [Google Scholar]
- Coling, D.E.; Yu, K.C.Y.; Somand, D.; Satar, B.; Bai, U.; Huang, T.-T.; Seidman, M.D.; Epstein, C.J.; Mhatre, A.N.; Lalwani, A.K. Effect of SOD1 overexpression on age- and noise-related hearing loss. Free Radic. Biol. Med. 2003, 34, 873–880. [Google Scholar] [CrossRef]
- Endo, T.; Nakagawa, T.; Iguchi, F.; Kita, T.; Okano, T.; Sha, S.-H.; Schacht, J.; Shiga, A.; Kim, T.-S.; Ito, J. Elevation of superoxide dismutase increases acoustic trauma from noise exposure. Free Radic. Biol. Med. 2005, 38, 492–498. [Google Scholar] [CrossRef]
- Alam, S.A.; Oshima, T.; Suzuki, M.; Kawase, T.; Takasaka, T.; Ikeda, K. The expression of apoptosis-related proteins in the aged cochlea of Mongolian gerbils. Laryngoscope 2001, 111, 528–534. [Google Scholar] [CrossRef]
- Huang, Q.; Xiong, H.; Yang, H.; Ou, Y.; Zhang, Z.; Chen, S.; Ye, Y.; Zheng, Y. Differential expression of Bcl-2 in the cochlea and auditory cortex of a mouse model of age-related hearing loss. Audiol. Neurotol. 2016, 21, 326–332. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Hajiani, M.; Razi, F.; Golestani, A.; Frouzandeh, M.; Owji, A.A.; Khaghani, S.; Ghannadian, N.; Shariftabrizi, A.; Pasalar, P. Time- and dose-dependent differential regulation of copper-zinc superoxide dismutase and manganese superoxide dismutase enzymatic activity and mRNA level by vitamin E in rat blood cells. Redox Rep. 2012, 17, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.N.; Niu, Z.Y.; Sun, T.T.; Wang, Z.P.; Jiao, P.X.; Zi, B.B.; Chen, P.P.; Tian, D.L.; Liu, F.Z. Vitamin E and vitamin C supplementation improves antioxidant status and immune function in oxidative-stressed breeder roosters by up-regulating expression of GSH-Px gene. Poult. Sci. 2018, 97, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Samson, J.; Wiktorek-Smagur, A.; Politanski, P.; Rajkowska, E.; Pawlaczyk-Luszczynska, M.; Dudarewicz, A.; Sha, S.-H.; Schacht, J.; Sliwinska-Kowalska, M. Noise-induced time-dependent changes in oxidative stress in the mouse cochlea and attenuation by d-methionine. Neuroscience 2008, 152, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.H.; Zhang, C.; Frye, M.D. Immune cells and non-immune cells with immune function in mammalian cochleae. Hear. Res. 2018, 362, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Weber, P.C.; Cunningham, C.D.; Schulte, B.A. Potassium recycling pathways in the human cochlea. Laryngoscope 2001, 111, 1156–1165. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, J.C.; Fuentes-Santamaría, V.; Melgar-Rojas, P.; Valero, M.L.; Gabaldón-Ull, M.C.; Miller, J.M.; Juiz, J.M. Synergistic effects of free radical scavengers and cochlear vasodilators: A new otoprotective strategy for age-related hearing loss. Front. Aging Neurosci. 2015, 7, 86. [Google Scholar] [CrossRef]
- Edlich, F. BCL-2 proteins and apoptosis: Recent insights and unknowns. Biochem. Biophys. Res. Commun. 2018, 500, 26–34. [Google Scholar] [CrossRef]
- Peña-Blanco, A.; García-Sáez, A.J. Bax, Bak and beyond—Mitochondrial performance in apoptosis. FEBS J. 2018, 285, 416–431. [Google Scholar] [CrossRef]
- Morrill, S.; He, D.Z.Z. Apoptosis in inner ear sensory hair cells. J. Otol. 2017, 12, 151–164. [Google Scholar] [CrossRef]



| Gene | Accesion No. | Sequence (5’–3’) | Genomic Location (Exons; FW–RV) | Bp | PCR Efficiency | R2 |
|---|---|---|---|---|---|---|
| Hprt1 | NM_012583.2 | FW:TCCCAGCGTCGTGATTAGTGA RV:CCTTCATGACATCTCGAGCAAG | 1/2–3 a | 152 | 97.3% | 0.9996 |
| Tbp | NM_001004198.1 | FW:CCCACATCACTGTTTCATGG RV:CCGTAAGGCATCATTGGACT | 1/2–3 | 215 | 99.2% | 0.9995 |
| Bax | NM_017059 | FW: CGAGCTGATCAGAACCATCA RV:CTCAGCCCATCTTCTTCCAG | 5–6 | 91 | 98.4% | 0.9994 |
| Bcl-2 | NM_016993 | FW:GAGCGTCAACAGGGAGATGT RV:CTCACTTGTGGCCCAGGTAT | 1–2 | 242 | 99.3% | 1.000 |
| Casp3 | NM_012922 | FW:GGCCCTGAAATACGAAGTCA RV:GGCAGTAGTCGCCTCTGAAG | 4–5 | 209 | 97.6% | 0.9986 |
| Cat | NM_012520 | FW:GAGGAAACGCCTGTGTGAGA RV:TTGGCAGCTATGTGAGAGCC | 11–13 | 201 | 98.8% | 0.9997 |
| Gpx1 | NM_030826 | FW:GTTTCCCGTGCAATCAGTTC RV:CATTCCGCAGGAAGGTAAAG | 1–2 | 71 | 99.3% | 0.9972 |
| Sod1 | NM_017050 | FW:CCACTGCAGGACCTCATTTT RV:CACCTTTGCCCAAGTCATCT | 3–5 | 216 | 99.1% | 0.9991 |
| Primary Antibody | Immunogen | Host | Code/Clone | Dilution | Manufacturer |
|---|---|---|---|---|---|
| Catalase | C-terminus of catalase of mouse origin | Goat | SC-34285 | 1:100 | Santacruz, Biotechnology, Inc., Dallas, TX, USA |
| GPX1 | Synthetic peptide conjugated to KLH derived from within residues 150 to the C-terminus of Human GPX1 | Rabbit | ab22604 | 1:100 | Abcam plc. Cambridge, UK |
| SOD1 | C-terminus of SOD-1 of human origin | Goat | SC-8637 | 1:100 | Santacruz, Biotechnology |
| Bcl-2 | N-terminus of Bcl-2 of human origin | Goat | SC-492 (N19) | 1:100 | Santacruz, Biotechnology |
| Groups | Frequencies (kHz) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | ||
| Threshold | ND-CTR | 48.5 ± 1.0 | 44.4 ± 1.1 | 37.9 ± 1.2 | 38.7 ± 1.4 | 37.3 ± 1.5 | 41.1 ± 1.7 | 43.9 ± 1.9 |
| ND-1D | 77.8 ± 1.7 | 78.3 ± 1.2 | 78.3 ± 1.2 | 79.4 ± 0.6 | 80.0 ± 0.0 | 78.9 ± 0.7 | 78.3 ± 0.8 | |
| ND-10D | 75.7 ± 1.4 | 76.1 ± 1.3 | 75.4 ± 1.9 | 78.2 ± 0.8 | 77.5 ± 1.0 | 76.8 ± 1.4 | 76.8 ± 1.0 | |
| ND-30D | 80.0 ± 0.0 | 80.0 ± 0.0 | 80.0 ± 0.0 | 80.0 ± 0.0 | 80.0 ± 0.0 | 79.3 ± 0.7 | 79.3 ± 0.7 | |
| ED-CTR | 47.4 ± 1.0 | 42.6 ± 1.1 | 37.1 ± 1.3 | 37.8 ± 1.3 | 37.4 ± 1.5 | 39.6 ± 1.3 | 42.4 ± 1.4 | |
| ED-1D | 72.9 ± 2.9 | 70.7 ± 3.4 | 69.3 ± 4.0 | 74.3 ± 1.7 | 73.6 ± 4.0 | 72.9 ± 3.9 | 73.6 ± 2.1 | |
| ED-10D | 62.0 ± 1.9 | 59.0 ± 2.1 | 54.0 ± 2.3 | 60.5 ± 2.3 | 60.0 ± 2.2 | 61.0 ± 1.9 | 60.5 ± 1.6 | |
| ED-30D | 61.7 ± 2.1 | 62.5 ± 1.1 | 55.0 ± 5.3 | 59.2 ± 3.0 | 61.7 ± 1.1 | 65.0 ± 1.8 | 64.2 ± 2.7 | |
| Anova: F(7,40) = | 46.9 (***) | 62.8 (***) | 29.2 (***) | 86.9 (***) | 66.8 (***) | 57.5 (***) | 84.3 (***) | |
| 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | ||
| Threshold Shift | ND-1D | 30.0 ± 1.7 | 36.7 ± 1.9 | 40.6 ± 1.9 | 44.4 ± 2.4 | 46.0 ± 1.8 | 42.8 ± 1.5 | 40.6 ± 1.3 |
| ND-10D | 22.5 ± 2.7 | 26.0 ± 4.0 | 32.5 ± 4.4 | 35.0 ± 3.2 | 37.5 ± 3.0 | 32.0 ± 2.7 | 30.8 ± 3.9 | |
| ND-30D | 25.5 ± 2.5 | 28.9 ± 3.6 | 35.5 ± 4.9 | 38.0 ± 2.8 | 40.0 ± 2.5 | 36.0 ± 3.2 | 31.8 ± 3.3 | |
| ED-1D | 21.4 ± 3.0 | 25.0 ± 3.8 | 30.9 ± 5.4 | 33.6 ± 3.6 | 30.7 ± 3.8 | 30.0 ± 4.5 | 29.3 ± 3.0 | |
| ED-10D | 15.5 ± 1.7 | 19.0 ± 2.4 | 20.0 ± 3.3 | 26.0 ± 1.9 | 25.5 ± 2.6 | 23.5 ± 2.8 | 23.0 ± 2.5 | |
| ED-30D | 17.0 ± 2.5 | 20.0 ± 2.4 | 19.0 ± 2.2 | 25.5 ± 2.7 | 26.5 ± 3.0 | 24.0 ± 2.3 | 22.0 ± 2.1 | |
| ANOVA: F (5,30 )= | 23.5 (***) | 5.6 (***) | 5.2 (***) | 8.6 (***) | 6.8 (***) | 5.8 (***) | 9.5 (***) | |
| Groups | Apical | Middle | Basal | |||||
|---|---|---|---|---|---|---|---|---|
| ND-CTR | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | |||||
| ND-1D | 92.04 ± 1.21 | 63.45 ± 1.68 | 73.12 ± 2.35 | |||||
| ND-10D | 90.16 ± 2.12 | 53.79 ± 2.93 | 67.43 ± 0.97 | |||||
| ND-30D | 81.73 ± 1.22 | 41.61 ± 2.20 | 61.28 ± 2.46 | |||||
| ED-CTR | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | |||||
| ED-1D | 97.20 ± 1.23 | 75.12 ± 1.16 | 86.89 ± 2.26 | |||||
| ED-10D | 96.73 ± 1.53 | 67.74 ± 2.46 | 80.80 ± 0.99 | |||||
| ED-30D | 93.46 ± 1.49 | 64.29 ± 1.29 | 78.22 ± 1.11 | |||||
| Anova: | F(7,48) = 13.8 (***) | F(7,48) = 97.9 (***) | F(7,48) = 41.9 (***) | |||||
| Significance levels | ||||||||
| Apical | ND-CTR | ED-CTR | ND-1D | ED-1D | ND-10D | ED-10D | ND-30D | ED-30D |
| ND-CTR | 1.000 | 0.311 | 0.989 | 0.025 | 0.971 | 0.000 | 0.406 | |
| ED-CTR | 1.000 | 0.269 | 0.982 | 0.020 | 0.957 | 0.000 | 0.357 | |
| ND-1D | 0.311 | 0.269 | 0.852 | 0.971 | 0.915 | 0.000 | 1.000 | |
| ED-1D | 0.989 | 0.982 | 0.852 | 0.230 | 1.000 | 0.000 | 0.915 | |
| ND-10D | 0.025 | 0.020 | 0.971 | 0.230 | 0.311 | 0.094 | 0.938 | |
| ED-10D | 0.971 | 0.957 | 0.915 | 1.000 | 0.311 | 0.000 | 0.957 | |
| ND-30D | 0.000 | 0.000 | 0.000 | 0.000 | 0.094 | 0.000 | 0.000 | |
| ED-30D | 0.406 | 0.357 | 1.000 | 0.915 | 0.938 | 0.957 | 0.000 | |
| Middle | ND-CTR | ED-CTR | ND-1D | ED-1D | ND-10D | ED-10D | ND-30D | ED-30D |
| ND-CTR | 1.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| ED-CTR | 1.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| ND-1D | 0.000 | 0.000 | 0.049 | 0.008 | 0.994 | 0.000 | 1.000 | |
| ED-1D | 0.000 | 0.000 | 0.049 | 0.000 | 0.415 | 0.000 | 0.046 | |
| ND-10D | 0.000 | 0.000 | 0.008 | 0.000 | 0.003 | 0.014 | 0.066 | |
| ED-10D | 0.000 | 0.000 | 0.994 | 0.415 | 0.003 | 0.000 | 0.976 | |
| ND-30D | 0.000 | 0.000 | 0.000 | 0.000 | 0.014 | 0.000 | 0.000 | |
| ED-30D | 0.000 | 0.000 | 1.000 | 0.046 | 0.066 | 0.976 | 0.000 | |
| Basal | ND-CTR | ED-CTR | ND-1D | ED-1D | ND-10D | ED-10D | ND-30D | ED-30D |
| ND-CTR | 0.992 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| ED-CTR | 0.992 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| ND-1D | 0.000 | 0.000 | 0.034 | 0.551 | 0.845 | 0.000 | 0.998 | |
| ED-1D | 0.000 | 0.000 | 0.034 | 0.000 | 0.644 | 0.000 | 0.194 | |
| ND-10D | 0.000 | 0.000 | 0.551 | 0.000 | 0.023 | 0.598 | 0.168 | |
| ED-10D | 0.000 | 0.000 | 0.845 | 0.644 | 0.023 | 0.000 | 0.995 | |
| ND-30D | 0.000 | 0.000 | 0.000 | 0.000 | 0.598 | 0.000 | 0.000 | |
| ED-30D | 0.000 | 0.000 | 0.998 | 0.194 | 0.168 | 0.995 | 0.000 | |
| Groups | Apical | Middle | Basal | |||||
|---|---|---|---|---|---|---|---|---|
| ND-CTR | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | |||||
| ND-1D | 92.04 ± 1.21 | 63.45 ± 1.68 | 73.12 ± 2.35 | |||||
| ND-10D | 90.16 ± 2.12 | 53.79 ± 2.93 | 67.43 ± 0.97 | |||||
| ND-30D | 81.73 ± 1.22 | 41.61 ± 2.20 | 61.28 ± 2.46 | |||||
| ED-CTR | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | |||||
| ED-1D | 97.20 ± 1.23 | 75.12 ± 1.16 | 86.89 ± 2.26 | |||||
| ED-10D | 96.73 ± 1.53 | 67.74 ± 2.46 | 80.80 ± 0.99 | |||||
| ED-30D | 93.46 ± 1.49 | 64.29 ± 1.29 | 78.22 ± 1.11 | |||||
| Anova: | F(7,48) = 13.8 (***) | F(7,48) = 97.9 (***) | F(7,48) = 41.9 (***) | |||||
| Significance levels | ||||||||
| Apical | ND-CTR | ED-CTR | ND-1D | ED-1D | ND-10D | ED-10D | ND-30D | ED-30D |
| ND-CTR | 1.000 | 0.311 | 0.989 | 0.025 | 0.971 | 0.000 | 0.406 | |
| ED-CTR | 1.000 | 0.269 | 0.982 | 0.020 | 0.957 | 0.000 | 0.357 | |
| ND-1D | 0.311 | 0.269 | 0.852 | 0.971 | 0.915 | 0.000 | 1.000 | |
| ED-1D | 0.989 | 0.982 | 0.852 | 0.230 | 1.000 | 0.000 | 0.915 | |
| ND-10D | 0.025 | 0.020 | 0.971 | 0.230 | 0.311 | 0.094 | 0.938 | |
| ED-10D | 0.971 | 0.957 | 0.915 | 1.000 | 0.311 | 0.000 | 0.957 | |
| ND-30D | 0.000 | 0.000 | 0.000 | 0.000 | 0.094 | 0.000 | 0.000 | |
| ED-30D | 0.406 | 0.357 | 1.000 | 0.915 | 0.938 | 0.957 | 0.000 | |
| Middle | ND-CTR | ED-CTR | ND-1D | ED-1D | ND-10D | ED-10D | ND-30D | ED-30D |
| ND-CTR | 1.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| ED-CTR | 1.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| ND-1D | 0.000 | 0.000 | 0.049 | 0.008 | 0.994 | 0.000 | 1.000 | |
| ED-1D | 0.000 | 0.000 | 0.049 | 0.000 | 0.415 | 0.000 | 0.046 | |
| ND-10D | 0.000 | 0.000 | 0.008 | 0.000 | 0.003 | 0.014 | 0.066 | |
| ED-10D | 0.000 | 0.000 | 0.994 | 0.415 | 0.003 | 0.000 | 0.976 | |
| ND-30D | 0.000 | 0.000 | 0.000 | 0.000 | 0.014 | 0.000 | 0.000 | |
| ED-30D | 0.000 | 0.000 | 1.000 | 0.046 | 0.066 | 0.976 | 0.000 | |
| Basal | ND-CTR | ED-CTR | ND-1D | ED-1D | ND-10D | ED-10D | ND-30D | ED-30D |
| ND-CTR | 0.992 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| ED-CTR | 0.992 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| ND-1D | 0.000 | 0.000 | 0.034 | 0.551 | 0.845 | 0.000 | 0.998 | |
| ED-1D | 0.000 | 0.000 | 0.034 | 0.000 | 0.644 | 0.000 | 0.194 | |
| ND-10D | 0.000 | 0.000 | 0.551 | 0.000 | 0.023 | 0.598 | 0.168 | |
| ED-10D | 0.000 | 0.000 | 0.845 | 0.644 | 0.023 | 0.000 | 0.995 | |
| ND-30D | 0.000 | 0.000 | 0.000 | 0.000 | 0.598 | 0.000 | 0.000 | |
| ED-30D | 0.000 | 0.000 | 0.998 | 0.194 | 0.168 | 0.995 | 0.000 | |
| Groups | Bax | Bcl-2 | Casp3 | |||||
|---|---|---|---|---|---|---|---|---|
| ND-CTR | 0.95 ± 0.04 | 1.02 ± 0.03 | 0.95 ± 0.03 | |||||
| ND-1D | 0.99 ± 0.04 | 1.00 ± 0.03 | 1.30 ± 0.05 | |||||
| ND-10D | 1.63 ± 0.09 | 1.55 ± 0.07 | 1.71 ± 0.07 | |||||
| ND-30D | 1.14 ± 0.04 | 1.13 ± 0.04 | 1.24 ± 0.07 | |||||
| ED-CTR | 1.02 ± 0.03 | 1.01 ± 0.04 | 1.00 ± 0.01 | |||||
| ED-1D | 0.89 ± 0.02 | 0.92 ± 0.04 | 1.22 ± 0.04 | |||||
| ED-10D | 1.42 ± 0.05 | 1.90 ± 0.08 | 1.32 ± 0.04 | |||||
| ED-30D | 0.86 ± 0.02 | 1.05 ± 0.04 | 1.06 ± 0.02 | |||||
| Anova: | F(7,274) = 35.7 (***) | F(7,254) = 45.8 (***) | F(7,266) = 35.8 (***) | |||||
| Significance levels | ||||||||
| Bax | ND-CTR | ED-CTR | ND-1D | ED-1D | ND-10D | ED-10D | ND-30D | ED-30D |
| ND-CTR | 0.922 | 0.999 | 0.984 | 0.000 | 0.000 | 0.161 | 0.886 | |
| ED-CTR | 0.922 | 1.000 | 0.346 | 0.000 | 0.000 | 0.793 | 0.162 | |
| ND-1D | 0.999 | 1.000 | 0.896 | 0.000 | 0.000 | 0.675 | 0.722 | |
| ED-1D | 0.984 | 0.346 | 0.896 | 0.000 | 0.000 | 0.014 | 1.000 | |
| ND-10D | 0.000 | 0.000 | 0.000 | 0.000 | 0.249 | 0.000 | 0.000 | |
| ED-10D | 0.000 | 0.000 | 0.000 | 0.000 | 0.249 | 0.006 | 0.000 | |
| ND-30D | 0.161 | 0.793 | 0.675 | 0.014 | 0.000 | 0.006 | 0.005 | |
| ED-30D | 0.886 | 0.162 | 0.722 | 1.000 | 0.000 | 0.000 | 0.005 | |
| Bcl-2 | ND-CTR | ED-CTR | ND-1D | ED-1D | ND-10D | ED-10D | ND-30D | ED-30D |
| ND-CTR | 1.000 | 1.000 | 0.954 | 0.000 | 0.000 | 0.946 | 1.000 | |
| ED-CTR | 1.000 | 1.000 | 0.952 | 0.000 | 0.000 | 0.886 | 1.000 | |
| ND-1D | 1.000 | 1.000 | 0.994 | 0.000 | 0.000 | 0.917 | 1.000 | |
| ED-1D | 0.954 | 0.952 | 0.994 | 0.000 | 0.000 | 0.373 | 0.823 | |
| ND-10D | 0.000 | 0.000 | 0.000 | 0.000 | 0.009 | 0.003 | 0.000 | |
| ED-10D | 0.000 | 0.000 | 0.000 | 0.000 | 0.009 | 0.000 | 0.000 | |
| ND-30D | 0.946 | 0.886 | 0.917 | 0.373 | 0.003 | 0.000 | 0.988 | |
| ED-30D | 1.000 | 1.000 | 1.000 | 0.823 | 0.000 | 0.000 | 0.988 | |
| Casp3 | ND-CTR | ED-CTR | ND-1D | ED-1D | ND-10D | ED-10D | ND-30D | ED-30D |
| ND-CTR | 0.981 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.486 | |
| ED-CTR | 0.981 | 0.000 | 0.004 | 0.000 | 0.000 | 0.003 | 0.951 | |
| ND-1D | 0.000 | 0.000 | 0.955 | 0.000 | 1.000 | 0.997 | 0.010 | |
| ED-1D | 0.000 | 0.004 | 0.955 | 0.000 | 0.745 | 1.000 | 0.222 | |
| ND-10D | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| ED-10D | 0.000 | 0.000 | 1.000 | 0.745 | 0.000 | 0.949 | 0.002 | |
| ND-30D | 0.000 | 0.003 | 0.997 | 1.000 | 0.000 | 0.949 | 0.149 | |
| ED-30D | 0.486 | 0.951 | 0.010 | 0.222 | 0.000 | 0.002 | 0.149 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarado, J.C.; Fuentes-Santamaría, V.; Melgar-Rojas, P.; Gabaldón-Ull, M.C.; Cabanes-Sanchis, J.J.; Juiz, J.M. Oral Antioxidant Vitamins and Magnesium Limit Noise-Induced Hearing Loss by Promoting Sensory Hair Cell Survival: Role of Antioxidant Enzymes and Apoptosis Genes. Antioxidants 2020, 9, 1177. https://doi.org/10.3390/antiox9121177
Alvarado JC, Fuentes-Santamaría V, Melgar-Rojas P, Gabaldón-Ull MC, Cabanes-Sanchis JJ, Juiz JM. Oral Antioxidant Vitamins and Magnesium Limit Noise-Induced Hearing Loss by Promoting Sensory Hair Cell Survival: Role of Antioxidant Enzymes and Apoptosis Genes. Antioxidants. 2020; 9(12):1177. https://doi.org/10.3390/antiox9121177
Chicago/Turabian StyleAlvarado, Juan C., Verónica Fuentes-Santamaría, Pedro Melgar-Rojas, María C. Gabaldón-Ull, José J. Cabanes-Sanchis, and José M. Juiz. 2020. "Oral Antioxidant Vitamins and Magnesium Limit Noise-Induced Hearing Loss by Promoting Sensory Hair Cell Survival: Role of Antioxidant Enzymes and Apoptosis Genes" Antioxidants 9, no. 12: 1177. https://doi.org/10.3390/antiox9121177
APA StyleAlvarado, J. C., Fuentes-Santamaría, V., Melgar-Rojas, P., Gabaldón-Ull, M. C., Cabanes-Sanchis, J. J., & Juiz, J. M. (2020). Oral Antioxidant Vitamins and Magnesium Limit Noise-Induced Hearing Loss by Promoting Sensory Hair Cell Survival: Role of Antioxidant Enzymes and Apoptosis Genes. Antioxidants, 9(12), 1177. https://doi.org/10.3390/antiox9121177






