Extracellular Vesicles from SOD3-Transduced Stem Cells Exhibit Improved Immunomodulatory Abilities in the Murine Dermatitis Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Construction of SOD3-Expressing Lentiviral Vector
2.2. Lentivirus Production and Transduction
2.3. hUCB-MSC Culture
2.4. Western Blotting
2.5. SOD3 Transduction and Detection
2.6. Cell Viability and Proliferation Assay
2.7. hUCB-MSC Differentiation
2.7.1. Osteogenic Differentiation
2.7.2. Adipogenic Differentiation
2.8. Flow Cytometric Analysis
2.9. Cytokine Array
2.10. Mixed Lymphocyte Reaction and Mitogen-Stimulated Proliferation Assay
2.11. Isolation and Culture of Human CD4+ Helper T Cells
2.12. CD4+ T-Cell Polarization
2.13. Macrophage Activation Assay
2.14. LAD-2 Cell Culture
2.15. LAD-2 Cell Degranulation and β-Hexosaminidase Analysis
2.16. HaCaT Cell and Human Dermal Fibroblast (HDF) Culture
2.17. Immunofluorescence
2.18. Chloro-2, 4-dinitrochlorobenzene (DNCB)-Induced Mice Model
2.19. Migration Assay
2.19.1. In Vitro
2.19.2. In Vivo
2.20. Histological Analysis
2.21. Isolation of EVs from Conditioned Medium of hUCB-MSCs
2.22. EV Uptake Assay
2.23. Statistical Analysis
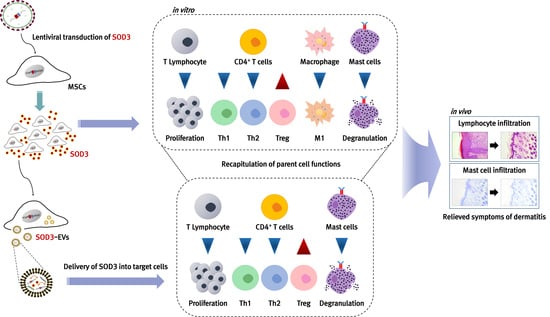
3. Results
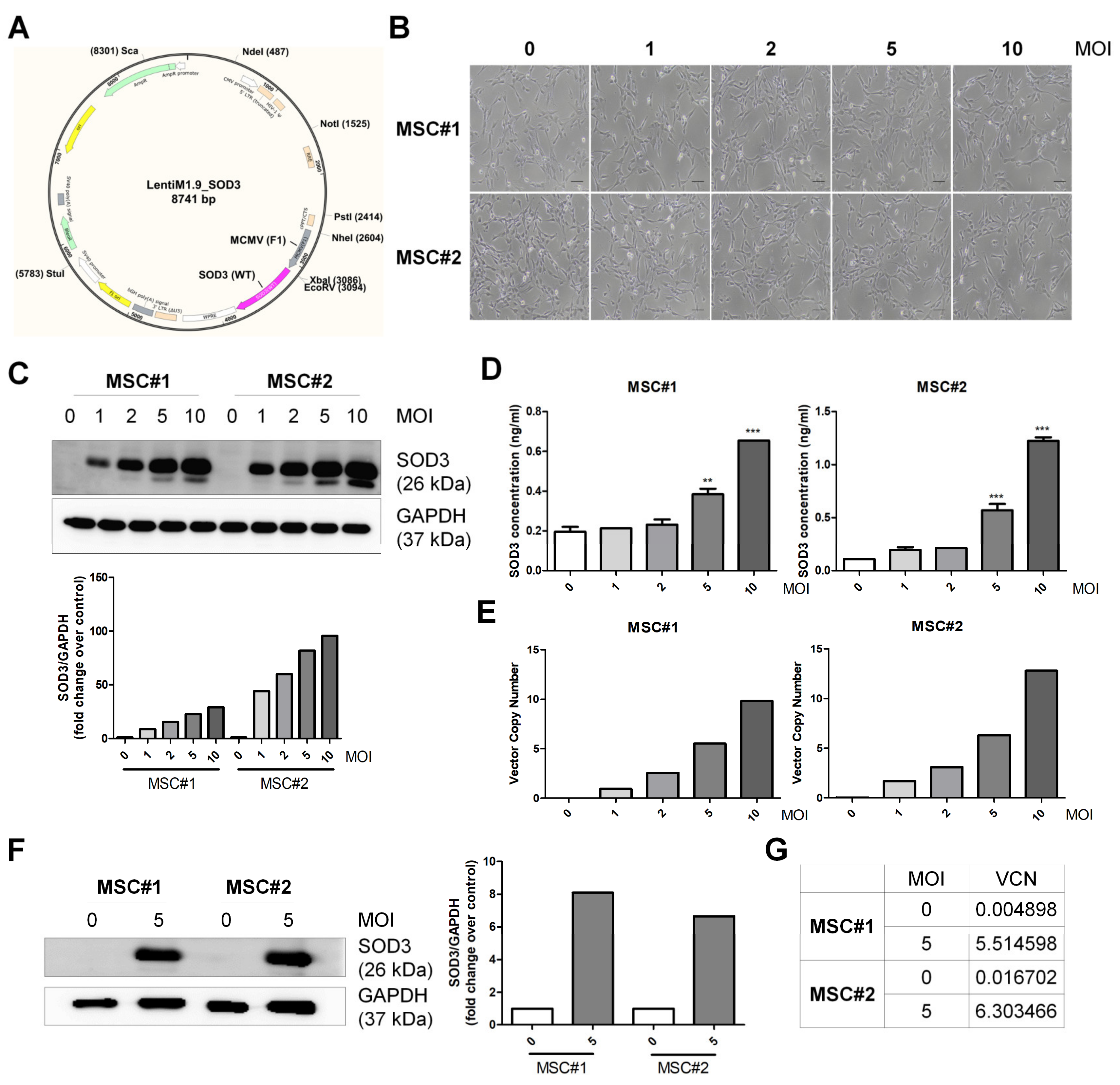
3.1. Vector Integration in hUCB-MSCs
3.2. Characterization of SOD3-Transduced MSCs
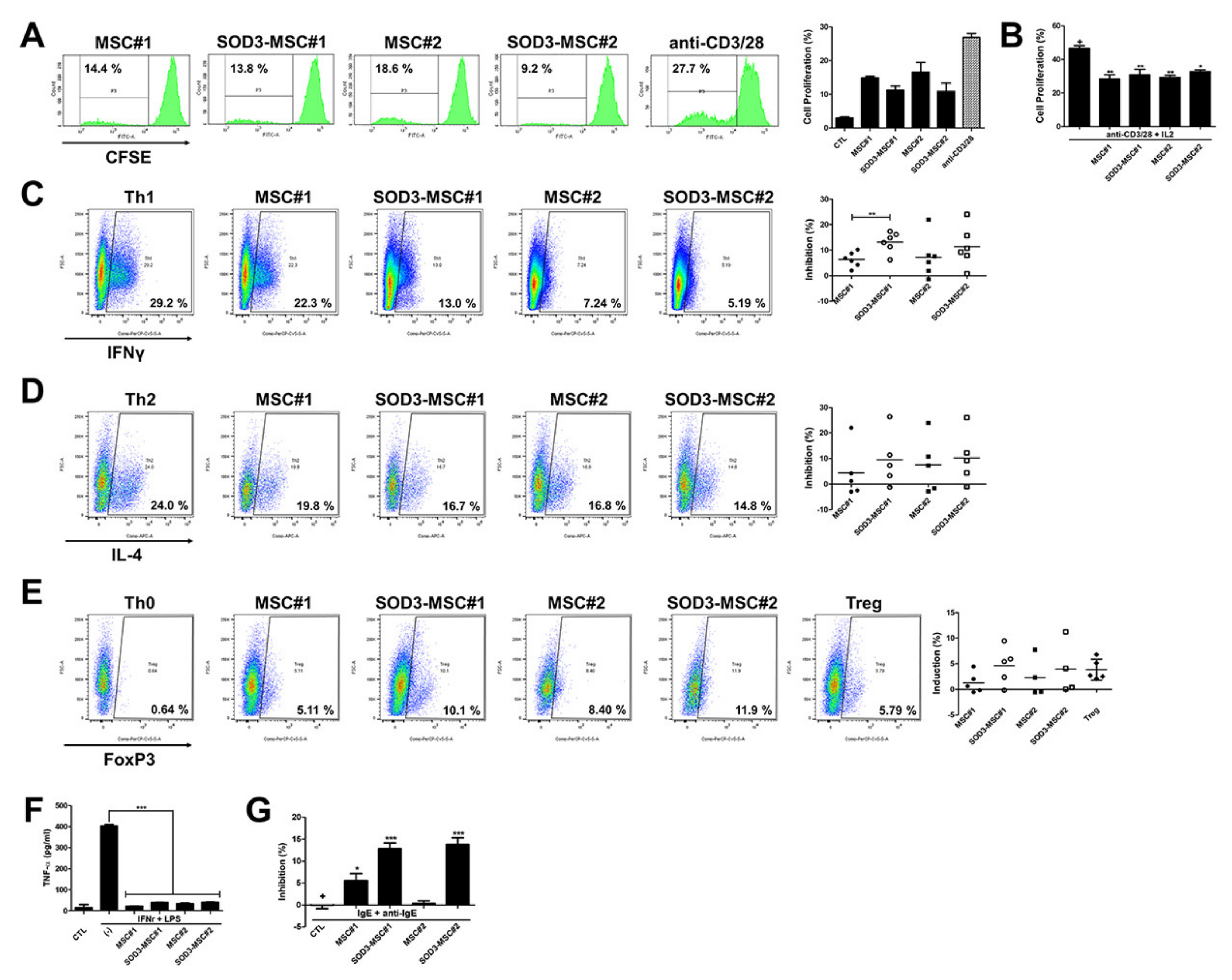
3.3. Immunomodulatory Effects of SOD3-MSCs on Human Immune Cells
3.4. Anti-Fibrotic Feature of SOD3-MSCs
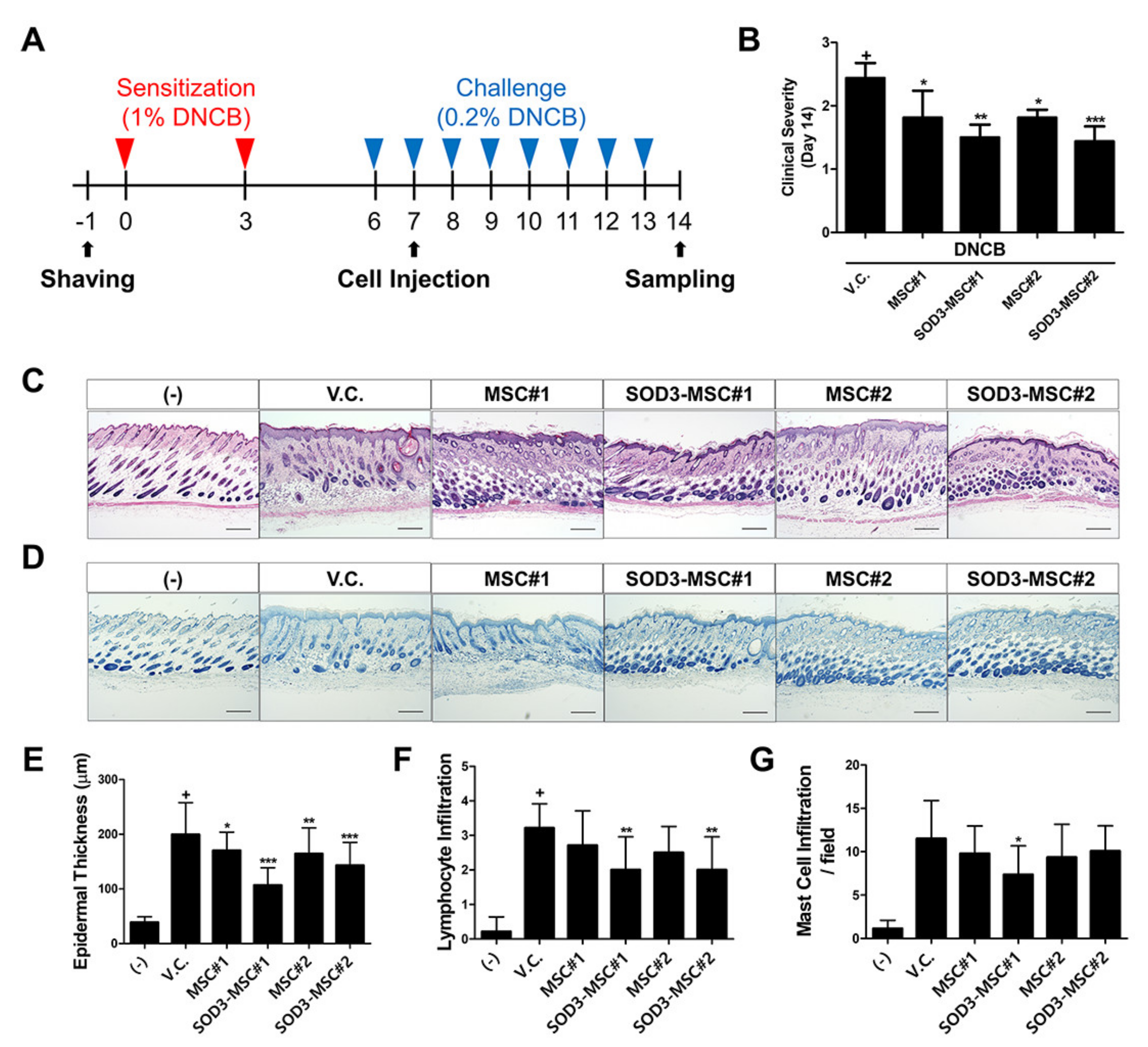
3.5. Therapeutic Efficacy of SOD3-MSCs in Murine Dermatitis Model
3.6. Migration of SOD3-MSCs to the Inflamed Site
3.7. Recapitulation of SOD3-MSC Functions by EVs
3.8. In Vivo Efficacy of SOD3-MSC-Derived EVs in Dermatitis Model
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kolf, C.M.; Cho, E.; Tuan, R.S. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: Regulation of niche, self-renewal and differentiation. Arthritis Res. Ther. 2007, 9, 204. [Google Scholar] [CrossRef][Green Version]
- Lee, M.; Jeong, S.Y.; Ha, J.; Kim, M.; Jin, H.J.; Kwon, S.-J.; Chang, J.W.; Choi, S.J.; Oh, W.; Yang, Y.S.; et al. Low immunogenicity of allogeneic human umbilical cord blood-derived mesenchymal stem cells in vitro and in vivo. Biochem. Biophys. Res. Commun. 2014, 446, 983–989. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Y.; Yang, D.; Luo, F.; Liang, W.; Guo, S.; Xu, J. The immunomodulatory activity of human umbilical cord blood-derived mesenchymal stem cellsin vitro. Immunology 2009, 126, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-Q.; Deng, M.-X.; He, J.; Zeng, Q.-X.; Wen, W.; Wong, D.S.; Tse, H.-F.; Xu, G.; Lian, Q.; Shi, J.; et al. Human Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Prevent Allergic Airway Inflammation in Mice. Stem Cells 2012, 30, 2692–2699. [Google Scholar] [CrossRef] [PubMed]
- Shin, T.-H.; Lee, B.-C.; Choi, S.W.; Shin, J.-H.; Kang, I.; Lee, J.Y.; Kim, J.-J.; Lee, H.-K.; Jung, J.-E.; Choi, Y.-W.; et al. Human adipose tissue-derived mesenchymal stem cells alleviate atopic dermatitis via regulation of B lymphocyte maturation. Oncotarget 2016, 8, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Shin, T.-H.; Kim, H.-S.; Kang, T.-W.; Lee, B.-C.; Lee, H.-Y.; Kim, Y.-J.; Shin, J.-H.; Seo, Y.; Choi, S.W.; Lee, S.; et al. Human umbilical cord blood-stem cells direct macrophage polarization and block inflammasome activation to alleviate rheumatoid arthritis. Cell Death Dis. 2016, 7, e2524. [Google Scholar] [CrossRef]
- Németh, K.; Leelahavanichkul, A.; Yuen, P.S.T.; Mayer, B.; Parmelee, A.; Doi, K.; Robey, P.G.; Leelahavanichkul, K.; Koller, B.H.; Brown, J.M.; et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E2–dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 2009, 15, 42–49. [Google Scholar] [CrossRef]
- Nemeth, K.; Keane-Myers, A.; Brown, J.M.; Metcalfe, D.D.; Gorham, J.D.; Bundoc, V.G.; Hodges, M.G.; Jelinek, I.; Madala, S.; Karpati, S.; et al. Bone marrow stromal cells use TGF- to suppress allergic responses in a mouse model of ragweed-induced asthma. Proc. Natl. Acad. Sci. USA 2010, 107, 5652–5657. [Google Scholar] [CrossRef]
- Lee, R.H.; Seo, M.J.; Reger, R.L.; Spees, J.L.; Pulin, A.A.; Olson, S.D.; Prockop, D.J. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc. Natl. Acad. Sci. USA 2006, 103, 17438–17443. [Google Scholar] [CrossRef]
- Le Blanc, K.; Rasmusson, I.; Sundberg, B.; Götherström, C.; Hassan, M.; Uzunel, M.; Ringdén, O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 2004, 363, 1439–1441. [Google Scholar] [CrossRef]
- Kim, H.-S.; Yun, J.-W.; Shin, T.-H.; Lee, S.-H.; Lee, B.-C.; Yu, K.-R.; Seo, Y.; Lee, S.; Kang, T.-W.; Choi, S.W.; et al. Human Umbilical Cord Blood Mesenchymal Stem Cell-Derived PGE2 and TGF-β1 Alleviate Atopic Dermatitis by Reducing Mast Cell Degranulation. Stem Cells 2015, 33, 1254–1266. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Shin, T.; Lee, B.; Yu, K.; Seo, Y.; Lee, S.; Seo, M.; Hong, I.; Choi, S.W.; Seo, K.; et al. Human Umbilical Cord Blood Mesenchymal Stem Cells Reduce Colitis in Mice by Activating NOD2 Signaling to COX2. Gastroenterology 2013, 145, 1392–1403.e8. [Google Scholar] [CrossRef] [PubMed]
- Sah, S.K.; Agrahari, G.; Nguyen, C.T.; Kim, Y.-S.; Kang, K.-S.; Kim, T.-Y. Enhanced therapeutic effects of human mesenchymal stem cells transduced with superoxide dismutase 3 in a murine atopic dermatitis-like skin inflammation model. Allergy 2018, 73, 2364–2376. [Google Scholar] [CrossRef] [PubMed]
- Regmi, S.; Pathak, S.; Thanh, T.P.; Nguyen, T.T.; Sung, J.-H.; Yook, S.; Kim, J.O.; Yong, C.S.; Choi, I.; Doh, K.-O.; et al. Intraportally delivered stem cell spheroids localize in the liver and protect hepatocytes against GalN/LPS-induced fulminant hepatic toxicity. Stem Cell Res. Ther. 2019, 10, 230. [Google Scholar] [CrossRef]
- Lee, B.-C.; Kim, J.-J.; Lee, J.Y.; Kang, I.; Shin, N.; Lee, S.-E.; Choi, S.W.; Cho, J.-Y.; Kim, H.-S.; Kang, K.-S. Disease-specific primed human adult stem cells effectively ameliorate experimental atopic dermatitis in mice. Theranostics 2019, 9, 3608–3621. [Google Scholar] [CrossRef]
- Ahn, J.-S.; Seo, Y.; Oh, S.-J.; Yang, J.W.; Shin, Y.Y.; Lee, B.-C.; Kang, K.-S.; Sung, E.-S.; Lee, B.-J.; Mohammadpour, H.; et al. The activation of NLRP3 inflammasome potentiates the immunomodulatory abilities of mesenchymal stem cells in a murine colitis model. BMB Rep. 2020, 53, 329–334. [Google Scholar] [CrossRef]
- Sivaranjani, N. Role of Reactive Oxygen Species and Antioxidants in Atopic Dermatitis. J. Clin. Diagn. Res. 2013, 7, 2683. [Google Scholar] [CrossRef]
- Tsukahara, H.; Shibata, R.; Ohshima, Y.; Todoroki, Y.; Sato, S.; Ohta, N.; Hiraoka, M.; Yoshida, A.; Nishima, S.; Mayumi, M. Oxidative stress and altered antioxidant defenses in children with acute exacerbation of atopic dermatitis. Life Sci. 2003, 72, 2509–2516. [Google Scholar] [CrossRef]
- Omata, N.; Tsukahara, H.; Ito, S.; Ohshima, Y.; Yasutomi, M.; Yamada, A.; Jiang, M.; Hiraoka, M.; Nambu, M.; Deguchi, Y.; et al. Increased oxidative stress in childhood atopic dermatitis. Life Sci. 2001, 69, 223–228. [Google Scholar] [CrossRef]
- Federico, A.; Morgillo, F.; Tuccillo, C.; Ciardiello, F.; Loguercio, C. Chronic inflammation and oxidative stress in human carcinogenesis. Int. J. Cancer 2007, 121, 2381–2386. [Google Scholar] [CrossRef]
- Chatterjee, S. Oxidative stress, inflammation, and disease. In Oxidative Stress and Biomaterials; Elsevier: Amsterdam, The Netherlands, 2016; pp. 35–58. [Google Scholar]
- Marklund, S.L.; Holme, E.; Hellner, L. Superoxide dismutase in extracellular fluids. Clin. Chim. Acta 1982, 126, 41–51. [Google Scholar] [CrossRef]
- Nozik-Grayck, E.; Suliman, H.B.; Piantadosi, C.A. Extracellular superoxide dismutase. Int. J. Biochem. Cell Biol. 2005, 37, 2466–2471. [Google Scholar] [CrossRef] [PubMed]
- Irshad, M.; Chaudhuri, P.S. Oxidant-antioxidant system: Role and significance in human body. Indian J. Exp. Biol. 2002, 40, 1233–1239. [Google Scholar] [PubMed]
- Nguyen, C.T.; Sah, S.K.; Zouboulis, C.C.; Kim, T.-Y. Inhibitory effects of superoxide dismutase 3 on Propionibacterium acnes-induced skin inflammation. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sah, S.K.; Park, K.H.; Yun, C.-O.; Kang, K.-S.; Kim, T.-Y. Effects of Human Mesenchymal Stem Cells Transduced with Superoxide Dismutase on Imiquimod-Induced Psoriasis-Like Skin Inflammation in Mice. Antioxidants Redox Signal. 2016, 24, 233–248. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Li, L.; Li, M.; Guo, C.; Yao, J.; Mi, S. Exosome and Exosomal MicroRNA: Trafficking, Sorting, and Function. Genom. Proteom. Bioinform. 2015, 13, 17–24. [Google Scholar] [CrossRef]
- Bang, C.; Thum, T. Exosomes: New players in cell–cell communication. Int. J. Biochem. Cell Biol. 2012, 44, 2060–2064. [Google Scholar] [CrossRef]
- Yang, J.; Liu, X.-X.; Fan, H.; Tang, Q.; Shou, Z.-X.; Zuo, D.-M.; Zou, Z.; Xu, M.; Chen, Q.-Y.; Peng, Y.; et al. Extracellular Vesicles Derived from Bone Marrow Mesenchymal Stem Cells Protect against Experimental Colitis via Attenuating Colon Inflammation, Oxidative Stress and Apoptosis. PLoS ONE 2015, 10, e0140551. [Google Scholar] [CrossRef]
- Wang, L.; Gu, Z.; Zhao, X.; Zhenyang, G.; Wang, F.; Deng, A.; Zhao, S.; Luo, L.; Wei, H.; Guan, L.; et al. Extracellular Vesicles Released from Human Umbilical Cord-Derived Mesenchymal Stromal Cells Prevent Life-Threatening Acute Graft-Versus-Host Disease in a Mouse Model of Allogeneic Hematopoietic Stem Cell Transplantation. Stem Cells Dev. 2016, 25, 1874–1883. [Google Scholar] [CrossRef]
- Song, Y.; Dou, H.; Li, X.; Zhao, X.; Li, Y.; Liu, D.; Ji, J.; Liu, F.; Ding, L.; Ni, Y.; et al. Exosomal miR-146a Contributes to the Enhanced Therapeutic Efficacy of Interleukin-1β-Primed Mesenchymal Stem Cells Against Sepsis. Stem Cells 2017, 35, 1208–1221. [Google Scholar] [CrossRef]
- Nojehdehi, S.; Soudi, S.; Hesampour, A.; Rasouli, S.; Soleimani, M.; Hashemi, S.M. Immunomodulatory effects of mesenchymal stem cell-derived exosomes on experimental type-1 autoimmune diabetes. J. Cell. Biochem. 2018, 119, 9433–9443. [Google Scholar] [CrossRef] [PubMed]
- Cosenza, S.; Toupet, K.; Maumus, M.; Luz-Crawford, P.; Blanc-Brude, O.; Jorgensen, C.; Noël, D. Mesenchymal stem cells-derived exosomes are more immunosuppressive than microparticles in inflammatory arthritis. Theranostics 2018, 8, 1399–1410. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-L.; Sung, P.-H.; Chen, K.-H.; Shao, P.-L.; Yang, C.-C.; Cheng, B.-C.; Lin, K.-C.; Chen, C.-H.; Chai, H.-T.; Chang, H.-W.; et al. Adipose-derived mesenchymal stem cell-derived exosomes alleviate overwhelming systemic inflammatory reaction and organ damage and improve outcome in rat sepsis syndrome. Am. J. Transl. Res. 2018, 10, 1053–1070. [Google Scholar] [PubMed]
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef]
- Vishnubhatla, I.; Corteling, R.; Stevanato, L.; Hicks, C.; Sinden, J. The development of stem cell-derived exosomes as a cell-free regenerative medicine. J. Circ. Biomark. 2014, 3. [Google Scholar] [CrossRef]
- Bieback, K.; Kern, S.; Kocaömer, A.; Ferlik, K.; Bugert, P. Comparing mesenchymal stromal cells from different human tissues: Bone marrow, adipose tissue and umbilical cord blood. Bio-Med. Mater. Eng. 2008, 18, 71–76. [Google Scholar]
- Krampera, M.; Glennie, S.; Dyson, J.; Scott, D.; Laylor, R.; Simpson, E.; Dazzi, F. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 2003, 101, 3722–3729. [Google Scholar] [CrossRef]
- Beyth, S.; Borovsky, Z.; Mevorach, D.; Liebergall, M.; Gazit, Z.; Aslan, H.; Galun, E.; Rachmilewitz, J. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood 2005, 105, 2214–2219. [Google Scholar] [CrossRef]
- Puissant, B.; Barreau, C.; Bourin, P.; Clavel, C.; Corre, J.; Bousquet, C.; Taureau, C.; Cousin, B.; Abbal, M.; Laharrague, P.; et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: Comparison with bone marrow mesenchymal stem cells. Br. J. Haematol. 2005, 129, 118–129. [Google Scholar] [CrossRef]
- Yañez, R.; Lamana, M.L.; García-Castro, J.; Colmenero, I.; Ramírez, M.; Bueren, J.A. Adipose Tissue-Derived Mesenchymal Stem Cells Have In Vivo Immunosuppressive Properties Applicable for the Control of the Graft-Versus-Host Disease. Stem Cells 2006, 24, 2582–2591. [Google Scholar] [CrossRef]
- Sato, K.; Ozaki, K.; Oh, I.; Meguro, A.; Hatanaka, K.; Nagai, T.; Muroi, K.; Ozawa, K. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood 2006, 109, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Tse, W.T.; Pendleton, J.D.; Beyer, W.M.; Egalka, M.C.; Guinan, E.C. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: Implications in transplantation. Transplantation 2003, 75, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Spergel, J.M.; Mizoguchi, E.; Oettgen, H.; Bhan, A.K.; Geha, R.S. Roles of TH1 and TH2 cytokines in a murine model of allergic dermatitis. J. Clin. Investig. 1999, 103, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.; Tilles, S.; Lio, P.; Boguniewicz, M.; Beck, L.; LeBovidge, J.; Novak, N.; Bernstein, D.; Blessing-Moore, J.; Khan, D.; et al. Atopic dermatitis: A practice parameter update 2012. J. Allergy Clin. Immunol. 2013, 131, 295–299.e27. [Google Scholar] [CrossRef]
- Biedermann, T.; Röcken, M.; Carballido, J.M. TH1 and TH2 Lymphocyte Development and Regulation of TH Cell–Mediated Immune Responses of the Skin. J. Investig. Dermatol. Symp. Proc. 2004, 9, 5–14. [Google Scholar] [CrossRef]
- Kawakami, T.; Ando, T.; Kimura, M.; Wilson, B.S.; Kawakami, Y. Mast cells in atopic dermatitis. Curr. Opin. Immunol. 2009, 21, 666–678. [Google Scholar] [CrossRef]
- Kasraie, S.; Werfel, T. Role of Macrophages in the Pathogenesis of Atopic Dermatitis. Mediat. Inflamm. 2013, 2013, 942375. [Google Scholar] [CrossRef]
- Proksch, E.; Fölster-Holst, R.; Jensen, J.-M. Skin barrier function, epidermal proliferation and differentiation in eczema. J. Dermatol. Sci. 2006, 43, 159–169. [Google Scholar] [CrossRef]
- Boguniewicz, M. Atopic Dermatitis: Beyond the Itch that Rashes. Immunol. Allergy Clin. N. Am. 2005, 25, 333–351. [Google Scholar] [CrossRef]
- Bucala, R.; Spiegel, L.A.; Chesney, J.; Hogan, M.; Cerami, A. Circulating Fibrocytes Define a New Leukocyte Subpopulation That Mediates Tissue Repair. Mol. Med. 1994, 1, 71–81. [Google Scholar] [CrossRef]
- Wu, Y.; Peng, Y.; Gao, D.; Feng, C.; Yuan, X.; Li, H.; Wang, Y.; Yang, L.; Huang, S.; Fu, X. Mesenchymal Stem Cells Suppress Fibroblast Proliferation and Reduce Skin Fibrosis Through a TGF-β3-Dependent Activation. Int. J. Low. Extremity Wounds 2015, 14, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Huang, S.; Enhe, J.; Ma, K.; Yang, S.; Sun, T.; Fu, X. Bone marrow-derived mesenchymal stem cell attenuates skin fibrosis development in mice. Int. Wound J. 2013, 11, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Trohatou, O.; Roubelakis, M.G. Mesenchymal Stem/Stromal Cells in Regenerative Medicine: Past, Present, and Future. Cell. Reprogram. 2017, 19, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-H.K. Aging of mesenchymal stem cells: Implication in regenerative medicine. Regen. Ther. 2018, 9, 120–122. [Google Scholar] [CrossRef]
- Mendt, M.; Kamerkar, S.; Sugimoto, H.; McAndrews, K.M.; Wu, C.-C.; Gagea, M.; Yang, S.; Blanko, E.V.R.; Peng, Q.; Ma, X.; et al. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Zhu, X.; Badawi, M.; Pomeroy, S.; Sutaria, D.S.; Xie, Z.; Baek, A.; Jiang, J.; Elgamal, O.A.; Mohamed, B.; La Perle, K.; et al. Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from HEK293T cells. J. Extracell. Vesicles 2017, 6, 1324730. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, H.; Jin, M.; Yang, X.; Ji, H.; Jiang, Y.; Zhang, H.; Wu, F.; Wu, G.; Lai, X.; et al. Exosomes from MiR-30d-5p-ADSCs Reverse Acute Ischemic Stroke-Induced, Autophagy-Mediated Brain Injury by Promoting M2 Microglial/Macrophage Polarization. Cell. Physiol. Biochem. 2018, 47, 864–878. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.; Yang, J.; Yu, Y.; Chai, J.; Wang, L.; Ma, L.; Yin, H. Exosome Derived From Human Umbilical Cord Mesenchymal Stem Cell Mediates MiR-181c Attenuating Burn-induced Excessive Inflammation. EBioMedicine 2016, 8, 72–82. [Google Scholar] [CrossRef]
- Sicco, C.L.; Reverberi, D.; Balbi, C.; Ulivi, V.; Principi, E.; Pascucci, L.; Becherini, P.; Bosco, M.C.; Varesio, L.; Franzin, C.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles as Mediators of Anti-Inflammatory Effects: Endorsement of Macrophage Polarization. Stem Cells Transl. Med. 2017, 6, 1018–1028. [Google Scholar] [CrossRef]
- Spinosa, M.; Lu, G.; Su, G.; Bontha, S.V.; Gehrau, R.; Salmon, M.D.; Smith, J.R.; Weiss, M.L.; Mas, V.R.; Upchurch, G.R.; et al. Human mesenchymal stromal cell-derived extracellular vesicles attenuate aortic aneurysm formation and macrophage activation via microRNA-147. FASEB J. 2018, 32, 6038–6050. [Google Scholar] [CrossRef]
- Ti, D.; Hao, H.; Tong, C.; Liu, J.; Dong, L.; Zheng, J.; Zhao, Y.; Liu, H.; Fu, X.-B.; Han, W. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J. Transl. Med. 2015, 13, 308. [Google Scholar] [CrossRef] [PubMed]
- Siegel, G.; Kluba, T.; Hermanutz-Klein, U.; Bieback, K.; Northoff, H.; Schäfer, R. Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC Med. 2013, 11, 146. [Google Scholar] [CrossRef] [PubMed]
- Kang, I.; Lee, B.-C.; Choi, S.W.; Lee, J.Y.; Kim, J.-J.; Kim, B.-E.; Kim, D.-H.; Lee, S.E.; Shin, N.; Seo, Y.; et al. Donor-dependent variation of human umbilical cord blood mesenchymal stem cells in response to hypoxic preconditioning and amelioration of limb ischemia. Exp. Mol. Med. 2018, 50, 1–15. [Google Scholar] [CrossRef]
- Phinney, D.G.; Kopen, G.; Righter, W.; Webster, S.; Tremain, N.; Prockop, D.J. Donor variation in the growth properties and osteogenic potential of human marrow stromal cells. J. Cell Biochem. 1999, 75, 424–436. [Google Scholar] [CrossRef]
- Siddappa, R.; Licht, R.; Van Blitterswijk, C.; De Boer, J. Donor variation and loss of multipotency during in vitro expansion of human mesenchymal stem cells for bone tissue engineering. J. Orthop. Res. 2007, 25, 1029–1041. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.W.; Seo, Y.; Shin, T.-H.; Ahn, J.-S.; Oh, S.-J.; Shin, Y.Y.; Kang, M.-J.; Lee, B.-C.; Lee, S.; Kang, K.-S.; et al. Extracellular Vesicles from SOD3-Transduced Stem Cells Exhibit Improved Immunomodulatory Abilities in the Murine Dermatitis Model. Antioxidants 2020, 9, 1165. https://doi.org/10.3390/antiox9111165
Yang JW, Seo Y, Shin T-H, Ahn J-S, Oh S-J, Shin YY, Kang M-J, Lee B-C, Lee S, Kang K-S, et al. Extracellular Vesicles from SOD3-Transduced Stem Cells Exhibit Improved Immunomodulatory Abilities in the Murine Dermatitis Model. Antioxidants. 2020; 9(11):1165. https://doi.org/10.3390/antiox9111165
Chicago/Turabian StyleYang, Ji Won, Yoojin Seo, Tae-Hoon Shin, Ji-Su Ahn, Su-Jeong Oh, Ye Young Shin, Min-Jung Kang, Byung-Chul Lee, Seunghee Lee, Kyung-Sun Kang, and et al. 2020. "Extracellular Vesicles from SOD3-Transduced Stem Cells Exhibit Improved Immunomodulatory Abilities in the Murine Dermatitis Model" Antioxidants 9, no. 11: 1165. https://doi.org/10.3390/antiox9111165
APA StyleYang, J. W., Seo, Y., Shin, T.-H., Ahn, J.-S., Oh, S.-J., Shin, Y. Y., Kang, M.-J., Lee, B.-C., Lee, S., Kang, K.-S., Hur, J., Kim, Y.-S., Kim, T.-Y., & Kim, H.-S. (2020). Extracellular Vesicles from SOD3-Transduced Stem Cells Exhibit Improved Immunomodulatory Abilities in the Murine Dermatitis Model. Antioxidants, 9(11), 1165. https://doi.org/10.3390/antiox9111165