Osteoprotective Roles of Green Tea Catechins
Abstract
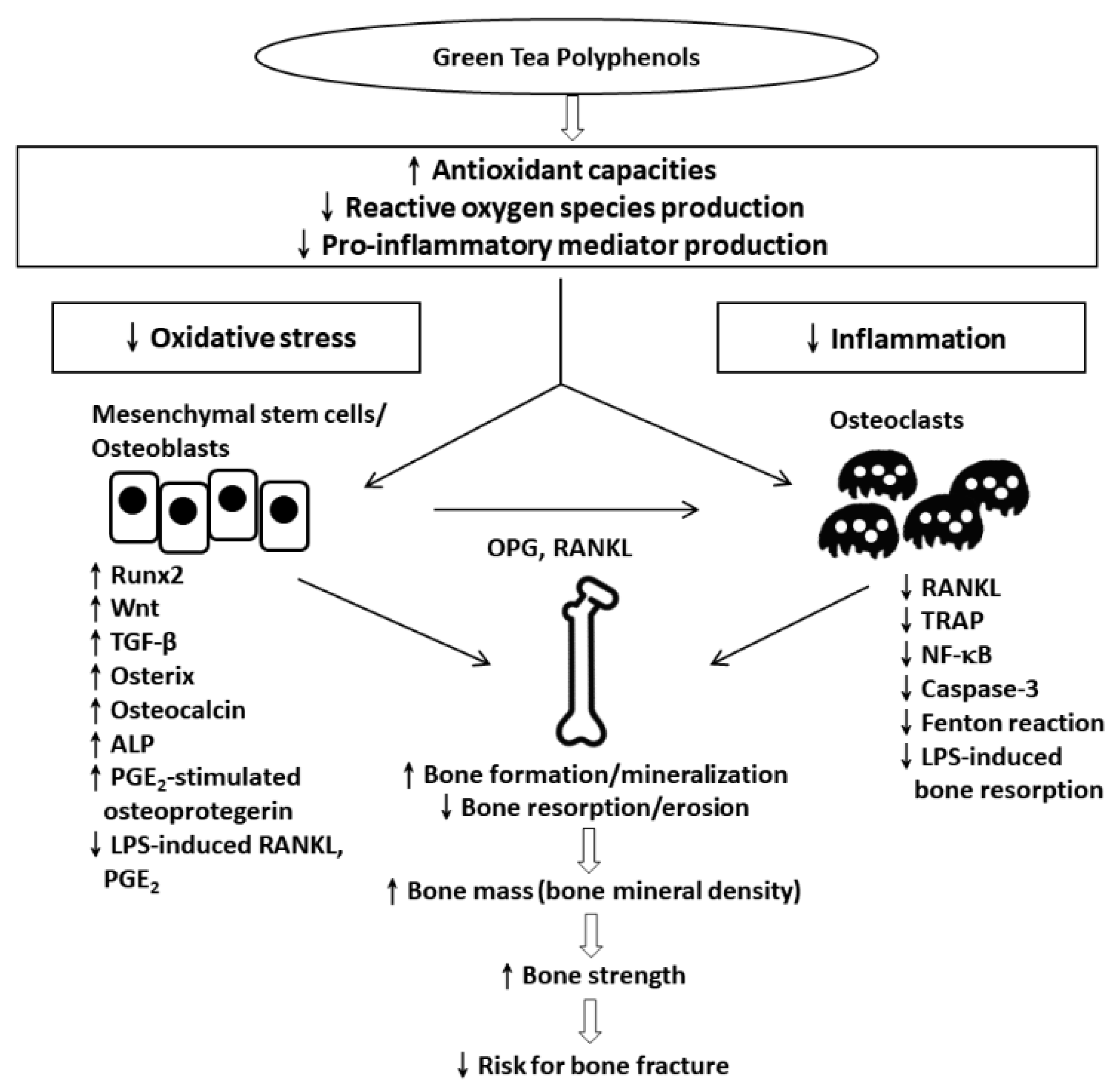
1. Osteoporosis
1.1. Molecular Regulation in Osteoporosis
1.2. Oxidative Stress is Related to Osteoporosis
2. Catechins
2.1. In Vitro Effect of Catechins
2.2. In Vivo Effects of Catechins
2.3. Human Studies of Catechins
2.3.1. Epidemiological Observational Studies
2.3.2. Human Clinical Trials
3. Conclusions and Prospective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Klibanski, A.; Adams-Campbell, L.; Bassford, T.L.; Blair, S.N.; Boden, S.D.; Dickersin, K.; Gifford, D.R.; Glasse, L.; Goldring, S.R.; Hruska, K.; et al. Osteoporosis prevention, diagnosis, and therapy. JAMA 2001, 285, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A. Diagnosis of osteoporosis and assessment of fracture risk. Lancet 2002, 359, 1929–1936. [Google Scholar] [CrossRef]
- Osteoporosis Australia. Available online: http://www.osteoporosis.org.au (accessed on 20 July 2020).
- Kanis, J.A.; Delmas, P.; Burckhardt, P.; Cooper, C.; Torgerson, D. Guidelines for diagnosis and management of osteoporosis. The European Foundation for Osteoporosis and Bone Disease. Osteoporos Int. 1997, 7, 390–406. [Google Scholar] [CrossRef] [PubMed]
- Tatangelo, G.; Watts, J.; Lim, K.; Connaughton, C.; Abimanyi-Ochom, J.; Borgstrom, F.; Nicholson, G.C.; Shore-Lorenti, C.; Stuart, A.L.; Iuliano-Burns, S.; et al. The Cost of Osteoporosis, Osteopenia, and Associated Fractures in Australia in 2017. J. Bone Miner. Res. 2019, 34, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Ducy, P.; Schinke, T.; Karsenty, G. The osteoblast: A sophisticated fibroblast under central surveillance. Science 2000, 289, 1501–1504. [Google Scholar] [CrossRef] [PubMed]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef]
- Manolagas, S.C. Birth and Death of Bone Cells: Basic Regulatory Mechanisms and Implications for the Pathogenesis and Treatment of Osteoporosis*. Endocr. Rev. 2000, 21, 115–137. [Google Scholar] [CrossRef]
- Harada, S.; Rodan, G.A. Control of osteoblast function and regulation of bone mass. Nature 2003, 423, 349–355. [Google Scholar] [CrossRef]
- Ducy, P.; Zhang, R.; Geoffroy, V.; Ridall, A.L.; Karsenty, G. Osf2/Cbfa1: A transcriptional activator of osteoblast differentiation. Cell 1997, 89, 747–754. [Google Scholar] [CrossRef]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef]
- DeLaurier, A.; Eames, B.F.; Blanco-Sánchez, B.; Peng, G.; He, X.; Swartz, M.E.; Ullmann, B.; Westerfield, M.; Kimmel, C.B. Zebrafish sp7:EGFP: A transgenic for studying otic vesicle formation, skeletogenesis, and bone regeneration. Genesis 2010, 48, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Corrado, A.; Cici, D.; Rotondo, C.; Maruotti, N.; Cantatore, F.P. Molecular Basis of Bone Aging. Int. J. Mol. Sci. 2020, 21, 3679. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.; Han, L.; Martin-Millan, M.; Plotkin, L.I.; Stewart, S.A.; Roberson, P.K.; Kousteni, S.; O’Brien, C.A.; Bellido, T.; Parfitt, A.M.; et al. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J. Biol. Chem. 2007, 282, 27285–27297. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhu, L.; Zhang, D.; Li, N.; Li, Q.; Dai, P.; Mao, Y.; Li, X.; Ma, J.; Huang, S. Oxidative Stress-Related Biomarkers in Postmenopausal Osteoporosis: A Systematic Review and Meta-Analyses. Dis. Markers 2016, 2016, 7067984. [Google Scholar] [CrossRef]
- Domazetovic, V.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Oxidative stress in bone remodeling: Role of antioxidants. Clin. Cases Miner. Bone Metab. 2017, 14, 209–216. [Google Scholar] [CrossRef]
- Manolagas, S.C.; Almeida, M. Gone with the Wnts: Beta-catenin, T-cell factor, forkhead box O, and oxidative stress in age-dependent diseases of bone, lipid, and glucose metabolism. Mol. Endocrinol. 2007, 21, 2605–2614. [Google Scholar] [CrossRef]
- Komori, T. Cell Death in Chondrocytes, Osteoblasts, and Osteocytes. Int. J. Mol. Sci. 2016, 17, 2045. [Google Scholar] [CrossRef]
- Cao, J.; Venton, L.; Sakata, T.; Halloran, B.P. Expression of RANKL and OPG correlates with age-related bone loss in male C57BL/6 mice. J. Bone Miner. Res. 2003, 18, 270–277. [Google Scholar] [CrossRef]
- Cao, J.J.; Wronski, T.J.; Iwaniec, U.; Phleger, L.; Kurimoto, P.; Boudignon, B.; Halloran, B.P. Aging increases stromal/osteoblastic cell-induced osteoclastogenesis and alters the osteoclast precursor pool in the mouse. J. Bone Miner. Res. 2005, 20, 1659–1668. [Google Scholar] [CrossRef]
- Shen, C.L.; Chyu, M.C. Tea flavonoids for bone health: From animals to humans. J. Investig. Med. 2016, 64, 1151–1157. [Google Scholar] [CrossRef]
- Garrett, I.R.; Boyce, B.F.; Oreffo, R.O.; Bonewald, L.; Poser, J.; Mundy, G.R. Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J. Clin. Invest. 1990, 85, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Banfi, G.; Iorio, E.L.; Corsi, M.M. Oxidative stress, free radicals and bone remodeling. Clin. Chem. Lab. Med. 2008, 46, 1550–1555. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, M.; Maeda, T.; Chatani, M.; Handa, K.; Yamakawa, T.; Kiyohara, S.; Negishi-Koga, T.; Kato, Y.; Takami, M.; Niida, S.; et al. A Delphinidin-Enriched Maqui Berry Extract Improves Bone Metabolism and Protects against Bone Loss in Osteopenic Mouse Models. Antioxidants 2019, 8, 386. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.C.; Lu, D.; Bai, J.; Zheng, H.; Ke, Z.Y.; Li, X.M.; Luo, S.Q. Oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF-kappaB. Biochem. Biophys. Res. Commun. 2004, 314, 197–207. [Google Scholar] [CrossRef]
- Manolagas, S.C. De-fense! De-fense! De-fense: Scavenging H2O2 while making cholesterol. Endocrinology 2008, 149, 3264–3266. [Google Scholar] [CrossRef]
- Yang, S.; Madyastha, P.; Bingel, S.; Ries, W.; Key, L. A new superoxide-generating oxidase in murine osteoclasts. J. Biol. Chem. 2001, 276, 5452–5458. [Google Scholar] [CrossRef]
- Kim, B.; Lee, S.H.; Song, S.J.; Kim, W.H.; Song, E.S.; Lee, J.C.; Lee, S.J.; Han, D.W.; Lee, J.H. Protective Effects of Melon Extracts on Bone Strength, Mineralization, and Metabolism in Rats with Ovariectomy-Induced Osteoporosis. Antioxidants 2019, 8, 306. [Google Scholar] [CrossRef]
- Milkovic, L.; Vukovic, T.; Zarkovic, N.; Tatzber, F.; Bisenieks, E.; Kalme, Z.; Bruvere, I.; Ogle, Z.; Poikans, J.; Velena, A.; et al. Antioxidative 1,4-Dihydropyridine Derivatives Modulate Oxidative Stress and Growth of Human Osteoblast-Like Cells In Vitro. Antioxidants 2018, 7, 123. [Google Scholar] [CrossRef]
- Oh, Y.; Ahn, C.B.; Cho, W.H.; Yoon, N.Y.; Je, J.Y. Anti-Osteoporotic Effects of Antioxidant Peptides PIISVYWK and FSVVPSPK from Mytilus edulis on Ovariectomized Mice. Antioxidants 2020, 9, 866. [Google Scholar] [CrossRef]
- Domazetovic, V.; Marcucci, G.; Falsetti, I.; Bilia, A.R.; Vincenzini, M.T.; Brandi, M.L.; Iantomasi, T. Blueberry Juice Antioxidants Protect Osteogenic Activity against Oxidative Stress and Improve Long-Term Activation of the Mineralization Process in Human Osteoblast-Like SaOS-2 Cells: Involvement of SIRT1. Antioxidants 2020, 9, 125. [Google Scholar] [CrossRef]
- Sanghani-Kerai, A.; Osagie-Clouard, L.; Blunn, G.; Coathup, M. The influence of age and osteoporosis on bone marrow stem cells from rats. Bone Jt. Res. 2018, 7, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.T.; Lee, M.J.; Chen, C.H.; Chuang, S.C.; Chang, L.F.; Ho, M.L.; Hung, S.H.; Fu, Y.C.; Wang, Y.H.; Wang, H.I.; et al. Proliferation and differentiation potential of human adipose-derived mesenchymal stem cells isolated from elderly patients with osteoporotic fractures. J. Cell Mol. Med. 2012, 16, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Chen, H.T.; Ho, M.L.; Chen, C.H.; Chuang, S.C.; Huang, S.C.; Fu, Y.C.; Wang, G.J.; Kang, L.; Chang, J.K. PPARgamma silencing enhances osteogenic differentiation of human adipose-derived mesenchymal stem cells. J. Cell Mol. Med. 2013, 17, 1188–1193. [Google Scholar] [CrossRef]
- Tyner, S.D.; Venkatachalam, S.; Choi, J.; Jones, S.; Ghebranious, N.; Igelmann, H.; Lu, X.; Soron, G.; Cooper, B.; Brayton, C.; et al. p53 mutant mice that display early ageing-associated phenotypes. Nature 2002, 415, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Luk, H.Y.; Appell, C.; Chyu, M.C.; Chen, C.H.; Wang, C.Y.; Yang, R.S.; Shen, C.L. Impacts of Green Tea on Joint and Skeletal Muscle Health: Prospects of Translational Nutrition. Antioxidants 2020, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.L.; Chyu, M.C.; Wang, J.S. Tea and bone health: Steps forward in translational nutrition. Am. J. Clin. Nutr. 2013, 98, 1694S–1699S. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.Y.; Zhao, C.N.; Xu, X.Y.; Gan, R.Y.; Cao, S.Y.; Liu, Q.; Shang, A.; Mao, Q.Q.; Li, H.B. Phytochemical Composition and Antioxidant Capacity of 30 Chinese Teas. Antioxidants 2019, 8, 180. [Google Scholar] [CrossRef]
- Liao, S.; Kao, Y.H.; Hiipakka, R.A. Green tea: Biochemical and biological basis for health benefits. Vitam. Horm. 2001, 62, 1–94. [Google Scholar]
- Zhao, C.N.; Tang, G.Y.; Cao, S.Y.; Xu, X.Y.; Gan, R.Y.; Liu, Q.; Mao, Q.Q.; Shang, A.; Li, H.B. Phenolic Profiles and Antioxidant Activities of 30 Tea Infusions from Green, Black, Oolong, White, Yellow and Dark Teas. Antioxidants 2019, 8, 215. [Google Scholar] [CrossRef]
- Katiyar, S.K.; Elmets, C.A. Green tea polyphenolic antioxidants and skin photoprotection (Review). Int. J. Oncol. 2001, 18, 1307–1313. [Google Scholar] [CrossRef]
- Kondo, K.; Kurihara, M.; Fukuhara, K. Mechanism of antioxidant effect of catechins. Methods Enzymol. 2001, 335, 203–217. [Google Scholar] [PubMed]
- Toronjo Urquiza, L.; James, D.C.; Nagy, T.; Falconer, R.J. Screening Naturally Occurring Phenolic Antioxidants for Their Suitability as Additives to CHO Cell Culture Media Used to Produce Monoclonal Antibodies. Antioxidants 2019, 8, 159. [Google Scholar] [CrossRef] [PubMed]
- Meneses-Gutierrez, C.L.; Hernandez-Damian, J.; Pedraza-Chaverri, J.; Guerrero-Legarreta, I.; Tellez, D.I.; Jaramillo-Flores, M.E. Antioxidant Capacity and Cytotoxic Effects of Catechins and Resveratrol Oligomers Produced by Enzymatic Oxidation against T24 Human Urinary Bladder Cancer Cells. Antioxidants 2019, 8, 214. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Azain, M.; Crowe-White, K.; Mumaw, J.; Grimes, J.A.; Schmiedt, C.; Barletta, M.; Rayalam, S.; Park, H.J. Effect of Acute Ingestion of Green Tea Extract and Lemon Juice on Oxidative Stress and Lipid Profile in Pigs Fed a High-Fat Diet. Antioxidants 2019, 8, 195. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, J.M.; Marques, B.M.; Novaes, V.C.N.; de Oliveira, F.L.P.; Matheus, H.R.; Fiorin, L.G.; Ervolino, E. Influence of adjuvant therapy with green tea extract in the treatment of experimental periodontitis. Arch. Oral Biol. 2019, 102, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Attanzio, A.; D’Anneo, A.; Pappalardo, F.; Bonina, F.P.; Livrea, M.A.; Allegra, M.; Tesoriere, L. Phenolic Composition of Hydrophilic Extract of Manna from Sicilian Fraxinus angustifolia Vahl and its Reducing, Antioxidant and Anti-Inflammatory Activity in Vitro. Antioxidants 2019, 8, 494. [Google Scholar] [CrossRef] [PubMed]
- Cheaib, D.; El Darra, N.; Rajha, H.N.; El-Ghazzawi, I.; Mouneimne, Y.; Jammoul, A.; Maroun, R.G.; Louka, N. Study of the Selectivity and Bioactivity of Polyphenols Using Infrared Assisted Extraction from Apricot Pomace Compared to Conventional Methods. Antioxidants 2018, 7, 174. [Google Scholar] [CrossRef]
- Rha, C.S.; Jung, Y.S.; Lee, J.D.; Jang, D.; Kim, M.S.; Lee, M.S.; Hong, Y.D.; Kim, D.O. Chemometric Analysis of Extracts and Fractions from Green, Oxidized, and Microbial Fermented Teas and Their Correlation to Potential Antioxidant and Anticancer Effects. Antioxidants 2020, 9, 1015. [Google Scholar] [CrossRef]
- Lee, S.; Yu, J.S.; Phung, H.M.; Lee, J.G.; Kim, K.H.; Kang, K.S. Potential Anti-Skin Aging Effect of (−)-Catechin Isolated from the Root Bark of Ulmus davidiana var. japonica in Tumor Necrosis Factor-alpha-Stimulated Normal Human Dermal Fibroblasts. Antioxidants 2020, 9, 981. [Google Scholar] [CrossRef]
- Kim, T.Y.; Leem, E.; Lee, J.M.; Kim, S.R. Control of Reactive Oxygen Species for the Prevention of Parkinson’s Disease: The Possible Application of Flavonoids. Antioxidants 2020, 9, 583. [Google Scholar] [CrossRef]
- Nakagawa, H.; Wachi, M.; Woo, J.T.; Kato, M.; Kasai, S.; Takahashi, F.; Lee, I.S.; Nagai, K. Fenton reaction is primarily involved in a mechanism of (−)-epigallocatechin-3-gallate to induce osteoclastic cell death. Biochem. Biophys. Res. Commun. 2002, 292, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.H.; Pang, E.K.; Kim, C.S.; Yoo, Y.J.; Cho, K.S.; Chai, J.K.; Kim, C.K.; Choi, S.H. Inhibitory effects of green tea polyphenol (−)-epigallocatechin gallate on the expression of matrix metalloproteinase-9 and on the formation of osteoclasts. J. Periodontal. Res. 2004, 39, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Oka, Y.; Iwai, S.; Amano, H.; Irie, Y.; Yatomi, K.; Ryu, K.; Yamada, S.; Inagaki, K.; Oguchi, K. Tea polyphenols inhibit rat osteoclast formation and differentiation. J. Pharmacol. Sci. 2012, 118, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, H.; Takai, S.; Hanai, Y.; Matsushima-Nishiwaki, R.; Hosoi, T.; Harada, A.; Ohta, T.; Kozawa, O. (−)-Epigallocatechin gallate suppresses endothelin-1-induced interleukin-6 synthesis in osteoblasts: Inhibition of p44/p42 MAP kinase activation. FEBS Lett. 2007, 581, 1311–1316. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, H.; Takai, S.; Matsushima-Nishiwaki, R.; Akamatsu, S.; Hanai, Y.; Hosoi, T.; Harada, A.; Ohta, T.; Kozawa, O. (--)-epigallocatechin gallate enhances prostaglandin F2alpha-induced VEGF synthesis via upregulating SAPK/JNK activation in osteoblasts. J. Cell Biochem. 2007, 100, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Morinobu, A.; Biao, W.; Tanaka, S.; Horiuchi, M.; Jun, L.; Tsuji, G.; Sakai, Y.; Kurosaka, M.; Kumagai, S. (−)-Epigallocatechin-3-gallate suppresses osteoclast differentiation and ameliorates experimental arthritis in mice. Arthritis Rheum. 2008, 58, 2012–2018. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jin, H.; Shim, H.E.; Kim, H.N.; Ha, H.; Lee, Z.H. Epigallocatechin-3-gallate inhibits osteoclastogenesis by down-regulating c-Fos expression and suppressing the nuclear factor-kappaB signal. Mol. Pharmacol. 2010, 77, 17–25. [Google Scholar] [CrossRef]
- Lin, R.; Chen, C.; Wang, Y.; Ho, M.; Hung, S.; Chen, I.; Wang, G. (−)-Epigallocatechin gallate inhibition of osteoclastic differentiation via NF-kappaB. Biochem. Biophys. Res. Commun. 2009, 379, 1033–1037. [Google Scholar] [CrossRef]
- Chen, C.H.; Ho, M.L.; Chang, J.K.; Hung, S.H.; Huang, H.T.; Wang, C.; Yeh, C.H.; Wang, G.J. Green tea catechins enhance the expression of osteoprotegerin(OPG) in pluripotent stem cells. J. Orthop Surg Taiwan 2003, 20, 178–183. [Google Scholar]
- Chen, S.T.; Kang, L.; Wang, C.Z.; Huang, P.J.; Huang, H.T.; Lin, S.Y.; Chou, S.H.; Lu, C.C.; Shen, P.C.; Lin, Y.S.; et al. (−)-Epigallocatechin-3-Gallate Decreases Osteoclastogenesis via Modulation of RANKL and Osteoprotegrin. Molecules 2019, 24, 156. [Google Scholar] [CrossRef]
- Kuroyanagi, G.; Tokuda, H.; Yamamoto, N.; Kainuma, S.; Fujita, K.; Ohguchi, R.; Kawabata, T.; Sakai, G.; Matsushima-Nishiwaki, R.; Harada, A.; et al. (−)-Epigallocatechin gallate synergistically potentiates prostaglandin E(2)-stimulated osteoprotegerin synthesis in osteoblasts. Prostaglandins Other Lipid Mediat. 2017, 128–129, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Tominari, T.; Matsumoto, C.; Watanabe, K.; Hirata, M.; Grundler, F.M.; Miyaura, C.; Inada, M. Epigallocatechin gallate (EGCG) suppresses lipopolysaccharide-induced inflammatory bone resorption, and protects against alveolar bone loss in mice. FEBS Open Bio. 2015, 5, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Ho, M.L.; Chang, J.K.; Hung, S.H.; Wang, G.J. Green tea catechin enhances osteogenesis in a bone marrow mesenchymal stem cell line. Osteoporos Int. 2005, 16, 2039–2045. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Kang, L.; Wang, C.Z.; Huang, H.H.; Cheng, T.L.; Huang, H.T.; Lee, M.J.; Lin, Y.S.; Ho, M.L.; Wang, G.J.; et al. (−)-Epigallocatechin-3-Gallate (EGCG) Enhances Osteogenic Differentiation of Human Bone Marrow Mesenchymal Stem Cells. Molecules 2018, 23, 3221. [Google Scholar] [CrossRef] [PubMed]
- Takai, S.; Matsushima-Nishiwaki, R.; Adachi, S.; Natsume, H.; Minamitani, C.; Mizutani, J.; Otsuka, T.; Tokuda, H.; Kozawa, O. (−)-Epigallocatechin gallate reduces platelet-derived growth factor-BB-stimulated interleukin-6 synthesis in osteoblasts: Suppression of SAPK/JNK. Mediators Inflamm. 2008, 2008, 291808. [Google Scholar] [CrossRef]
- Vali, B.; Rao, L.G.; El-Sohemy, A. Epigallocatechin-3-gallate increases the formation of mineralized bone nodules by human osteoblast-like cells. J. Nutr. Biochem. 2007, 18, 341–347. [Google Scholar] [CrossRef]
- Rawadi, G. Wnt signaling and potential applications in bone diseases. Curr. Drug Targets 2008, 9, 581–590. [Google Scholar] [CrossRef]
- Matsuno, M.; Kozawa, O.; Suzuki, A.; Tokuda, H.; Kaida, T.; Matsuno, H.; Niwa, M.; Uematsu, T. Involvement of protein kinase C activation in endothelin-1-induced secretion of interleukin-6 in osteoblast-like cells. Cell Signal. 1998, 10, 107–111. [Google Scholar] [CrossRef]
- Tokuda, H.; Takai, S.; Hanai, Y.; Matsushima-Nishiwaki, R.; Yamauchi, J.; Harada, A.; Hosoi, T.; Ohta, T.; Kozawa, O. (−)-Epigallocatechin gallate inhibits basic fibroblast growth factor-stimulated interleukin-6 synthesis in osteoblasts. Horm. Metab. Res. 2008, 40, 674–678. [Google Scholar] [CrossRef]
- Hayashi, K.; Takai, S.; Matsushima-Nishiwaki, R.; Hanai, Y.; Kato, K.; Tokuda, H.; Kozawa, O. (−)-Epigallocatechin gallate reduces transforming growth factor beta-stimulated HSP27 induction through the suppression of stress-activated protein kinase/c-Jun N-terminal kinase in osteoblasts. Life Sci. 2008, 82, 1012–1017. [Google Scholar] [CrossRef]
- Lu, T.X.; Rothenberg, M.E. MicroRNA. J. Allergy Clin. Immunol. 2018, 141, 1202–1207. [Google Scholar] [CrossRef] [PubMed]
- Feurer, E.; Kan, C.; Croset, M.; Sornay-Rendu, E.; Chapurlat, R. Lack of Association Between Select Circulating miRNAs and Bone Mass, Turnover, and Fractures: Data From the OFELY Cohort. J. Bone Miner. Res. 2019, 34, 1074–1085. [Google Scholar] [CrossRef] [PubMed]
- Hadjiargyrou, M.; Komatsu, D.E. The Therapeutic Potential of MicroRNAs as Orthobiologics for Skeletal Fractures. J. Bone Miner. Res. 2019, 34, 797–809. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hao, X.; Yin, M.; Xu, T.; Guo, F. Long non-coding RNA in osteogenesis: A new world to be explored. Bone Jt. Res. 2019, 8, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Takahara, S.; Lee, S.Y.; Iwakura, T.; Oe, K.; Fukui, T.; Okumachi, E.; Waki, T.; Arakura, M.; Sakai, Y.; Nishida, K.; et al. Altered expression of microRNA during fracture healing in diabetic rats. Bone Jt. Res. 2018, 7, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Kelch, S.; Balmayor, E.R.; Seeliger, C.; Vester, H.; Kirschke, J.S.; van Griensven, M. miRNAs in bone tissue correlate to bone mineral density and circulating miRNAs are gender independent in osteoporotic patients. Sci. Rep. 2017, 7, 15861. [Google Scholar] [CrossRef]
- Chen, J.; Li, K.; Pang, Q.; Yang, C.; Zhang, H.; Wu, F.; Cao, H.; Liu, H.; Wan, Y.; Xia, W.; et al. Identification of suitable reference gene and biomarkers of serum miRNAs for osteoporosis. Sci. Rep. 2016, 6, 36347. [Google Scholar] [CrossRef]
- Qiu, Y.; Chen, Y.; Zeng, T.; Guo, W.; Zhou, W.; Yang, X. EGCG ameliorates the hypoxia-induced apoptosis and osteogenic differentiation reduction of mesenchymal stem cells via upregulating miR-210. Mol. Biol. Rep. 2016, 43, 183–193. [Google Scholar] [CrossRef]
- Rao, N.C.; Barsky, S.H.; Terranova, V.P.; Liotta, L.A. Isolation of a tumor cell laminin receptor. Biochem. Biophys. Res. Commun. 1983, 111, 804–808. [Google Scholar] [CrossRef]
- Malinoff, H.L.; Wicha, M.S. Isolation of a cell surface receptor protein for laminin from murine fibrosarcoma cells. J. Cell Biol. 1983, 96, 1475–1479. [Google Scholar] [CrossRef]
- Lesot, H.; Kuhl, U.; Mark, K. Isolation of a laminin-binding protein from muscle cell membranes. EMBO J. 1983, 2, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.N.; Castronovo, V.; Schmitt, M.C.; Wewer, U.M.; Claysmith, A.P.; Liotta, L.A.; Sobel, M.E. Evidence for a precursor of the high-affinity metastasis-associated murine laminin receptor. Biochemistry 1989, 28, 7476–7486. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, H.; Koga, K.; Fujimura, Y.; Yamada, K. A receptor for green tea polyphenol EGCG. Nat. Struct. Mol. Biol. 2004, 11, 380–381. [Google Scholar] [CrossRef]
- Tachibana, H. Green tea polyphenol sensing. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2011, 87, 66–80. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.L.; Wang, P.; Guerrieri, J.; Yeh, J.K.; Wang, J.S. Protective effect of green tea polyphenols on bone loss in middle-aged female rats. Osteoporos Int. 2008, 19, 979–990. [Google Scholar] [CrossRef]
- Shen, C.L.; Yeh, J.K.; Stoecker, B.J.; Chyu, M.C.; Wang, J.S. Green tea polyphenols mitigate deterioration of bone microarchitecture in middle-aged female rats. Bone 2009, 44, 684–690. [Google Scholar] [CrossRef]
- Shen, C.L.; Cao, J.J.; Dagda, R.Y.; Tenner, T.E., Jr.; Chyu, M.C.; Yeh, J.K. Supplementation with green tea polyphenols improves bone microstructure and quality in aged, orchidectomized rats. Calcif. Tissue Int. 2011, 88, 455–463. [Google Scholar] [CrossRef]
- Shen, C.L.; Han, J.; Wang, S.; Chung, E.; Chyu, M.C.; Cao, J.J. Green tea supplementation benefits body composition and improves bone properties in obese female rats fed with high-fat diet and caloric restricted diet. Nutr. Res. 2015, 35, 1095–1105. [Google Scholar] [CrossRef]
- Cao, J.J. Effects of obesity on bone metabolism. J. Orthop Surg. Res. 2011, 6, 30. [Google Scholar] [CrossRef]
- Xi, J.; Li, Q.; Luo, X.; Li, J.; Guo, L.; Xue, H.; Wu, G. Epigallocatechin-3-gallate protects against secondary osteoporosis in a mouse model via the Wnt/β-catenin signaling pathway. Mol. Med. Rep. 2018, 18, 4555–4562. [Google Scholar] [CrossRef]
- Smith, B.J.; Lerner, M.R.; Bu, S.Y.; Lucas, E.A.; Hanas, J.S.; Lightfoot, S.A.; Postier, R.G.; Bronze, M.S.; Brackett, D.J. Systemic bone loss and induction of coronary vessel disease in a rat model of chronic inflammation. Bone 2006, 38, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Hienz, S.A.; Paliwal, S.; Ivanovski, S. Mechanisms of Bone Resorption in Periodontitis. J. Immunol. Res. 2015, 2015, 615486. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Kang, L.; Lin, R.W.; Fu, Y.C.; Lin, Y.S.; Chang, J.K.; Chen, H.T.; Chen, C.H.; Lin, S.Y.; Wang, G.J.; et al. (−)-Epigallocatechin-3-gallate improves bone microarchitecture in ovariectomized rats. Menopause 2013, 20, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Kan, J.Y.; Lu, C.C.; Huang, H.H.; Cheng, T.L.; Huang, H.T.; Ho, C.J.; Lee, T.C.; Chuang, S.C.; Lin, Y.S.; et al. Green Tea Catechin (−)-Epigallocatechin-3-Gallate (EGCG) Facilitates Fracture Healing. Biomolecules 2020, 10, 620. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Kang, L.; Chen, J.C.; Wang, C.Z.; Huang, H.H.; Lee, M.J.; Cheng, T.L.; Chang, C.F.; Lin, Y.S.; Chen, C.H. (−)-Epigallocatechin-3-gallate (EGCG) enhances healing of femoral bone defect. Phytomedicine 2019, 55, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, L.; Mu, S.; Fu, Q. Epigallocatechin-3-Gallate Ameliorates Glucocorticoid-Induced Osteoporosis of Rats in Vivo and in Vitro. Front. Pharmacol. 2018, 9, 447. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.L.; Yeh, J.K.; Cao, J.J.; Tatum, O.L.; Dagda, R.Y.; Wang, J.S. Green tea polyphenols mitigate bone loss of female rats in a chronic inflammation-induced bone loss model. J. Nutr. Biochem. 2010, 21, 968–974. [Google Scholar] [CrossRef]
- Shen, C.L.; Yeh, J.K.; Samathanam, C.; Cao, J.J.; Stoecker, B.J.; Dagda, R.Y.; Chyu, M.C.; Dunn, D.M.; Wang, J.S. Green tea polyphenols attenuate deterioration of bone microarchitecture in female rats with systemic chronic inflammation. Osteoporos Int. 2011, 22, 327–337. [Google Scholar] [CrossRef]
- Shen, C.L.; Yeh, J.K.; Cao, J.J.; Tatum, O.L.; Dagda, R.Y.; Wang, J.S. Synergistic effects of green tea polyphenols and alphacalcidol on chronic inflammation-induced bone loss in female rats. Osteoporos Int. 2010, 21, 1841–1852. [Google Scholar] [CrossRef]
- Shen, C.L.; Cao, J.J.; Dagda, R.Y.; Chanjaplammootil, S.; Lu, C.; Chyu, M.C.; Gao, W.; Wang, J.S.; Yeh, J.K. Green tea polyphenols benefits body composition and improves bone quality in long-term high-fat diet-induced obese rats. Nutr. Res. 2012, 32, 448–457. [Google Scholar] [CrossRef]
- Shen, C.L.; Chyu, M.C.; Cao, J.J.; Yeh, J.K. Green tea polyphenols improve bone microarchitecture in high-fat-diet-induced obese female rats through suppressing bone formation and erosion. J. Med. Food 2013, 16, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Ukai, T.; Yoshimura, A.; Kozuka, Y.; Yoshioka, H.; Yoshinaga, Y.; Abe, Y.; Hara, Y. Green tea catechin inhibits lipopolysaccharide-induced bone resorption in vivo. J. Periodontal. Res. 2010, 45, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, Y.; Ukai, T.; Nakatsu, S.; Kuramoto, A.; Nagano, F.; Yoshinaga, M.; Montenegro, J.L.; Shiraishi, C.; Hara, Y. Green tea extract inhibits the onset of periodontal destruction in rat experimental periodontitis. J. Periodontal. Res. 2014, 49, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Chen, Z.; Liu, H.; Xuan, Y.; Wang, X.; Luan, Q. Green tea epigallocatechin-3-gallate alleviates Porphyromonas gingivalis-induced periodontitis in mice. Int. Immunopharmacol. 2015, 29, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Gennaro, G.; Claudino, M.; Cestari, T.M.; Ceolin, D.; Germino, P.; Garlet, G.P.; de Assis, G.F. Green Tea Modulates Cytokine Expression in the Periodontium and Attenuates Alveolar Bone Resorption in Type 1 Diabetic Rats. PLoS ONE 2015, 10, e0134784. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Park, J.Y.; Hong, J.M.; Kim, T.H.; Shin, H.I.; Park, E.K.; Kim, S.Y. Inhibitory effect of (−)-epigallocatechin gallate on titanium particle-induced TNF-alpha release and in vivo osteolysis. Exp. Mol. Med. 2011, 43, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Iwaniec, U.T.; Turner, R.T.; Koo, S.I.; Kaur, R.; Ho, E.; Wong, C.P.; Bruno, R.S. Consumption of green tea extract results in osteopenia in growing male mice. J. Nutr. 2009, 139, 1914–1919. [Google Scholar] [CrossRef]
- Shen, C.L.; Yeh, J.K.; Samathanam, C.; Cao, J.J.; Stoecker, B.J.; Dagda, R.Y.; Chyu, M.C.; Wang, J.S. Protective actions of green tea polyphenols and alfacalcidol on bone microstructure in female rats with chronic inflammation. J. Nutr. Biochem. 2011, 22, 673–680. [Google Scholar] [CrossRef]
- WHO Study Group. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ. Tech. Rep. Ser. 1994, 843, 1–129. [Google Scholar]
- Marshall, D.; Johnell, O.; Wedel, H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 1996, 312, 1254–1259. [Google Scholar] [CrossRef]
- Bouxsein, M.L.; Eastell, R.; Lui, L.Y.; Wu, L.A.; de Papp, A.E.; Grauer, A.; Marin, F.; Cauley, J.A.; Bauer, D.C.; Black, D.M.; et al. Change in Bone Density and Reduction in Fracture Risk: A Meta-Regression of Published Trials. J. Bone Miner. Res. 2019, 34, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Li, N.; Wang, Q.; Feng, J.; Sun, D.; Zhang, Q.; Huang, J.; Wen, Q.; Hu, R.; Wang, L.; et al. The Prevalence of Osteoporosis in China, a Nationwide, Multicenter DXA Survey. J. Bone Miner. Res. 2019, 34, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Cummings, S.R.; Bates, D.; Black, D.M. Clinical use of bone densitometry: Scientific review. JAMA 2002, 288, 1889–1897. [Google Scholar] [CrossRef] [PubMed]
- Anastasilakis, A.D.; Papapoulos, S.E.; Polyzos, S.A.; Appelman-Dijkstra, N.M.; Makras, P. Zoledronate for the Prevention of Bone Loss in Women Discontinuing Denosumab Treatment. A Prospective 2-Year Clinical Trial. J. Bone Miner. Res. 2019, 34, 2220–2228. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.; Libanati, C.; Lin, C.J.F.; Brown, J.P.; Cosman, F.; Czerwinski, E.; de Gregomicronrio, L.H.; Malouf-Sierra, J.; Reginster, J.Y.; Wang, A.; et al. Relationship Between Bone Mineral Density T-Score and Nonvertebral Fracture Risk Over 10 Years of Denosumab Treatment. J. Bone Miner. Res. 2019, 34, 1033–1040. [Google Scholar] [CrossRef]
- Kim, T.Y.; Bauer, D.C.; McNabb, B.L.; Schafer, A.L.; Cosman, F.; Black, D.M.; Eastell, R. Comparison of BMD Changes and Bone Formation Marker Levels 3 Years After Bisphosphonate Discontinuation: FLEX and HORIZON-PFT Extension I Trials. J. Bone Miner. Res. 2019, 34, 810–816. [Google Scholar] [CrossRef]
- Leder, B.Z.; Zapalowski, C.; Hu, M.Y.; Hattersley, G.; Lane, N.E.; Singer, A.J.; Dore, R.K. Fracture and Bone Mineral Density Response by Baseline Risk in Patients Treated With Abaloparatide Followed by Alendronate: Results From the Phase 3 ACTIVExtend Trial. J. Bone Miner. Res. 2019, 34, 2213–2219. [Google Scholar] [CrossRef]
- Wu, C.H.; Chang, Y.F.; Chen, C.H.; Lewiecki, E.M.; Wüster, C.; Reid, I.; Tsai, K.S.; Matsumoto, T.; Mercado-Asis, L.B.; Chan, D.C.; et al. Consensus Statement on the Use of Bone Turnover Markers for Short-Term Monitoring of Osteoporosis Treatment in the Asia-Pacific Region. J. Clin. Densitom 2019. In press. [Google Scholar] [CrossRef]
- Crandall, C.J.; Vasan, S.; LaCroix, A.; LeBoff, M.S.; Cauley, J.A.; Robbins, J.A.; Jackson, R.D.; Bauer, D.C. Bone Turnover Markers Are Not Associated With Hip Fracture Risk: A Case-Control Study in the Women’s Health Initiative. J. Bone Miner. Res. 2018, 33, 1199–1208. [Google Scholar] [CrossRef]
- Hegarty, V.M.; May, H.M.; Khaw, K.T. Tea drinking and bone mineral density in older women. Am. J. Clin. Nutr. 2000, 71, 1003–1007. [Google Scholar] [CrossRef]
- Hoover, P.A.; Webber, C.E.; Beaumont, L.F.; Blake, J.M. Postmenopausal bone mineral density: Relationship to calcium intake, calcium absorption, residual estrogen, body composition, and physical activity. Can. J. Physiol. Pharmacol. 1996, 74, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Pettinger, M.B.; Ritenbaugh, C.; LaCroix, A.Z.; Robbins, J.; Caan, B.J.; Barad, D.H.; Hakim, I.A. Habitual tea consumption and risk of osteoporosis: A prospective study in the women’s health initiative observational cohort. Am. J. Epidemiol 2003, 158, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Yang, Y.C.; Yao, W.J.; Lu, F.H.; Wu, J.S.; Chang, C.J. Epidemiological evidence of increased bone mineral density in habitual tea drinkers. Arch. Intern. Med. 2002, 162, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Johnell, O.; Gullberg, B.; Kanis, J.A.; Allander, E.; Elffors, L.; Dequeker, J.; Dilsen, G.; Gennari, C.; Lopes Vaz, A.; Lyritis, G.; et al. Risk factors for hip fracture in European women: The MEDOS Study. Mediterranean Osteoporosis Study. J. Bone Miner. Res. 1995, 10, 1802–1815. [Google Scholar] [CrossRef]
- Kanis, J.; Johnell, O.; Gullberg, B.; Allander, E.; Elffors, L.; Ranstam, J.; Dequeker, J.; Dilsen, G.; Gennari, C.; Vaz, A.L.; et al. Risk factors for hip fracture in men from southern Europe: The MEDOS study. Mediterranean Osteoporosis Study. Osteoporos Int. 1999, 9, 45–54. [Google Scholar] [CrossRef]
- Vestergaard, P.; Hermann, A.P.; Gram, J.; Jensen, L.B.; Eiken, P.; Abrahamsen, B.; Brot, C.; Kolthoff, N.; Sorensen, O.H.; Beck Nielsen, H.; et al. Evaluation of methods for prediction of bone mineral density by clinical and biochemical variables in perimenopausal women. Maturitas 2001, 40, 211–220. [Google Scholar] [CrossRef]
- Hossein-nezhad, A.; Zh, M.; Shafaie, A.R.; Javadi, E.; Larijani, B. Relationship between Tea drinking and Bone Mineral Density in Iranian population. Iran. J. Public Health 2007, 36, 57–62. [Google Scholar]
- Hamdi Kara, I.; Aydin, S.; Gemalmaz, A.; Akturk, Z.; Yaman, H.; Bozdemir, N.; Kurdak, H.; Sitmapinar, K.; Devran Sencar, I.; Basak, O.; et al. Habitual tea drinking and bone mineral density in postmenopausal Turkish women: Investigation of prevalence of postmenopausal osteoporosis in Turkey (IPPOT Study). Int. J. Vitam Nutr. Res. 2007, 77, 389–397. [Google Scholar] [CrossRef]
- Muraki, S.; Yamamoto, S.; Ishibashi, H.; Oka, H.; Yoshimura, N.; Kawaguchi, H.; Nakamura, K. Diet and lifestyle associated with increased bone mineral density: Cross-sectional study of Japanese elderly women at an osteoporosis outpatient clinic. J. Orthop Sci. 2007, 12, 317–320. [Google Scholar] [CrossRef]
- Keramat, A.; Patwardhan, B.; Larijani, B.; Chopra, A.; Mithal, A.; Chakravarty, D.; Adibi, H.; Khosravi, A. The assessment of osteoporosis risk factors in Iranian women compared with Indian women. BMC Musculoskelet Disord 2008, 9, 28. [Google Scholar] [CrossRef]
- Devine, A.; Hodgson, J.M.; Dick, I.M.; Prince, R.L. Tea drinking is associated with benefits on bone density in older women. Am. J. Clin. Nutr. 2007, 86, 1243–1247. [Google Scholar] [CrossRef] [PubMed]
- Myers, G.; Prince, R.L.; Kerr, D.A.; Devine, A.; Woodman, R.J.; Lewis, J.R.; Hodgson, J.M. Tea and flavonoid intake predict osteoporotic fracture risk in elderly Australian women: A prospective study. Am. J. Clin. Nutr. 2015, 102, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Tavani, A.; Negri, E.; La Vecchia, C. Coffee intake and risk of hip fracture in women in northern Italy. Prev. Med. 1995, 24, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Kreiger, N.; Gross, A.; Hunter, G. Dietary factors and fracture in postmenopausal women: A case-control study. Int. J. Epidemiol. 1992, 21, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Birong, D.; Changquan, H.; Hongmei, W.; Yanling, Z.; Wen, Z.; Li, L. Association of osteoporotic fracture with smoking, alcohol consumption, tea consumption and exercise among Chinese nonagenarians/centenarians. J. Nutr. Health Aging 2011, 15, 327–331. [Google Scholar] [CrossRef]
- Zeng, F.F.; Wu, B.H.; Fan, F.; Xie, H.L.; Xue, W.Q.; Zhu, H.L.; Chen, Y.M. Dietary patterns and the risk of hip fractures in elderly Chinese: A matched case-control study. J. Clin. Endocrinol. Metab. 2013, 98, 2347–2355. [Google Scholar] [CrossRef]
- Jha, R.M.; Mithal, A.; Malhotra, N.; Brown, E.M. Pilot case-control investigation of risk factors for hip fractures in the urban Indian population. BMC Musculoskelet Disord 2010, 11, 49. [Google Scholar] [CrossRef]
- Huang, H.; Han, G.Y.; Jing, L.P.; Chen, Z.Y.; Chen, Y.M.; Xiao, S.M. Tea Consumption Is Associated with Increased Bone Strength in Middle-Aged and Elderly Chinese Women. J. Nutr. Health Aging 2018, 22, 216–221. [Google Scholar] [CrossRef]
- Shen, Q.; Yu, C.; Guo, Y.; Bian, Z.; Zhu, N.; Yang, L.; Chen, Y.; Luo, G.; Li, J.; Qin, Y.; et al. Habitual Tea Consumption and Risk of Fracture in 0.5 Million Chinese Adults: A Prospective Cohort Study. Nutrients 2018, 10, 1633. [Google Scholar] [CrossRef]
- Li, X.; Qiao, Y.; Yu, C.; Guo, Y.; Bian, Z.; Yang, L.; Chen, Y.; Yan, S.; Xie, X.; Huang, D.; et al. Tea consumption and bone health in Chinese adults: A population-based study. Osteoporos Int. 2019, 30, 333–341. [Google Scholar] [CrossRef]
- Shen, C.L.; Chyu, M.C.; Yeh, J.K.; Zhang, Y.; Pence, B.C.; Felton, C.K.; Brismée, J.M.; Arjmandi, B.H.; Doctolero, S.; Wang, J.S. Effect of green tea and Tai Chi on bone health in postmenopausal osteopenic women: A 6-month randomized placebo-controlled trial. Osteoporos Int. 2012, 23, 1541–1552. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.L.; Chyu, M.C.; Pence, B.C.; Yeh, J.K.; Zhang, Y.; Felton, C.K.; Doctolero, S.; Wang, J.S. Green tea polyphenols supplementation and Tai Chi exercise for postmenopausal osteopenic women: Safety and quality of life report. BMC Complement. Altern Med. 2010, 10, 76. [Google Scholar] [CrossRef] [PubMed]
- Qian, G.; Xue, K.; Tang, L.; Wang, F.; Song, X.; Chyu, M.C.; Pence, B.C.; Shen, C.L.; Wang, J.S. Mitigation of oxidative damage by green tea polyphenols and Tai Chi exercise in postmenopausal women with osteopenia. PLoS ONE 2012, 7, e48090. [Google Scholar] [CrossRef] [PubMed]
- Samavat, H.; Dostal, A.M.; Wang, R.; Bedell, S.; Emory, T.H.; Ursin, G.; Torkelson, C.J.; Gross, M.D.; Le, C.T.; Yu, M.C.; et al. The Minnesota Green Tea Trial (MGTT), a randomized controlled trial of the efficacy of green tea extract on biomarkers of breast cancer risk: Study rationale, design, methods, and participant characteristics. Cancer Causes Control 2015, 26, 1405–1419. [Google Scholar] [CrossRef] [PubMed]
- Dostal, A.M.; Samavat, H.; Bedell, S.; Torkelson, C.; Wang, R.; Swenson, K.; Le, C.; Wu, A.H.; Ursin, G.; Yuan, J.M.; et al. The safety of green tea extract supplementation in postmenopausal women at risk for breast cancer: Results of the Minnesota Green Tea Trial. Food Chem. Toxicol. 2015, 83, 26–35. [Google Scholar] [CrossRef]
- Dostal, A.M.; Arikawa, A.; Espejo, L.; Kurzer, M.S. Long-Term Supplementation of Green Tea Extract Does Not Modify Adiposity or Bone Mineral Density in a Randomized Trial of Overweight and Obese Postmenopausal Women. J. Nutr. 2016, 146, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Elsalmawy, A.H.; Ish-Shalom, S.; Lim, S.J.; Al-Ali, N.S.; Cunha-Borges, J.L.; Yang, H.; Casas, N.; Altan, L.; Moll, T.; et al. Study description and baseline characteristics of the population enrolled in a multinational, observational study of teriparatide in postmenopausal women with osteoporosis: The Asia and Latin America Fracture Observational Study (ALAFOS). Curr. Med. Res. Opin 2019, 35, 1041–1049. [Google Scholar] [CrossRef]
- Chen, C.H.; Lim, S.J.; Oh, J.K.; Huang, T.W.; Zeng, Y.H.; Wu, M.T.; Yang, H.L.; Cheung, J.P.; Kim, J.W.; Han, J.H.; et al. Teriparatide in East Asian Postmenopausal Women with Osteoporosis in a Real-World Setting: A Baseline Analysis of the Asia and Latin America Fracture Observational Study (ALAFOS). Clin. Interv. Aging 2020, 15, 111–121. [Google Scholar] [CrossRef]
- Wu, C.-H.; Hung, W.-C.; Chang, I.-L.; Tsai, T.-T.; Chang, Y.-F.; McCloskey, E.V.; Watts, N.B.; McClung, M.R.; Huang, C.-F.; Chen, C.-H.; et al. Pharmacologic intervention for prevention of fractures in osteopenic and osteoporotic postmenopausal women: Systemic review and meta-analysis. Bone Rep. 2020, 13, 100729. [Google Scholar] [CrossRef]
- Bliuc, D.; Tran, T.; van Geel, T.; Adachi, J.D.; Berger, C.; van den Bergh, J.; Eisman, J.A.; Geusens, P.; Goltzman, D.; Hanley, D.A.; et al. Reduced Bone Loss Is Associated With Reduced Mortality Risk in Subjects Exposed to Nitrogen Bisphosphonates: A Mediation Analysis. J. Bone Miner. Res. 2019, 34, 2001–2011. [Google Scholar] [CrossRef]
- Borhan, S.; Papaioannou, A.; Gajic-Veljanoski, O.; Kennedy, C.; Ioannidis, G.; Berger, C.; Goltzman, D.; Josse, R.; Kovacs, C.S.; Hanley, D.A.; et al. Incident Fragility Fractures Have a Long-Term Negative Impact on Health-Related Quality of Life of Older People: The Canadian Multicentre Osteoporosis Study. J. Bone Miner. Res. 2019, 34, 838–848. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Huang, P.J.; Huang, H.T.; Lin, S.Y.; Wang, H.Y.; Fang, T.J.; Lin, Y.C.; Ho, C.J.; Lee, T.C.; Lu, Y.M.; et al. Impact of orthogeriatric care, comorbidity, and complication on 1-year mortality in surgical hip fracture patients: An observational study. Medicine 2019, 98, e17912. [Google Scholar] [CrossRef] [PubMed]
- Kjorholt, K.E.; Johnsen, S.P.; Kristensen, N.R.; Prieto-Alhambra, D.; Pedersen, A.B. Increasing Risk of Hospital-Treated Infections and Community-Based Antibiotic Use After Hip Fracture Surgery: A Nationwide Study 2005-2016. J. Bone Miner. Res. 2019, 34, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Diem, S.J.; Vo, T.N.; Langsetmo, L.; Schousboe, J.T.; Yaffe, K.; Ensrud, K.E.; Study of Osteoporotic Fractures Research, G. Impact of Competing Risk of Mortality on Association of Cognitive Impairment With Risk of Hip Fracture in Older Women. J. Bone Miner. Res. 2018, 33, 1595–1602. [Google Scholar] [CrossRef] [PubMed]
- Parsons, N.; Griffin, X.L.; Achten, J.; Chesser, T.J.; Lamb, S.E.; Costa, M.L. Modelling and estimation of health-related quality of life after hip fracture: A re-analysis of data from a prospective cohort study. Bone Jt. Res. 2018, 7, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, J.T.; Michou, L.; Vaillancourt, F.; Pelet, S.; Simonyan, D.; Belzile, E.L. Prevalence and Characteristics of Atypical Periprosthetic Femoral Fractures. J. Bone Miner. Res. 2019, 34, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Compston, J.E.; McClung, M.R.; Leslie, W.D. Osteoporosis. Lancet 2019, 393, 364–376. [Google Scholar] [CrossRef]
- Paschalis, E.P.; Krege, J.H.; Gamsjaeger, S.; Eriksen, E.F.; Burr, D.B.; Disch, D.P.; Stepan, J.J.; Fahrleitner-Pammer, A.; Klaushofer, K.; Marin, F.; et al. Teriparatide Treatment Increases Mineral Content and Volume in Cortical and Trabecular Bone of Iliac Crest: A Comparison of Infrared Imaging With X-Ray-Based Bone Assessment Techniques. J. Bone Miner. Res. 2018, 33, 2230–2235. [Google Scholar] [CrossRef]
| First Author, Year | Experimental Design and Treatments | Results |
|---|---|---|
| Nakagawa, 2002 [52] | Model: primary cultured Crude murine osteoclast-like multinucleated cells (OCLs) Treatments: EGCG (25–100 μM) for 24 h | EGCG treatments: ↑ apoptotic cell death of osteoclast-like multinucleated cells, whereas osteoblasts affected the Fenton reaction primarily involved in EGCG-induced osteoclastic cell death |
| Chen, 2003 [60] | Model: The pluripotent mesenchymal cell, D1, cloned from mouse bone marrow cells Treatments: EC, ECG, EGC, and EGCG at a concentration of 1 μM, the achievable concentration with one cup of tea drinking, 10 μM and 100 μM for 24 and 48 h, respectively. | Compared to vehicle group, EGCG groups: ↑ mRNA expression of OPG |
| Yun, 2004 [53] | Model: co-culture system of mouse bone marrow cells and calvarial primary osteoblastic cells Treatments: EGCG (20 μM) in the presence of sonicated P. gingivalis extracts. | EGCG treatments: ↓expression of MMP-9 mRNA ↓ osteoclast formation |
| Chen, 2005 [64] | Model: The pluripotent mesenchymal cell, D1, cloned from mouse bone marrow cells Treatments: vehicle, EGCG (1 and 10 μM) for 48 h | Compared to vehicle group, EGCG groups: ↑ mRNA expression of Runx2, osterix, osteocalcin, ALP after 48 h ↑ ALP activity after 4 d, 7 d, and 14 d ↑ mineralization after 2–4 w ↓ thymidine incorporation |
| Tokuda, 2007 [55] | Model: osteoblastic cell line MC3T3-E1 Treatments: EGCG in a dose-dependent manner in the range between 1 and 100 μM | EGCG treatments: ↓ ET-1-induced IL-6 synthesis ↔ ET-1-induced phosphorylation of p38 MAP kinase ↓ phosphorylation of p44/p42 MAP kinase and 12-O-tetradecanoylphorbol 13-acetate (TPA), a direct activator of PKC induced by ET-1 |
| Tokuda, 2007 [56] | Model: osteoblastic cell line MC3T3-E1 Treatments: EGCG in a dose-dependent manner in the range between 1 and 100 μM | EGCG treatments: ↑ PGF(2alpha)-induced VEGF synthesis ↔ PGF(2alpha)-induced phosphorylation of p44/p42 MAP kinase ↑ phosphorylation of SAPK/JNK induced by PGF(2alpha) ↔ PGF(2alpha)-induced phosphorylation of p38 MAP kinase ↑ PGF(2alpha)-induced phosphorylation of c-Jun |
| Vali, 2007 [67] | Model: the formation of mineralized bone nodules by SaOS-2 human osteoblast (HOB)-like cells Treatments: EGCG at concentrations of 1–5 μM for 48 h | EGCG treatments: ↑ number and area of mineralized bone nodules ↑ alkaline phosphatase activity ↓ protein levels of Runx2 |
| Hayashi, 2008 [71] | Model: osteoblast-like MC3T3-E1 cells Treatments: the cells were pretreated with various doses of EGCG (0, 10, and 30 μM) for 48 h | EGCG treatments: ↓ HSP27 induction stimulated by TGF-β ↔ HSP70 levels↔ advanced oxidation protein products ↔ TGF-β-induced phosphorylation of p38 MAP kinase and ERK1/2 ↓ phosphorylation of both MKK4 and TAK1 induced by TGF-β ↓ TGF-β-induced phosphorylation of SAPK/JNK without affecting the phosphorylation of Smad2 |
| Morinobu, 2008 [57] | Model: Mononuclear cells were isolated from peripheral blood obtained from healthy donors. Treatments: For osteoclast differentiation, the cells were cultured in the presence of M-CSF (50 ng/mL) and RANKL (100 ng/mL) for 6 d EGCG or other chemicals were added throughout the culture, and half the medium was replaced every 2–3 d | EGCG treatments: ↓ generation of TRAP-positive multinucleated cells, bone resorption activity, and osteoclast-specific gene expression ↓ expression of nuclear factor of activated T cells c1 (NF-ATc1), but not of NF-κB, c-Fos, and c-Jun ↔ cell viability |
| Takai, 2008 [66] | Model: osteoblast-like MC3T3-E1 cells; primary-cultured osteoblasts were obtained from the calvaria of newborn (1 or 2-day old) BALB/c mice Treatments: EGCG (0–30 μM) for 24 h | EGCG treatments: ↓ IL-6 synthesis and IL-6 mRNA expression stimulated by PDGF-BB ↓PDGF-BB-induced phosphorylation of SAPK/JNK ↔ levels of osteocalcin and osteoprotegerin in MC3T3-E1 cells ↔ PDGF-BB-induced autophosphorylation of PDGF receptor β ↔ PDGF-BB-induced phosphorylation of p44/p42 MAP kinase and p38 MAP kinase ↔ PDGF-BB-induced phosphorylation of Akt and p70 S6 kinase |
| Tokuda, 2008 [70] | Model: osteoblast-like MC3T3-E1 cells Treatments: EGCG (0–30 μM) for 24 h | EGCG treatments: ↓ IL-6 synthesis stimulated by FGF-2 in a dose-dependent manner ↓ FGF-2-induced phosphorylation of p44/p42 MAP kinase and p38 MAP kinase |
| Lin, 2009 [59] | Model: murine preosteoclast cells (RAW 264.7); bone marrow macrophages (BMMs) Treatments: RANKL (100 ng/mL), RANKL+EGCG (0, 10, 20, 50, and 100 μM) for 24 h | Compared to RANKL group, EGCG treatment: ↓ differentiation of osteoclasts and the formation of pits ↓ RANKL-induced NF-κB transcriptional activity and nuclear translocation |
| Lee, 2010 [58] | Model: bone marrow macrophages (BMMs) Treatments: Pretreatment with EGCG under RANKL-induced osteoclastogenesis | EGCG treatments: ↓ RANKL-induced the gene expression of c-Fos and nuclear factor of activated T-cells (NFATc1) ↓ RANKL-induced activation of c-Jun N-terminal protein kinase (JNK) pathway, among the three well known mitogen-activated protein kinases ↓ RANKL-induced phosphorylation of the NF-κB p65 subunit at Ser276 and NF-κB transcriptional activity ↔ degradation of IκBα and NF-κB DNA-binding in BMMs |
| Oka, 2012 [54] | Model: rat osteoclast precursors cells and mature osteoclasts Treatments: vehicle, EGCG (10 and 100 μM) for 12 or 24 h | Compared to vehicle group, EGCG groups: ↓ formation and differentiation of osteoclasts via inhibition of MMPs ↓actin ring formation |
| Tominari, 2015 [63] | Model: Primary osteoblastic cells were isolated from newborn mouse calvariae Treatments: cells were treated with LPS (1μg/mL) with and without EGCG (30, 60, or 90 μM), and further cultured for 24 h for measurement of PGE2 | EGCG treatments: ↓LPS-induced expression of RANKL mRNA in osteoblasts at 12–24 h ↓LPS-induces PGE2 production in osteoblasts at 12–24 h |
| Qiu, 2016 [79] | Model: human bone marrow-derived MSCs | EGCG treatments: ↑miR-210 ↑RUNX2, BMP-2, ALP, and PINP |
| Kuroyanagi, 2017 [62] | Model: Cloned osteoblast-like MC3T3-E1 cells Treatments: cells were pretreated with EGCG (0, 10, 30, and 50 μM) for 60 min, and then stimulated by 10 μM of PGE2 | EGCG treatments: ↑PGE2-stimulated mRNA and protein expression of osteoprotegerin 48 h after treatment |
| Lin, 2018 [65] | Model: human bone marrow stem cells Treatments: EGCG with concentrations of 1 μM and 10 μM | Compared to control group, EGCG groups: ↑ mRNA expression of BMP2, Runx2, alkaline phosphatase (ALP), osteonectin, and osteocalcin 48 h after treatment ↑ ALP activity both 7 and 14 days after treatment. ↑ mineralization two weeks after treatment |
| Chen, 2019 [61] | Model: Murine bone marrow stromal ST2 cells; Murine RAW 264.7 cells Treatments: Cells were treated by 1 μM and 10 μM of EGCG | Compared to control group, EGCG groups: ↓ RANKL/OPG ratio in both mRNA expression and secretory protein levels ↓ osteoclastogenesis via the RANK/RANKL/OPG pathway |
| First Author, Year | Experimental Design and Treatments | Results |
|---|---|---|
| Shen, 2008 [86] | Model: a mouse model of OVX-induced bone loss 14-mo-old female rats (n = 10/group) Treatments: A 16-week study of a 2 (SHAM vs. OVX) × 3 (no GTP, 0.1% GTP, and 0.5% GTP in drinking water) factorial design | ↔ femur bone mineral density between baseline and the SHAM+0.5% GTP group. In OVX group: ↓ liver glutathione peroxidase activity, serum estradiol, and bone mineral density group. GTP supplementation: ↑ urinary epigallocatechin and epicatechin concentrations, liver glutathione peroxidase activity, and femur bone mineral density decreased urinary 8-hydroxy-2’-deoxyguanosine and urinary calcium levels. ↔ serum estradiol and blood chemistry levels. |
| Iwaniec, 2009 [108] | Model: a mouse model of obese Male leptin-deficient (ob/ob) obese mice and male C57Bl/6 WT littermates (4-week-old) Treatments: Each group had equal numbers of obese and lean mice. Groups included 0% GTE (control), 1% GTE, and 2% GTE for 6 w | Compared to control group, GTE groups: Neither genotype affected femoral bone mineral density ↓ femur length, volume, mineral content, cortical volume, cortical thickness, cancellous bone volume/tissue volume, and trabecular thickness in lumbar vertebrae in GTE groups. |
| Shen, 2009 [87] | Model: a mouse model of OVX-induced bone loss 14-month-old female rats Treatments: A 16-w study of 2 (SHAM vs. OVX) × 3 (no GTP, 0.1% GTP, and 0.5% GTP in drinking water) factorial design | GTP supplementation: ↑ trabecular volume, thickness, number, and bone formation of proximal tibia, periosteal bone formation rate of tibia shaft, and cortical thickness and area of femur ↓ trabecular separation and bone erosion of proximal tibia, and endocortical bone eroded surface of tibia shaft |
| Shen, 2010 [98] | Model: a mouse model of LPS-induced chronic inflammation and bone loss Virgin CD female rats (3 months old) Treatments: placebo implantation (P), lipopolysaccharide (LPS) administration (L), P+0.5% GTP (PG) and LPS+0.5% GTP (LG) for 12 w 2 (placebo vs. LPS administration) × 2 (no GTP vs. 0.5% GTP in drinking water) factorial design | Neither LPS administration nor GTP levels affected body weight and femoral bone area throughout the study period. LPS administration: ↓ femur BMC and BMD, and serum OC levels ↑ serum TRAP, urinary 8-OHdG, and spleen mRNA expression of TNF-α and COX-2 levels GTP supplementation: ↑values for femur BMC, BMD, and serum OC ↓values for serum TRAP, urinary 8-OHdG, and spleen mRNA expression of TNF-α and COX-2 levels |
| Shen, 2010 [100] | Model: a mouse model of LPS-induced chronic inflammation and bone loss Virgin 3-month-old CD female rats Treatments: Among the LPS treated rats, a 2 (no GTP vs. 0.5% GTP in drinking water) × 2 (0 vs. 0.05 μg/kg body weight 1-α-OH-vitamin D3) factorial design enabled evaluation of effects of GTP, 1-α-OH-vitamin D3, and GTP × 1-α-OH-vitamin D3 interaction on chronic inflammation-induced bone loss along with related mechanism(s). In addition, a group receiving placebo administration only (the P group) was used. | LPS administration: ↓ Values for bone mass ↑ Values for serum tartrate-resistant acid phosphatase (TRAP), urinary 8-hydroxy-2′-deoxyguanosine, and mRNA expression of tumor necrosis factor-α and cyclooxygenase-2 in spleen GTP supplementation: ↑ urinary epigallocatechin and epicatechin concentrations A synergistic effect of GTP and alphacalcidol was observed in these parameters Neither GTP nor alphacalcidol affected femoral bone area or serum osteocalcin. |
| Shen, 2011 [99] | Model: a mouse model of LPS-induced chronic inflammation and bone loss 3-month-old virgin Sprague Dawley (SD) female rats Treatments: placebo implantation (P), lipopolysaccharide (LPS) administration (L), P+0.5% GTP (PG), or LPS+0.5% GTP (LG) for 12 w 2 (placebo vs. LPS administration) × 2 (no GTP vs. 0.5% GTP in drinking water) factorial design | LPS group: ↓ trabecular volume fraction, thickness, and bone formation in proximal tibia ↑osteoclast number and surface perimeter in proximal tibia and eroded surface in endocortical tibial shafts GTP group: ↑ trabecular volume fraction and number in both femur and tibia and periosteal bone formation rate in tibial shafts ↓trabecular separation in proximal tibia and eroded surface in endocortical tibial shafts ↑ strength of femur ↓ TNF-α expression in tibia |
| Shen, 2011 [109] | Model: a mouse model of LPS-induced chronic inflammation Virgin CD female rats (3 months old) Treatments: (1) LPS administration (L, n = 10), (2) LPS + 1-α-OH-vitamin D3 (LD, n = 10), (3) LPS + GTP (LG, n = 10), and (4) LPS + GTP + 1-α-OH-vitamin D3 (LGD, n = 10) for 12 w | Compared to LPS group, Both GTP and alfacalcidol: ↑ femoral mass, trabecular volume, thickness, and number in proximal tibia and femur, and periosteal bone formation rate in tibial shafts ↓ trabecular separation and osteoclast number in proximal tibia and eroded surface in endocortical tibial shafts ↑ femoral strength ↓ TNF-α expression in proximal tibia |
| Shen, 2011 [88] | Model: a mouse model of orchidectomy-induced male osteoporosis Virgin male F344 rats (15 months old) Treatments: A 2 (sham vs. orchidectomy) × 2 (no GTP and 0.5% GTP in drinking water) factorial design was studied for 16 w | Compared to orchidectomy group, GTP supplementation: ↑ serum osteocalcin concentrations, bone mineral density, and trabecular volume, number, and strength of femur ↑ trabecular volume and thickness and bone formation in both the proximal tibia and periosteal tibial shaft ↓ eroded surface in the proximal tibia and endocortical tibial shaft ↓ liver glutathione peroxidase activity |
| Oka, 2012 [54] | Model: cultures of rat osteoclast precursors cells and mature osteoclasts Treatments: the black tea polyphenol, theaflavin-3,3’-digallate (TFDG), or EGCG (10 and 100 µM) was added | Compared to control group, TFDG or EGCG treatment: ↓ numbers of multinucleated osteoclasts and actin rings ↓ MMP-2 and MMP-9 activities ↓ MMP-9 mRNA levels |
| Shen, 2012 [101] | Model: a mouse model of high-fat (HF) diet-induced obese female 3-month-old Sprague Dawley female rats Treatments: After low-fat (LF) (10% energy as fat) (n = 12) or HF diet (45% energy as fat) (n = 24) ad libitum for 4 m, whereas those in the HF diet group were randomly divided into two groups: with (the HF + GTP group, n = 12) or without GTP (HF group, n = 12) in drinking water, in addition to an HF diet for another 4 m | Compared to HF group, GTP supplementation: ↑ percentage of fat-free mass, bone mineral density and strength, and GPX protein expression ↓ percentage of fat mass, serum insulin–like growth factor I, leptin, adiponectin, and proinflammatory cytokines in the obese rats |
| Chen, 2013 [94] | Model: a mouse model of OVX-induced bone loss Twelve-week-old female Sprague-Dawley rats Treatments: (1) sham-operated controls (SHAM; n = 8); (2) ovariectomized controls (OVX; n = 14); (3) OVX with EGCG 0.34 mg/kg/day (OVX + 1 EGCG; n = 12; estimated peak serum concentration, 1 μM); and (4) OVX with EGCG 3.4 mg/kg/day (OVX + 10 EGCG; n = 14; estimated peak serum concentration, 10 μM). Three months after OVX, EGCG was given intraperitoneally for 12 w | Compared to untreated control group, EGCG treatment: ↑ bone volume, trabecular thickness, trabecular numbness, and trabecular separation. Similar ↑ in bone volume and trabecular thickness in third lumbar spine. ↑ bone volume in tibial cortex ↑ trabecular number and trabecular volume in histology |
| Shen, 2013 [102] | Model: a mouse model of high-fat diet (HFD)-induced obese female 3-month-old virgin Sprague-Dawley female rats Treatments: After 4 m of HF diet, they were randomly divided into two groups, with GTP supplement in drinking water (the HFD + GTP group, n = 12) or without GTP (the HFD group, n = 12), in addition to the same HFD for another 4 m | Compared to HF group, GTP supplementation: ↑ BMD at the femur, a greater trabecular volume, thickness, and number at the proximal tibia, a larger cortical area and thickness at the tibial shaft, and a greater trabecular volume and thickness at the femur and the lumbar vertebrae ↓Tb.Sp, MAR, bone formation rate, and eroded surface at the tibia |
| Yoshinaga, 2014 [104] | Model: a mouse model of LPS-induced PE 9-week-old male Lewis rats Treatments: LPS group (n = 12); the green tea extract group (n = 12); and the phosphate buffered saline (PBS) group (n = 6) for 20 d | Compared to LPS group, GTE group: ↓loss of attachment, level of alveolar bone, inflammatory cell infiltration and RANKL expression |
| Cai, 2015 [105] | Model: a mouse PE model induced by P. gingivalis infection Female BALB/c mice (8-w-old) Treatments: EGCG group (0.02%) or drinking water group both infected with P. gingivalis every 2 days for 15 w | Compared to water group, EGCG group: ↓ reduction in bone loss ↓ inflammatory serum mediators ↓ high positive areas of IL-17 and IL-1β ↓ IL-1β, IL-6, IL-17, TNF-α, and other mediators, but not IL-23 |
| Gennaro, 2015 [106] | Model: a mouse models of type 1 diabetes Male Wistar rats (8–10-w-old) Treatments: Groups included the Diab group (type 1 diabetes) and Ctr (control group). Each group was further divided into two control groups (water and green tea-treated) and two diabetic groups (water and green-tea treated) measured at 15, 30, 60, and 90 d | ↓ number of cells expressing RANKL and TNF-α in diabetic rats treated with green tea. ↑ cells positive for OPG, RUNX-2, and IL-10 in diabetic rats |
| Shen, 2015 [89] | Model: obese rats fed a high-fat diet (HFD) or a caloric restricted diet (CRD) Sprague Dawley female rats (3-mo-old) Treatments: rats were fed with an HFD diet ad libitum for 4 m Then, based on body weight, the animals were assigned to one of the four groups ((HFD vs CRD with 35% caloric deficit) × (0% vs 0.5% GTP in drinking water)) in a two-factorial study for another 4 m | In CRD: ↓ percent fat mass; bone mass and trabecular number of tibia, femur, and lumbar vertebrae; femoral strength; trabecular and cortical thickness of tibia; insulin-like growth factor-I and leptin ↑ percent fat-free mass; trabecular separation of tibia and femur; eroded surface of tibia; bone formation rate and erosion rate at tibia shaft; and adiponectin GTP supplementation: ↑ femoral mass and strength (p = 0.026), trabecular thickness (p = 0.012) and number (p = 0.019), and cortical thickness of tibia (p < 0.001) ↓ trabecular separation (p = 0.021), formation rate (p < 0.001), eroded surface (p < 0.001) at proximal tibia, and insulin-like growth factor-I and leptin. There were significant interactions (diet type × GTP) on osteoblast surface/bone surface, mineral apposition rate at periosteal and endocortical bones, periosteal bone formation rate, and trabecular thickness at femur and lumbar vertebrate (p < 0.05). |
| Tominari, 2015 [63] | Model: LPS-induced alveolar bone loss in mice Treatments: injected LPS with or without EGCG (0.5 mg/mouse) into the gingiva of the lower mandibles of mice. After seven days of the first injection, alveolar bone was collected from mouse | EGCG treatment ↓ LPS-induced bone resorption and alveolar bone loss in mice |
| Liu, 2018 [97] | Model: a mouse model of steroid-induced bone loss Female experimental SD rats (8-week-old) Treatments: control group, dexamethasone (DEX) groups, and DEX with EGCG (5 mg/kg/day) | ↑Osteoblast cell viability, ALP, and SOD activities ↑11β-HSD activity ↑Nrf2/HO-1 Signaling ↓DEX-Induced Oxidative Stress, DEX-Induced apoptosis of Osteoblasts ↑Osteogenic differentiation in primary osteoblasts ↑Improve microarchitecture |
| Xi, 2018 [91] | Model: a mouse model of dexamethasone-induced osteoporosis Male C57BLKS/J mice (6-week-old) Treatments: Groups included control, model, and EGCG (0.5 mg/kg/day) for 4 weeks. Model and EGCG groups were injected with dexamethasone (5 mg/kg/day) to establish the osteoporosis model. | ↓ serum calcium, urinary calcium, body weight, and body fat ↑ leptin in mice with secondary osteoporosis Inhibited structure score of articular cartilage and cancellous bone in proximal tibia metaphysis in mice with secondary osteoporosis. ↓ alkaline phosphatase activity, runt related transcription factor 2, and osterix mRNA expression. ↑ protein expression of cyclin D1, Wnt, and β catenin. ↓ peroxisome proliferator activated receptor γ protein expression in mice with secondary osteoporosis. |
| de Almeida, 2019 [46] | Model: a mouse model of ligature-induced experimental periodontitis (EP) Male Wistar rats (3-month-old) Treatments: control (no EP), EP (ligature induction), SRP (SRP given after 7 d of EP), and SRP/GT (SRP and GTE given after 7 d of EP), measured at 14, 22, and 37 d. | Compared to control group, SRP/GT group: ↓ inflammatory process ↓ immunolabeling pattern of IL-1ß and TNF-α ↑ immunolabeling pattern of IL-10 ↓ TRAP-positive multinucleated osteoclasts ↑ PBF |
| Lin, 2019 [96] | Model: A defect on left distal femur was created by using a 0.5 mm cone-shape hand drill Male Sprague-Dawley (SD) rats aged 12 w Treatments: vehicle and EGCG were applied locally by percutaneous local injection 2 d after defect creation for 2 w | Compared to control group, EGCG groups: ↑ de novo bone formation by increasing bone volume ↑ mechanical properties including max load, break point, stiffness, area under the max load curve, area under the break point curve, and ultimate stress |
| Lin, 2020 [95] | Model: a mouse model of tibia fracture Male Sprague–Dawley (SD) rats at 12 weeks Treatments: vehicle treatment as control group (Ctrl) (n = 28) and fracture with treatment of EGCG (EGCG) (n = 28). EGCG, 40 μL at 10 μM, with a total dose of 0.52 μg/kg/time treated daily with EGCG or vehicle by percutaneous local injection for 2 w | Compared to control group, EGCG groups: ↑ callus formation by increasing the bone volume ↑ mechanical properties of the tibial bone, including the maximal load, break load, stiffness, and Young’s modulus ↑ bone matrix formation and stronger expression of BMP-2 |
| First Author, Year | Experimental Design and Treatments | Results |
|---|---|---|
| Kreiger, 1992 [135] | Model: a case-control study examined the effect of diet on the risk of postmenopausal fracture of the hip and wrist. Treatments: Cases, women aged 50–84 y, were admitted to one of four Metropolitan Toronto hospitals during the period September 1983 through May 1985. Controls were women of the same age, admitted to the same hospitals, and seen for orthopedic or general surgical complaints. | Coffee and tea consumption appeared to be unrelated to fracture risk. |
| Johnell, 1995 [125] | Model: a multicenter study to determine common international risk factors for hip fracture in women aged 50 y or more. Treatments: Women aged 50 y or more selected from the neighborhood or population registers served as controls. Cases and controls were interviewed using a structured questionnaire on work, physical activity, exposure to sunlight, reproductive, gynecologic status and history, height, weight, mental score, and consumption of tobacco, alcohol, calcium, coffee, and tea. | Significant risk factors identified by univariate analysis included low body mass index (BMI), short fertile period, low physical activity, lack of sunlight exposure, low milk consumption, no consumption of tea, and a poor mental score. A late menarche, poor mental score, low BMI and physical activity, low exposure to sunlight, and a low consumption of calcium and tea remained independent risk factors after multivariate analysis, accounting for 70% of hip fractures. |
| Tavani, 1995 [134] | Model: 279 cases of hip fracture and 1061 controls in hospital for acute, nonneoplastic nontraumatic, and non-hormone-related diseases Treatments: consumption of coffee and other methylxanthine-containing beverages | ↔ hip fractures |
| Hoover, 1996 [122] | Model: Physical and lifestyle data were collected from 62 postmenopausal women who had declined hormone replacement therapy. Treatments: Tea drinking was assessed by self-completed questionnaire and women were categorized as tea drinkers or non-tea drinkers. | Compared to non-tea drinkers, tea drinkers: ↑ associated with both bone density ↑ femoral BMD, lumbar BMD, and lean body mass |
| Kanis, 1999 [126] | Model: a multicenter study to identify risk factors for hip fracture in men aged 50 y or more. Treatments: 730 men with hip fracture from 14 centers, and 1132 age-stratified controls selected from the neighborhood or population registers. questionnaire examined aspects of work, physical activity past and present, diseases and drugs, height, weight, indices of co-morbidity, and consumption of tobacco, alcohol, calcium, coffee, and tea. | Of the potentially ‘reversible’ risk factors, BMI, leisure exercise, exposure to sunlight, and consumption of tea and alcohol and tobacco remained independent risk factors after multivariate analysis, accounting for 54% of hip fractures |
| Hegarty, 2000 [121] | Model: measured BMD at the lumbar spine, femoral neck, greater trochanter, and Ward’s triangle in 1256 free-living women aged 65–76 y in Cambridge, United Kingdom. Treatments: Tea drinking was assessed by self-completed questionnaire and women were categorized as tea drinkers or non-tea drinkers | Compared to non-tea drinkers, tea drinkers: ↑ mean BMD measurements, adjusted for age and body mass index, at the lumbar spine (0.033 g/cm2; p = 0.03), greater trochanter (0.028 g/cm2; p = 0.004), and Ward’s triangle (0.025 g/cm2; p = 0.02). ↔ Differences at the femoral neck (0.013 g/cm2) |
| Vestergaard, 2001 [127] | Model: to predict spinal and femoral bone mineral density (BMD) in perimenopausal women from simple clinical and biochemical variables. Treatments: 2016 women 3–24 months after last menstrual bleeding. Mean age 50.1 ± 2.8 years. Independent factors: age, height, weight, number of full-term pregnancies, weekly hours of physical activity, sunbathing habits, use of sun bed, daily intake of calcium and vitamin D, smoking habits, and consumption of alcohol, coffee, and tea. | Conclusions: Simple clinical and biochemical variables are not useful to predict spinal and femoral BMD in the individual perimenopausal woman |
| Wu, 2002 [124] | Model: an epidemiological survey study Treatments: 497 men and 540 women, 30 y and older. All subjects were questioned about their habit of tea consumption and other lifestyle characteristics by means of a structured questionnaire. | Compared to non-tea drinkers, tea drinkers: ↑ lumbar spine BMDs the duration of tea consumption was the only independent determinant for the BMDs |
| Chen, 2003 [123] | Model: investigating associations of habitual drinking of regular tea with bone mineral density and fracture risk. Treatments: Study participants were a multiethnic postmenopausal cohort (n = 91,465) from the nationwide Women’s Health Initiative Observational Study. These women were recruited in the United States and aged 50-79 years at the time of enrollment (1994–1998). The average follow-up time was 4.1 y. Habitual consumption of regular tea was assessed with a structured questionnaire at baseline. | Multivariate analyses suggested a positive trend of increased total body bone mineral density with tea drinking (p < 0.05). Cox proportional hazard models did not show any significant association between tea drinking and the risk of fractures at the hip and forearm/wrist. |
| Devine, 2007 [132] | Model: Using both cross-sectional and longitudinal study designs, we examined the relation of tea consumption with hip structure. Treatments: Randomly selected women (n = 1500) aged 70–85 y participated in a 5-y prospective trial to evaluate whether oral calcium supplements prevent osteoporotic fractures. | In the cross-sectional analysis, tea drinkers: ↑ total hip areal bone mineral density (aBMD) In the prospective analysis over 4 y, tea drinkers: ↓ loss of total hip aBMD |
| Hossein-Nezhad, 2007 [128] | Model: BMD was measured at the lumbar spine and hip, in 830 men and women living in Tehran, all aged between 20 and 76 y. Treatments: The degree of tea consumption was assessed by questionnaire, and subjects were categorized as either tea drinkers (more than five cups of tea per day) or non-tea drinkers (equal to or less than five cups of tea per day) | Compared to non-tea drinkers, tea drinkers: ↑ BMD in the hip of female tea drinkers |
| Hamdi Kara, 2007 [129] | Model: investigation of prevalence of postmenopausal osteoporosis in Turkey (IPPOT Study) Treatments: 742 women were included in the study. The mean age was 57.6 ± 9.6 y, and mean age at natural menopause was 46.4 ± 5.6 y. A semi-structured questionnaire was completed by face-to-face interview, consisting of closed- and open-ended questions about demographic characteristics, nutritional status, and habits with two or more choices as possible responses. | Compared to non-tea drinkers, tea drinkers: ↑ T-scores, and BMD |
| Muraki, 2007 [130] | Model: to identify lifestyle factors associated with BMD. Treatments: A total of 632 women age > or = 60 y enrolled in this study. Subjects were interviewed about their lifestyle by means of a questionnaire regarding the consumption pattern of dietary items | Compared to smoking and cheese consumption, green tea drinking: ↑ BMD |
| Keramat, 2008 [131] | Model: a multicenter interview-based study conducted in selected hospitals and health centers from urban areas in Iran and India. Sample sizes included a total of 363 subjects from Iran (178 osteoporotic and 185 normal) and a total of 354 subjects from India (203 osteoporotic and 151 normal). Treatments: The case group included postmenopausal osteoporotic women, and the controls were chosen from postmenopausal women with normal bone density | Compared to non-tea drinkers, tea drinkers: ↑ significant protective factors in Iran. |
| Jha, 2010 [138] | Model: a case control investigation comprising 100 case subjects (57 women and 43 men) admitted with a first hip fracture into one of three hospitals across New Delhi. Treatments: The 100 controls were age- and sex-matched subjects who were either healthy visitors not related to the case patients or hospital staff. Information from all subjects was obtained through a questionnaire-based interview. | Tea and other caffeinated beverages were significant risk factors. Tea drinkers: ↑ risk of hip fracture (OR 22.8; 95% CI 3.73-139.43) |
| Du, 2011 [136] | Model: a cross-sectional study conducted in Dujiangyan Sichuan China. Treatments: 703 unrelated Chinese nonagenarians and centenarians (67.76% women, mean age 93.48 y) resident in Dujiangyan. Medical history of osteoporosis and the statement of fracture and habits (current and former) of smoking, alcohol consumption, tea consumption, and exercise were collected. | In summary, among nonagenarians and centenarians, among habits (current and former) of smoking, alcohol consumption, tea consumption, and exercise, there seems to be significant association of osteoporotic fracture only with current or former habits of alcohol consumption, former habit of exercise. |
| Shen, 2010 [143] Shen, 2012 [142] Qian, 2012 [144] | Model: a 6-month randomized placebo-controlled trial. Postmenopausal women with osteopenia received green tea polyphenols (GTP) supplement and/or Tai Chi (TC) exercise for 6 months. Treatments: A total of 171 postmenopausal osteopenic women were randomly assigned to four groups: (1) placebo (500 mg starch/day), (2) GTP (500 mg GTP/day), (3) placebo + TC (placebo plus TC training at 60 min/session, three sessions/week), and (4) GTP + TC (GTP plus TC training). | Compared to control group, GTP intake, and TC groups: ↑ bone-specific alkaline phosphatase (BAP) level ↑ BAP/TRAP ratio ↑ muscle strength ↔ serum TRAP, serum and urinary calcium, and inorganic phosphate Compared to control placebo group, GTP groups: ↓ urinary 8-OHdG concentrations ↓ oxidative damage biomarker |
| Zeng, 2013 [137] | Model: Face-to-face interviews to examined the association of dietary patterns with the risk of hip fractures in elderly Chinese. Treatments: A total of 581 pairs of hip fracture incident cases and controls (71 ± 7 y) were studied. | No significant association was found between the traditional dietary pattern (with a high intake of Chinese herbal tea, double stewed soup, processed meat and fish, and organ meat) and hip fracture risk. |
| Myers, 2015 [133] | Model: A total of 1188 women were assessed for habitual dietary intake with a food-frequency and beverage questionnaire. Treatments: Incidence of osteoporotic fracture requiring hospitalization was determined through the Western Australian Hospital Morbidity Data system | In comparison with the lowest tea intake category (≤1 cup/wk), consumption of ≥3 cups/d: ↓ 30% in the risk of any osteoporotic fracture compared with women in the lowest tertile of total flavonoid intake (from tea and diet): ↓risk of any osteoporotic fracture (HR: 0.65; 95% CI: 0.47, 0.88), major osteoporotic fracture (HR: 0.66; 95% CI: 0.45, 0.95), and hip fracture (HR: 0.58; 95% CI: 0.36, 0.95). |
| Dostal, 2016 [147] | Model: a randomized, double-blind, placebo-controlled clinical trial. This substudy was conducted in 121 overweight/obese participants [body mass index (BMI) (kg/m2) ≥ 25.0]. Treatments: placebo-controlled clinical trial in 937 postmenopausal women (aged 50–70 y) assigned to receive either GTE containing 843 mg (−)-epigallocatechin-3-gallate or placebo | Compared to control group, GTE groups: ↔ BMI, total fat mass), percentage of body fat, or BMD ↔ circulating leptin, ghrelin, adiponectin, or insulin concentrations ↓ tissue %fat during the intervention as baseline BMI increased |
| Huang, 2018 [139] | Model: Cross-sectional study Treatments: A total of 1495 Chinese women aged more than 40 years were included. Tea consumption, socio-demographic information, and lifestyle habits were collected by a face-to-face questionnaire. | Compared to non-consumers, tea consumption group: ↑ approximately 1.9% higher BMD ↓ 3.6% lower BR |
| Shen, 2018 [140] | Model: A Prospective Cohort Study Treatments: 453,625 participants from the China Kadoorie Biobank (CKB). Tea consumption was self-reported at baseline. Hospitalized fractures were ascertained through linkage with local health insurance claim databases. | Compared to non-consumers, tea consumption group: ↓ risk of any fracture in daily tea consumption ↓ risk of hip fracture in those who had drunk tea for more than 30 years |
| Li, 2019 [141] | Model: a population-based study Treatments: 20,643 participants from the China Kadoorie Biobank (CKB), who have finished both baseline survey (2004–2008) and a re-survey (2013–2014). They were aged 38–86 y at re-survey. Tea consumption was self-reported at both baseline and re-survey. | Compared to non-consumers, prolonged weekly tea consumers: ↑ calcaneus BMD ↔ BMD measures with the amount of tea leaves added ↔ Tea consumption was not associated with calcaneus BMD measures in men |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.-T.; Cheng, T.-L.; Lin, S.-Y.; Ho, C.-J.; Chyu, J.Y.; Yang, R.-S.; Chen, C.-H.; Shen, C.-L. Osteoprotective Roles of Green Tea Catechins. Antioxidants 2020, 9, 1136. https://doi.org/10.3390/antiox9111136
Huang H-T, Cheng T-L, Lin S-Y, Ho C-J, Chyu JY, Yang R-S, Chen C-H, Shen C-L. Osteoprotective Roles of Green Tea Catechins. Antioxidants. 2020; 9(11):1136. https://doi.org/10.3390/antiox9111136
Chicago/Turabian StyleHuang, Hsuan-Ti, Tsung-Lin Cheng, Sung-Yen Lin, Cheng-Jung Ho, Joanna Y. Chyu, Rong-Sen Yang, Chung-Hwan Chen, and Chwan-Li Shen. 2020. "Osteoprotective Roles of Green Tea Catechins" Antioxidants 9, no. 11: 1136. https://doi.org/10.3390/antiox9111136
APA StyleHuang, H.-T., Cheng, T.-L., Lin, S.-Y., Ho, C.-J., Chyu, J. Y., Yang, R.-S., Chen, C.-H., & Shen, C.-L. (2020). Osteoprotective Roles of Green Tea Catechins. Antioxidants, 9(11), 1136. https://doi.org/10.3390/antiox9111136







