Antioxidant and Cytoprotective Effect of Quinoa (Chenopodium quinoa Willd.) with Pressurized Hot Water Extraction (PHWE)
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Quinoa Seeds Materials
2.2. XRF Determination of Quinoa Seeds
2.3. PHWE System
2.4. LC/UV/MS Profiling of Quinoa Seed
2.5. Determination of DPPH Radical Scavenging Activities
2.6. ABTS Antioxidant Capacity Assay
2.7. Cell-Culture and Cytoprotective Effects
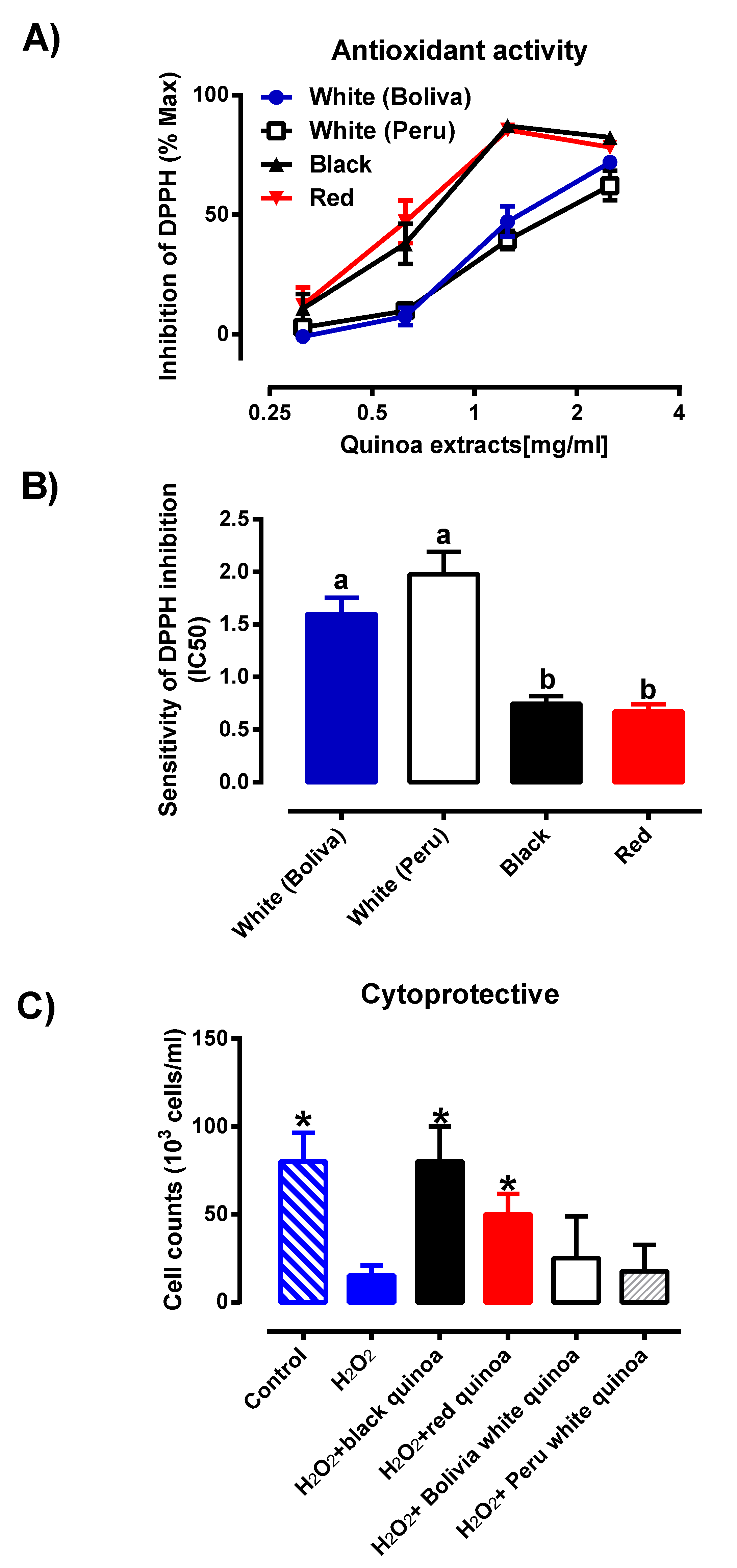
3. Results and Discussion
3.1. Characterization of Minerals Present in Quinoa Seeds by XRF
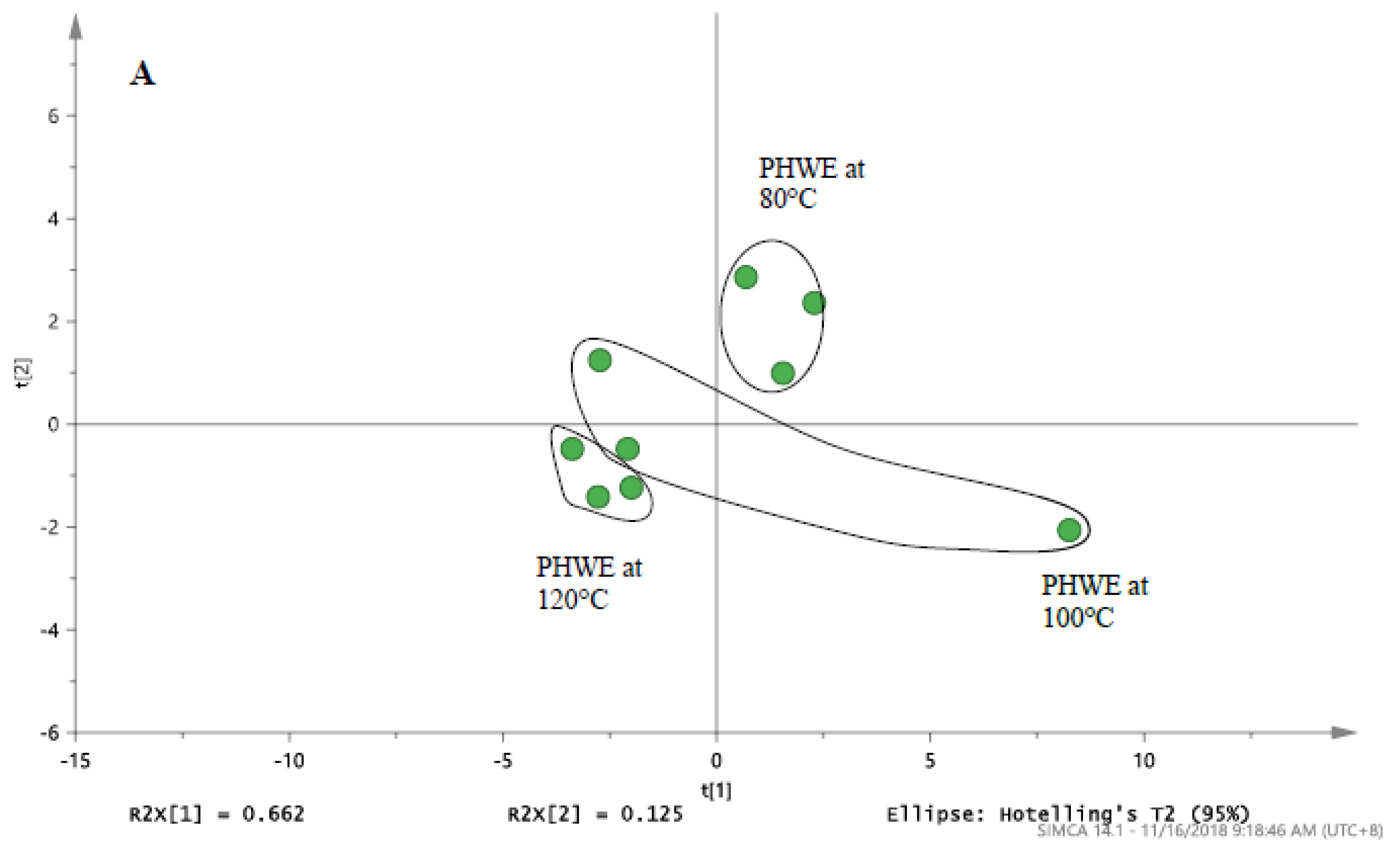
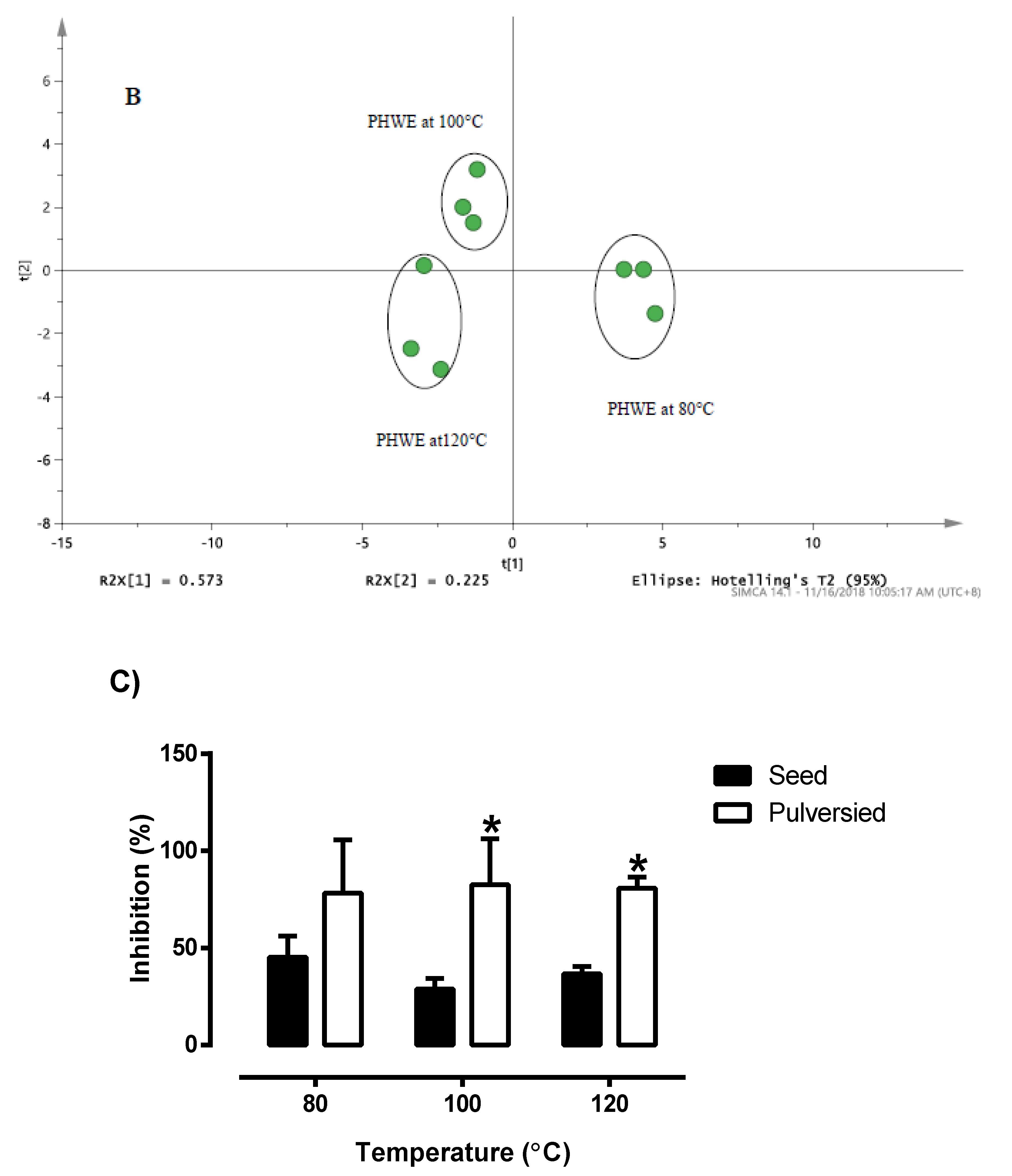
3.2. Optimization of PHWE for Pulverized and Non-Pulverized Seeds
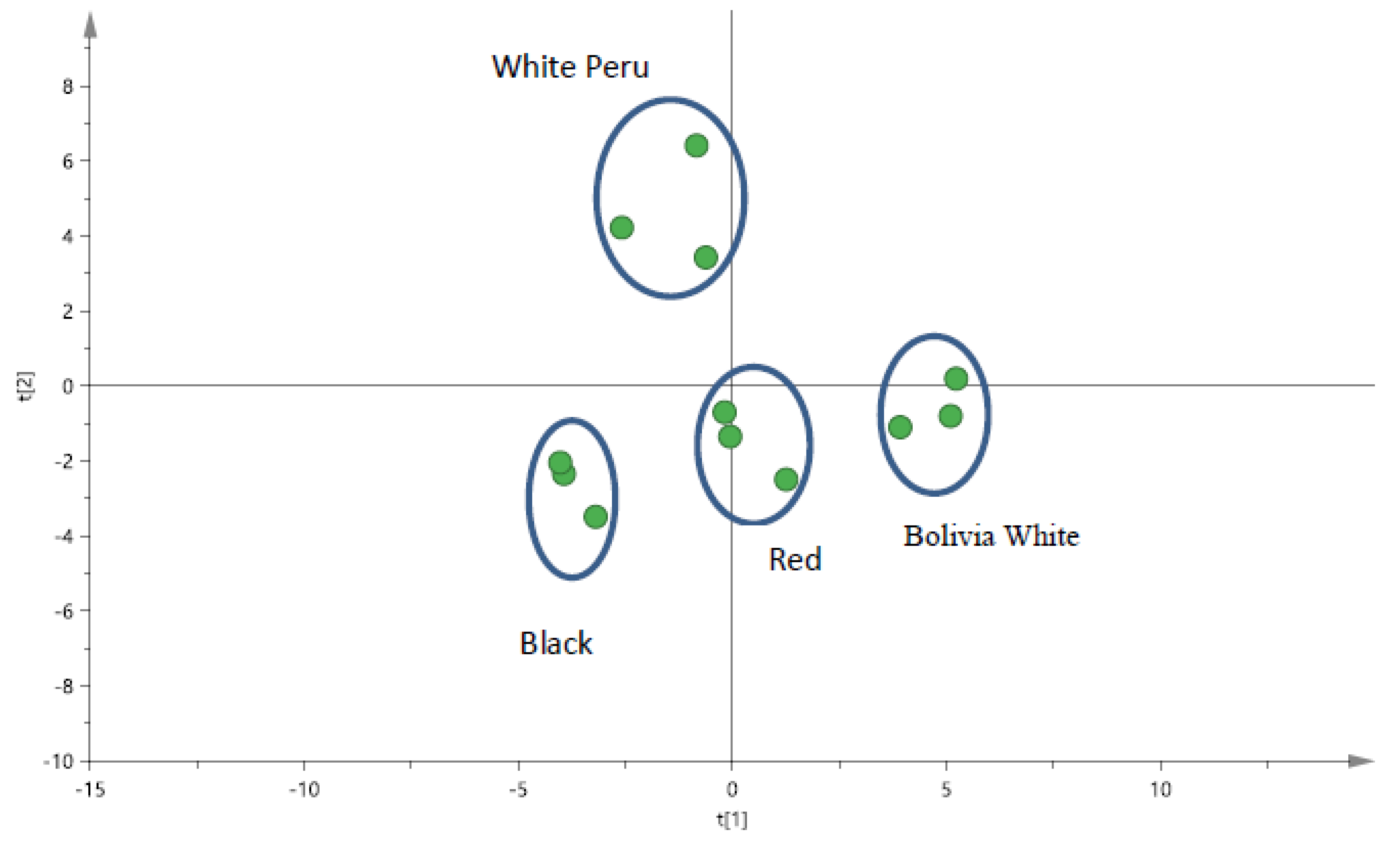
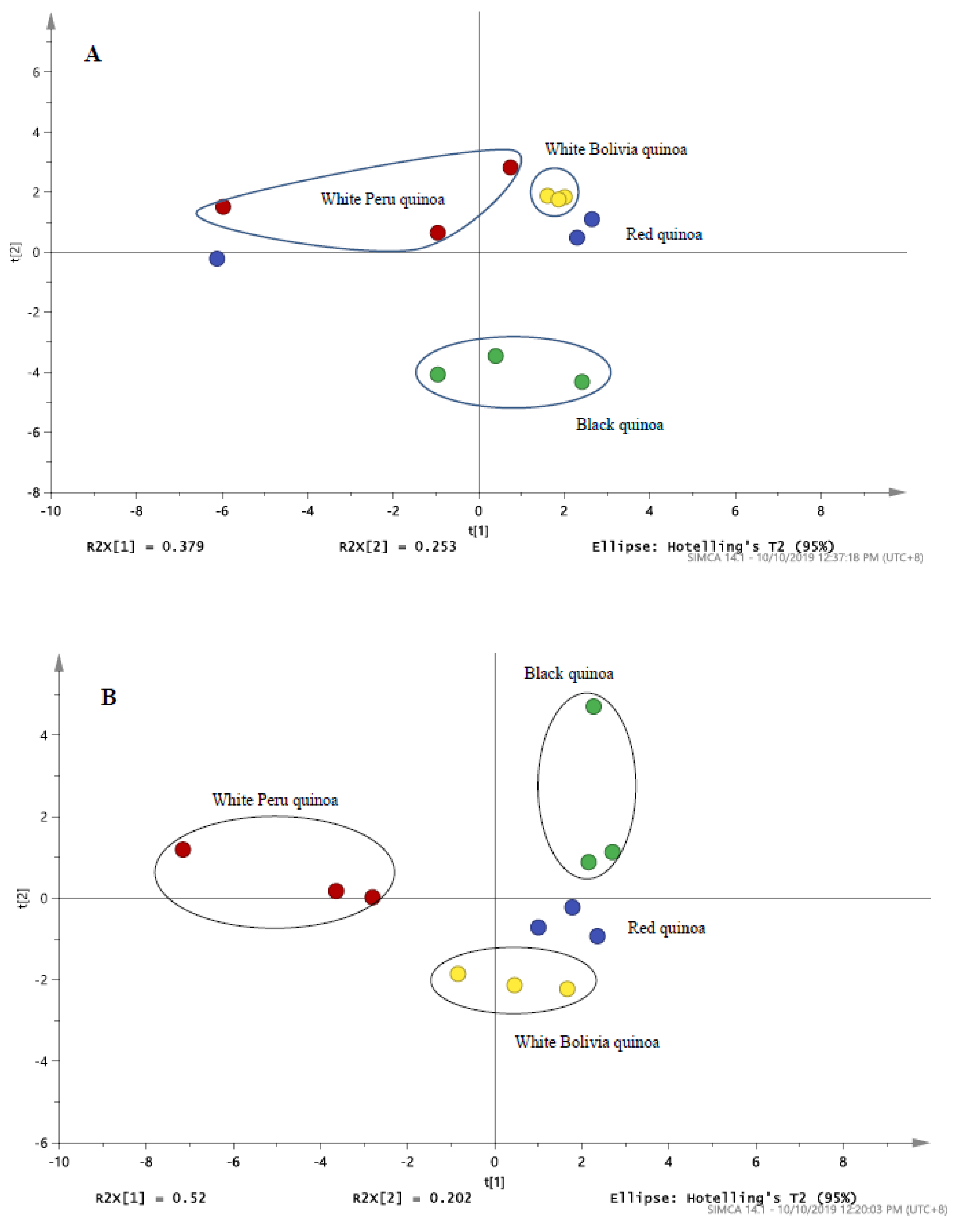
3.3. Analysis of Black, Red and White Quinoa by LC/UV/MS
3.4. Cytoprotective Effects of Plant Extracts against HMEC-1 Cell Line
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Filho, A.M.; Pirozi, M.R.; Borges, J.T.; Pinheiro, S.A.H.M.; Chaves, J.B.; Coimbra, J.S. Quinoa: Nutritional, functional, and antinutritional aspects. Crit. Rev. Food Sci. Nutr. 2017, 57, 1618–1630. [Google Scholar] [CrossRef] [PubMed]
- Nowak, V.; Du, J.; Charrondière, U.R. Assessment of the nutritional composition of quinoa (Chenopodium quinoa Willd.). Food Chem. 2016, 193, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, X.; Chen, P.X.; Zhang, B.; Hernandez, M.; Zhang, H.; Marcone, M.F.; Liu, R.; Tsao, R. Characterisation of fatty acid, carotenoid, tocopherol/tocotrienol compositions and antioxidant activities in seeds of three Chenopodium quinoa Willd. genotypes. Food Chem. 2015, 174, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, X.; Zhang, B.; Chen, P.X.; Liu, R.; Tsao, R. Characterisation of phenolics, betanins and antioxidant activities in seeds of three Chenopodium quinoa Willd. genotypes. Food Chem. 2015, 166, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Tsao, R. Phytochemicals in quinoa and amaranth grains and their antioxidant, anti-inflammatory, and potential health beneficial effects: A review. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Leo, C.H.; Hart, J.L.; Woodman, O.L. Impairment of both nitric oxide-mediated and EDHF-type relaxation in small mesenteric arteries from rats with streptozotocin-induced diabetes. Br. J. Pharm. 2011, 162, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.H.; Leo, C.H.; O’Sullivan, K.; Alexander, S.A.; Davies, M.J.; Schiesser, C.H.; Parry, L.J. 1,4-Anhydro-4-seleno-d-talitol (SeTal) protects endothelial function in the mouse aorta by scavenging superoxide radicals under conditions of acute oxidative stress. Biochem. Pharm. 2017, 128, 34–45. [Google Scholar] [CrossRef]
- Ng, H.H.; Leo, C.H.; Prakoso, D.; Qin, C.; Ritchie, R.H.; Parry, L.J. Serelaxin treatment reverses vascular dysfunction and left ventricular hypertrophy in a mouse model of Type 1 diabetes. Sci. Rep. 2017, 7, 39604. [Google Scholar] [CrossRef]
- Qin, C.X.; Anthonisz, J.; Leo, C.H.; Kahlberg, N.; Velagic, A.; Li, M.; Jap, E.; Woodman, O.L.; Parry, L.J.; Horowitz, J.D.; et al. NO• resistance, induced in the myocardium by diabetes is circumvented by the NO redox sibling, nitroxyl. Antioxid. Redox Signal. 2020, 32, 60–77. [Google Scholar] [CrossRef]
- Jelinic, M.; Kahlberg, N.; Leo, C.H.; Ng, H.H.; Rosli, S.; Deo, M.; Li, M.; Finlayson, S.; Walsh, J.; Parry, L.J.; et al. Annexin-A1 deficiency exacerbates pathological remodelling of the mesenteric vasculature in insulin-resistant, but not insulin-deficient, mice. Br. J. Pharm. 2020, 177, 1677–1691. [Google Scholar] [CrossRef]
- Marshall, S.A.; Qin, C.X.; Jelinic, M.; O’Sullivan, K.; Deo, M.; Walsh, J.; Li, M.; Parry, L.J.; Ritchie, R.H.; Leo, C.H. The Novel Small-molecule Annexin-A1 Mimetic, Compound 17b, Elicits Vasoprotective Actions in Streptozotocin-induced Diabetic Mice. Int. J. Mol. Sci. 2020, 21, 1384. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S.A.; Leo, C.H.; Girling, J.E.; Tare, M.; Beard, S.; Hannan, N.J.; Parry, L.J. Relaxin treatment reduces angiotensin II-induced vasoconstriction in pregnancy and protects against endothelial dysfunction. Biol. Reprod 2017, 96, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Rana, I.; Badoer, E.; Alahmadi, E.; Leo, C.H.; Woodman, O.L.; Stebbing, M.J. Microglia Are Selectively Activated in Endocrine and Cardiovascular Control Centres in Streptozotocin-Induced Diabetic Rats. J. Neuroendocr. 2014, 26, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Carciochi, R.A.; Manrique, G.D.; Dimitrov, K. Optimization of antioxidant phenolic compounds extraction from quinoa (Chenopodium quinoa) seeds. J. Food Sci. Technol. 2015, 52, 4396–4404. [Google Scholar] [CrossRef]
- Dzikia, D.; Różyłob, R.; Gawlik-Dzikic, U.; Świecac, M. Current trends in the enhancement of antioxidant activity of wheat bread by the addition of plant materials rich in phenolic compounds. Trends Food Sci. Technol. 2014, 10, 48–61. [Google Scholar] [CrossRef]
- Hemalatha, P.; Bomzan, D.P.; Sathyendra Rao, B.V.; Sreerama, Y.N. Distribution of phenolic antioxidants in whole and milled fractions of quinoa and their inhibitory effects on α-amylase and α-glucosidase activities. Food Chem. 2016, 199, 330–338. [Google Scholar] [CrossRef]
- Heng, M.Y.; Katayama, S.; Mitani, T.; Ong, E.S.; Nakamura, S. Solventless extraction methods for immature fruits: Evaluation of their antioxidant and cytoprotective activities. Food Chem. 2017, 221, 1388–1393. [Google Scholar] [CrossRef]
- Teo, C.C.; Tan, S.N.; Yong, J.W.; Hew, C.S.; Ong, E.S. Pressurized hot water extraction (PHWE). J. Chromatogr. A 2010, 1217, 2484–2494. [Google Scholar] [CrossRef]
- Teo, C.C.; Tan, S.N.; Yong, J.W.; Hew, C.S.; Ong, E.S. Validation of green-solvent extraction combined with chromatographic chemical fingerprint to evaluate quality of Stevia rebaudiana Bertoni. J. Sep. Sci. 2009, 32, 613–622. [Google Scholar] [CrossRef]
- Gil-Ramirez, A.; Salas-Veizaga, D.M.; Grey, C.; Karlsson, E.N.; Rodriguez-Meizoso, I.; Linares-Pastén, J.A. Integrated process for sequential extraction of saponins, xylan and cellulose from quinoa stalks (Chenopodium quinoa Willd.). Ind. Crop. Prod. 2018, 121, 54–65. [Google Scholar] [CrossRef]
- Liu, F.; Ong, E.S.; Li, S.F. A green and effective approach for characterisation and quality control of chrysanthemum by pressurized hot water extraction in combination with HPLC with UV absorbance detection. Food Chem. 2013, 141, 1807–1813. [Google Scholar] [CrossRef] [PubMed]
- Hawrył, A.; Hawrył, M.; Waksmundzka-Hajnos, M. Liquid chromatography fingerprint analysis and antioxidant activity of selected lavender species with chemometric calculations. PLoS ONE 2019, 14, e0218974. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pérez, C.; Zengin, G.; Segura-Carretero, A.; Lobine, D.; Mahomoodally, M.F. Chemical fingerprint and bioactivity evaluation of Globularia orientalis L. and Globularia trichosantha Fisch. & C. A. Mey. using non-targeted HPLC-ESI-QTOF-MS approach. Phytochem. Anal. 2019, 30, 237–252. [Google Scholar]
- Zhou, S.T.; Luan, K.; Ni, L.L.; Wang, Y.; Yuan, S.M.; Che, Y.H.; Yang, Z.Z.; Zhang, C.G.; Yang, Z.B. A Strategy for Quality Control of Vespa magnifica (Smith) Venom Based on HPLC Fingerprint Analysis and Multi-Component Separation Combined with Quantitative Analysis. Molecules 2019, 24, 2920. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, H.; Wu, H.; Pan, Y.; Wang, K.; Jin, Y.; Zhang, C. Characterization and Quantification by LC-MS/MS of the Chemical Components of the Heating Products of the Flavonoids Extract in Pollen Typhae for Transformation Rule Exploration. Molecules 2015, 20, 18352–18366. [Google Scholar] [CrossRef] [PubMed]
- Taira, J.; Toyoshima, R.; Ameku, N.; Iguchi, A.; Tamaki, Y. Vanillin production by biotransformation of phenolic compounds in fungus, Aspergillus luchuensis. AMB Express 2018, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Li, Q.S.; Han, Q.Z.; Zou, L.L.; Lv, L.; Zhou, Q.; Li, N. LC-MS/MS for Simultaneous Determination of Four Major Active Catechins of Tea Polyphenols in Rat Plasma and Its Application to Pharmacokinetics. Chin. Herb. Med. 2010, 2, 289–296. [Google Scholar]
- Leo, C.H.; Jelinic, M.; Parkington, H.C.; Tare, M.; Parry, L.J. Acute intravenous injection of serelaxin (recombinant human relaxin-2) causes rapid and sustained bradykinin-mediated vasorelaxation. J. Am. Heart Assoc. 2014, 3, e000493. [Google Scholar] [CrossRef]
- Marshall, S.A.; O’Sullivan, K.; Ng, H.H.; Bathgate, R.A.D.; Parry, L.J.; Hossain, M.A.; Leo, C.H. B7-33 replicates the vasoprotective functions of human relaxin-2 (serelaxin). Eur. J. Pharm. 2017, 807, 190–197. [Google Scholar] [CrossRef]
- Ng, H.H.; Jelinic, M.; Parry, L.J.; Leo, C.H. Increased superoxide production and altered nitric oxide-mediated relaxation in the aorta of young but not old male relaxin-deficient mice. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H285–H296. [Google Scholar] [CrossRef]
- Leo, C.H.; Jelinic, M.; Ng, H.H.; Tare, M.; Parry, L.J. Time-dependent activation of prostacyclin and nitric oxide pathways during continuous i.v. infusion of serelaxin (recombinant human H2 relaxin). Br. J. Pharm. 2016, 173, 1005–1017. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S.A.; Leo, C.H.; Senadheera, S.N.; Girling, J.E.; Tare, M.; Parry, L.J. Relaxin deficiency attenuates pregnancy-induced adaptation of the mesenteric artery to angiotensin II in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R847–R857. [Google Scholar] [CrossRef] [PubMed]
- Leo, C.H.; Ng, H.H.; Marshall, S.A.; Jelinic, M.; Rupasinghe, T.; Qin, C.; Roessner, U.; Ritchie, R.H.; Tare, M.; Parry, L.J. Relaxin reduces endothelium-derived vasoconstriction in hypertension: Revealing new therapeutic insights. Br. J. Pharm. 2020, 177, 217–233. [Google Scholar] [CrossRef] [PubMed]
- ICH Expert Working Group. ICH Harmonised Tripartite Guideline Impurities in New Drug Products Q3B(R2). Int. Conf. Harmon. Tech. Requir. Regist. Pharm. Hum. Use 2006. Available online: http://academy.gmp-compliance.org/guidemgr/files/ICH%20Q3B%20R2 (accessed on 1 November 2020).
- Law, W.S.; Huang, P.Y.; Ong, E.S.; Ong, C.N.; Li, S.F.; Pasikanti, K.K.; Chan, E.C. Metabonomics investigation of human urine after ingestion of green tea with gas chromatography/mass spectrometry, liquid chromatography/mass spectrometry and (1)H NMR spectroscopy. Rapid Commun. Mass Spectrom. 2008, 22, 2436–2446. [Google Scholar] [CrossRef] [PubMed]
- Leo, C.H.; Woodman, O.L. Flavonols in the Prevention of Diabetes-induced Vascular Dysfunction. J. Cardiovasc. Pharm. 2015, 65, 532–544. [Google Scholar] [CrossRef]
- Leo, C.H.; Hart, J.L.; Woodman, O.L. 3′,4′-dihydroxyflavonol restores endothelium dependent relaxation in small mesenteric artery from rats with type 1 and type 2 diabetes. Eur. J. Pharm. 2011, 659, 193–198. [Google Scholar] [CrossRef]
- Leo, C.H.; Hart, J.L.; Woodman, O.L. 3′,4′-dihydroxyflavonol reduces superoxide and improves nitric oxide function in diabetic rat mesenteric arteries. PLoS ONE 2011, 6, e20813. [Google Scholar] [CrossRef]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef]

| Name of Compound | [M-H]- | MSMS | Normalised Peak Area | |||
|---|---|---|---|---|---|---|
| m/z | m/z | Black | Red | White (Peru) | White (Bolivia) | |
| Vanillic acid | 167 | 151, 125, 106, 80, 58 | 0.00695 ± 0.00245 | 0.0085 ± 0.00251 | 0.00152 ± 0.000946 | 0.00468 ± 0.000122 |
| Vanillic acid glucoside | 329 | 151, 133, 109 | 0.0108 ± 0.00292 | 0.0488 ± 0.0254 | 0.0179 ± 0.00829 | 0.0726 ± 0.00478 |
| Vanillin | 151 | 108, 80, 66, 61 | 0.0173 ± 0.00561 | 0.0165 ± 0.0144 | 0.235 ± 0.317 | 0.0547 ± 0.00677 |
| p-coumaric acid | 163 | 99, 91, 73, 71, 69, 57 | 0.102 ± 0.0272 | 0.0272 ± 0.0136 | 0.0166 ± 0.00801 | 0.0482 ± 0.00109 |
| Ferulic acid | 193 | 147, 128, 113, 89, 77,75, 71, 67, 59, 57, 55 | 0.0162 ± 0.00252 | 0.0145 ± 0.00840 | 0.0399 ± 0.00928 | 0.0288 ± 0.00197 |
| Caffeic acid | 179 | 89, 71, 59, 57 | 0.285 ± 0.0592 | 0.268 ± 0.0309 | 0.267 ± 0.125 | 0.230 ± 0.0175 |
| Catechin | 289 | 171, 157, 145, 133,127, 111, 106 | 0.0667 ± 0.0178 | 0.0318 ± 0.0166 | 0.0354 ± 0.0153 | 0.104 ± 0.00879 |
| Daidzein | 263 | 230, 166, 146, 124, 110, 107, 102 | 0.00564 ± 0.00183 | 0.00167 ± 0.000792 | 0.00145 ± 0.000543 | 0.0022 ± 5.8 E-05 |
| Genistein | 269 | 197, 173, 157, 143, 139, 121 | 0.0106 ± 0.00223 | 0.00768 ± 0.00396 | 0.00835 ± 0.00299 | 0.0137 ± 0.00157 |
| Quercetin | 301 | 193, 168, 150, 125, 107 | 0.00436 ± 0.00152 | 0.00448 ± 0.00244 | 0.00290 ± 0.00135 | 0.005 ± 0.000557 |
| Quercetin 3-rutinoside | 609 | 300, 285, 287, 257, 137, 114 | 0.0348 ± 0.00984 | 0.0570 ± 0.0301 | 0.0041 ± 0.00156 | 0.0528 ± 0.013 |
| Unknown | 431 | 0.185 ± 0.0174 | 0.135 ± 0.0736 | 0.142 ± 0.0701 | 0.157 ± 0.00459 | |
| Unknown | 319 | 242, 206, 189, 160, 133, 125 | 0.0216 ± 0.00346 | 0.0205 ± 0.0105 | 0.0151 ± 0.00692 | 0.0304 ± 0.00331 |
| Unknown | 726 | 726, 284 | 0.00663 ± 0.00140 | 0.00483 ± 0.0006 | 0.0551 ± 0.0241 | 0.0288 ± 0.00412 |
| Unkownn | 479 | 389, 318, 258, 139 | 0.00226 ± 0.000549 | 0.00613 ± 0.00342 | 0.00505 ± 0.00208 | 0.00732 ± 0.000860 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ong, E.S.; Pek, C.J.N.; Tan, J.C.W.; Leo, C.H. Antioxidant and Cytoprotective Effect of Quinoa (Chenopodium quinoa Willd.) with Pressurized Hot Water Extraction (PHWE). Antioxidants 2020, 9, 1110. https://doi.org/10.3390/antiox9111110
Ong ES, Pek CJN, Tan JCW, Leo CH. Antioxidant and Cytoprotective Effect of Quinoa (Chenopodium quinoa Willd.) with Pressurized Hot Water Extraction (PHWE). Antioxidants. 2020; 9(11):1110. https://doi.org/10.3390/antiox9111110
Chicago/Turabian StyleOng, Eng Shi, Charlene Jia Ning Pek, Joseph Choon Wee Tan, and Chen Huei Leo. 2020. "Antioxidant and Cytoprotective Effect of Quinoa (Chenopodium quinoa Willd.) with Pressurized Hot Water Extraction (PHWE)" Antioxidants 9, no. 11: 1110. https://doi.org/10.3390/antiox9111110
APA StyleOng, E. S., Pek, C. J. N., Tan, J. C. W., & Leo, C. H. (2020). Antioxidant and Cytoprotective Effect of Quinoa (Chenopodium quinoa Willd.) with Pressurized Hot Water Extraction (PHWE). Antioxidants, 9(11), 1110. https://doi.org/10.3390/antiox9111110






