Salvia sclarea L. Essential Oil Extract and Its Antioxidative Phytochemical Sclareol Inhibit Oxytocin-Induced Uterine Hypercontraction Dysmenorrhea Model by Inhibiting the Ca2+–MLCK–MLC20 Signaling Cascade: An Ex Vivo and In Vivo Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagent Preparation
2.2. Ultra-Performance Liquid Chromatography–Mass Spectrometry (UPLC–MS) Analysis of Total Sclareol Content of Salvia sclarea Essential Oil
2.3. Cell Culture
2.4. Reactive Oxygen Species (ROS) Measurement
2.5. Animals
2.6. Uterine Tissue Preparations and Measurement of Uterine Contraction Ex Vivo
2.7. Measurement of Uterine Contraction Pressure in the In Vivo Study
2.8. Measurement of Uterine Tissue under Ca2+-Dependent Contractions
2.9. Acetic Acid-Induced Writhing Test
2.10. Oxytocin-Induced Writhing Test
2.11. Lipid Peroxidation Determination
2.12. Western Blotting Analysis
2.13. Statistical Analysis
3. Results
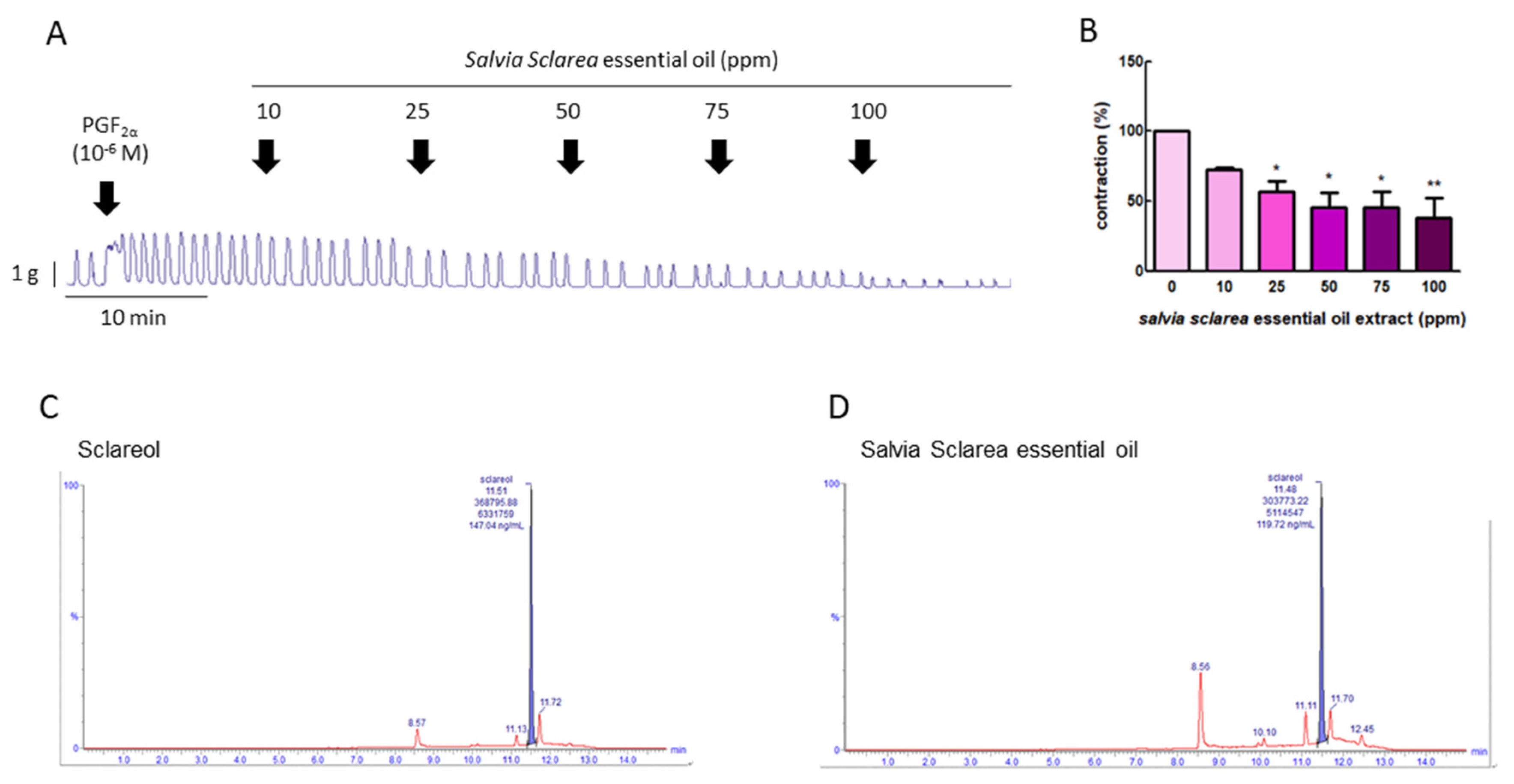
3.1. Effect of Salvia sclarea L. Essential Oil on PGF2α-Induced Uterine Contractions
3.2. Total Sclareol Content of Salvia sclarea L. Essential Oil
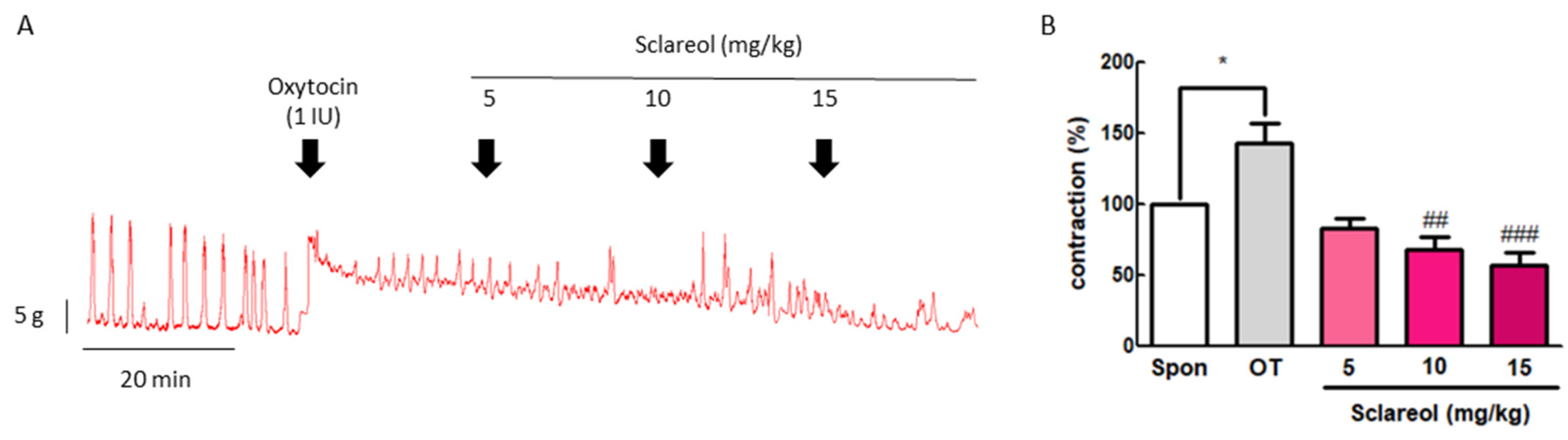
3.3. Effect of Sclareol on Oxytocin-Induced Uterine Contraction In Vivo
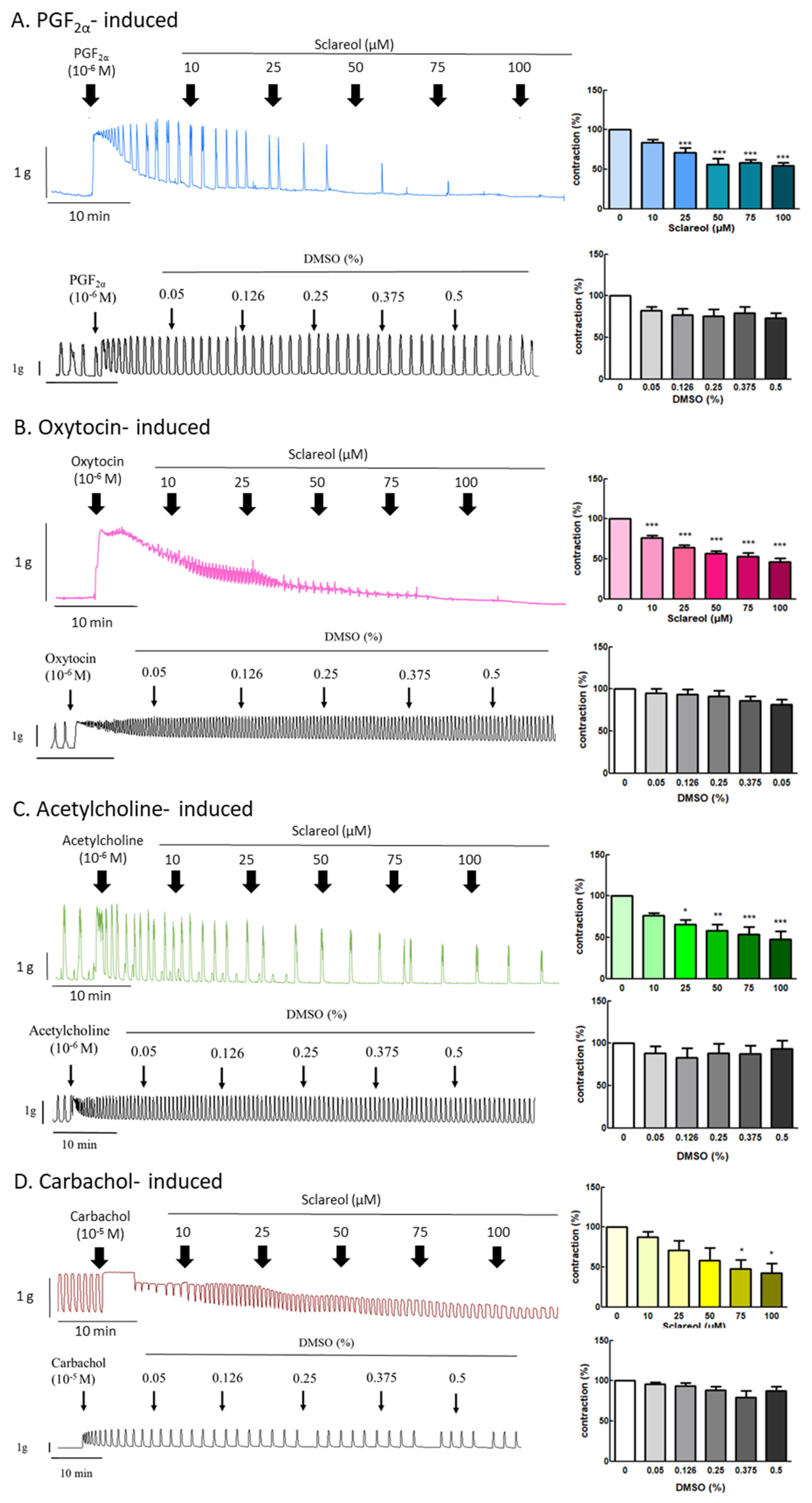
3.4. Effect of Sclareol on PGF2α-, Oxytocin-, Acetylcholine-, and Carbachol-Induced Uterine Contractions
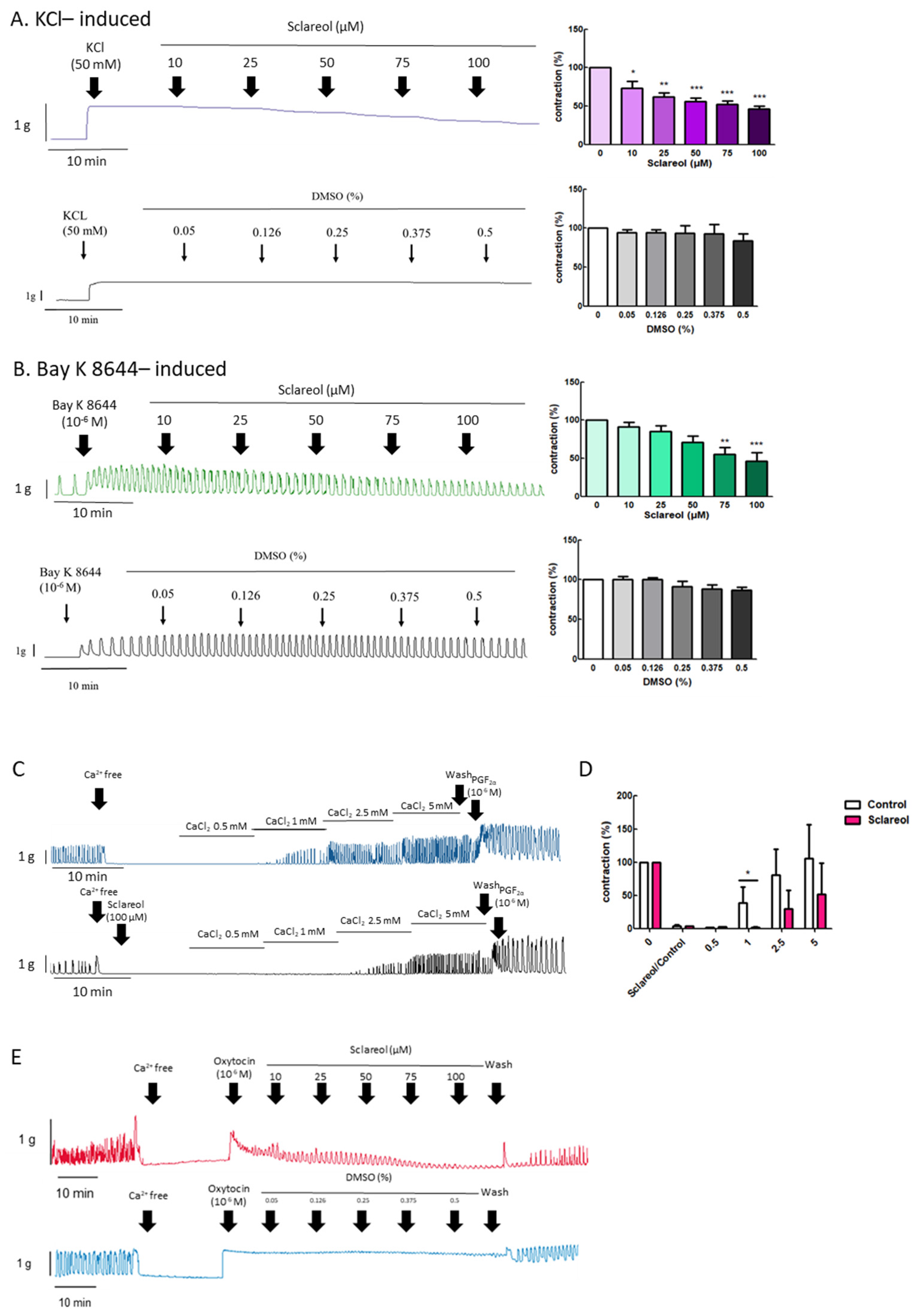
3.5. Effect of Sclareol on KCl- and Bay K 8644-Induced Uterine Contractions
3.6. Effect of Sclareol on Ca2+-Dependent Contractions
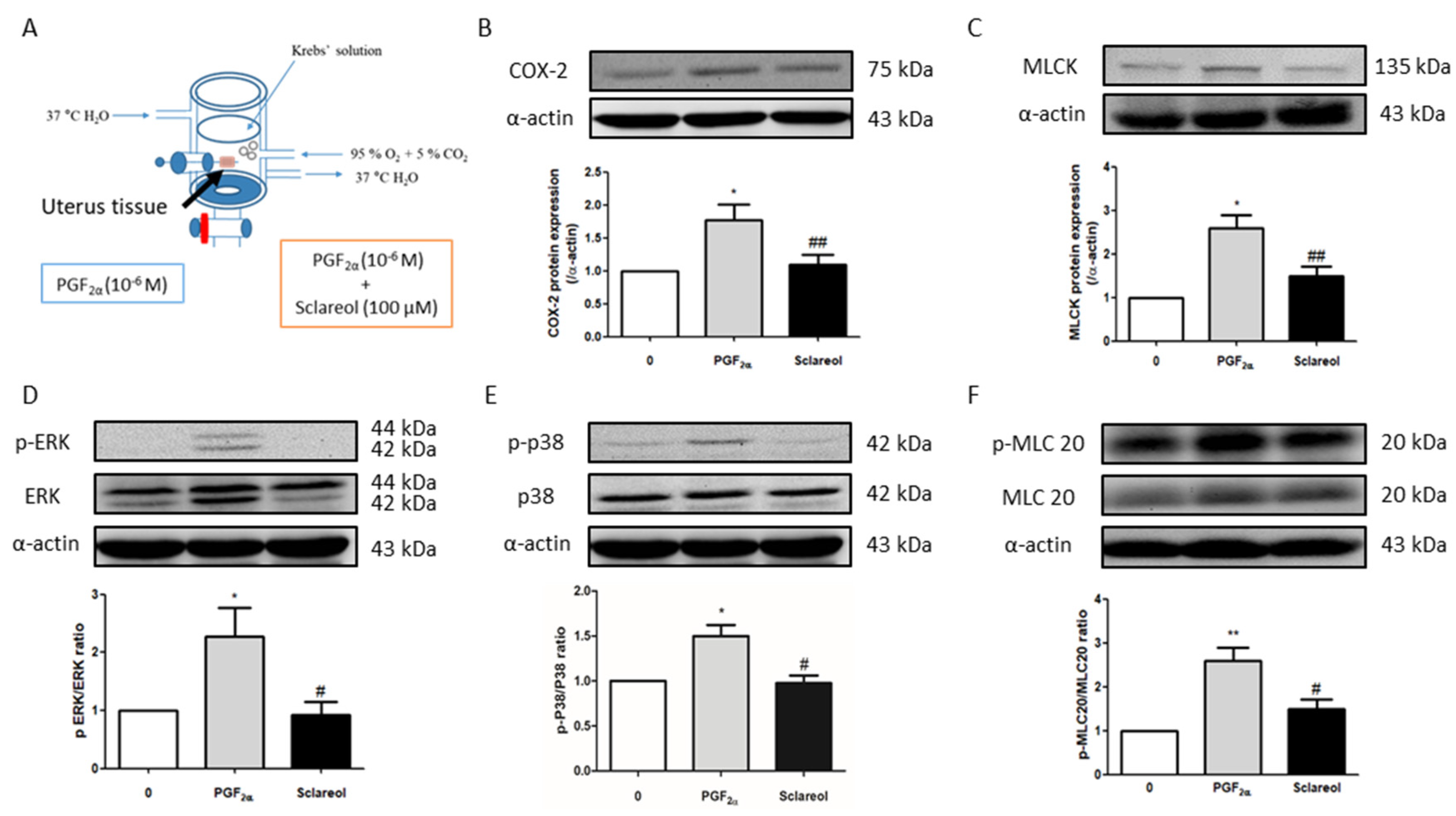
3.7. Effect of Sclareol on PGF2α-Induced Uterine Contraction-Related Protein Expression
3.8. Effect of Sclareol on Acetic Acid-Induced Writhing Test
3.9. Effect of Sclareol on Oxytocin-Induced Writhing Test
3.10. Effect of Sclareol on Oxidative Stress in Uterine Smooth Muscle Cell and Dysmenorrhea Mice
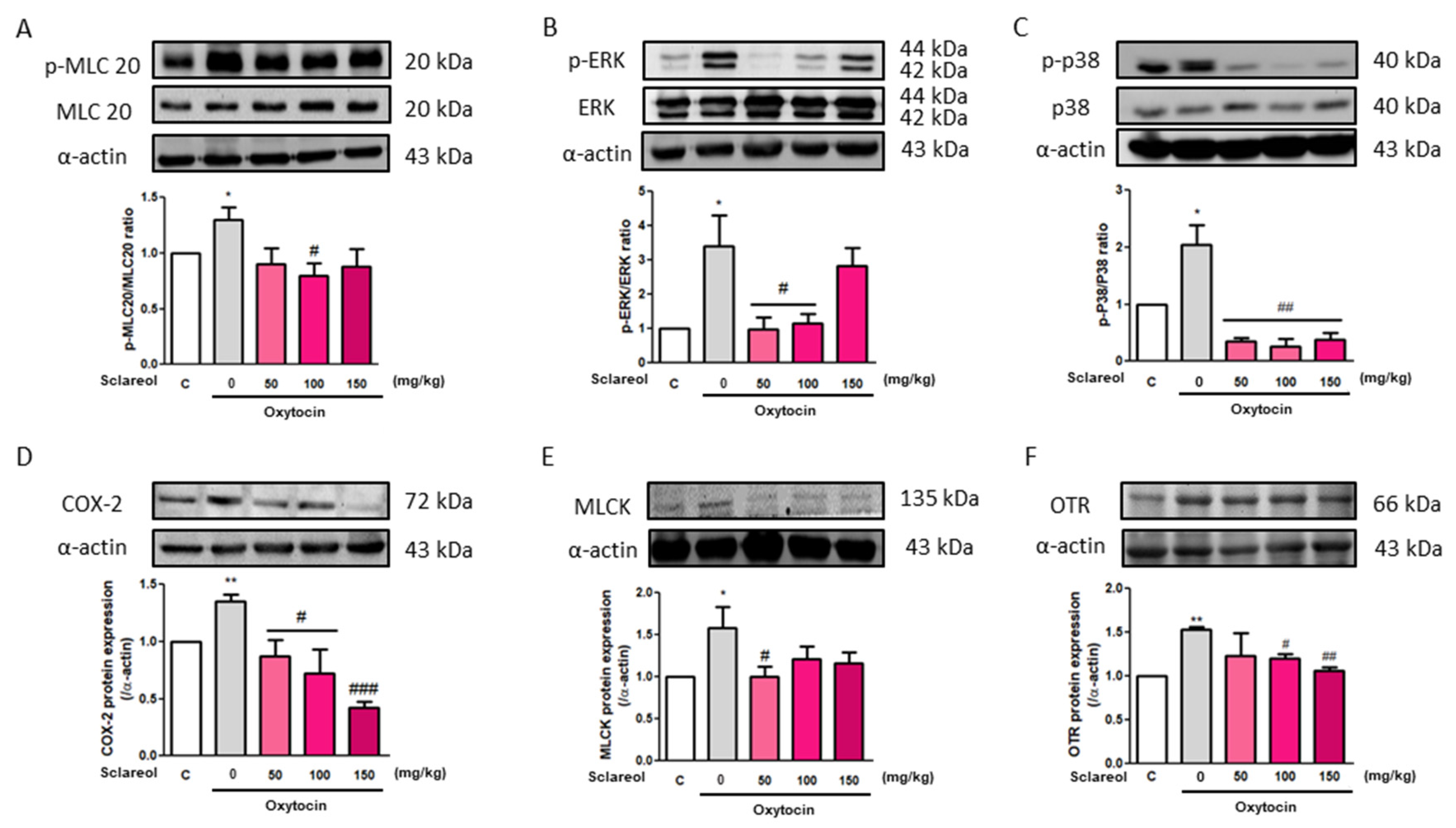
3.11. Effect of Sclareol on Oxytocin-Induced Uterine Contraction-Related Protein Expression
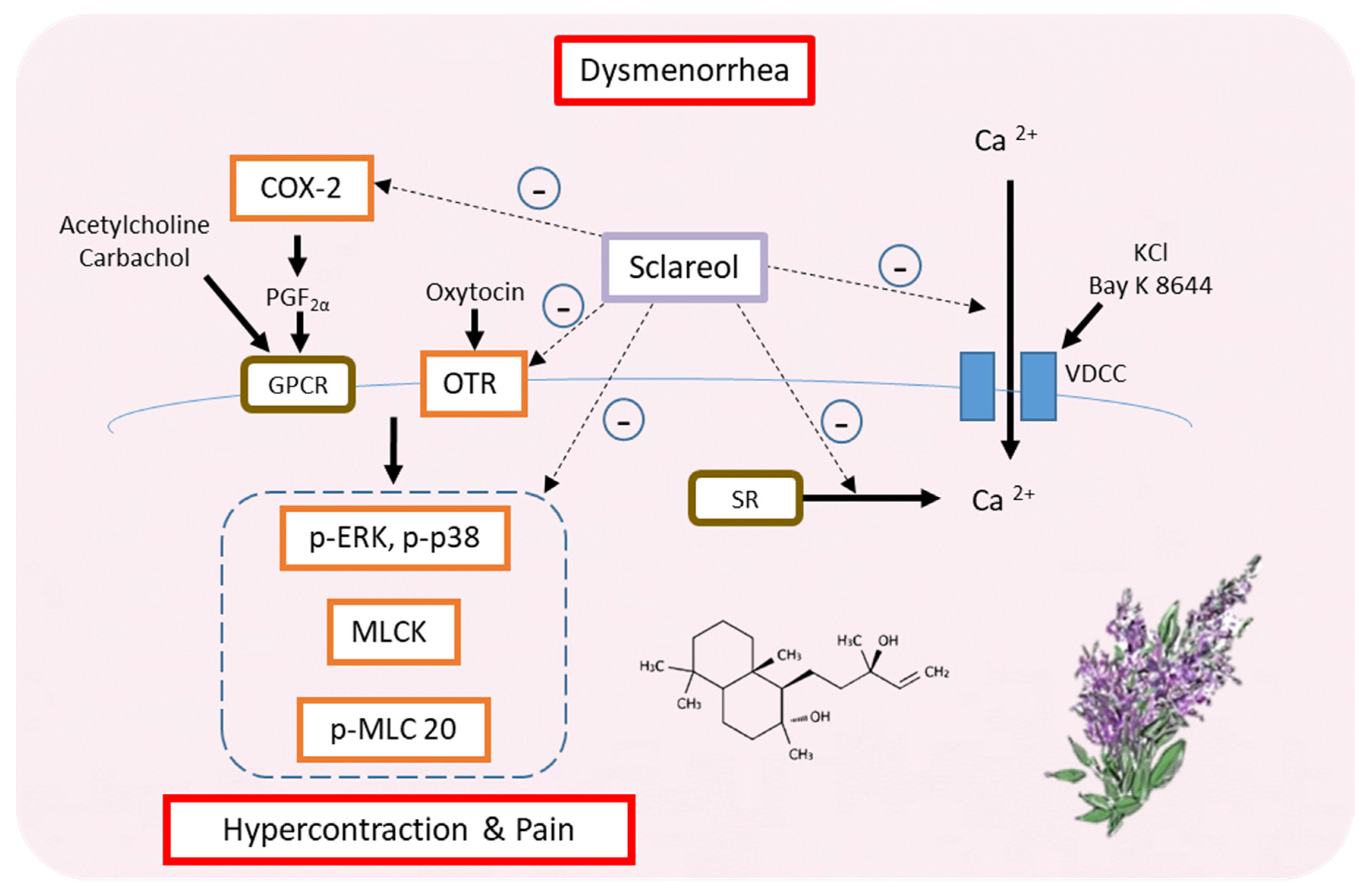
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ju, H.; Jones, M.; Mishra, G. The Prevalence and Risk Factors of Dysmenorrhea. Epidemiologic Rev. 2014, 36, 104–113. [Google Scholar] [CrossRef] [PubMed]
- French, L. Dysmenorrhea. Am. Fam. Physician 2005, 71, 285–291. [Google Scholar] [PubMed]
- Dawood, M. Nonsteroidal anti-inflammatory drugs and changing attitudes toward dysmenorrhea. Am. J. Med. 1988, 84, 23–29. [Google Scholar] [CrossRef]
- Burnett, M.; Lemyre, M. No. 345-Primary Dysmenorrhea Consensus Guideline. J. Obstet. Gynaecol. Can. 2017, 39, 585–595. [Google Scholar] [CrossRef]
- Iacovides, S.; Avidon, I.; Baker, F.C. What we know about primary dysmenorrhea today: A critical review. Hum. Reprod. Updat. 2015, 21, 762–778. [Google Scholar] [CrossRef]
- Hanna, V.S.; Hafez, E.A.A. Synopsis of arachidonic acid metabolism: A review. J. Adv. Res. 2018, 11, 23–32. [Google Scholar] [CrossRef]
- Chen, L.; Yang, G.; Grosser, T. Prostanoids and inflammatory pain. Prostaglandins Other Lipid Mediat. 2013, 104–105, 58–66. [Google Scholar] [CrossRef]
- Wray, S.; Jones, K.; Kupittayanant, S.; Li, Y.; Matthew, A.; Monir-Bishty, E.; Noble, K.; Pierce, S.J.; Quenby, S.; Shmygol, A.V. Calcium signaling and uterine contractility. J. Soc. Gynecol. Investig. 2003, 10, 252–264. [Google Scholar] [CrossRef]
- Ulrich, C.C.; Quillici, D.R.; Schegg, K.; Woolsey, R.; Nordmeier, A.; Buxton, I.L.O. Uterine smooth muscle S-nitrosylproteome in pregnancy. Mol. Pharmacol. 2011, 81, 143–153. [Google Scholar] [CrossRef]
- Ou, M.-C.; Hsu, T.-F.; Lai, A.C.; Lin, Y.-T.; Lin, C.-C. Pain relief assessment by aromatic essential oil massage on outpatients with primary dysmenorrhea: A randomized, double-blind clinical trial. J. Obstet. Gynaecol. Res. 2012, 38, 817–822. [Google Scholar] [CrossRef]
- Zhong, Y.; Huang, Y.; Santoso, M.B.; Wu, L.D. Sclareol exerts anti-osteoarthritic activities in interleukin-1beta-induced rabbit chondrocytes and a rabbit osteoarthritis model. Int. J. Clin. Exp. Pathol. 2015, 8, 2365–2374. [Google Scholar]
- Hsieh, Y.-H.; Deng, J.-S.; Pan, H.-P.; Liao, J.-C.; Huang, S.-S.; Huang, G.-J. Sclareol ameliorate lipopolysaccharide-induced acute lung injury through inhibition of MAPK and induction of HO-1 signaling. Int. Immunopharmacol. 2017, 44, 16–25. [Google Scholar] [CrossRef]
- Tsai, S.-W.; Hsieh, M.-C.; Li, S.; Lin, S.-C.; Wang, S.-P.; Lehman, C.W.; Lien, C.Z.; Lin, C.-C. Therapeutic Potential of Sclareol in Experimental Models of Rheumatoid Arthritis. Int. J. Mol. Sci. 2018, 19, 1351. [Google Scholar] [CrossRef] [PubMed]
- Noori, S.; Hassan, Z.M.; Mohammadi, M.; Habibi, Z.; Sohrabi, N.; Bayanolhagh, S. Sclareol modulates the Treg intra-tumoral infiltrated cell and inhibits tumor growth in vivo. Cell. Immunol. 2010, 263, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, T.; Cai, P. Sclareol inhibits cell proliferation and sensitizes cells to the antiproliferative effect of bortezomib via upregulating the tumor suppressor caveolin-1 in cervical cancer cells. Mol. Med. Rep. 2017, 15, 3566–3574. [Google Scholar] [CrossRef] [PubMed]
- Peritore, A.F.; Siracusa, R.; Crupi, R.; Cuzzocrea, S. Therapeutic Efficacy of Palmitoylethanolamide and Its New Formulations in Synergy with Different Antioxidant Molecules Present in Diets. Nutrients 2019, 11, 2175. [Google Scholar] [CrossRef]
- Fusco, R.; D’Amico, R.; Cordaro, M.; Gugliandolo, E.; Siracusa, R.; Peritore, A.F.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. Absence of formyl peptide receptor 1 causes endometriotic lesion regression in a mouse model of surgically-induced endometriosis. Oncotarget 2018, 9, 31355–31366. [Google Scholar] [CrossRef]
- Liang, J.; Bonvino, N.P.; Hung, A.; Karagiannis, T.C. In silico characterisation of olive phenolic compounds as potential cyclooxygenase modulators. Part 1. J. Mol. Graph. Model 2020, 101, 107719. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Carpena, M.; Lourenço-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.M.; Barba, F.J.; Prieto, M.A.; Simal-Gandara, J. Bioactive Compounds and Quality of Extra Virgin Olive Oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef]
- Angeloni, C.; Malaguti, M.; Barbalace, M.C.; Hrelia, S. Bioactivity of Olive Oil Phenols in Neuroprotection. Int. J. Mol. Sci. 2017, 18, 2230. [Google Scholar] [CrossRef]
- Hsia, S.M.; Wang, K.L.; Wang, P.S. Effects of resveratrol, a grape polyphenol, on uterine contraction and Ca²+ mobilization in rats in vivo and in vitro. Endocrinology 2011, 152, 2090–2099. [Google Scholar] [CrossRef]
- Wu, C.-H.; Shieh, T.-M.; Wang, K.-L.; Huang, T.-C.; Hsia, S.-M. Quercetin, a main flavonoid in onion, inhibits the PGF2α-induced uterine contraction in vitro and in vivo. J. Funct. Foods 2015, 19, 495–504. [Google Scholar] [CrossRef]
- Hsu, C.S.; Yang, J.K.; Yang, L.L. Effect of “Dang-Qui-Shao-Yao-San” a Chinese medicinal prescription for dysmenorrhea on uterus contractility in vitro. Phytomedicine 2006, 13, 94–100. [Google Scholar] [CrossRef]
- Zendehdel, M.; Torabi, Z.; Hassanpour, S. Antinociceptive mechanisms of Bunium persicum essential oil in the mouse writhing test: Role of opioidergic and histaminergic systems. Veterinární Med. 2016, 60, 63–70. [Google Scholar] [CrossRef]
- Peng, Y.; Zheng, X.; Fan, Z.; Zhou, H.; Zhu, X.; Wang, G.; Liu, Z. Paeonol alleviates primary dysmenorrhea in mice via activating CB2R in the uterus. Phytomedicine 2020, 68, 153151. [Google Scholar] [CrossRef]
- Yang, L.; Cao, Z.; Yu, B.; Chai, C. An in vivo mouse model of primary dysmenorrhea. Exp Anim. 2015, 64, 295–303. [Google Scholar] [CrossRef]
- Chen, H.L.; Gong, J.Y.; Lin, S.-C.; Li, S.; Chiang, Y.-C.; Hung, J.-H.; Yen, C.-C.; Lin, C.-C. Effects of Sclareol Against Small Cell Lung Carcinoma and the Related Mechanism: In Vitro and In Vivo Studies. Anticancer Res. 2020, 40, 4947–4960. [Google Scholar] [CrossRef]
- Li, W.; Ping, Z.; Xuemei, G.; Minglian, L.; Hongjuan, M.; Yi, H.; Zhongxiang, Z. Naturally Occurring Sclareol Diterpene Augments the Chemosensitivity of Human Hela Cervical Cancer Cells by Inducing Mitochondrial Mediated Programmed Cell Death, S-Phase Cell Cycle Arrest and Targeting Mitogen-Activated Protein Kinase (MAPK)/Extracellular-Signal-Regulated Kinase (ERK) Signaling Pathway. Med. Sci. Monit. 2020, 26, e920248. [Google Scholar]
- Mahboubi, M. Clary sage essential oil and its biological activities. Adv. Tradit. Med. 2020, 1–12. [Google Scholar] [CrossRef]
- Han, S.-H.; Hur, M.-H.; Buckle, J.; Choi, J.; Lee, M.S. Effect of Aromatherapy on Symptoms of Dysmenorrhea in College Students: A Randomized Placebo-Controlled Clinical Trial. J. Altern. Complement. Med. 2006, 12, 535–541. [Google Scholar] [CrossRef]
- Sun, L.; Liu, L.-N.; Li, J.-C.; Lv, Y.-Z.; Zong, S.-B.; Zhou, J.; Wang, Z.-Z.; Kou, J.-P.; Xiao, W. The essential oil from the twigs of Cinnamomum cassia Presl inhibits oxytocin-induced uterine contraction in vitro and in vivo. J. Ethnopharmacol. 2017, 206, 107–114. [Google Scholar] [CrossRef]
- Kitazawa, T.; Hirama, R.; Masunaga, K.; Nakamura, T.; Asakawa, K.; Cao, J.; Teraoka, H.; Unno, T.; Komori, S.-I.; Yamada, M.; et al. Muscarinic receptor subtypes involved in carbachol-induced contraction of mouse uterine smooth muscle. Naunyn Schmiedeberg Arch. Pharmacol. 2007, 377, 503–513. [Google Scholar] [CrossRef]
- Alotaibi, M.F. The effect of cinnamon extract on isolated rat uterine strips. Reprod. Biol. 2016, 16, 27–33. [Google Scholar] [CrossRef]
- Ratz, P.H.; Berg, K.M.; Urban, N.H.; Miner, A.S. Regulation of smooth muscle calcium sensitivity: KCl as a calcium-sensitizing stimulus. Am. J. Physiol. Physiol. 2005, 288, C769–C783. [Google Scholar] [CrossRef]
- Deng, M.; Ding, W.; Min, X.; Xia, Y. MLCK-independent phosphorylation of MLC20 and its regulation by MAP kinase pathway in human bladder smooth muscle cells. Cytoskeleton 2010, 68, 139–149. [Google Scholar] [CrossRef]
- Tanimura, S.; Takeda, K. ERK signalling as a regulator of cell motility. J. Biochem. 2017, 162, 145–154. [Google Scholar] [CrossRef]
- Signoretto, E.; Laufer, S.A.; Lang, F. Stimulating Effect of Sclareol on Suicidal Death of Human Erythrocytes. Cell Physiol. Biochem. 2016, 39, 554–564. [Google Scholar] [CrossRef]
- Proctor, M.; Farquhar, C. Diagnosis and management of dysmenorrhoea. BMJ 2006, 332, 1134–1138. [Google Scholar] [CrossRef]
- Kumar, S.; Boehm, J.; Lee, J.C. p38 MAP kinases: Key signalling molecules as therapeutic targets for inflammatory diseases. Nat. Rev. Drug Discov. 2003, 2, 717–726. [Google Scholar] [CrossRef]
- Ho, Y.-L.; Chang, Y.-S. Studies on the antinociceptive, anti-inflammatory and antipyretic effects of Isatis indigotica root. Phytomedicine 2002, 9, 419–424. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Rai, G. Experimental evaluation of analgesic and anti-inflammatory potential of Oyster mushroom Pleurotus florida. Indian J. Pharmacol. 2013, 45, 66–70. [Google Scholar] [CrossRef]
- Munir, M.A.; Enany, N.; Zhang, J.-M. Nonopioid Analgesics. Med. Clin. N. Am. 2007, 91, 97–111. [Google Scholar] [CrossRef]
- Ma, H.; Su, S.-L.; Duan, J.-A.; Tang, Y.-P.; Zhou, J.; Guo, J.-M.; Zhan, Z. Evaluation of the analgesic activities of the crude aqueous extract and fractions of Shao Fu Zhu Yu decoction. Pharm. Biol. 2010, 49, 137–145. [Google Scholar] [CrossRef]
- Nissenson, R.; Flouret, G.; Hechter, O. Opposing effects of estradiol and progesterone on oxytocin receptors in rabbit uterus. Proc. Natl. Acad. Sci. USA 1978, 75, 2044–2048. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |

| Model | Acetic Acid- Induced | Oxytocin-Induced(Pretreat Sclareol) | |||
|---|---|---|---|---|---|
| Group | Writhing Times /30 min | Analgesia (%) | Writhing Times /30 min | Analgesia (%) | |
| Control | 0.0 ± 0.0 | - | 0.0 ± 0.0 | - | |
| Model control (MC) | 62.5 ± 18.6 *** | - | 17.4 ± 5.6 *** | - | |
| Sclareol (mg/kg) | 50 | 24.6 ± 16.5 ## | 60.7 | 4.0 ± 3.3 ### | 77 |
| 100 | 30.4 ± 11.0 ## | 51.3 | 3.5 ± 2.8 ### | 79.9 | |
| 150 | 36.2 ± 19.8 # | 42.1 | 1.8 ± 1.7 ### | 89.9 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, J.; Chiang, Y.-F.; Shih, Y.-H.; Chiu, C.-H.; Chen, H.-Y.; Shieh, T.-M.; Wang, K.-L.; Huang, T.-C.; Hong, Y.-H.; Hsia, S.-M. Salvia sclarea L. Essential Oil Extract and Its Antioxidative Phytochemical Sclareol Inhibit Oxytocin-Induced Uterine Hypercontraction Dysmenorrhea Model by Inhibiting the Ca2+–MLCK–MLC20 Signaling Cascade: An Ex Vivo and In Vivo Study. Antioxidants 2020, 9, 991. https://doi.org/10.3390/antiox9100991
Wong J, Chiang Y-F, Shih Y-H, Chiu C-H, Chen H-Y, Shieh T-M, Wang K-L, Huang T-C, Hong Y-H, Hsia S-M. Salvia sclarea L. Essential Oil Extract and Its Antioxidative Phytochemical Sclareol Inhibit Oxytocin-Induced Uterine Hypercontraction Dysmenorrhea Model by Inhibiting the Ca2+–MLCK–MLC20 Signaling Cascade: An Ex Vivo and In Vivo Study. Antioxidants. 2020; 9(10):991. https://doi.org/10.3390/antiox9100991
Chicago/Turabian StyleWong, Jennifer, Yi-Fen Chiang, Yin-Hwa Shih, Chun-Hui Chiu, Hsin-Yuan Chen, Tzong-Ming Shieh, Kai-Lee Wang, Tsui-Chin Huang, Yong-Han Hong, and Shih-Min Hsia. 2020. "Salvia sclarea L. Essential Oil Extract and Its Antioxidative Phytochemical Sclareol Inhibit Oxytocin-Induced Uterine Hypercontraction Dysmenorrhea Model by Inhibiting the Ca2+–MLCK–MLC20 Signaling Cascade: An Ex Vivo and In Vivo Study" Antioxidants 9, no. 10: 991. https://doi.org/10.3390/antiox9100991
APA StyleWong, J., Chiang, Y.-F., Shih, Y.-H., Chiu, C.-H., Chen, H.-Y., Shieh, T.-M., Wang, K.-L., Huang, T.-C., Hong, Y.-H., & Hsia, S.-M. (2020). Salvia sclarea L. Essential Oil Extract and Its Antioxidative Phytochemical Sclareol Inhibit Oxytocin-Induced Uterine Hypercontraction Dysmenorrhea Model by Inhibiting the Ca2+–MLCK–MLC20 Signaling Cascade: An Ex Vivo and In Vivo Study. Antioxidants, 9(10), 991. https://doi.org/10.3390/antiox9100991








