Synergistic Effects of Regular Walking and Alkaline Electrolyzed Water on Decreasing Inflammation and Oxidative Stress, and Increasing Quality of Life in Individuals with Type 2 Diabetes: A Community Based Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
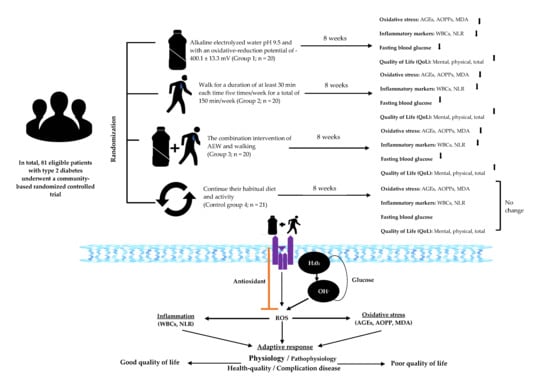
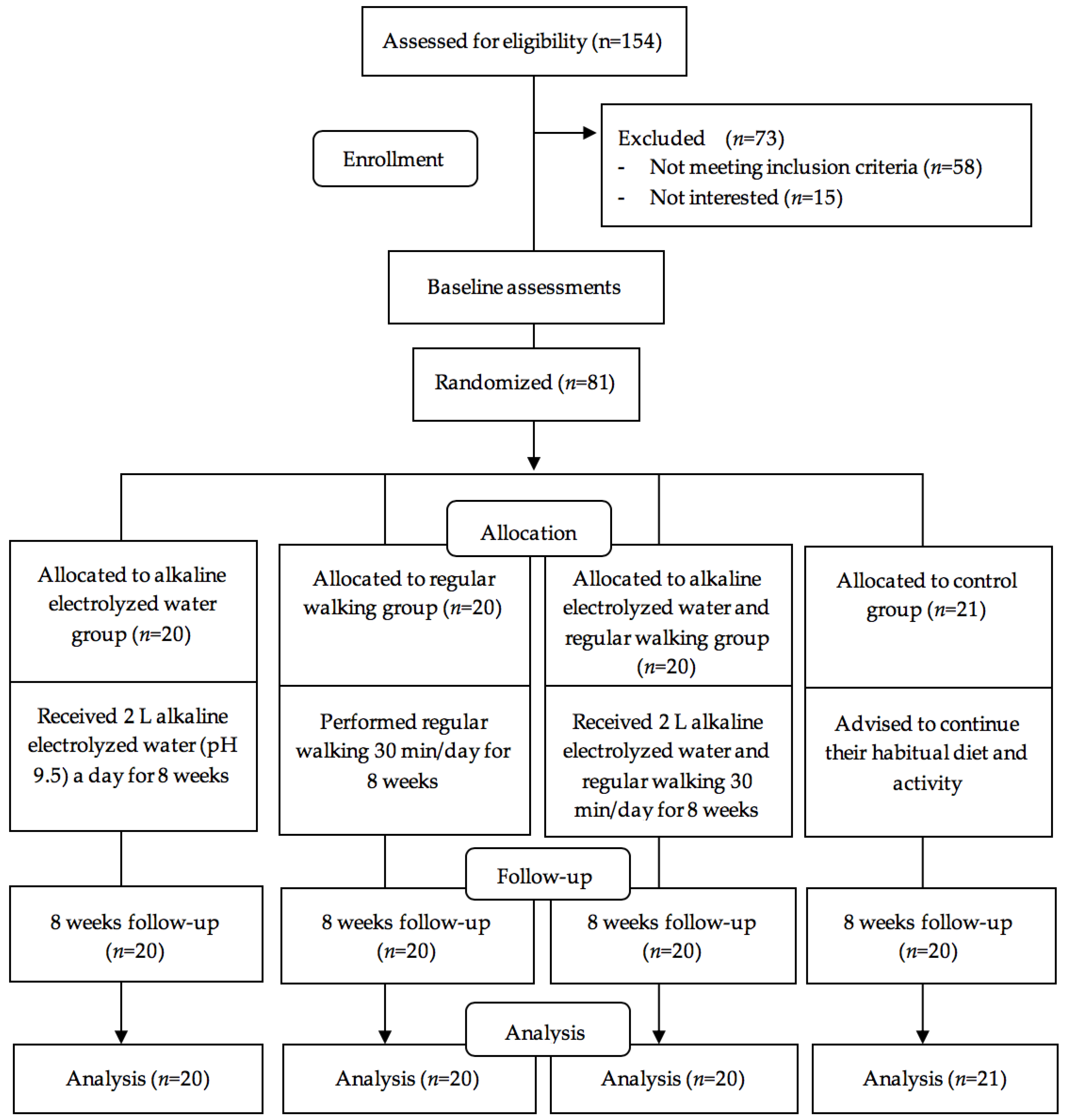
2.1. Research Ethic and Sample Size
2.2. Study Design and Setting
2.3. Randomization and Masking
2.4. Interventions
2.4.1. Alkaline Electrolyzed Water Group
2.4.2. Walking Group
2.4.3. Measurements of Electrolyzed Water and Walking Group
2.4.4. Control Group
2.5. Instruments and Measurements
2.5.1. Measurements of Oxidative Stress and Inflammatory Markers
2.5.2. Measurements of Quality of Life
2.5.3. Measurements by a Food Frequency Questionnaire
2.5.4. Measurements of Depression, Anxiety and Stress
2.5.5. Measurements of Incidence of Adverse Events
2.6. Study Fidelity
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- American Diabetes Association. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2019. Diabetes Care 2019, 42, S13–S28. [Google Scholar] [CrossRef] [PubMed]
- Halim, M.; Halim, A. The effects of inflammation, aging and oxidative stress on the pathogenesis of diabetes mellitus (type 2 diabetes). Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Oguntibeju, O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45. [Google Scholar]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Rias, Y.A.; Kurniasari, M.D.; Traynor, V.; Niu, S.F.; Wiratama, B.S.; Chang, C.W.; Tsai, H.T. Synergistic Effect of Low Neutrophil–Lymphocyte Ratio With Physical Activity on Quality of Life in Type 2 Diabetes Mellitus: A Community-Based Study. Biol. Res. Nurs. 2020. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Chawla, D.; Siddarth, M.; Banerjee, B.D.; Madhu, S.V.; Tripathi, A.K. A study on serum advanced glycation end products and its association with oxidative stress and paraoxonase activity in type 2 diabetic patients with vascular complications. Clin. Biochem. 2013, 46, 109–114. [Google Scholar] [CrossRef]
- Lee, C.T.C.; Harris, S.B.; Retnakaran, R.; Gerstein, H.C.; Perkins, B.A.; Zinman, B.; Hanley, A.J. White blood cell subtypes, insulin resistance and β-cell dysfunction in high-risk individuals—The PROMISE cohort. Clin. Endocrinol. 2014, 81, 536–541. [Google Scholar] [CrossRef]
- Imtiaz, F.; Shafique, K.; Mirza, S.S.; Ayoob, Z.; Vart, P.; Rao, S. Neutrophil lymphocyte ratio as a measure of systemic inflammation in prevalent chronic diseases in Asian population. Int. Arch. Med. 2012, 5, 2. [Google Scholar] [CrossRef]
- Öztürk, Z.A.; Kuyumcu, M.E.; Yesil, Y.; Savas, E.; Yildiz, H.; Kepekçi, Y.; Arioğul, S. Is there a link between neutrophil-lymphocyte ratio and microvascular complications in geriatric diabetic patients? J. Endocrinol. Investig. 2013, 36, 593–599. [Google Scholar] [CrossRef]
- Kang, D.H.; Rice, M.; Park, N.J.; Turner-Henson, A.; Downs, C. Stress and inflammation: A biobehavioral approach for nursing research. West. J. Nurs. Res. 2010, 32, 730–760. [Google Scholar] [CrossRef]
- Dinçer, Ş.; Altan, M.; Terzioğlu, D.; Uslu, E.; Karşidağ, K.; Batu, Ş.; Metin, G. Effects of a regular exercise program on biochemical parameters of type 2 diabetes mellitus patients. J. Sports Med. Phys. Fit. 2016, 56, 1384–1391. [Google Scholar]
- Magalhães, P.M.; Appell, H.; Duarte, J.A. Involvement of advanced glycation end products in the pathogenesis of diabetic complications: The protective role of regular physical activity. Eur. Rev. Aging Phys. Act. 2008, 5, 17–29. [Google Scholar] [CrossRef]
- Kanter, M.; Aksu, F.; Takir, M.; Kostek, O.; Kanter, B.; Oymagil, A. Effects of low intensity exercise against apoptosis and oxidative stress in Streptozotocin-induced diabetic rat heart. Exp. Clin. Endocrinol. Diabetes 2017, 125, 583–591. [Google Scholar] [CrossRef]
- Kim, T.J.; Pyo, J.H.; Lee, H.; Baek, S.Y.; Ahn, S.H.; Min, Y.W.; Min, B.H.; Lee, J.H.; Son, H.J.; Rhee, P.L. Lack of association between helicobacter pylori infection and various markers of systemic inflammation in asymptomatic adults. Korean J. Gastroenterol. 2018, 72, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.Q.; Zhao, D.; Zhu, M.; Wang, Z.M.; Gao, W.; Zhao, H.; Zhang, D.G.; Yang, Z.J.; Wang, L.S. The influence of regular walking at different times of day on blood lipids and inflammatory markers in sedentary patients with coronary artery disease. Prev. Med. 2014, 58, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Pengpid, S.; Peltzer, K. The Prevalence of Underweight, Overweight/Obesity and Their Related Lifestyle Factors in Indonesia, 2014–2015. AIMS Public Health 2017, 4, 633–649. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.O. Walking and Type 2 Diabetes. Diabetes Care 2005, 28, 1524. [Google Scholar] [CrossRef] [PubMed]
- Hamasaki, T.; Harada, G.; Nakamichi, N.; Kabayama, S.; Teruya, K.; Fugetsu, B.; Gong, W.; Sakata, I.; Shirahata, S. Electrochemically reduced water exerts superior reactive oxygen species scavenging activity in HT1080 cells than the equivalent level of hydrogen-dissolved water. PLoS ONE 2017, 12, e0171192. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, H.K. Anti-diabetic effects of electrolyzed reduced water in streptozotocin-induced and genetic diabetic mice. Life Sci. 2006, 79, 2288–2292. [Google Scholar] [CrossRef]
- Novikasari, S.; Rias, Y.A.; Choriah, N. Effect of Alkaline Water pH 8 Against Fibroblasts and Collagen In Rattus norvegicus with Wound Conditions Hyperglycemia. In Proceedings of the 3rd AIPNEMA International Nusing Conference, East Java, Indonesia, 20–21 May 2017. [Google Scholar]
- Ogawa, S.; Shimizu, M.; Nako, K.; Okamura, M.; Ohsaki, Y.; Kabayama, S.; Tabata, K.; Tanaka, Y.; Ito, S. A Clinical Study on the Insulin Resistance Improvement Effects of Electrolyzed Hydrogen Rich Water in Type 2 Diabetes Patients: A Multicenter Prospective Double-Blind Randomized Control Trial. SSRN 2019. [Google Scholar] [CrossRef]
- Kajiyama, S.; Hasegawa, G.; Asano, M.; Hosoda, H.; Fukui, M.; Nakamura, N.; Kitawaki, J.; Imai, S.; Nakano, K.; Ohta, M. Supplementation of hydrogen-rich water improves lipid and glucose metabolism in patients with type 2 diabetes or impaired glucose tolerance. Nutr. Res. 2008, 28, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hamasaki, T.; Nakamichi, N.; Kashiwagi, T.; Komatsu, T.; Ye, J.; Teruya, K.; Abe, M.; Yan, H.; Kinjo, T.; et al. Suppressive effects of electrolyzed reduced water on alloxan-induced apoptosis and type 1 diabetes mellitus. Cytotechnology 2011, 63, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Siswantoro, E.; Purwanto, N.H. Effectiveness of Alkali Water Consumption to Reduce Blood Sugar Levels in Diabetes Mellitus Type 2. J. Diabetes Mellit. 2017, 7, 249. [Google Scholar] [CrossRef]
- LeBaron, T.W.; Singh, R.B.; Fatima, G.; Kartikey, K.; Sharma, J.P.; Ostojic, S.M.; Gvozdjakova, A.; Kura, B.; Noda, M.; Mojto, V. The Effects of 24-Week, High-Concentration Hydrogen-Rich Water on Body Composition, Blood Lipid Profiles and Inflammation Biomarkers in Men and Women with Metabolic Syndrome: A Randomized Controlled Trial. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 889. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.A.; Lee, D.H.; Cho, D.Y.; Lee, Y.J. Outcomes Assessment of Sustainable and Innovatively Simple Lifestyle Modification at the Workplace-Drinking Electrolyzed-Reduced Water (OASIS-ERW): A Randomized, Double-Blind, Placebo-Controlled Trial. Antioxidants 2020, 9, 564. [Google Scholar] [CrossRef] [PubMed]
- Gadek, Z.; Li, Y.; Shirahata, S. Influence of natural reduced water on relevant tests parameters and reactive oxygen species concentration in blood of 320 diabetes patients in the prospective observation procedure. In Animal Cell Technology: Basic & Applied Aspects; Springer: Dordrecht, The Netherlands, 2006; pp. 377–385. [Google Scholar] [CrossRef]
- Kang, K.M.; Kang, Y.N.; Choi, I.B.; Gu, Y.; Kawamura, T.; Toyoda, Y.; Nakao, A. Effects of drinking hydrogen-rich water on the quality of life of patients treated with radiotherapy for liver tumors. Med. Gas Res. 2011, 1, 11. [Google Scholar] [CrossRef]
- Dobashi, S.; Takeuchi, K.; Koyama, K. Hydrogen-rich water suppresses the reduction in blood total antioxidant capacity induced by 3 consecutive days of severe exercise in physically active males. Med. Gas Res. 2020, 10, 21. [Google Scholar] [CrossRef]
- Sung, K.; Bae, S. Effects of a regular walking exercise program on behavioral and biochemical aspects in elderly people with type II diabetes. Nurs. Health Sci. 2012, 14, 438–445. [Google Scholar] [CrossRef]
- Buse, J.B.; Wexler, D.J.; Tsapas, A.; Rossing, P.; Mingrone, G.; Mathieu, C.; D’Alessio, D.A.; Davies, M.J. 2019 update to: Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2020, 63, 221–228. [Google Scholar] [CrossRef]
- Sigal, R.J.; Armstrong, M.J.; Bacon, S.L.; Boulé, N.G.; Dasgupta, K.; Kenny, G.P.; Riddell, M.C. Physical activity and diabetes. Can. J. Diabetes 2018, 42, S54–S63. [Google Scholar] [CrossRef]
- Houjeghani, S.; Kheirouri, S.; Faraji, E.; Jafarabadi, M.A. l-Carnosine supplementation attenuated fasting glucose, triglycerides, advanced glycation end products, and tumor necrosis factor–α levels in patients with type 2 diabetes: A double-blind placebo-controlled randomized clinical trial. Nutr. Res. 2018, 49, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Sanaei, M.; Ebrahimi, M.; Banazadeh, Z.; Shafiee, G.; Khatami, F.; Ahadi, Z.; Heshmat, R. Consequences of AphanizomenonFlos-aqua e (AFA) extract (Stemtech TM) on metabolic profile of patients with type 2 diabetes. J. Diabetes Metab. Disord. 2015, 14, 50. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hays, R.D.; Morales, L.S. The RAND-36 measure of health-related quality of life. Ann. Med. 2001, 33, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Salim, S.; Yamin, M.; Alwi, I.; Setiati, S. Validity and Reliability of the Indonesian Version of SF-36 Quality of Life Questionnaire on Patients with Permanent Pacemakers. Acta Med. Indones. 2017, 49, 10–16. [Google Scholar] [PubMed]
- Candriasih, P. Estimation of Energy Intake Using Food Frequency Questionnaire (FFQ). Food Record and 24 Hours Food Recall at Primary School Children in Palu, Central Sulawesi. Master’s Thesis, Universitas Gadjah Mada, Yogyakarta, Indonesia, 2007. [Google Scholar]
- Sudargo, T.; Pertiwi, S.; Alexander, R.A.; Siswati, T.; Ernawati, Y. The relationship between fried food consumption and physical activity with diabetes mellitus in Yogyakarta, Indonesia. Int. J. Community Med. Public Health 2016, 4, 38–44. [Google Scholar] [CrossRef][Green Version]
- Lovibond, P.F.; Lovibond, S.H. The structure of negative emotional states: Comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav. Res. Ther. 1995, 33, 335–343. [Google Scholar] [CrossRef]
- Oei, T.P.S.; Sawang, S.; Goh, Y.W.; Mukhtar, F. Using the Depression Anxiety Stress Scale 21 (DASS-21) across cultures. Int. J. Psychol. 2013, 48, 1018–1029. [Google Scholar] [CrossRef]
- Shirinzadeh, M.; Afshin-Pour, B.; Angeles, R.; Gaber, J.; Agarwal, G. The effect of community-based programs on diabetes prevention in low-and middle-income countries: A systematic review and meta-analysis. Glob. Health 2019, 15, 10. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Ramulu, P.Y. Objectively measured physical activity and inflammatory markers among US adults with diabetes: Implications for attenuating disease progression. Mayo Clin. Proc. 2013, 88, 942–951. [Google Scholar] [CrossRef]
- Kurban, S.; Mehmetoglu, I.; Yerlikaya, H.F.; Gönen, S.; Erdem, S. Effect of chronic regular exercise on serum ischemia-modified albumin levels and oxidative stress in type 2 diabetes mellitus. Endocr. Res. 2011, 36, 116–123. [Google Scholar] [CrossRef]
- Teixeira-Lemos, E.; Nunes, S.; Teixeira, F.; Reis, F. Regular physical exercise training assists in preventing type 2 diabetes development: Focus on its antioxidant and anti-inflammatory properties. Cardiovasc. Diabetol. 2011, 10, 12. [Google Scholar] [CrossRef]
- Handelsman, Y.; Bloomgarden, Z.T.; Grunberger, G.; Umpierrez, G.; Zimmerman, R.S.; Bailey, T.S.; Blonde, L.; Bray, G.A.; Cohen, A.J.; Dagogo-Jack, S. American Association of Clinical Endocrinologists and American College of Endocrinology–clinical practice guidelines for developing a diabetes mellitus comprehensive care plan–2015. Endocr. Pract. 2015, 21, 1–87. [Google Scholar] [CrossRef]
- Kalousova, M.; Skrha, J.; Zima, T. Advanced glycation end-products and advanced oxidation protein products in patients with diabetes mellitus. Physiol. Res. 2002, 51, 597–604. [Google Scholar] [PubMed]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care 2016, 39, 2065–2079. [Google Scholar] [CrossRef] [PubMed]
- Eckert, K. Impact of physical activity and bodyweight on health-related quality of life in people with type 2 diabetes. Diabetes Metab. Syndr. Obes. Targets Ther. 2012, 5, 303. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.; Dressler, N.; Ben-Shushan, R.S.; Meerson, A.; LeBaron, T.W.; Tamir, S. Effects of alkaline-electrolyzed and hydrogen-rich water, in a high-fat-diet nonalcoholic fatty liver disease mouse model. World J. Gastroenterol. 2018, 24, 5095. [Google Scholar] [CrossRef] [PubMed]
- Fujita, R.; Tanaka, Y.; Saihara, Y.; Yamakita, M.; Ando, D.; Koyama, K. Effect of Molecular Hydrogen Saturated Alkaline Electrolyzed Water on Disuse Muscle Atrophy in Gastrocnemius Muscle. J. Physiol. Anthropol. 2011, 30, 195–201. [Google Scholar] [CrossRef][Green Version]
- Ohta, S. Molecular hydrogen is a novel antioxidant to efficiently reduce oxidative stress with potential for the improvement of mitochondrial diseases. Biochim. Biophys. Acta Gen. Subj. 2012, 1820, 586–594. [Google Scholar] [CrossRef]
- Kamimura, N.; Nishimaki, K.; Ohsawa, I.; Ohta, S. Molecular Hydrogen Improves Obesity and Diabetes by Inducing Hepatic FGF21 and Stimulating Energy Metabolism in db/db Mice. Obesity 2011, 19, 1396–1403. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Saihara, Y.; Izumotani, K.; Nakamura, H. Daily ingestion of alkaline electrolyzed water containing hydrogen influences human health, including gastrointestinal symptoms. Med. Gas Res. 2018, 8, 160–166. [Google Scholar] [CrossRef]
- Weidman, J.; Holsworth, R.E.; Brossman, B.; Cho, D.J.; Cyr, J.S.; Fridman, G. Effect of electrolyzed high-pH alkaline water on blood viscosity in healthy adults. J. Int. Soc. Sports Nutr. 2016, 13, 45. [Google Scholar] [CrossRef] [PubMed]
| Sociodemographic and Clinical Data | AEW Group (n = 20), n (%) | Walking Group (n = 20), n (%) | AEW and Walking Group (n = 20), n (%) | Control Group (n = 21), n (%) | p Value (x2) a |
|---|---|---|---|---|---|
| Age (years), (mean, SD) | 57.50 (5.48) | 54.70 (4.87) | 56.15 (4.96) | 55.71 (4.97) | 0.359 (1.089) b |
| Diabetes duration (years), (mean, SD) | 4.30 (1.87) | 4.25 (1.62) | 4.20 (1.51) | 4.19 (1.54) | 0.996 (0.019) b |
| Gender | |||||
| Female | 11 (55.0) | 13 (65.0) | 13 (65.0) | 13 (61.9) | 0.904 (0.565) |
| Male | 9 (45.0) | 7 (35.0) | 7 (35.0) | 8 (38.1) | |
| Marital status | |||||
| Married | 11 (55.0) | 9 (45.0) | 11 (55.0) | 13 (61.9) | 0.754 (1.194) |
| Non-married | 9 (45.0) | 11 (55.0) | 9 (45.0) | 8 (38.1) | |
| Income (IDR) | |||||
| Low income | 8 (40.0) | 10 (50.0) | 10 (50.0) | 12 (57.1) | 0.749 (1.216) |
| High income | 12 (60.0) | 10 (50.0) | 10 (50.0) | 9 (42.9) | |
| Education | |||||
| ISCED < 3 | 9 (45.0) | 8 (40.0) | 9 (45.0) | 12 (57.1) | 0.723 (1.325) |
| ISCED ≥ 3 | 11 (55.0) | 12 (60.0) | 11 (55.0) | 9 (42.9) | |
| Smoking status | |||||
| Smoker | 7 (35.0) | 5 (25.0) | 6 (30.0) | 7 (33.3) | 0.961 (1.484) |
| Secondhand smoking | 7 (35.0) | 9 (45.0) | 10 (50.0) | 9 (42.9) | |
| Non-smoker | 6 (30.0) | 6 (30.0) | 4 (20.0) | 5 (23.8) | |
| DASS-21 stress | |||||
| Yes (≥15 score) | 12 (60.0) | 12 (60.0) | 11 (55.0) | 12 (57.1) | 0.986 (0.146) |
| No (<15 score) | 8 (40.0) | 8 (40.0) | 9 (45.0) | 9 (42.9) | |
| DASS-21 anxiety | |||||
| Yes (≥8 score) | 11 (55.0) | 11 (55.0) | 11 (55.0) | 12 (57.1) | 0.999 (0.029) |
| No (<8 score) | 9 (45.0) | 9 (45.0) | 9 (45.0) | 9 (42.9) | |
| DASS-21 depression | |||||
| Yes (≥10 score) | 12 (60.0) | 10 (50.0) | 11 (55.0) | 12 (57.1) | 0.933 (0.434) |
| No (<10 score) | 8 (40.0) | 10 (50.0) | 9 (45.0) | 9 (42.9) | |
| Carbohydrate consumption score | |||||
| <17 | 10 (50.0) | 9 (45.0) | 9 (45.0) | 12 (57.1) | 0.846 (0.816) |
| ≥17 | 10 (50.0) | 11 (55.0) | 11 (55.0) | 9 (42.9) | |
| Protein consumption score | |||||
| <18 | 10 (50.0) | 8 (40.0) | 8 (40.0) | 11 (52.4) | 0.789 (1.051) |
| ≥18 | 10 (50.0) | 12 (60.0) | 12 (60.0) | 10 (47.6) | |
| Fat consumption score | |||||
| <20 | 10 (50.0) | 9 (45.0) | 9 (45.0) | 10 (47.6) | 0.987 (0.140) |
| ≥20 | 10 (50.0) | 11 (55.0) | 11 (55.0) | 11 (52.4) | |
| Fast food consumption score | |||||
| <9 | 12 (60.0) | 8 (40.0) | 7 (35.0) | 12 (57.1) | 0.293 (3.723) |
| ≥ 9 | 8 (40.0) | 12 (60.0) | 13 (65.0) | 9 (42.9) | |
| Fiber consumption score | |||||
| ≥2 | 9 (45.0) | 7 (35.0) | 8 (40.0) | 12 (57.1) | 0.521 (2.256) |
| <2 | 11 (55.0) | 13 (65.0) | 12 (60.0) | 9 (42.9) |
| Sociodemographic and Clinical Data | AEW Group (n = 20), Mean (SD) | Walking Group (n = 20), Mean (SD) | AEW and Walking Group (n = 20), Mean (SD) | Control Group (n = 21), Mean (SD) | p Value (F) a |
|---|---|---|---|---|---|
| AOPPs (ng/ml) | 0.38 (0.57) | 0.35 (0.43) | 0.53 (0.70) | 0.44 (0.65) | 0.794 (0.343) |
| AGEs (ng/L) | 0.35 (0.54) | 0.27 (0.32) | 0.46 (0.59) | 0.39 (0.58) | 0.715 (0.454) |
| MDA (nmol/ml) | 0.77 (0.42) | 0.85 (0.69) | 0.97 (0.67) | 0.96 (0.62) | 0.691 (0.489) |
| FBG (mg/dl) | 298.95 (48.84) | 303.95 (67.95) | 324.60 (59.63) | 287.24 (80.43) | 0.331 (1.159) |
| WBCs (103/µL) | 7.92 (0.51) | 8.19 (0.44) | 8.48 (0.93) | 8.19 (1.16) | 0.199 (1.589) |
| NLR | 2.63 (0.22) | 2.55 (0.18) | 2.61 (0.22) | 2.62 (0.23) | 0.691 (0.488) |
| SF36-PCS | 45.16 (6.34) | 45.16 (6.34) | 44.94 (6.82) | 43.99 (5.75) | 0.922 (0.161) |
| SF36-MCS | 46.52 (8.12) | 47.50 (9.01) | 46.43 (6.67) | 46.66 (8.99) | 0.976 (0.070) |
| SF36-Total QoL | 45.84 (6.22) | 46.33 (6.63) | 45.69 (4.64) | 45.33 (5.20) | 0.955 (0.107) |
| Variables | AEW Group (n = 20), Mean (SD) | Walking Group (n = 20), mean (SD) | AEW and Walking Group (n = 20), Mean (SD) | Control Group (n = 21), Mean (SD) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 8-Week | Diff (95% CI), p Value | Baseline | 8-Week | Diff (95% CI), p Value | Baseline | 8-Week | Diff (95% CI), p Value | Baseline | 8-Week | Diff (95% CI), p Value | |
| AOPPs (ng/ml) | 0.38 (0.57) | 0.28 (0.47) | −0.101 (−0.151~−0.045) ** | 0.35 (0.43) | 0.26 (0.32) | −0.089 (−0.145~−0.322) ** | 0.53 (0.70) | 0.30 (0.42) | −0.225 (−0.369~−0.080) ** | 0.44 (0.65) | 0.54 (0.67) | 0.83 (0.001~0.165) * |
| AGEs (ng/L) | 0.35 (0.54) | 0.27 (0.47) | −0.077 (−0.121~−0.029) ** | 0.27 (0.32) | 0.20 (0.26) | −0.072 (−0.110~−0.034) ** | 0.46 (0.59) | 0.28 (0.37) | −0.180 (−0.287~−0.073) ** | 0.39 (0.58) | 0.60 (0.80) | 0.218 (0.101~0.335) ** |
| MDA (nmol/ml) | 0.77 (0.42) | 0.46 (0.41) | −0.312 (−0.352~−0.272) ** | 0.85 (0.69) | 0.53 (0.58) | −0.321 (−0.386~−0.256) ** | 0.97 (0.67) | 0.47 (0.45) | −0.499 (−0.628~−0.370) ** | 0.96 (0.62) | 1.21 (0.75) | 0.253 (0.154~0.351) ** |
| FBG (mg/dl) | 298.95 (48.84) | 278.00 (49.03) | −20.950 (−24.470~−17.430) ** | 303.95 (67.95) | 288.00 (67.70) | −15.950 (−16.637~−15.263) ** | 324.60 (59.63) | 297.85 (58.89) | −26.750 (−27.697~−25.803) ** | 287.24 (80.43) | 331.19 (124.16) | 43.952 (−5.688~93.592) |
| WBCs (103/µL) | 7.92 (0.51) | 5.70 (0.51) | −2.220 (−2.473~−1.966) ** | 8.19 (0.44) | 6.34 (0.55) | −1.855 (−2.064~−1.645) ** | 8.48 (0.93) | 5.86 (0.78) | −2.620 (−2.832~−2.407) ** | 8.19 (1.16) | 8.42 (1.12) | 0.233 (0.121~0.346) ** |
| NLR | 2.63 (0.22) | 1.39 (0.29) | −1.235 (−1.297~−1.173) ** | 2.55 (0.18) | 1.36 (0.18) | −1.193 (−1.200~−1.186) ** | 2.61 (0.22) | 1.21 (0.16) | −1.392 (−1.437~−1.347) ** | 2.62 (0.23) | 3.09 (0.30) | 0.471 (0.329~0.612) ** |
| Variables | AEW Group (n = 20), Mean (SD) | Walking Group (n = 20), Mean (SD) | AEW and Walking Group (n = 20), Mean (SD) | Control Group (n = 21), mean (SD) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 8-Week | Diff (95% CI), p Value | Baseline | 8-Week | Diff (95% CI), p Value | Baseline | 8-Week | Diff (95% CI), p Value | Baseline | 8-Week | Diff (95% CI), p Value | |
| SF36- PCS | 45.16 (6.34) | 57.09 (4.84) | 11.938 (9.687~14.188) ** | 45.16 (6.34) | 60.81 (3.24) | 15.656 (12.966~18.346) ** | 44.94 (6.82) | 67.69 (3.10) | 22.750 (20.242~25.258) ** | 43.99 (5.75) | 42.11 (6.57) | −1.875 (−4.107~0.357) |
| SF36- MCS | 46.52 (8.12) | 47.21 (7.89) | 0.694 (−0.090~1.447) | 47.50 (9.01) | 61.37 (12.53) | 13.869 (10.397~17.341) ** | 46.43 (6.67) | 73.54 (5.81) | 27.113 (24.089~30.136) ** | 46.66 (8.99) | 47.84 (12.51) | 1.183 (−2.602~4.967) |
| SF36-Total QoL | 45.84 (6.22) | 52.16 (5.38) | 6.315 (5.108~7.523) ** | 46.33 (6.63) | 61.09 (7.15) | 14.763 (12.364~17.163) ** | 45.69 (4.64) | 70.62 (3.25) | 24.933 (23.090~26.776) ** | 45.33 (5.20) | 44.98 (8.33) | −0.346(−2.602~1.909) |
| Variable | Within-time Ref: Baseline ß (95% CI), p Value | Between Group | Interaction Group (AEW) × Time, Ref: (CG) × Time ß (95% CI), p Value | Interaction Group (Walking) × Time, Ref: (CG) × Time ß (95% CI), p Value | Interaction Group (both AEW and Walking) × Time, Ref: (CG) × Time ß (95% CI), p Value | ||
|---|---|---|---|---|---|---|---|
| AEW vs. CG ß (95% CI), p Value | Walking vs. CG ß (95% CI), p Value | AWE and Walking vs. CG ß (95% CI), p Value | |||||
| AOPPs (ng/ml) | 0.081 (−0.020~0.182) | −0.017 (−0.392~0.359) | −0.151 (−0.482~0.181) | 0.020 (−0.355~0.395) | −0.185 (−0.302~−0.068) * | −0.184 (−0.313~−0.055) * | −0.264 (−0.454~0.074) * |
| AGEs (ng/L) | 0.214 (0.094~0.333) ** | 0.006 (−0.338~0.350) | −0.157 (−0.444~0.130) | 0.028 (−0.305~0.362) | −0.298 (−0.433~−0.163) ** | −0.304 (−0.445~−0.163) ** | −0.364 (−0.546~−0.182) ** |
| MDA (nmol/ml) | 0.253 (0.144~0.362) ** | −0.138 (−0.467~0.191) | −0.152 (−0.524~0.220) | −0.040 (−0.400~0.321) | −0.566 (−0.691~−0.440) ** | −0.579 (−0.727~−0.431) ** | −0.716 (−0.909~−0.524) ** |
| FBG (mg/dl) | 36.878 (−2.639~76.396) | 10.640 (−15.734~37.013) | 3.200 (−21.894~28.294) | 20.499 (−10.877~51.874) | −54.583 (−96.085~−13.080) * | −49.680 (−92.196~−13.080) * | −57.223 (−99.111~−7.163) * |
| WBCs (103/µL) | 0.234 (0.098~0.369) ** | −0.395 (−0.977~0.187) | −0.032 (−0.601~0.538) | 0.269 (−0.404~0.941) | −2.406 (−2.687~−2.125) ** | −2.020 (−2.289~−1.752) ** | −2.833 (−3.119~−2.547) ** |
| NLR | 0.440 (0.353~0.526) ** | 0.025 (−0.101~0.152) | −0.091 (−0.221~0.039) | −0.034 (−0.163~0.095) | −1.666 (−1.771~−1.560) ** | −1.623 (−1.713~−1.533) ** | −1.798 (−1.897~−1.897) ** |
| Variable | Within-Time Ref: Baseline ß (95% CI), p Value | Between Group | Interaction Group (AEW) × Time, Ref: (CG) × Time ß (95% CI), p Value | Interaction Group (Walking) × Time, Ref: (CG) × Time ß (95% CI), p Value | Interaction Group (both AEW & walking) × Time, Ref: (CG) × Time ß (95% CI), p Value | ||
|---|---|---|---|---|---|---|---|
| AEW vs. CGß (95% CI), p Value | Walking vs. CGß (95% CI), p Value | Both vs. CGß (95% CI), p Value | |||||
| SF36- PCS | −1.794 (−4.301~0.714) | 1.366 (−2.335~5.068) | 0.908 (−2.892~4.708) | 0.670 (−3.174~4.514) | 14.034 (10.727~17.342) ** | 18.017 (14.170~21.865) ** | 24.483 (20.881~28.085) ** |
| SF36- MCS | 1.753 (−2.526~6.032) | 0.505 (−4.082~5.092) | 1.604 (−3.428~6.637) | 0.782 (−3.969~5.533) | −0.378 (−4.846~4.089) | 13.360 (7.522~19.198) ** | 25.649 (20.310~30.988) ** |
| SF36-Total QoL | −0.021 (−2.463~2.422) | 0.936 (−2.223~2.422) | 1.257 (−2.225~4.738) | 0.727 (−2.332~3.786) | 6.828 (4.099~9.557) ** | 15.689 (11.964~19.414) ** | 25.068 (21.961~28.175) ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rias, Y.A.; Kurniawan, A.L.; Chang, C.W.; Gordon, C.J.; Tsai, H.T. Synergistic Effects of Regular Walking and Alkaline Electrolyzed Water on Decreasing Inflammation and Oxidative Stress, and Increasing Quality of Life in Individuals with Type 2 Diabetes: A Community Based Randomized Controlled Trial. Antioxidants 2020, 9, 946. https://doi.org/10.3390/antiox9100946
Rias YA, Kurniawan AL, Chang CW, Gordon CJ, Tsai HT. Synergistic Effects of Regular Walking and Alkaline Electrolyzed Water on Decreasing Inflammation and Oxidative Stress, and Increasing Quality of Life in Individuals with Type 2 Diabetes: A Community Based Randomized Controlled Trial. Antioxidants. 2020; 9(10):946. https://doi.org/10.3390/antiox9100946
Chicago/Turabian StyleRias, Yohanes Andy, Adi Lukas Kurniawan, Ching Wen Chang, Christopher James Gordon, and Hsiu Ting Tsai. 2020. "Synergistic Effects of Regular Walking and Alkaline Electrolyzed Water on Decreasing Inflammation and Oxidative Stress, and Increasing Quality of Life in Individuals with Type 2 Diabetes: A Community Based Randomized Controlled Trial" Antioxidants 9, no. 10: 946. https://doi.org/10.3390/antiox9100946
APA StyleRias, Y. A., Kurniawan, A. L., Chang, C. W., Gordon, C. J., & Tsai, H. T. (2020). Synergistic Effects of Regular Walking and Alkaline Electrolyzed Water on Decreasing Inflammation and Oxidative Stress, and Increasing Quality of Life in Individuals with Type 2 Diabetes: A Community Based Randomized Controlled Trial. Antioxidants, 9(10), 946. https://doi.org/10.3390/antiox9100946