Abstract
Oxidative stress and inflammation play crucial roles in the development and progression of retinal diseases. Retinal damage by various etiologies can result in retinopathy of prematurity (ROP), diabetic retinopathy (DR), and age-related macular degeneration (AMD). n-3 fatty acids are essential fatty acids and are necessary for homeostasis. They are important retinal membrane components and are involved in energy storage. n-3 fatty acids also have antioxidant and anti-inflammatory properties, and their suppressive effects against ROP, DR, and AMD have been previously evaluated. α-linolenic acid (ALA), eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and their metabolites have been shown to alleviate retinal oxidative stress and inflammation involving various biological signaling pathways. In this review, we summarize the current understanding of the n-3 fatty acids effects on the mechanisms of these retinal diseases and how they exert their therapeutic effects, focusing on ALA, EPA, DHA, and their metabolites. This knowledge may provide new remedial strategies for n-3 fatty acids in the prevention and treatment of retinal diseases associated with oxidative stress and inflammation.
1. Introduction
Oxidative stress is defined as a disturbance of the balance between the production of free radicals and reactive metabolites: reactive oxygen species (ROS) and the protective mechanism by antioxidants, respectively. The products of oxidation or nitrosylation by ROS reduce biological activity and lead to the loss of energy metabolism, cell signaling, transport, and other key cellular functions. These altered products also target proteasome degradation, further reducing cellular function [1]. In addition, ROS are important signaling molecules that play an essential role in the progression of inflammation [2]. Oxidative stress induces the production of oxidized proteins and glycated products as well as lipid peroxidation, and results in an inflammatory response. In inflammation, hypoxia-inducible factor (HIF) is stabilized, which induces the sequential growth of blood vessels and enables the downstream transcription of angiogenic factors, including vascular endothelial growth factor (VEGF), activation of nuclear factor-kappa B (NF-κB), augmentation of cyclooxygenase (COX)-2 expression, and increased levels of proinflammatory cytokines, like tumor necrosis factor α (TNF-α) and interleukin (IL)-6 [3,4,5,6,7]. Therefore, ROS are involved in a wide spectrum of diseases, including Alzheimer’s disease, Parkinson’s disease, cancer, and diabetes mellitus [8,9,10,11,12,13]. Among ocular tissues, the retina has the highest oxygen consumption per gram of tissue in the human body and requires large amounts of adenosine triphosphate to support cellular functions. However, because of the high metabolism, the retina is vulnerable to oxidative stress damage [14]. For this reason, the retina can be a major site of ROS production and, thus, ROS have been reportedly involved in a variety of retinal diseases, including retinopathy of prematurity (ROP) [15], diabetic retinopathy (DR) [16], and age-related macular degeneration (AMD) [17]. However, the roles of oxidative stress in these disorders remain elusive and a therapeutic strategy has not been established.
Lipids provide energy storage and serve as structural components of cell membranes, ensuring the homeostasis of these barriers. Lipids can also act as signaling molecules, influencing many processes, including gene expression [18,19]. Long-chain n-3 (also called omega-3) fatty acids are particularly essential for the normal growth and neural development of the brain and eye [20,21]. Human bodies cannot efficiently synthesize n-3 fatty acids and, therefore, we need to consume foods that provide adequate amounts of n-3 fatty acids, including fish and fish oil products [22,23,24]. Several randomized controlled trials have shown that oral consumption of n-3 fatty acids is effective in improving inflammation, cardiovascular disease, and peripheral arterial disease [25,26,27,28]. Furthermore, n-3 fatty acids supplementation was recently shown to be beneficial in suppressing ocular diseases, possibly via antioxidant and anti-inflammatory effects [29].
In this review, we summarize current knowledge regarding the therapeutic effects of n-3 fatty acids and their mechanisms, focusing on the pathogenesis of retinal diseases associated with oxidative stress and inflammation.
2. The Metabolism of n-3 Fatty Acids and Their Suppressive Effects against Oxidative Stress and Inflammation
Eicosanoids generation by COX, lipoxygenase, and cytochrome P450 enzymes from arachidonic acid (AA) indicate that the eicosanoids can induce oxidative stress, inflammation, and vascular function as lipid mediators [30]. Although AA is also an essential component of cell membranes and plays an integral role in growth during fetal development [31], n-6 fatty acids, including AA, have been strongly linked to inflammation [32]. By contrast, n-3 fatty acids suppress oxidative stress and inflammation, and could even inhibit the generation of AA-derived eicosanoids [33].
Biological roles of n-3 fatty acids have been investigated with a focus on α-linolenic acid (ALA), docosahexaenoic acid (DHA), and eicosapentaenoic acid (EPA). Their multiple roles in homeostasis and suppressive effects on various diseases have been elucidated by recent studies [34,35,36]. DHA, one of the n-3 fatty acids, accounts for approximately 20% of the retinal weight and affects both the survival and development of neurons and retinal vascular cells [37,38,39,40]. EPA, one of the major dietary n-3 fatty acids, is present in the blood. Only a small amount of EPA is stored in human tissues, because EPA is rapidly metabolized in the biosynthesis of DHA and eicosanoids. Previous reports have implied that EPA suppresses AA-derived eicosanoids, which is associated with retinal neovascular abnormalities, vascular permeability, and inflammation [29]. ALA is present in vegetable oils like canola and soybean oil, nuts, and some green leafy vegetables, and is an essential component of human diets. Only a small amount of EPA and DHA can be synthesized from ALA by the human body because there are large amounts of n-6 fatty acids in the diet, which compete for the same enzymes [41,42,43]. DHA and EPA are produced by algae and other aquatic plants and are abundant in fish. Because it is difficult to obtain an adequate DHA and EPA intake through diet alone [44], DHA and EPA supplementation rather than of ALA is recommended [45].
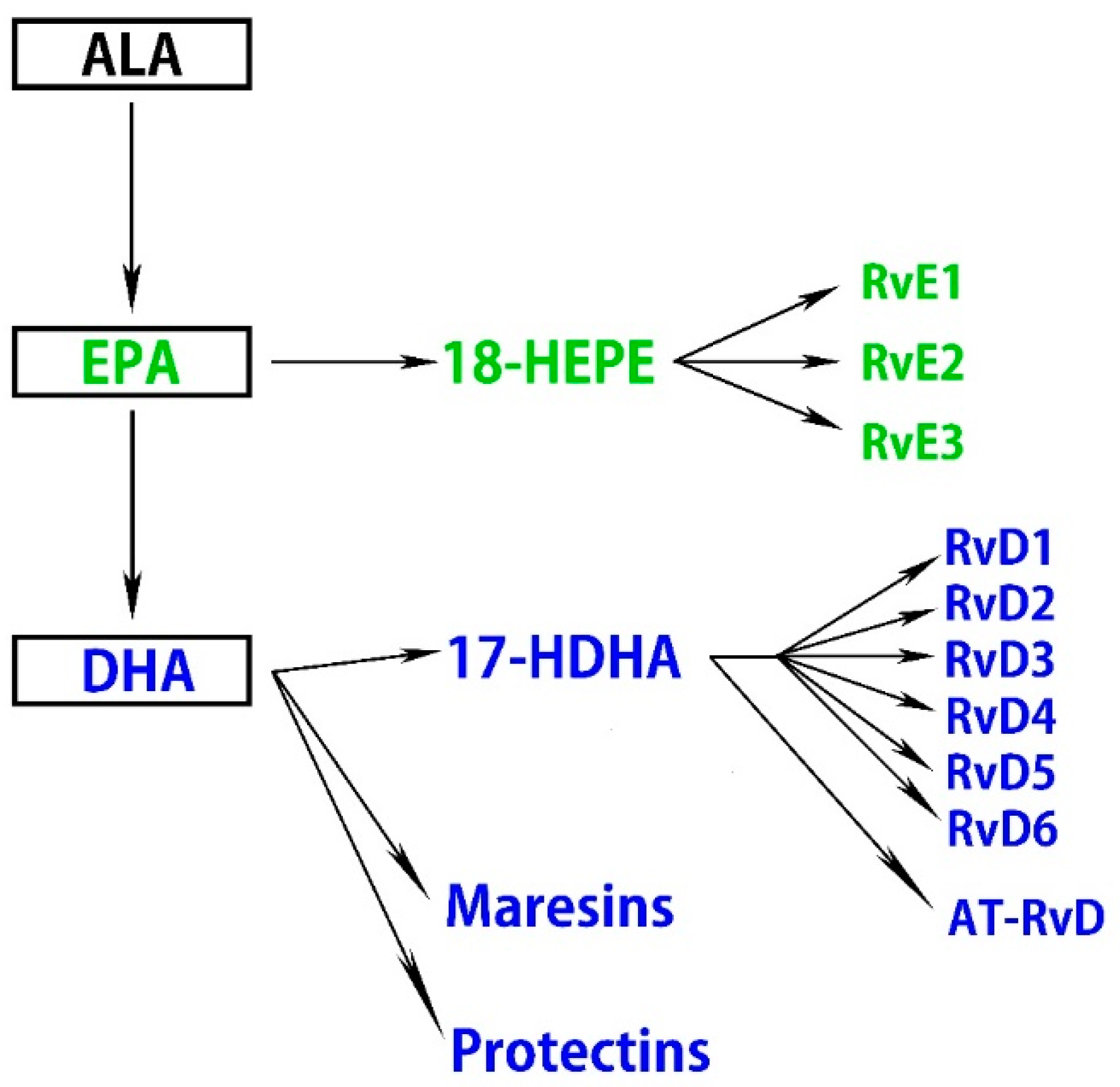
A high intake of n-3 fatty acids ameliorates retinal pigmented epithelial (RPE) and photoreceptor degeneration and N-retinyl-N-retinylidene ethanolamine (A2E) accumulation, partly by reducing the production of inflammatory eicosanoids, including prostaglandin E2 (PGE2) and leukotriene B4 (LTB4) [46]. Moreover, n-3 fatty acids attenuate ischemia-induced COX-2 and HIF expression, associated with alleviating neuronal damage [47]. ALA suppresses ROS production, chelates metal ions, inhibits NF-κB activation independent of its antioxidant function, and reduces the oxidized forms of other antioxidants, including vitamin C, vitamin E, and glutathione [48]. Some therapeutic effects of oral ALA administration have been reported, and it may have health benefits independent of its metabolites. ALA also exhibits retinal neuroprotective effects against oxidative stress and nuclear factor (erythroid-derived 2)-like 2 (Nrf2) signaling in retinal neurons [49]. DHA and EPA downregulate insulin-like growth factor-1 (IGF-1), attenuate the activation of NF-κB, IL-1β, VEGF, and TNF-α, and suppress inflammation in cytokine-stimulated human retinal vascular endothelial cells [50,51,52,53]. Several lines of evidence indicate that DHA and EPA improve nitric oxide (NO) bioavailability and decrease ROS production, which correlates with the suppression of VEGF-mediated angiogenic signaling and Nrf2 activation in a dose-dependent manner [54,55,56]. Another study demonstrated that DHA protects retinal photoreceptors from oxidative stress-induced apoptosis [57]. Furthermore, these anti-inflammatory effects of DHA are partly accomplished by downregulating sphingomyelinases; enzymes that catalyze the hydrolysis of sphingomyelin to proinflammatory ceramide [58]. Metabolites of n-3 fatty acids in addition to DHA and EPA also have bioactivity. Specialized proresolving mediators (SPMs) are part of a larger group of proresolving molecules. SPMs include maresins, protectins, and resolvins metabolized from DHA and EPA via 17-hydroxy docosahexaenoic acid (17-HDHA) and 18-hydroxyeicosapentaenoic acid (18-HEPE), respectively [59] (Figure 1).
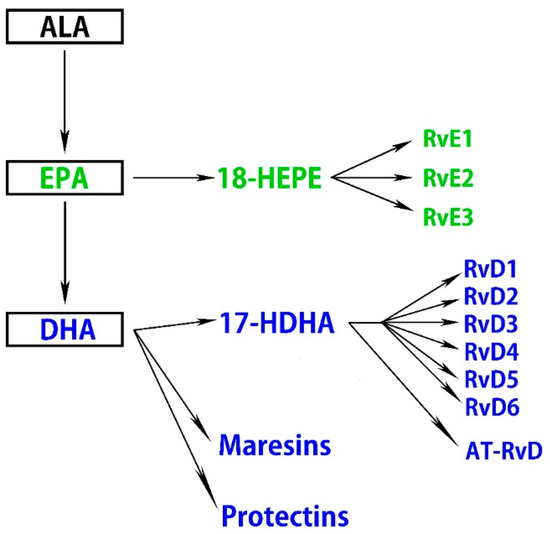
Figure 1.
n-3 fatty acids are metabolized to specialized proresolving mediators (SPMs).
It was previously confirmed in in vitro experiments that 17-HDHA and 18-HEPE inhibit TNF-α formation in the macrophage [60]. Maresin 1 (7R,14S-dihydroxy-docosa-4Z,8E,10E,12Z,16Z,19Z-hexaenoic acid), a recently identified lipid mediator generated endogenously by macrophage enzymes, is synthesized from DHA. It can regulate proinflammatory cytokines, including NF-κB, IL-1β, IL-6, and TNF-α [61]. Resolvins are produced from DHA and EPA and are denoted as the D-series (RvD1–RvD6) and E-series (RvE1–RvE3), respectively, while certain isomers of RvDs termed aspirin-triggered resolvin Ds (AT-RvDs) require acetylated COX-2 in the presence of aspirin or other nonsteroid anti-inflammatory drugs for their synthesis [62]. Resolvins exhibit their proresolving effects through specific G-protein coupled receptors (GPR). Currently, several receptors have been identified that recognize members of the D- and E-series of resolvins, including, D resolvin receptor 1 (DRV1)/GPR32, lipoxin A4 receptor/formyl peptide receptor 2, DRV2/GPR18, resolvin E1 receptor/ChemR23, and leukotriene B4 receptor 1 (BLT1) [63,64].
RvD1 inhibits ROS generation and suppresses oxidative stress-induced apoptosis of macrophages through the repression of NAPDH oxidase (NOX)2 activation and upregulation of anti-apoptotic protein expression [65]. RvD1 and RvD2 alleviate the expression of inflammatory cytokines, including TNF-α, IL-1α, IL-1β, CC-chemokine ligand 2 (CCL2), and IL-6, in addition to NF-κB and activator protein-1 [66]. AT-RvD1 reduces oxidative stress, lipid peroxidation, apoptosis, and NF-κB signaling in mice after hyperoxia [67]. It also displays an antioxidant effect via Nrf2 elevation [68]. RvE antagonizes BLT1; a proinflammatory receptor for LTB4 expressed in ocular macrophages and retinal glial and endothelial cells [69,70]. Furthermore, because BLT1 regulates ROS release and apoptosis, RvE1 might contribute to the inhibition of apoptosis as an antagonist of BLT1 [70]. Indeed, RvE1 modulates ROS generation by suppressing NOX2 activation [71]. It also attenuates proinflammatory signals, including NF-kB activation, and enhances phagocytosis [72]. Additionally, RvE1 serves as an agonist for ChemR23, which is present on the surface of retinal microglia, regulating TNF-α production as one of the potent regulators of angiogenesis [73,74].
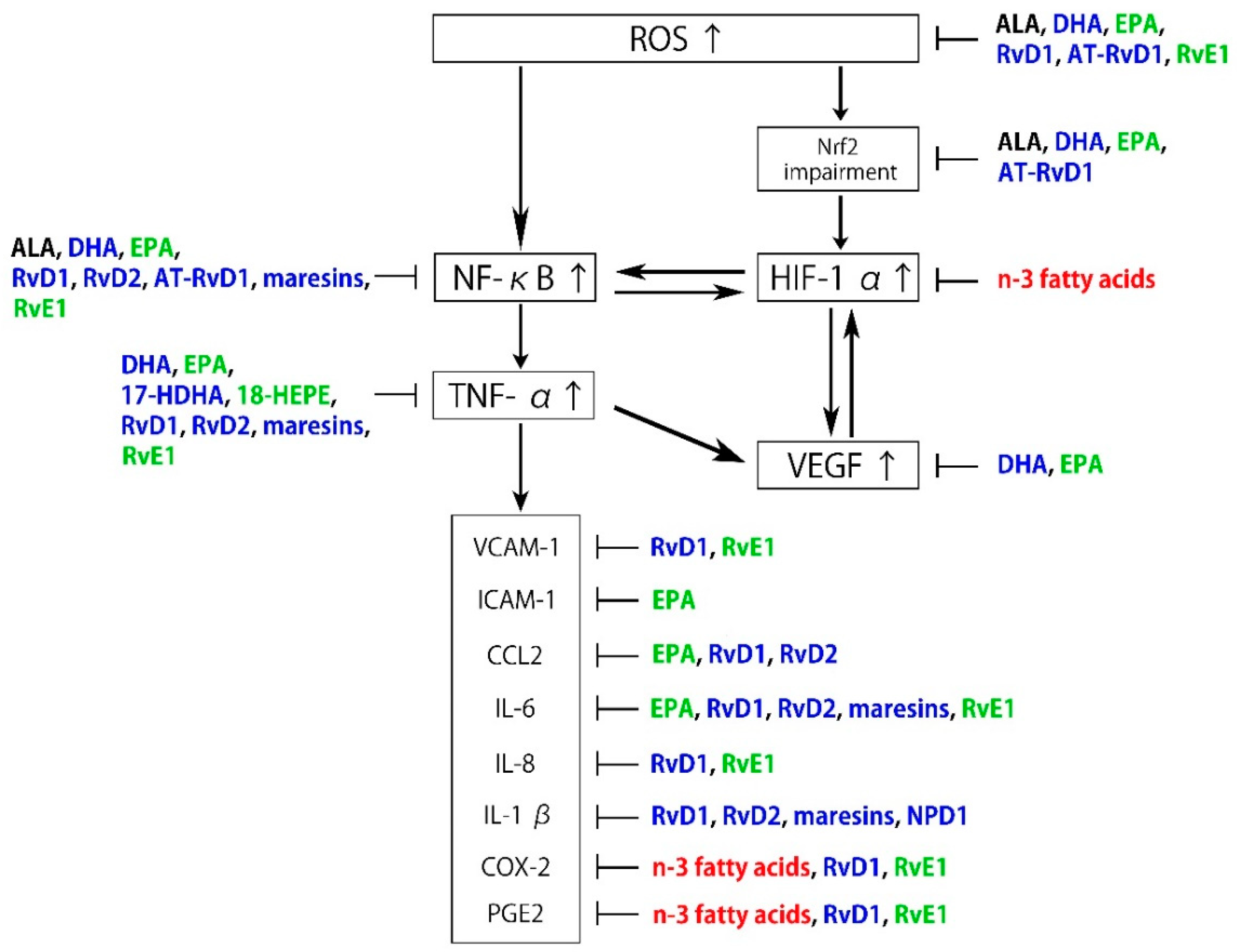
Neuroprotectin D1 (NPD1) is biosynthesized from DHA and also exhibits anti-inflammatory and neuroprotective activities [75], partly by interacting with GPR32 [76]. It has been proposed that NPD1 protects RPE cells from oxidative stress-induced apoptosis by inducing the phosphatidylinositol 3-kinase/Akt pathway and inhibiting IL-1β-stimulated COX-2 expression [77,78,79]. NPD1 synthesized in RPE cells also protects photoreceptors and retinal ganglion cells (RGCs) [80,81]. As described above, n-3 fatty acids and their metabolites are associated with the regulation of the pathogenesis of ROS and inflammation (Figure 2).
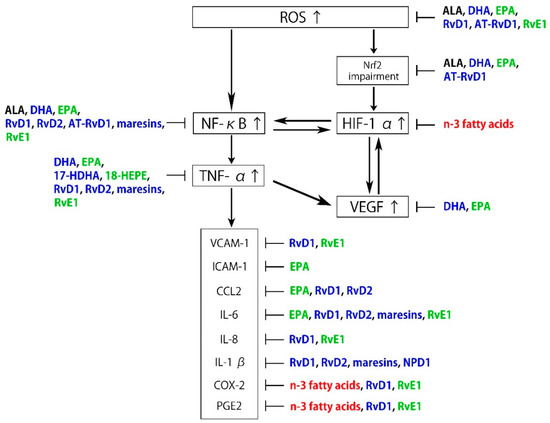
Figure 2.
Schematic representation of the inhibitory effects of n-3 fatty acids and specialized proresolving mediator (SPM) on reactive oxygen species (ROS) and inflammation. Black: α-linolenic acid (ALA), Blue: docosahexaenoic acid (DHA) and its metabolites, Green: eicosapentaenoic acid (EPA) and its metabolites, Red: n-3 fatty acids.
3. Pathology of Retinopathy of Prematurity (ROP) and Its Relationship to n-3 Fatty Acids
3.1. Pathogenesis of Retinopathy of Prematurity (ROP)
ROP is a retinal vascular disease leading to visual impairment and blindness in premature infants. Globally, approximately 190,000 preterm newborns are affected, 20,000 of whom become severely visually impaired or blind from ROP annually [82]. In the first stage of ROP, the normal retinal blood vessels that would grow in the womb are disrupted and a relative peroxidation state occurs when a premature infant is exposed to high oxygen in the incubator after birth [83]. The postnatal hyperoxic environment for premature neonates induces severe growth attenuation and vasodilation in tissues that have not yet fully developed; it reduces VEGF expression partly due to hyperoxia-induced HIF downregulation [84,85]. It is widely accepted that the use of supplemental oxygen, oxygen concentration and duration, and prolonged mechanical ventilation are risk factors, which contribute to ROP severity. Downregulated VEGF regresses the developing retinal vessels. The relative hypoxia that occurs as the infant is returned to normoxia after discontinuation of oxygen therapy worsens as the infant grows, resulting in hypoxic retinal damage. The neonatal retina becomes relatively hypoxic, leading to excessive VEGF production via HIF signaling [85,86,87]. Although HIF/VEGF signaling is particularly essential for fetal growth and vascular development under physiological hypoxia condition, excessive and/or ectopic HIF/VEGF causes retinal neovascularization sprouting towards the vitreous cavity. Retinal neovascularization causes tractional retinal detachment and retinal hemorrhage, which can lead to blindness [88]. These proliferative stages of ROP are strongly associated with IGF-1 [89,90]. IGF-1, which is sufficient to allow vessel growth in womb, is not maintained at in womb levels on premature birth, and vascular growth halts. As the premature infant matures, the retina without mature or sufficient vascularization suffers from hypoxia. When the IGF-1 concentration increases sufficiently to allow activation of the VEGF pathways, VEGF-driven endothelial cell proliferation may proceed and induce retinal pathological neovascularization [91].
The pathogenesis of ROP involves multiple signaling pathways induced by oxidative stress. NO production in the retina is increased in hypoxia by NF-κB, whereas administration of nitric oxide synthase (NOS) inhibitors and gene deletion of endothelial NOS effectively reduced ROP severity, suggesting that endogenous NO plays an important role in ROP [92,93,94]. Additionally, NOX is involved in retinal angiogenesis through ROS generation [95]. NOX4 is highly expressed in retinal endothelial cells and regulates VEGF-induced ROS in an animal model representative of ROP, enhancing endothelial cell proliferation [96]. Furthermore, the overproduced ROS causes neovascularization in the retina by activating the Janus kinase-signal transducer/activator of the transcription signaling pathway [97,98,99].
In addition to these oxidative stresses, inflammation is a key modulator in the development and progression of ROP [100,101]. Animal studies have proposed that retinal inflammation leads to abnormal retinal vascular development, suggesting that inflammation is involved in ROP pathogenesis [102,103,104]. In addition, recent investigations have demonstrated that the IL-1 family, consisting of both pro- and anti-inflammatory cytokines, is pivotal in ROP pathogenesis, and that increased complement activation induced microglia activation, which leads to increased inflammation [105,106,107]. Additionally, the NF-κB, IL-6, and TNF-α expression levels were significantly elevated in the retinas of oxygen-induced retinopathy (OIR) rats, which is a widely used ROP model [108].
3.2. Therapeutic Effects of n-3 Fatty Acids on Retinopathy of Prematurity (ROP)
Numerous investigations using the OIR model have shown that n-3 fatty acids administration during the neovascular phase significantly reduces neovascularization without altering vasodilation or normal vessel regeneration [109]. Their supplementation is a potent modifier of IGF-1 and reduces OIR severity [110]. n-3 fatty acids suppressed TNF-α expression in the OIR model, decreasing the size of the retinal avascular area [74,111].
Additionally, DHA administration reduced lipid peroxidation markers in a piglet model of severe hypoxia-reoxygenation, indicating the established benefit of neuroprotection from oxidative stress [112]. Regarding the SPMs, NPD1, RvD1, and RvE1, they presented significant protection from vaso-obliteration and neovascularization through TNF-α expression regulation in OIR model mice [74]. These studies suggest that n-3 fatty acids may contribute directly to protective effects against the molecular pathogenesis of ROP.
n-3 fatty acids are deficient in preterm infants because of their maternal origin [113,114]. Therefore, exogenous administration of n-3 fatty acids is necessary for such infants. In a double-blind parallel clinical trial, preterm infants with a birth weight between 1000 and 1500 g were assessed to define the effect of DHA on ROP. Infants receiving 75 mg DHA/kg/d displayed significantly reduced severe (stage 3) ROP compared with those receiving high oleic sunflower oil, which is rich in n-6 fatty acids [115]. Another randomized double-blinded controlled trial enrolling 160 premature infants with a gestational age of less than 32 weeks and birth weight of <1500 g found that 300 mg/d n-3 fatty acids could lower ROP frequency and severity [116]. Furthermore, n-3 fatty acid administration resulted in less laser treatment against retinal pathological neovascularization, suggesting that n-3 fatty acids may prevent the development of aggressive ROP [117,118]. By contrast, intravenous administration of fish-oil-based lipid emulsion (SmofLipid®, Fresenius Kabi), a blend of plant- and animal-based lipid emulsions with a higher n-6/n-3 fatty acids ratio than in the diet, resulted in less ROP but did not alter the requirement for laser treatment or the ROP frequency [119,120]. These conflicting results may stem from the optimal ratio of n-6 fatty acids, such as AA, to n-3 fatty acids for the developmental stage in preterm infants [121]. Recent randomized controlled clinical trials have studied the effect of n-3 fatty acid or a combination of DHA and AA on ROP outcome and may help to solve these problems (ClinicalTrials.gov Identifier: NCT02486042, NCT03201588).
4. Pathology of Diabetic Retinopathy (DR) and Its Relationship to n-3 Fatty Acids
4.1. Pathogenesis of Diabetic Retinopathy (DR)
Diabetes mellitus is a chronic degenerative disease characterized by hyperglycemia and is one of the most major public health challenges, with a global prevalence approaching 400 million [122]. It is associated with macrovascular and microvascular complications, including coronary artery disease, stroke, neuropathy, nephropathy, and retinopathy [123]. DR is a potentially blinding complication of diabetes and a significant cause globally of visual impairment [124]. The retina because of its anatomical and physiological specialization required for vision is subject to specific constraints compared with other nervous system tissues and may be vulnerable to diabetes mellitus, in which neurotransmitter production is inhibited and a proapoptotic or proinflammatory response is induced [125]. Approximately 33% of diabetic patients display signs of DR and 10% have vision-threatening stages of retinopathy [124,126].
Chronic hyperglycemia is a significant risk factor for the long-term progression of DR according to the Wisconsin Epidemiologic Study of Diabetic Retinopathy, the United Kingdom Prospective Diabetes Study, and the Barbados Eye Studies [127,128,129]. However, notably, observational follow-up of the Epidemiology of Diabetes Interventions and Complications study found that hemoglobin A1c levels did not necessarily correlate with the risk for further retinopathy four years after the completion of the Diabetes Control and Complications Trial [130]. Furthermore, recent studies have reported that small glutamyl transpeptidase -binding proteins and “glycemic memory”, which can persist for a long time even after the blood glucose concentration returns to normal, play a crucial role in DR [131,132]. Therefore, the involvement of another pathological factor is implicated in addition to hyperglycemia.
Recent studies have shown that oxidative stress is an influential factor in diabetic complications [133,134]. Metabolic abnormalities caused by hyperglycemia can lead to excessive ROS production, and ROS accumulation induces oxidative stress, damaging the tissues in and around retinal blood vessels and ultimately leading to DR. NOX2 is the predominant source of cytosolic ROS and the cytosolic ROS signaling is activated in the early stages of diabetes, leading to mitochondrial damage [135,136,137]. Mitochondria are a major source of ROS and mitochondrial ROS overproduction results in four classical mechanisms; the polyol, hexosamine, protein kinase C, and advanced glycation end-product pathways [138,139,140,141,142].
The activation of various pathways, including the renin-angiotensin system (RAS), inflammatory pathways mediated by NF-κB and HIF-1, and Nrf2 antioxidant defense system, have been studied and found to be involved in apoptosis and angiogenesis. RAS regulates pathological angiogenesis and inflammation as well as the systemic blood pressure, which is termed “tissue RAS” [143,144]. Recently, the involvement of the (pro)renin receptor ((P)RR) in the activation of the retinal RAS and its intracellular signaling has been elucidated, and it has been suggested that selective (P)RR targeting in particular may be a promising objective for DR therapy [145]. Additionally, angiotensin II (ANG-II) is the key product of RAS and stimulates NOX-derived ROS formation, directly damaging endothelial cells [95,146]. In the diabetic retina, upregulated ANG-II production, which leads to neuronal extracellular-signal-regulated kinase (ERK) activation, results in synaptophysin degradation, suggesting that RAS also directly affects neurons [147]. Regarding NF-κB, recent studies have reported that not only ROS but also ANG-II activates NF-κB, which in turn promotes the expression of VEGF and proinflammatory mediators, including intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), CCL2, and COX-2 [148,149,150]. In addition, several lines of evidence indicate that HIF-1 regulates VEGF expression, which is a crucial factor affecting angiogenesis in DR pathogenesis [151]. Nrf2 initiates the transcription and expression of various downstream detoxification and antioxidant enzymes, which is impaired under the diabetic environment [152]. Recently, it was determined that Nrf2 is expressed in human retina, and diabetic Nrf2-knockout mice displayed aggravated retina-blood barrier dysfunction, leading to the release of inflammatory factors and exacerbated neuronal dysfunction [153]. Therefore, the suppression of ROS production by these pathways as a novel therapeutic strategy has been investigated in several studies.
4.2. Therapeutic Effects of n-3 Fatty Acids on Diabetic Retinopathy (DR)
Several studies using streptozotocin (STZ)-induced diabetic rats, which is a widely used diabetic animal model, concluded that ALA supplementation can prevent pericyte loss, ameliorate oxidative stress, normalize NF-κB activation, and reduce VEGF expression in the diabetic retina [154,155,156]. Additionally, STZ-induced diabetic rats have significantly higher IL-6 levels and lower brain-derived neurotrophic factor (BDNF) levels in the serum, both of which are recovered to near normal by ALA administration, indicating that it provides both, anti-inflammatory and neuroprotective effects [157]. Furthermore, ALA inhibits RGCs loss and reduces the thinning of the inner and outer retinal layers in STZ-induced diabetic mice [158]. Various studies demonstrated reduced levels of neurotrophic factors, including BDNF, which are essential for retinal neuronal cell survival in diabetic patients as well as in diabetic animals with increased oxidative stress [159,160,161]. Consistent with these results, intravitreal 18-HEPE administration also induced BDNF upregulation and led to neuroprotection in early-stage DR [162].
DHA also has suppressive effects against DR pathogenesis. The oil mixture of DHA and EPA protected RPE cells against the high glucose-induced reduction of mitochondrial dehydrogenase activity and ROS and TNF-α overproduction [163]. A single EPA administration improved vascular endothelial function in type 2 diabetic Otsuka Long-Evans Tokushima fatty rats by inhibiting ERK, decreasing NF-κB activation, and reducing COX-2 expression [164]. In addition, oral EPA administration alleviated retinal neurodegeneration via BDNF in Müller glia cells in the early DR stages [162]. Clinically, a randomized controlled trial conducted in patients with diabetic macular edema (DME), which was induced by ROS and DR inflammation, found that the combined administration of oral DHA and intravitreal anti-VEGF significantly improved DME compared with a single treatment with anti-VEGF drugs. This anatomical improvement in central retinal thickness was accompanied by an amelioration of visual acuity [165]. Another clinical trial concluded that EPA administration in type 2 diabetic patients increases endogenous antioxidant enzymes, including superoxide dismutase and glutathione peroxidase, and decreases the level of malondialdehyde, a classic biomarker of oxidative stress [166].
n-3 fatty acids also displayed DR attenuation, partly by suppressing rod photoreceptor and inner retinal dysfunction in STZ-induced diabetic rats [167]. In the leptin-receptor-deficient (db/db) mouse, which is a major rodent model of type 2 diabetes mellitus, n-3 fatty acids significantly preserved retinal function, whereas retinal function gradually deteriorated in db/db mice on an n-6 fatty-acidsrich diet [168]. Moreover, it was reported that a low tissue n-3/n-6 fatty acids ratio can be a high-risk factor for many diseases, with one previous study showing a decreasing trend in the n-3/n-6 fatty acids ratio in human donor retinas with DR in comparison with age-matched control retinas [169,170]. Interestingly, the n-3/n-6 fatty acids ratios in retina and serum of Nile rat and Akita mouse, type 2 and type 1 diabetes mellitus animal models, respectively, improved significantly by dietary supplementation of n-3 fatty acids [170]. In a randomized controlled trial, a six-year follow-up analysis in individuals with type 2 diabetes mellitus elucidated that a dietary intake of a minimum of 500 mg/d n-3 fatty acids could reduce the risk for developing DR by 48% [171]. The results from a cross-sectional study evaluating multiethnic Asian adults with type 2 diabetes mellitus showed that higher fish consumption, and thus n-3 fatty acids intake, was significantly associated with reduced odds for severe DR and was correlated with wider retinal vascular caliber in diabetes mellitus patients without retinopathy [172]. Given previous reports describing that narrower retinal vascular diameters are associated with an increased risk for DR, n-3 fatty acids may be effective not only in halting DR progression but also in preventing DR preclinically [173,174].
Maresin 1 can inhibit ROS generation induced by high glucose in a dose-dependent manner [175,176,177]. In addition, maresin-like mediators (14,22-dihydroxy-docosa-4Z, 7Z, 10Z, 12E, 16Z, 19Z-hexaenoic acids) ameliorated impaired macrophage function by high glucose, suppressing the chronic inflammation in diabetic wounds [176]. RvD1 also decreased NF-κB levels in photoreceptors stimulated with high glucose and modified VEGF content in exosomes released by photoreceptors [178]. Additionally, BLT1 expression was increased in diabetic mice retina and retinal glial cells exposed to high glucose also demonstrated enhanced BLT1 expression, whose antagonist RvE1 attenuated its ROS pathway and apoptosis signaling [70,179]. It was also elucidated that intravitreal RvD1 injection suppresses NF-κB activation and downregulates the retinal IL-1β levels of the STZ-induced diabetic rats [180].
5. Pathology of Age-Related Macular Degeneration (AMD) and Its Relationship to n-3 Fatty Acids
5.1. Pathogenesis of Age-Related Macular Degeneration
AMD is the leading cause of irreversible blindness in patients aged over 50 years in developed countries, affecting 170 million globally [181]. Because aging is one of the greatest risk factors, the disease prevalence is expected to increase with the aging of society [182]. There are two types of AMD: “dry” and “wet”. Dry AMD is a chronic disease that results in vision loss because of the “geographic atrophic” death of photoreceptors and RPE cells [183]. Wet, or neovascular, AMD can also cause significant vision loss by the formation of choroidal neovascularization (CNV), as a result of pathological angiogenesis [184]. In both types, oxidative stress is strongly implicated in their pathogenesis [185,186]. While an adequate oxygen supply to the retina is necessary to maintain retinal functions, a high supply also induces retinal ROS production. Increased ROS levels and attenuated antioxidant cellular defense systems in RPE cells lead to AMD pathogenesis [187,188,189].
These sources of ROS include oxidative loads from cigarette smoking and high-energy light exposure. Several systematic reviews have found smoking to be a major risk factor for AMD [190,191,192]. Additionally, recent in vitro studies have shown that exposure to cigarette smoking induces a dose- and time-dependent increases in endoplasmic reticulum stress markers, enhanced ROS, and apoptosis of RPE cells [193,194,195]. Furthermore, sunlight exposure is a significant risk for AMD [196]. Indeed, RPE cells are particularly susceptible to wavelengths within the blue region of the solar spectrum and blue light is the most energetic radiation reaching the macula, resulting in RPE cell apoptosis and necrosis [197,198,199].
Drusen formation is a manifestation of early-stage AMD, but little is known about its origin. Some hypotheses suggest that a significant amount of drusen originates from blood, while others suggest that it is derived from cellular debris processed from photoreceptor outer segments and RPE cells [200]. The photoreceptor is an abundant source of DHA-containing phospholipids, which are highly susceptible to damage by increased ROS and oxidative stress levels [201,202]. Active phagocytosis of photoreceptor outer segments by the RPE cells removes oxidatively damaged photoreceptor discs, but impairment of these functions by aging involve the accumulation of toxic proteins, including lipofuscin and extracellular drusen [203,204,205]. A2E is one of the retinoid components of lipofuscin and is involved in blue light-induced RPE cell apoptosis, inflammatory changes, and inducing VEGF expression [206,207,208,209,210]. Chronic inflammation, a prolonged response that can result in tissue damage when the protective response becomes dysfunctional, is also thought to be a contributory factor to AMD. Among proangiogenic factors and inflammatory cytokines, VEGF, CCL2, HIF, and IL-8 are reportedly crucial factors associated with CNV formation [211]. In CNV formation, VEGF induces the proliferation of vascular endothelial cells and promotes macrophage migration [212]. Macrophages/microglia which infiltrate the CNV regions secrete IL-6 and TNF-α, and the attenuation of macrophage migration by blocking CCL2 and downregulating the HIF-1α/VEGF pathway suppresses the leakage and reduce the area of laser-induced CNV, a widely used animal model of neovascular AMD [213,214]. Surgically removed human CNV membranes also indicated that TNF-α derived from macrophages facilitates pathologic angiogenesis in AMD [215]. A previous study reported elevated CCL2, IL-6, and IL-8 levels in the intraocular sample from neovascular AMD patients, and the levels were related to CNV lesion size [216]. Moreover, levels of ICAM-1 and VCAM-1, which contribute to CNV development by means of strong leukocyte-endothelial interactions and cell migration, were elevated in patients with neovascular AMD [217]. Additionally, NF-κB, an essential regulator of IL-6 and PGE2, and COX-2, whose selective antagonist inhibits subretinal fibrosis, are both further crucial regulators of CNV. [19,218,219,220]. Compelling evidence also suggests that ocular infiltration of a specific type of macrophages (M2 macrophage) via the LTB4-BLT1 signaling pathway is intimately involved in the development of laser-induced CNV [69].
5.2. Therapeutic Effects of n-3 Fatty Acids on Age-Related Macular Degeneration (AMD)
EPA application substantially reduced ICAM-1 and CCL2 expression in endothelial cells and VEGF and IL-6 expression in macrophages [221]. EPA-fed mice exhibited prominently decreased ICAM-1, CCL2, VEGF, and IL-6 expression and production in the RPE-choroid, resulting in significant suppression of laser-induced CNV. A previous study using the Ccl2(−/−)/Cx3cr1(−/−) mouse exhibiting focal deep retinal lesions, abnormal RPE, photoreceptor degeneration, and A2E accumulation, demonstrated that a high n-3 fatty acid diet slowed the progression of AMD-like retinal lesions and reduced retinal TNF-α and IL-6 expression levels [46]. A previous study using the Ccl2(−/−) mouse revealed that n-3 fatty acids administration increased EPA and AA in the blood and retina, and decreased retinal NF-κB expression, accompanied by increased outer layer thickness [222].
DHA selectively enhances NPD1 synthesis and release through the apical surface of RPE cells [223]. Additionally, NPD1 reduced the leakage area and vascular endothelial cell volume in a laser-induced mouse model of CNV concomitant with the redistribution and ramification of microglia [224,225]. Regarding other SPMs, RvD1 and RvE1 reduced the expression of IL-8, IL-6, PGE2, COX-2, and VCAM-1 induced in choroid–retinal endothelial cells and leukocytes after inflammatory stimulation [226].
An epidemiological study concluded that high plasma total n-3 fatty acids significantly reduced the probability of developing late AMD by 38% [227]. Given that low n-3/n-6 fatty acids ratios occur in human retinas with macular degenerations and the imbalance of n-3/n-6 fatty acids may be involved in AMD pathogenesis, appropriate oral intake of lipids may contribute to AMD management because the n-3 fatty acids concentration in the retina can be modified by dietary composition [228,229]. A recent prospective clinical trial reported that n-3 fatty acids supplementation significantly lowered intravitreal VEGF-A levels in neovascular AMD patients [230]. A prospective cohort study with a mean follow-up time of 4.6 years showed that higher n-3 fatty acids intake was associated with a lower risk for AMD progression [231]. Another large prospective cohort study demonstrated that regular DHA and EPA intake reduced the 10-year incidence of visually significant AMD by 35–45%, indicating that dietary n-3 fatty acids intake may be advantageous for the primary prevention of AMD [232]. With respect to the protective effect on early AMD, the Blue Mountains Eye Study indicated that the dietary intake of n-3 fatty-acid-rich fish at least once weekly was associated with a reduced risk for developing early-stage AMD [233]. Another large prospective cohort study with a 24–28-year follow-up also indicated that a high DHA or EPA intake was correlated with a 17–40% reduction in the risk for visually significant intermediate AMD [234]. Moreover, in the previous meta-analysis and systematic review evaluating 4202 cases with 128,988 individuals from eight cohort studies, a linear association was revealed between n-3 fatty-acid-rich fish consumption and risk for AMD, and higher fish consumption was found to be associated with a lower risk for both, early and late AMD [235]. These results were consistent with the conclusion of the Age-Related Eye Disease Study (AREDS), which retrospectively evaluated the effect of dietary n-3 fatty acids intake on AMD severity and indicated that higher n-3 fatty acids and fish intake was associated with lower odds for neovascular AMD [236].
Some clinical investigations have failed to verify any impact of n-3 fatty acids on progression to advanced AMD. The AREDS2, a randomized, double-masked, controlled trial, was a follow-up study from AREDS designed to prospectively evaluate the impact of n-3 fatty acids supplementation on AMD progression. Participants enrolled in the study were at high risk for late AMD progression, ranging from bilateral large drusen to large drusen in one eye and late AMD in the fellow eye. DHA and EPA supplementation plus the AREDS formulation (antioxidant vitamins C and E, beta carotene, and zinc) displayed no statistically significant reduction in progression to advanced AMD [237]. Correspondingly, another clinical study also concluded that high DHA and EPA intakes, which may prevent or delay the occurrence of visually significant intermediate AMD, were not associated with a reduction in the risk for advanced AMD [234]. The Nutritional AMD treatment-2 (NAT-2) study, conducted in patients with early lesions of age-related maculopathy in the study eye and neovascular AMD in the fellow eye, indicated that oral DHA and EPA supplementation did not modify the rate of visual acuity changes or the time to onset or incidence of CNV in the study eye over the three-year study period [238].
Similarly, the post-hoc subgroup analysis from the NAT-2 study reported that DHA supplementation was not significantly associated with drusen count, total diameter, or total area progression on fundus photographs [239]. The divergence in the results of these studies may be influenced by the difference in an uncontrolled baseline diet or basal nutritional status of the participants. Additional randomized controlled trials with low rates of loss to follow-up and consistently good adherence to treatment regimens are required to examine the benefit of n-3 fatty acids for AMD.
6. Future Perspectives
Experimental studies and clinical investigations suggest the therapeutic effects of n-3 fatty acids on pathological stages of ROP, DR, and AMD (Table 1). More detailed investigations to elucidate the complex interaction between oxidative stress/inflammation and lipids are required. A better understanding of the mechanisms of lipids acting on the retina and in retinal disorders may allow the establishment of more effective n-3 fatty acids administration.

Table 1.
The therapeutic effects of the different n-3 fatty acids, the models in which they are tested, and the mechanisms targeted.
Author Contributions
Conceptualization, A.S., R.T., and H.K.; writing—original draft preparation, A.S. and R.T.; writing—review and editing, H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We thank Robert Blakytny, DPhil, from Edanz Group (https://en-author-services.edanzgroup.com/ac) for editing a draft of this manuscript. This work was partially supported by Grants-in-Aid for Scientific Research (C) (H.K., 19K09988) from JSPS KAKENHI (http://www.jsps.go.jp/), the Eye Research Foundation for the Aged (ERFA, H.K.), the Charitable Trust Fund for Ophthalmic Research in Commemoration of Santen Pharmaceutical’s Founder (H.K.), the Bayer Retina Award Foundation (H.K.), and Ichihara International Scholarship Foundation (H.K.).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| 17-HDHA | 17-hydroxy docosahexaenoic acid |
| 18-HEPE | 18-hydroxyeicosapentaenoic acid |
| A2E | N-retinyl-N-retinylidene ethanolamine |
| AA | arachidonic acid |
| ALA | α-linolenic acid |
| AMD | age-related macular degeneration |
| ANG-II | angiotensin II |
| AREDS | the Age-Related Eye Disease Study |
| AT-RvD | aspirin-triggered resolvin D |
| BDNF | brain-derived neurotrophic factor |
| BLT1 | leukotriene B4 receptor 1 |
| CCL2 | CC-chemokine ligand 2 |
| CNV | choroidal neovascularization |
| COX-2 | cyclooxygenase-2 |
| db/db | leptin-receptor-deficient |
| DHA | docosahexaenoic acid |
| DM | diabetes mellitus |
| DME | diabetic macular edema |
| DR | diabetic retinopathy |
| DRV1 | D resolvin receptor 1 |
| EPA | eicosapentaenoic acid |
| ERK | extracellular-signal-regulated kinase |
| GPR | G-protein coupled receptors |
| HIF | hypoxia-inducible factor |
| ICAM-1 | intercellular adhesion molecule-1 |
| IGF-1 | insulin-like growth factor-1 |
| IL | interleukin |
| LTB4 | leukotriene B4 |
| NAT-2 study | the Nutritional AMD treatment-2 study |
| NO | nitric oxide |
| NOS | nitric oxide synthase |
| NOX | NAPDH oxidase |
| NPD1 | neuroprotectin D1 |
| Nrf2 | nuclear factor (erythroid-derived 2)-like 2 |
| NSAIDs | nonsteroidal anti-inflammatory drugs |
| OIR | oxygen-induced retinopathy |
| PGE2 | prostaglandin E2 |
| (P)RR | (pro)renin receptor |
| RAS | renin-angiotensin system |
| RGC | retinal ganglion cell |
| ROP | retinopathy of prematurity |
| ROS | reactive oxygen species |
| RPE | retinal pigmented epithelial |
| SPM | specialized proresolving mediator |
| STZ | streptozotocin |
| TNF-α | tumor necrosis factor α |
| VCAM-1 | vascular cell adhesion molecule-1 |
| VEGF | vascular endothelial growth factor |
References
- Vincent, A.M.; Russell, J.W.; Low, P.; Feldman, E.L. Oxidative Stress in the Pathogenesis of Diabetic Neuropathy. Endocr. Rev. 2004, 25, 612–628. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.L.; Jiang, B.; Rue, E.A.; Semenza, G.L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 1995, 92, 5510–5514. [Google Scholar] [CrossRef] [PubMed]
- Hartnett, M.E. Pathophysiology and mechanisms of severe retinopathy of prematurity. Ophthalmology 2014, 122, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxidative Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed]
- Michiels, C.; Minet, E.; Mottet, D.; Raes, M. Regulation of gene expression by oxygen: NF-κB and HIF-1, two extremes. Free Radic. Biol. Med. 2002, 33, 1231–1242. [Google Scholar] [CrossRef]
- Yang, C.-T.; Yang, Z.; Zhang, M.; Dong, Q.; Wang, X.; Lan, A.; Zeng, F.; Chen, P.; Wang, C.; Feng, J. Hydrogen Sulfide Protects against Chemical Hypoxia-Induced Cytotoxicity and Inflammation in HaCaT Cells through Inhibition of ROS/NF-κB/COX-2 Pathway. PLoS ONE 2011, 6, e21971. [Google Scholar] [CrossRef]
- Dey, A.; Bhattacharya, R.; Mukherjee, A.; Pandey, D.K. Natural products against Alzheimer’s disease: Pharmaco-therapeutics and biotechnological interventions. Biotechnol. Adv. 2017, 35, 178–216. [Google Scholar] [CrossRef]
- Lan, A.P.; Chen, J.; Chai, Z.F.; Hu, Y. The neurotoxicity of iron, copper and cobalt in Parkinson’s disease through ROS-mediated mechanisms. BioMetals 2016, 29, 665–678. [Google Scholar] [CrossRef]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K. Reactive oxygen species (ROS) and cancer: Role of antioxidative nutraceuticals. Cancer Lett. 2017, 387, 95–105. [Google Scholar] [CrossRef]
- Idelchik, M.D.P.S.; Begley, U.; Begley, T.J.; Melendez, J.A. Mitochondrial ROS control of cancer. Semin. Cancer Biol. 2017, 47, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.W.; Byzova, T.V. Oxidative stress in angiogenesis and vascular disease. Blood 2014, 123, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, P.; Cruzat, V.F.; Keane, K.N.; Carlessi, R.; De Bittencourt, P.I.H.J.; De Bittencourt, P.I.H. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem. J. 2016, 473, 4527–4550. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive oxygen species in living systems: Source, biochemistry, and role in human disease. Am. J. Med. 1991, 91, S14–S22. [Google Scholar] [CrossRef]
- Stone, W.L. Fbsbioscience.Org Retinopathy of prematurity an oxidative stress neonatal disease. Front. Biosci. 2016, 21, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Calderon, G.D.; Olguín, H.J.; Hernandez, G.E.; Punzo, S.M.; De La Cruz, Z.D. Oxidative stress and diabetic retinopathy: Development and treatment. Eye 2017, 31, 1122–1130. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Pawlowska, E.; Szczepanska, J.; Jablkowska, A.; Blasiak, J. Role of Mitochondrial DNA Damage in ROS-Mediated Pathogenesis of Age-Related Macular Degeneration (AMD). Int. J. Mol. Sci. 2019, 20, 2374. [Google Scholar] [CrossRef]
- Papsdorf, K.; Brunet, A. Linking Lipid Metabolism to Chromatin Regulation in Aging. Trends Cell Biol. 2019, 29, 97–116. [Google Scholar] [CrossRef]
- Terao, R.; Kaneko, H. Lipid Signaling in Ocular Neovascularization. Int. J. Mol. Sci. 2020, 21, 4758. [Google Scholar] [CrossRef]
- Innis, S.M. Dietary omega 3 fatty acids and the developing brain. Brain Res. 2008, 1237, 35–43. [Google Scholar] [CrossRef]
- Rogers, L.K.; Valentine, C.J.; Keim, S.A. DHA supplementation: Current implications in pregnancy and childhood. Pharmacol. Res. 2013, 70, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Neff, L.M.; Culiner, J.; Cunningham-Rundles, S.; Seidman, C.; Meehan, D.; Maturi, J.; Wittkowski, K.M.; Levine, B.; Breslow, J.L. Algal docosahexaenoic acid affects plasma lipoprotein particle size distribution in overweight and obese adults. J. Nutr. 2011, 141, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Taylor, D.S.; Yu-Poth, S.; Huth, P.; Moriarty, K.; Fishell, V.; Hargrove, R.L.; Zhao, G.; Etherton, T.D. Polyunsaturated fatty acids in the food chain in the United States. Am. J. Clin. Nutr. 2000, 71, 179S–188S. [Google Scholar] [CrossRef] [PubMed]
- Holub, D.J.; Holub, B.J. Omega-3 fatty acids from fish oils and cardiovascular disease. Mol. Cell. Biochem. 2004, 263, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Bouwens, M.; Van De Rest, O.; Dellschaft, N.S.; Bromhaar, M.G.; De Groot, L.C.P.G.M.; Geleijnse, J.M.; Muller, M.; Afman, L.A. Fish-oil supplementation induces antiinflammatory gene expression profiles in human blood mononuclear cells. Am. J. Clin. Nutr. 2009, 90, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.; Mobarhan, M.G.-; Rezaiean, S.; Hoseini, M.; Parizade, S.; Farhoudi, F.; Hosseininezhad, S.; Tavallaei, S.; Vejdani, A.; Azimi-Nezhad, M.; et al. Omega-3 fatty acid supplements improve the cardiovascular risk profile of subjects with metabolic syndrome, including markers of inflammation and auto-immunity. Acta Cardiol. 2009, 64, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Tavazzi, L.; Maggioni, A.P.; Marchioli, R.; Barlera, S.; Franzosi, M.G.; Latini, R.; Lucci, D.; Nicolosi, G.L.; Porcu, M.; Tognoni, G.; et al. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): A randomised, double-blind, placebo-controlled trial. Lancet 2008, 372, 1223–1230. [Google Scholar] [CrossRef]
- Casanova, M.A.; Medeiros, F.; Trindade, M.; Cohen, C.; Oigman, W.; Neves, M.F. Omega-3 fatty acids supplementation improves endothelial function and arterial stiffness in hypertensive patients with hypertriglyceridemia and high cardiovascular risk. J. Am. Soc. Hypertens. 2017, 11, 10–19. [Google Scholar] [CrossRef]
- SanGiovanni, J.P.; Chew, E.Y. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog. Retin. Eye Res. 2005, 24, 87–138. [Google Scholar] [CrossRef]
- Wang, M.-H.; Hsiao, G.; Al-Shabrawey, M. Eicosanoids and Oxidative Stress in Diabetic Retinopathy. Antioxidants 2020, 9, 520. [Google Scholar] [CrossRef]
- Carlson, S.E.; Werkman, S.H.; Peeples, J.M.; Cooke, R.J.; Tolley, E.A. Arachidonic acid status correlates with first year growth in preterm infants. Proc. Natl. Acad. Sci. USA 1993, 90, 1073–1077. [Google Scholar] [CrossRef] [PubMed]
- Innes, J.K.; Calder, P.C. Omega-6 fatty acids and inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2018, 132, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, G.; Ecker, J. The opposing effects of n-3 and n-6 fatty acids. Prog. Lipid. Res. 2008, 47, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.H.; Peterson, S.J.; Bellner, L.; Choudhary, A.; Levy, L.; Gancz, L.; Sasson, A.; Trainer, J.; Rezzani, R.; Resnick, A.; et al. Cold-Pressed. Antioxidants 2020, 9, 489. [Google Scholar] [CrossRef]
- Fan, R.; Koehler, K.; Chung, S. Adaptive thermogenesis by dietary n-3 polyunsaturated fatty acids: Emerging evidence and mechanisms. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2019, 1864, 59–70. [Google Scholar] [CrossRef]
- Fiala, M.; Kooij, G.; Wagner, K.; Hammock, B.; Pellegrini, M. Modulation of innate immunity of patients with Alzheimer’s disease by omega-3 fatty acids. FASEB J. 2017, 31, 3229–3239. [Google Scholar] [CrossRef]
- Innis, S.M. Essential fatty acid transfer and fetal development. Placenta 2005, 26 (Suppl. A), S70–S75. [Google Scholar] [CrossRef]
- Bazan, N.G. Cell survival matters: Docosahexaenoic acid signaling, neuroprotection and photoreceptors. Trends. Neurosci. 2006, 29, 263–271. [Google Scholar] [CrossRef]
- Litman, B.J.; Mitchell, D.C. A role for phospholipid polyunsaturation in modulating membrane protein function. Lipids 1996, 31, S193–S197. [Google Scholar] [CrossRef]
- Politi, L.; Rotstein, N.; Carri, N. Effects of docosahexaenoic acid on retinal development: Cellular and molecular aspects. Lipids 2001, 36, 927–935. [Google Scholar] [CrossRef]
- Rajaram, S. Health benefits of plant-derived α-linolenic acid. Am. J. Clin. Nutr. 2014, 100 (Suppl. 1), 443S–448S. [Google Scholar] [CrossRef] [PubMed]
- Liou, Y.A.; King, D.J.; Zibrik, D.; Innis, S.M. Decreasing Linoleic Acid with Constant α-Linolenic Acid in Dietary Fats Increases (n-3) Eicosapentaenoic Acid in Plasma Phospholipids in Healthy Men. J. Nutr. 2007, 137, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Barceló-Coblijn, G.; Murphy, E.J. Alpha-linolenic acid and its conversion to longer chain n-3 fatty acids: Benefits for human health and a role in maintaining tissue n-3 fatty acid levels. Prog. Lipid. Res. 2009, 48, 355–374. [Google Scholar] [CrossRef] [PubMed]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Grieger, J.A.; Etherton, T.D. Dietary reference intakes for DHA and EPA. Prostaglandins Leukot. Essent. Fat. Acids 2009, 81, 99–104. [Google Scholar] [CrossRef]
- Tuo, J.; Ross, R.J.; Herzlich, A.A.; Shen, D.; Ding, X.; Zhou, M.; Coon, S.L.; Hussein, N.; Salem, N.; Chan, C.C. A high omega-3 fatty acid diet reduces retinal lesions in a murine model of macular degeneration. Am. J. Pathol. 2009, 175, 799–807. [Google Scholar] [CrossRef]
- Zendedel, A.; Habib, P.; Dang, J.; Lammerding, L.; Hoffmann, S.; Beyer, C.; Slowik, A. Omega-3 polyunsaturated fatty acids ameliorate neuroinflammation and mitigate ischemic stroke damage through interactions with astrocytes and microglia. J. Neuroimmunol. 2015, 278, 200–211. [Google Scholar] [CrossRef]
- Golbidi, S.; Badran, M.; Laher, I. Diabetes and alpha lipoic Acid. Front. Pharmacol. 2011, 2, 69. [Google Scholar] [CrossRef]
- Koriyama, Y.; Nakayama, Y.; Matsugo, S.; Kato, S. Protective effect of lipoic acid against oxidative stress is mediated by Keap1/Nrf2-dependent heme oxygenase-1 induction in the RGC-5 cellline. Brain Res. 2013, 1499, 145–157. [Google Scholar] [CrossRef]
- Yang, Y.C.; Lii, C.K.; Wei, Y.L.; Li, C.C.; Lu, C.Y.; Liu, K.L.; Chen, H.W. Docosahexaenoic acid inhibition of inflammation is partially via cross-talk between Nrf2/heme oxygenase 1 and IKK/NF-κB pathways. J. Nutr. Biochem. 2013, 24, 204–212. [Google Scholar] [CrossRef]
- Coyne, G.S.; Kenny, D.A.; Waters, S.M. Effect of dietary n-3 polyunsaturated fatty acid supplementation on bovine uterine endometrial and hepatic gene expression of the insulin-like growth factor system. Theriogenology 2011, 75, 500–512. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Esselman, W.J.; Jump, D.B.; Busik, J.V. Anti-inflammatory effect of docosahexaenoic acid on cytokine-induced adhesion molecule expression in human retinal vascular endothelial cells. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4342–4347. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Joshi-Barve, S.; Barve, S.; Chen, L.H. Eicosapentaenoic acid prevents LPS-induced TNF-alpha expression by preventing NF-kappaB activation. J. Am. Coll. Nutr. 2004, 23, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Okuda, Y.; Kawashima, K.; Sawada, T.; Tsurumaru, K.; Asano, M.; Suzuki, S.; Soma, M.; Nakajima, T.; Yamashita, K. Eicosapentaenoic acid enhances nitric oxide production by cultured human endothelial cells. Biochem. Biophys. Res. Commun. 1997, 232, 487–491. [Google Scholar] [CrossRef]
- Matesanz, N.; Park, G.; McAllister, H.; Leahey, W.; Devine, A.; McVeigh, G.E.; Gardiner, T.A.; McDonald, D.M. Docosahexaenoic acid improves the nitroso-redox balance and reduces VEGF-mediated angiogenic signaling in microvascular endothelial cells. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6815–6825. [Google Scholar] [CrossRef]
- Zgórzyńska, E.; Dziedzic, B.; Gorzkiewicz, A.; Stulczewski, D.; Bielawska, K.; Su, K.P.; Walczewska, A. Omega-3 polyunsaturated fatty acids improve the antioxidative defense in rat astrocytes via an Nrf2-dependent mechanism. Pharmacol. Rep. 2017, 69, 935–942. [Google Scholar] [CrossRef]
- Rotstein, N.P.; Politi, L.E.; German, O.L.; Girotti, R. Protective effect of docosahexaenoic acid on oxidative stress-induced apoptosis of retina photoreceptors. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2252–2259. [Google Scholar] [CrossRef]
- Opreanu, M.; Lydic, T.A.; Reid, G.E.; McSorley, K.M.; Esselman, W.J.; Busik, J.V. Inhibition of cytokine signaling in human retinal endothelial cells through downregulation of sphingomyelinases by docosahexaenoic acid. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3253–3263. [Google Scholar] [CrossRef]
- Basil, M.C.; Levy, B.D. Specialized pro-resolving mediators: Endogenous regulators of infection and inflammation. Nat. Rev. Immunol. 2016, 16, 51–67. [Google Scholar] [CrossRef]
- Weylandt, K.H.; Krause, L.F.; Gomolka, B.; Chiu, C.Y.; Bilal, S.; Nadolny, A.; Waechter, S.F.; Fischer, A.; Rothe, M.; Kang, J.X. Suppressed liver tumorigenesis in fat-1 mice with elevated omega-3 fatty acids is associated with increased omega-3 derived lipid mediators and reduced TNF-α. Carcinogenesis 2011, 32, 897–903. [Google Scholar] [CrossRef]
- Tang, S.; Wan, M.; Huang, W.; Stanton, R.C.; Xu, Y. Maresins: Specialized Proresolving Lipid Mediators and Their Potential Role in Inflammatory-Related Diseases. Mediat. Inflamm. 2018, 2018, 2380319. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y. Immuno-Resolving Ability of Resolvins, Protectins, and Maresins Derived from Omega-3 Fatty Acids in Metabolic Syndrome. Mol. Nutr. Food Res. 2020, 64, e1900824. [Google Scholar] [CrossRef] [PubMed]
- Pirault, J.; Bäck, M. Lipoxin and Resolvin Receptors Transducing the Resolution of Inflammation in Cardiovascular Disease. Front. Pharmacol. 2018, 9, 1273. [Google Scholar] [CrossRef]
- Arita, M.; Ohira, T.; Sun, Y.P.; Elangovan, S.; Chiang, N.; Serhan, C.N. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J. Immunol. 2007, 178, 3912–3917. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.N.; Surh, Y.J. Resolvin D1-mediated NOX2 inactivation rescues macrophages undertaking efferocytosis from oxidative stress-induced apoptosis. Biochem. Pharmacol. 2013, 86, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Makino, Y.; Miyahara, T.; Nitta, J.; Miyahara, K.; Seo, A.; Kimura, M.; Suhara, M.; Akai, A.; Akagi, D.; Yamamoto, K.; et al. Proresolving Lipid Mediators Resolvin D1 and Protectin D1 Isomer Attenuate Neointimal Hyperplasia in the Rat Carotid Artery Balloon Injury Model. J. Surg. Res. 2019, 233, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.; Phillips, O.; Fukumoto, J.; Fukumoto, I.; Parthasarathy, P.T.; Arias, S.; Cho, Y.; Lockey, R.F.; Kolliputi, N. Enhanced Resolution of Hyperoxic Acute Lung Injury as a result of Aspirin Triggered Resolvin D1 Treatment. Am. J. Respir. Cell. Mol. Biol. 2015, 53, 422–435. [Google Scholar] [CrossRef]
- Shu, Y.; Liu, Y.; Li, X.; Cao, L.; Yuan, X.; Li, W.; Cao, Q. Aspirin-Triggered Resolvin D1 Inhibits TGF-β1-Induced EndMT through Increasing the Expression of Smad7 and Is Closely Related to Oxidative Stress. Biomol. Ther. 2016, 24, 132–139. [Google Scholar] [CrossRef]
- Sasaki, F.; Koga, T.; Ohba, M.; Saeki, K.; Okuno, T.; Ishikawa, K.; Nakama, T.; Nakao, S.; Yoshida, S.; Ishibashi, T.; et al. Leukotriene B4 promotes neovascularization and macrophage recruitment in murine wet-type AMD models. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Talahalli, R.; Zarini, S.; Sheibani, N.; Murphy, R.C.; Gubitosi-Klug, R.A. Increased synthesis of leukotrienes in the mouse model of diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1699–1708. [Google Scholar] [CrossRef]
- Takamiya, R.; Fukunaga, K.; Arita, M.; Miyata, J.; Seki, H.; Minematsu, N.; Suematsu, M.; Asano, K. Resolvin E1 maintains macrophage function under cigarette smoke-induced oxidative stress. FEBS Open Bio 2012, 2, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Kytikova, O.; Novgorodtseva, T.; Denisenko, Y.; Antonyuk, M.; Gvozdenko, T. Pro-Resolving Lipid Mediators in the Pathophysiology of Asthma. Medicina 2019, 55, 284. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, M.; Hjorth, E.; Cortés-Toro, V.; Eyjolfsdottir, H.; Graff, C.; Nennesmo, I.; Palmblad, J.; Eriksdotter, M.; Sambamurti, K.; et al. Resolution of inflammation is altered in Alzheimer’s disease. Alzheimers Dement. 2015, 11, 40–50.e2. [Google Scholar] [CrossRef] [PubMed]
- Connor, K.M.; SanGiovanni, J.P.; Lofqvist, C.; Aderman, C.M.; Chen, J.; Higuchi, A.; Hong, S.; Pravda, E.A.; Majchrzak, S.; Carper, D.; et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat. Med. 2007, 13, 868–873. [Google Scholar] [CrossRef]
- Serhan, C.N. Resolution phase of inflammation: Novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu. Rev. Immunol. 2007, 25, 101–137. [Google Scholar] [CrossRef]
- Bang, S.; Xie, Y.K.; Zhang, Z.J.; Wang, Z.; Xu, Z.Z.; Ji, R.R. GPR37 regulates macrophage phagocytosis and resolution of inflammatory pain. J. Clin. Investig. 2018, 128, 3568–3582. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Marcheselli, V.L.; Serhan, C.N.; Bazan, N.G. Neuroprotectin D1: A docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc. Natl. Acad. Sci. USA 2004, 101, 8491–8496. [Google Scholar] [CrossRef]
- Calandria, J.M.; Marcheselli, V.L.; Mukherjee, P.K.; Uddin, J.; Winkler, J.W.; Petasis, N.A.; Bazan, N.G. Selective survival rescue in 15-lipoxygenase-1-deficient retinal pigment epithelial cells by the novel docosahexaenoic acid-derived mediator, neuroprotectin D1. J. Biol. Chem. 2009, 284, 17877–17882. [Google Scholar] [CrossRef]
- Faghiri, Z.; Bazan, N.G. PI3K/Akt and mTOR/p70S6K pathways mediate neuroprotectin D1-induced retinal pigment epithelial cell survival during oxidative stress-induced apoptosis. Exp. Eye Res. 2010, 90, 718–725. [Google Scholar] [CrossRef]
- Fliesler, S.J. Lipids and lipid metabolism in the eye. J. Lipid Res. 2010, 51, 1–3. [Google Scholar] [CrossRef]
- Qin, Q.; Patil, K.A.; Gronert, K.; Sharma, S.C. Neuroprotectin D1 inhibits retinal ganglion cell death following axotomy. Prostaglandins Leukot. Essent. Fat. Acids 2008, 79, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Blencowe, H.; Lawn, J.E.; Vazquez, T.; Fielder, A.; Gilbert, C. Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr. Res. 2013, 74 (Suppl. 1), 35–49. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.E. Pathogenesis of retinopathy of prematurity. Growth Horm. IGF Res. 2004, 14 (Suppl. A), S140–S144. [Google Scholar] [CrossRef] [PubMed]
- Pierce, E.A.; Foley, E.D.; Smith, L.E. Regulation of vascular endothelial growth factor by oxygen in a model of retinopathy of prematurity. Arch. Ophthalmol. 1996, 114, 1219–1228. [Google Scholar] [CrossRef]
- Sears, J.E.; Hoppe, G.; Ebrahem, Q.; Anand-Apte, B. Prolyl hydroxylase inhibition during hyperoxia prevents oxygen-induced retinopathy. Proc. Natl. Acad. Sci. USA 2008, 105, 19898–19903. [Google Scholar] [CrossRef]
- Werdich, X.Q.; McCollum, G.W.; Rajaratnam, V.S.; Penn, J.S. Variable oxygen and retinal VEGF levels: Correlation with incidence and severity of pathology in a rat model of oxygen-induced retinopathy. Exp. Eye Res. 2004, 79, 623–630. [Google Scholar] [CrossRef]
- Hoppe, G.; Yoon, S.; Gopalan, B.; Savage, A.R.; Brown, R.; Case, K.; Vasanji, A.; Chan, E.R.; Silver, R.B.; Sears, J.E. Comparative systems pharmacology of HIF stabilization in the prevention of retinopathy of prematurity. Proc. Natl. Acad. Sci. USA 2016, 113, E2516–E2525. [Google Scholar] [CrossRef]
- Rivera, J.C.; Sapieha, P.; Joyal, J.S.; Duhamel, F.; Shao, Z.; Sitaras, N.; Picard, E.; Zhou, E.; Lachapelle, P.; Chemtob, S. Understanding retinopathy of prematurity: Update on pathogenesis. Neonatology 2011, 100, 343–353. [Google Scholar] [CrossRef]
- Hellström, A.; Engström, E.; Hård, A.L.; Albertsson-Wikland, K.; Carlsson, B.; Niklasson, A.; Löfqvist, C.; Svensson, E.; Holm, S.; Ewald, U.; et al. Postnatal serum insulin-like growth factor I deficiency is associated with retinopathy of prematurity and other complications of premature birth. Pediatrics 2003, 112, 1016–1020. [Google Scholar] [CrossRef]
- Pérez-Muñuzuri, A.; Couce-Pico, M.L.; Baña-Souto, A.; López-Suárez, O.; Iglesias-Deus, A.; Blanco-Teijeiro, J.; Fernández-Lorenzo, J.R.; Fraga-Bermúdez, J.M. Preclinical screening for retinopathy of prematurity risk using IGF1 levels at 3 weeks post-partum. PLoS ONE 2014, 9, e88781. [Google Scholar] [CrossRef]
- Hellstrom, A.; Perruzzi, C.; Ju, M.; Engstrom, E.; Hard, A.L.; Liu, J.L.; Albertsson-Wikland, K.; Carlsson, B.; Niklasson, A.; Sjodell, L.; et al. Low IGF-I suppresses VEGF-survival signaling in retinal endothelial cells: Direct correlation with clinical retinopathy of prematurity. Proc. Natl. Acad. Sci. USA 2001, 98, 5804–5808. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.E.; Gu, X.; Samuel, S.; Marcus, D.M.; Bartoli, M.; Huang, P.L.; Caldwell, R.B. Reduced severity of oxygen-induced retinopathy in eNOS-deficient mice. Investig. Ophthalmol. Vis. Sci. 2001, 42, 222–228. [Google Scholar]
- Kaur, C.; Sivakumar, V.; Foulds, W.S.; Luu, C.D.; Ling, E.A. Cellular and vascular changes in the retina of neonatal rats after an acute exposure to hypoxia. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5364–5374. [Google Scholar] [CrossRef] [PubMed]
- Rathnasamy, G.; Sivakumar, V.; Rangarajan, P.; Foulds, W.S.; Ling, E.A.; Kaur, C. NF-κB-mediated nitric oxide production and activation of caspase-3 cause retinal ganglion cell death in the hypoxic neonatal retina. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5878–5889. [Google Scholar] [CrossRef]
- Wilkinson-Berka, J.L.; Rana, I.; Armani, R.; Agrotis, A. Reactive oxygen species, Nox and angiotensin II in angiogenesis: Implications for retinopathy. Clin. Sci. 2013, 124, 597–615. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, Z.; Jiang, Y.; Hartnett, M.E. Endothelial NADPH oxidase 4 mediates vascular endothelial growth factor receptor 2-induced intravitreal neovascularization in a rat model of retinopathy of prematurity. Mol. Vis. 2014, 20, 231–241. [Google Scholar]
- Byfield, G.; Budd, S.; Hartnett, M.E. The role of supplemental oxygen and JAK/STAT signaling in intravitreous neovascularization in a ROP rat model. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3360–3365. [Google Scholar] [CrossRef]
- Hartnett, M.E. The effects of oxygen stresses on the development of features of severe retinopathy of prematurity: Knowledge from the 50/10 OIR model. Doc. Ophthalmol. 2010, 120, 25–39. [Google Scholar] [CrossRef]
- Wang, H.; Byfield, G.; Jiang, Y.; Smith, G.W.; McCloskey, M.; Hartnett, M.E. VEGF-mediated STAT3 activation inhibits retinal vascularization by downregulating local erythropoietin expression. Am. J. Pathol. 2012, 180, 1243–1253. [Google Scholar] [CrossRef]
- Lee, J.; Dammann, O. Perinatal infection, inflammation, and retinopathy of prematurity. Semin. Fetal Neonatal Med. 2012, 17, 26–29. [Google Scholar] [CrossRef]
- Goldstein, G.P.; Leonard, S.A.; Kan, P.; Koo, E.B.; Lee, H.C.; Carmichael, S.L. Prenatal and postnatal inflammation-related risk factors for retinopathy of prematurity. J. Perinatol. 2019, 39, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, S.; Miloudi, K.; Chaychi, S.; Favret, S.; Binet, F.; Polosa, A.; Lachapelle, P.; Chemtob, S.; Sapieha, P. Systemic inflammation perturbs developmental retinal angiogenesis and neuroretinal function. Investig. Ophthalmol. Vis. Sci. 2013, 54, 8125–8139. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.K.; Lee, H.J.; Ko, J.H.; Park, J.H.; Park, J.Y.; Choi, C.W.; Yoon, C.H.; Ahn, S.J.; Park, K.H.; Woo, S.J.; et al. Neonatal systemic inflammation in rats alters retinal vessel development and simulates pathologic features of retinopathy of prematurity. J. Neuroinflamm. 2014, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Deliyanti, D.; Talia, D.M.; Zhu, T.; Maxwell, M.J.; Agrotis, A.; Jerome, J.R.; Hargreaves, E.M.; Gerondakis, S.; Hibbs, M.L.; Mackay, F.; et al. Foxp3. Nat. Commun. 2017, 8, 748. [Google Scholar] [CrossRef] [PubMed]
- Rivera, J.C.; Sitaras, N.; Noueihed, B.; Hamel, D.; Madaan, A.; Zhou, T.; Honoré, J.C.; Quiniou, C.; Joyal, J.S.; Hardy, P.; et al. Microglia and interleukin-1β in ischemic retinopathy elicit microvascular degeneration through neuronal semaphorin-3A. Arter. Thromb. Vasc. Biol. 2013, 33, 1881–1891. [Google Scholar] [CrossRef]
- Wooff, Y.; Man, S.M.; Aggio-Bruce, R.; Natoli, R.; Fernando, N. IL-1 Family Members Mediate Cell Death, Inflammation and Angiogenesis in Retinal Degenerative Diseases. Front. Immunol. 2019, 10, 1618. [Google Scholar] [CrossRef]
- Rathi, S.; Jalali, S.; Patnaik, S.; Shahulhameed, S.; Musada, G.R.; Balakrishnan, D.; Rani, P.K.; Kekunnaya, R.; Chhablani, P.P.; Swain, S.; et al. Abnormal Complement Activation and Inflammation in the Pathogenesis of Retinopathy of Prematurity. Front. Immunol. 2017, 8, 1868. [Google Scholar] [CrossRef]
- Cai, M.; Zhang, X.; Li, Y.; Xu, H. Toll-like receptor 3 activation drives the inflammatory response in oxygen-induced retinopathy in rats. Br. J. Ophthalmol. 2015, 99, 125–132. [Google Scholar] [CrossRef]
- Stahl, A.; Sapieha, P.; Connor, K.M.; Sangiovanni, J.P.; Chen, J.; Aderman, C.M.; Willett, K.L.; Krah, N.M.; Dennison, R.J.; Seaward, M.R.; et al. Short communication: PPAR gamma mediates a direct antiangiogenic effect of omega 3-PUFAs in proliferative retinopathy. Circ. Res. 2010, 107, 495–500. [Google Scholar] [CrossRef]
- Beharry, K.D.; Cai, C.L.; Siddiqui, F.; Chowdhury, S.; D’Agrosa, C.; Valencia, G.B.; Aranda, J.V. Comparative Effects of Coenzyme Q10 or n-3 Polyunsaturated Fatty Acid Supplementation on Retinal Angiogenesis in a Rat Model of Oxygen-Induced Retinopathy. Antioxidants 2018, 7, 160. [Google Scholar] [CrossRef]
- Gardiner, T.A.; Gibson, D.S.; de Gooyer, T.E.; de la Cruz, V.F.; McDonald, D.M.; Stitt, A.W. Inhibition of tumor necrosis factor-alpha improves physiological angiogenesis and reduces pathological neovascularization in ischemic retinopathy. Am. J. Pathol. 2005, 166, 637–644. [Google Scholar] [CrossRef]
- Solberg, R.; Longini, M.; Proietti, F.; Perrone, S.; Felici, C.; Porta, A.; Saugstad, O.D.; Buonocore, G. DHA Reduces Oxidative Stress after Perinatal Asphyxia: A Study in Newborn Piglets. Neonatology 2017, 112, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Neuringer, M.; Anderson, G.J.; Connor, W.E. The essentiality of n-3 fatty acids for the development and function of the retina and brain. Annu. Rev. Nutr. 1988, 8, 517–541. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Baack, M.L. Beyond building better brains: Bridging the docosahexaenoic acid (DHA) gap of prematurity. J. Perinatol. 2015, 35, 1–7. [Google Scholar] [CrossRef]
- Bernabe-García, M.; Villegas-Silva, R.; Villavicencio-Torres, A.; Calder, P.C.; Rodríguez-Cruz, M.; Maldonado-Hernández, J.; Macías-Loaiza, D.; López-Alarcón, M.; Inda-Icaza, P.; Cruz-Reynoso, L. Enteral Docosahexaenoic Acid and Retinopathy of Prematurity: A Randomized Clinical Trial. J. Parenter. Enter. Nutr. 2019, 43, 874–882. [Google Scholar] [CrossRef]
- Khalesi, N.; Bordbar, A.; Khosravi, N.; Kabirian, M.; Karimi, A. The Efficacy of Omega-3 Supplement on Prevention of Retinopathy of Prematurity in Premature Infants: A Randomized Double-blinded Controlled trial. Curr. Pharm. Des. 2018, 24, 1845–1848. [Google Scholar] [CrossRef]
- Pawlik, D.; Lauterbach, R.; Walczak, M.; Hurkała, J.; Sherman, M.P. Fish-oil fat emulsion supplementation reduces the risk of retinopathy in very low birth weight infants: A prospective, randomized study. J. Parenter. Enter. Nutr. 2014, 38, 711–716. [Google Scholar] [CrossRef]
- Pawlik, D.; Lauterbach, R.; Turyk, E. Fish-oil fat emulsion supplementation may reduce the risk of severe retinopathy in VLBW infants. Pediatrics 2011, 127, 223–228. [Google Scholar] [CrossRef]
- Beken, S.; Dilli, D.; Fettah, N.D.; Kabataş, E.U.; Zenciroğlu, A.; Okumuş, N. The influence of fish-oil lipid emulsions on retinopathy of prematurity in very low birth weight infants: A randomized controlled trial. Early Hum. Dev. 2014, 90, 27–31. [Google Scholar] [CrossRef]
- Najm, S.; Löfqvist, C.; Hellgren, G.; Engström, E.; Lundgren, P.; Hård, A.L.; Lapillonne, A.; Sävman, K.; Nilsson, A.K.; Andersson, M.X.; et al. Effects of a lipid emulsion containing fish oil on polyunsaturated fatty acid profiles, growth and morbidities in extremely premature infants: A randomized controlled trial. Clin. Nutr. ESPEN 2017, 20, 17–23. [Google Scholar] [CrossRef]
- Pallot, C.; Mazzocco, J.; Meillon, C.; Semama, D.S.; Chantegret, C.; Ternoy, N.; Martin, D.; Donier, A.; Gregoire, S.; Creuzot-Garcher, C.P.; et al. Alteration of erythrocyte membrane polyunsaturated fatty acids in preterm newborns with retinopathy of prematurity. Sci. Rep. 2019, 9, 7930. [Google Scholar] [CrossRef] [PubMed]
- Nathan, D.M. Diabetes: Advances in Diagnosis and Treatment. JAMA 2015, 314, 1052–1062. [Google Scholar] [CrossRef] [PubMed]
- Skyler, J.S.; Bakris, G.L.; Bonifacio, E.; Darsow, T.; Eckel, R.H.; Groop, L.; Groop, P.H.; Handelsman, Y.; Insel, R.A.; Mathieu, C.; et al. Differentiation of Diabetes by Pathophysiology, Natural History, and Prognosis. Diabetes 2017, 66, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Antonetti, D.A.; Klein, R.; Gardner, T.W. Diabetic retinopathy. N. Engl. J. Med. 2012, 366, 1227–1239. [Google Scholar] [CrossRef]
- Antonetti, D.A.; Barber, A.J.; Bronson, S.K.; Freeman, W.M.; Gardner, T.W.; Jefferson, L.S.; Kester, M.; Kimball, S.R.; Krady, J.K.; LaNoue, K.F.; et al. Diabetic retinopathy: Seeing beyond glucose-induced microvascular disease. Diabetes 2006, 55, 2401–2411. [Google Scholar] [CrossRef]
- Lamoureux, E.L.; Wong, T.Y. Diabetic retinopathy in 2011: Further insights from new epidemiological studies and clinical trials. Diabetes Care 2011, 34, 1066–1067. [Google Scholar] [CrossRef]
- Klein, R.; Klein, B.E.; Moss, S.E.; Cruickshanks, K.J. Relationship of hyperglycemia to the long-term incidence and progression of diabetic retinopathy. Arch. Intern. Med. 1994, 154, 2169–2178. [Google Scholar] [CrossRef]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998, 352, 837–853. [Google Scholar] [CrossRef]
- Leske, M.C.; Wu, S.Y.; Hennis, A.; Hyman, L.; Nemesure, B.; Yang, L.; Schachat, A.P.; Barbados Eye Study Group. Hyperglycemia, blood pressure, and the 9-year incidence of diabetic retinopathy: The Barbados Eye Studies. Ophthalmology 2005, 112, 799–805. [Google Scholar] [CrossRef]
- Lachin, J.M.; Genuth, S.; Cleary, P.; Davis, M.D.; Nathan, D.M.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N. Engl. J. Med. 2000, 342, 381–389. [Google Scholar] [CrossRef]
- Lu, Q.Y.; Chen, W.; Lu, L.; Zheng, Z.; Xu, X. Involvement of RhoA/ROCK1 signaling pathway in hyperglycemia-induced microvascular endothelial dysfunction in diabetic retinopathy. Int.J. Clin. Exp. Pathol. 2014, 7, 7268–7277. [Google Scholar] [PubMed]
- Nathan, D.M.; Cleary, P.A.; Backlund, J.Y.; Genuth, S.M.; Lachin, J.M.; Orchard, T.J.; Raskin, P.; Zinman, B.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N. Engl. J. Med. 2005, 353, 2643–2653. [Google Scholar] [CrossRef] [PubMed]
- Pitocco, D.; Tesauro, M.; Alessandro, R.; Ghirlanda, G.; Cardillo, C. Oxidative stress in diabetes: Implications for vascular and other complications. Int. J. Mol. Sci. 2013, 14, 21525–21550. [Google Scholar] [CrossRef]
- Sifuentes-Franco, S.; Pacheco-Moisés, F.P.; Rodríguez-Carrizalez, A.D.; Miranda-Díaz, A.G. The Role of Oxidative Stress, Mitochondrial Function, and Autophagy in Diabetic Polyneuropathy. J. Diabetes Res. 2017, 2017, 1673081. [Google Scholar] [CrossRef] [PubMed]
- Frey, R.S.; Ushio-Fukai, M.; Malik, A.B. NADPH oxidase-dependent signaling in endothelial cells: Role in physiology and pathophysiology. Antioxid. Redox Signal. 2009, 11, 791–810. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Kowluru, A.; Veluthakal, R.; Mohammad, G.; Syed, I.; Santos, J.M.; Mishra, M. TIAM1-RAC1 signalling axis-mediated activation of NADPH oxidase-2 initiates mitochondrial damage in the development of diabetic retinopathy. Diabetologia 2014, 57, 1047–1056. [Google Scholar] [CrossRef]
- Veluthakal, R.; Kumar, B.; Mohammad, G.; Kowluru, A.; Kowluru, R.A. Tiam1-Rac1 Axis Promotes Activation of p38 MAP Kinase in the Development of Diabetic Retinopathy: Evidence for a Requisite Role for Protein Palmitoylation. Cell. Physiol. Biochem. 2015, 36, 208–220. [Google Scholar] [CrossRef]
- El-Remessy, A.B.; Abou-Mohamed, G.; Caldwell, R.W.; Caldwell, R.B. High glucose-induced tyrosine nitration in endothelial cells: Role of eNOS uncoupling and aldose reductase activation. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3135–3143. [Google Scholar] [CrossRef]
- Du, X.L.; Edelstein, D.; Rossetti, L.; Fantus, I.G.; Goldberg, H.; Ziyadeh, F.; Wu, J.; Brownlee, M. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc. Natl. Acad. Sci. USA 2000, 97, 12222–12226. [Google Scholar] [CrossRef]
- Geraldes, P.; Hiraoka-Yamamoto, J.; Matsumoto, M.; Clermont, A.; Leitges, M.; Marette, A.; Aiello, L.P.; Kern, T.S.; King, G.L. Activation of PKC-delta and SHP-1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nat. Med. 2009, 15, 1298–1306. [Google Scholar] [CrossRef]
- Stitt, A.W.; Li, Y.M.; Gardiner, T.A.; Bucala, R.; Archer, D.B.; Vlassara, H. Advanced glycation end products (AGEs) co-localize with AGE receptors in the retinal vasculature of diabetic and of AGE-infused rats. Am. J. Pathol. 1997, 150, 523–531. [Google Scholar] [PubMed]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Iwao, H. Molecular and cellular mechanisms of angiotensin II-mediated cardiovascular and renal diseases. Pharmacol. Rev. 2000, 52, 11–34. [Google Scholar] [PubMed]
- Choudhary, R.; Kapoor, M.S.; Singh, A.; Bodakhe, S.H. Therapeutic targets of renin-angiotensin system in ocular disorders. J. Curr. Ophthalmol. 2017, 29, 7–16. [Google Scholar] [CrossRef]
- Kanda, A.; Ishida, S. (Pro)renin receptor: Involvement in diabetic retinopathy and development of molecular targeted therapy. J. Diabetes Investig. 2019, 10, 6–17. [Google Scholar] [CrossRef]
- Dimmeler, S.; Zeiher, A.M. Reactive oxygen species and vascular cell apoptosis in response to angiotensin II and pro-atherosclerotic factors. Regul. Pept. 2000, 90, 19–25. [Google Scholar] [CrossRef]
- Ozawa, Y.; Kurihara, T.; Sasaki, M.; Ban, N.; Yuki, K.; Kubota, S.; Tsubota, K. Neural degeneration in the retina of the streptozotocin-induced type 1 diabetes model. Exp. Diabetes. Res. 2011, 2011, 108328. [Google Scholar] [CrossRef]
- Cecilia, O.M.; José Alberto, C.G.; José, N.P.; Ernesto Germán, C.M.; Ana Karen, L.C.; Luis Miguel, R.P.; Ricardo Raúl, R.R.; Adolfo Daniel, R.C. Oxidative Stress as the Main Target in Diabetic Retinopathy Pathophysiology. J. Diabetes Res. 2019, 2019, 8562408. [Google Scholar] [CrossRef]
- Nagai, N.; Izumi-Nagai, K.; Oike, Y.; Koto, T.; Satofuka, S.; Ozawa, Y.; Yamashiro, K.; Inoue, M.; Tsubota, K.; Umezawa, K.; et al. Suppression of diabetes-induced retinal inflammation by blocking the angiotensin II type 1 receptor or its downstream nuclear factor-kappaB pathway. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4342–4350. [Google Scholar] [CrossRef]
- Tao, L.; Qiu, Y.; Fu, X.; Lin, R.; Lei, C.; Wang, J.; Lei, B. Angiotensin-converting enzyme 2 activator diminazene aceturate prevents lipopolysaccharide-induced inflammation by inhibiting MAPK and NF-κB pathways in human retinal pigment epithelium. J. Neuroinflamm. 2016, 13, 35. [Google Scholar] [CrossRef]
- Campochiaro, P.A. Ocular neovascularization. J. Mol. Med. 2013, 91, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Negi, G.; Kumar, A.; Joshi, R.P.; Sharma, S.S. Oxidative stress and Nrf2 in the pathophysiology of diabetic neuropathy: Old perspective with a new angle. Biochem. Biophys. Res. Commun. 2011, 408, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wei, Y.; Gong, J.; Cho, H.; Park, J.K.; Sung, E.R.; Huang, H.; Wu, L.; Eberhart, C.; Handa, J.T.; et al. NRF2 plays a protective role in diabetic retinopathy in mice. Diabetologia 2014, 57, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Odenbach, S. Effect of long-term administration of alpha-lipoic acid on retinal capillary cell death and the development of retinopathy in diabetic rats. Diabetes 2004, 53, 3233–3238. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Bierhaus, A.; Bugert, P.; Dietrich, N.; Feng, Y.; Vom Hagen, F.; Nawroth, P.; Brownlee, M.; Hammes, H.P. Effect of R-(+)-alpha-lipoic acid on experimental diabetic retinopathy. Diabetologia 2006, 49, 1089–1096. [Google Scholar] [CrossRef]
- Lee, S.G.; Lee, C.G.; Yun, I.H.; Hur, D.Y.; Yang, J.W.; Kim, H.W. Effect of lipoic acid on expression of angiogenic factors in diabetic rat retina. Clin. Exp. Ophthalmol. 2012, 40, e47–e57. [Google Scholar] [CrossRef]
- Shen, J.H.; Ma, Q.; Shen, S.R.; Shen, S.G.; Xu, G.T.; Das, U.N. Effect of α-linolenic acid on streptozotocin-induced diabetic retinopathy indices in vivo. Arch. Med. Res. 2013, 44, 514–520. [Google Scholar] [CrossRef]
- Kan, E.; Alici, Ö.; Kan, E.K.; Ayar, A. Effects of alpha-lipoic acid on retinal ganglion cells, retinal thicknesses, and VEGF production in an experimental model of diabetes. Int. Ophthalmol. 2017, 37, 1269–1278. [Google Scholar] [CrossRef]
- Wu, A.; Ying, Z.; Gomez-Pinilla, F. Dietary omega-3 fatty acids normalize BDNF levels, reduce oxidative damage, and counteract learning disability after traumatic brain injury in rats. J. Neurotrauma 2004, 21, 1457–1467. [Google Scholar] [CrossRef]
- Ola, M.S.; Nawaz, M.I.; El-Asrar, A.A.; Abouammoh, M.; Alhomida, A.S. Reduced levels of brain derived neurotrophic factor (BDNF) in the serum of diabetic retinopathy patients and in the retina of diabetic rats. Cell. Mol. Neurobiol. 2013, 33, 359–367. [Google Scholar] [CrossRef]
- Bathina, S.; Srinivas, N.; Das, U.N. Streptozotocin produces oxidative stress, inflammation and decreases BDNF concentrations to induce apoptosis of RIN5F cells and type 2 diabetes mellitus in Wistar rats. Biochem. Biophys. Res. Commun. 2017, 486, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Suzumura, A.; Kaneko, H.; Funahashi, Y.; Takayama, K.; Nagaya, M.; Ito, S.; Okuno, T.; Hirakata, T.; Nonobe, N.; Kataoka, K.; et al. n-3 Fatty Acid and Its Metabolite 18-HEPE Ameliorate Retinal Neuronal Cell Dysfunction by Enhancing Müller BDNF in Diabetic Retinopathy. Diabetes 2020, 69, 724–735. [Google Scholar] [CrossRef] [PubMed]
- Dutot, M.; de la Tourrette, V.; Fagon, R.; Rat, P. New approach to modulate retinal cellular toxic effects of high glucose using marine epa and dha. Nutr. Metab. 2011, 8, 39. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Matsumoto, T.; Nakayama, N.; Ishida, K.; Kobayashi, T.; Kamata, K. Eicosapentaenoic acid improves imbalance between vasodilator and vasoconstrictor actions of endothelium-derived factors in mesenteric arteries from rats at chronic stage of type 2 diabetes. J. Pharmacol. Exp. Ther. 2009, 329, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Lafuente, M.; Ortín, L.; Argente, M.; Guindo, J.L.; López-Bernal, M.D.; López-Román, F.J.; García, M.J.; Domingo, J.C.; Lajara, J. Combined Intravitreal Ranibizumab and Oral Supplementation with Docosahexaenoic Acid and Antioxidants for Diabetic Macular Edema: Two-Year Randomized Single-Blind Controlled Trial Results. Retina 2017, 37, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudabadi, M.M.; Rahbar, A.R. Effect of EPA and vitamin C on superoxide dismutase, glutathione peroxidase, total antioxidant capacity and malondialdehyde in type 2 diabetic patients. Oman Med. J. 2014, 29, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Yee, P.; Weymouth, A.E.; Fletcher, E.L.; Vingrys, A.J. A role for omega-3 polyunsaturated fatty acid supplements in diabetic neuropathy. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1755–1764. [Google Scholar] [CrossRef]
- Sapieha, P.; Chen, J.; Stahl, A.; Seaward, M.R.; Favazza, T.L.; Juan, A.M.; Hatton, C.J.; Joyal, J.S.; Krah, N.M.; Dennison, R.J.; et al. Omega-3 polyunsaturated fatty acids preserve retinal function in type 2 diabetic mice. Nutr. Diabetes 2012, 2, e36. [Google Scholar] [CrossRef]
- Kang, J.X. The omega-6/omega-3 fatty acid ratio in chronic diseases: Animal models and molecular aspects. World Rev. Nutr. Diet. 2011, 102, 22–29. [Google Scholar] [CrossRef]
- Gorusupudi, A.; Chang, F.Y.; Nelson, K.; Hageman, G.S.; Bernstein, P.S. n-3 PUFA Supplementation Alters Retinal Very-Long-Chain-PUFA Levels and Ratios in Diabetic Animal Models. Mol. Nutr. Food Res. 2019, 63, e1801058. [Google Scholar] [CrossRef]
- Sala-Vila, A.; Díaz-López, A.; Valls-Pedret, C.; Cofán, M.; García-Layana, A.; Lamuela-Raventós, R.M.; Castañer, O.; Zanon-Moreno, V.; Martinez-Gonzalez, M.A.; Toledo, E.; et al. Dietary Marine ω-3 Fatty Acids and Incident Sight-Threatening Retinopathy in Middle-Aged and Older Individuals With Type 2 Diabetes: Prospective Investigation From the PREDIMED Trial. JAMA Ophthalmol. 2016, 134, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Chua, J.; Chia, A.R.; Chee, M.L.; Man, R.E.K.; Tan, G.S.W.; Lamoureux, E.L.; Wong, T.Y.; Chong, M.F.; Schmetterer, L. The relationship of dietary fish intake to diabetic retinopathy and retinal vascular caliber in patients with type 2 diabetes. Sci. Rep. 2018, 8, 730. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Cheung, C.Y.; Ikram, M.K.; Zheng, Y.F.; Cheng, C.Y.; Lamoureux, E.L.; Tai, E.S.; Subramaniam, T.; Wong, T.Y. Early retinal arteriolar changes and peripheral neuropathy in diabetes. Diabetes Care 2012, 35, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.K.; Cheung, C.Y.; Lorenzi, M.; Klein, R.; Jones, T.L.; Wong, T.Y.; NIH/JDRF Workshop on Retinal Biomarker for Diabetes Group. Retinal vascular caliber as a biomarker for diabetes microvascular complications. Diabetes Care 2013, 36, 750–759. [Google Scholar] [CrossRef]
- Viola, J.R.; Lemnitzer, P.; Jansen, Y.; Csaba, G.; Winter, C.; Neideck, C.; Silvestre-Roig, C.; Dittmar, G.; Döring, Y.; Drechsler, M.; et al. Resolving Lipid Mediators Maresin 1 and Resolvin D2 Prevent Atheroprogression in Mice. Circ. Res. 2016, 119, 1030–1038. [Google Scholar] [CrossRef]
- Hong, S.; Lu, Y.; Tian, H.; Alapure, B.V.; Wang, Q.; Bunnell, B.A.; Laborde, J.M. Maresin-like lipid mediators are produced by leukocytes and platelets and rescue reparative function of diabetes-impaired macrophages. Chem. Biol. 2014, 21, 1318–1329. [Google Scholar] [CrossRef]
- Tang, S.; Gao, C.; Long, Y.; Huang, W.; Chen, J.; Fan, F.; Jiang, C.; Xu, Y. Maresin 1 Mitigates High Glucose-Induced Mouse Glomerular Mesangial Cell Injury by Inhibiting Inflammation and Fibrosis. Mediat. Inflamm. 2017, 2017, 2438247. [Google Scholar] [CrossRef] [PubMed]
- Maisto, R.; Trotta, M.C.; Petrillo, F.; Izzo, S.; Cuomo, G.; Alfano, R.; Hermenean, A.; Barcia, J.M.; Galdiero, M.; Platania, C.B.M.; et al. Resolvin D1 Modulates the Intracellular VEGF-Related miRNAs of Retinal Photoreceptors Challenged With High Glucose. Front. Pharmacol. 2020, 11, 235. [Google Scholar] [CrossRef]
- Gubitosi-Klug, R.A.; Talahalli, R.; Du, Y.; Nadler, J.L.; Kern, T.S. 5-Lipoxygenase, but not 12/15-lipoxygenase, contributes to degeneration of retinal capillaries in a mouse model of diabetic retinopathy. Diabetes 2008, 57, 1387–1393. [Google Scholar] [CrossRef]
- Yin, Y.; Chen, F.; Wang, W.; Wang, H.; Zhang, X. Resolvin D1 inhibits inflammatory response in STZ-induced diabetic retinopathy rats: Possible involvement of NLRP3 inflammasome and NF-κB signaling pathway. Mol. Vis. 2017, 23, 242–250. [Google Scholar]
- Pennington, K.L.; DeAngelis, M.M. Epidemiology of age-related macular degeneration (AMD): Associations with cardiovascular disease phenotypes and lipid factors. Eye Vis. 2016, 3, 34. [Google Scholar] [CrossRef]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, H.; Dridi, S.; Tarallo, V.; Gelfand, B.D.; Fowler, B.J.; Cho, W.G.; Kleinman, M.E.; Ponicsan, S.L.; Hauswirth, W.W.; Chiodo, V.A.; et al. DICER1 deficit induces Alu RNA toxicity in age-related macular degeneration. Nature 2011, 471, 325–330. [Google Scholar] [CrossRef] [PubMed]
- de Jong, P.T. Age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1474–1485. [Google Scholar] [CrossRef] [PubMed]
- Cano, M.; Thimmalappula, R.; Fujihara, M.; Nagai, N.; Sporn, M.; Wang, A.L.; Neufeld, A.H.; Biswal, S.; Handa, J.T. Cigarette smoking, oxidative stress, the anti-oxidant response through Nrf2 signaling, and Age-related Macular Degeneration. Vis. Res. 2010, 50, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Holekamp, N.M.; Bouck, N.; Volpert, O. Pigment epithelium-derived factor is deficient in the vitreous of patients with choroidal neovascularization due to age-related macular degeneration. Am. J. Ophthalmol. 2002, 134, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.Y.; Cringle, S.J. Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease. Prog. Retin. Eye Res. 2001, 20, 175–208. [Google Scholar] [CrossRef]
- Tokarz, P.; Kaarniranta, K.; Blasiak, J. Role of antioxidant enzymes and small molecular weight antioxidants in the pathogenesis of age-related macular degeneration (AMD). Biogerontology 2013, 14, 461–482. [Google Scholar] [CrossRef]
- Minasyan, L.; Sreekumar, P.G.; Hinton, D.R.; Kannan, R. Protective Mechanisms of the Mitochondrial-Derived Peptide Humanin in Oxidative and Endoplasmic Reticulum Stress in RPE Cells. Oxidative Med. Cell. Longev. 2017, 2017, 1675230. [Google Scholar] [CrossRef]
- Thornton, J.; Edwards, R.; Mitchell, P.; Harrison, R.A.; Buchan, I.; Kelly, S.P. Smoking and age-related macular degeneration: A review of association. Eye (Lond.) 2005, 19, 935–944. [Google Scholar] [CrossRef]
- Neuner, B.; Komm, A.; Wellmann, J.; Dietzel, M.; Pauleikhoff, D.; Walter, J.; Busch, M.; Hense, H.W. Smoking history and the incidence of age-related macular degeneration--results from the Muenster Aging and Retina Study (MARS) cohort and systematic review and meta-analysis of observational longitudinal studies. Addict. Behav. 2009, 34, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, U.; Wong, T.Y.; Fletcher, A.; Piault, E.; Evans, C.; Zlateva, G.; Buggage, R.; Pleil, A.; Mitchell, P. Clinical risk factors for age-related macular degeneration: A systematic review and meta-analysis. BMC Ophthalmol. 2010, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Cano, M.; Wang, J.J.; Li, J.; Huang, C.; Yu, Q.; Herbert, T.P.; Handa, J.T.; Zhang, S.X. Role of unfolded protein response dysregulation in oxidative injury of retinal pigment epithelial cells. Antioxid. Redox Signal. 2014, 20, 2091–2106. [Google Scholar] [CrossRef] [PubMed]
- Kunchithapautham, K.; Atkinson, C.; Rohrer, B. Smoke exposure causes endoplasmic reticulum stress and lipid accumulation in retinal pigment epithelium through oxidative stress and complement activation. J. Biol. Chem. 2014, 289, 14534–14546. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, J.J.; Ma, J.H.; Jin, C.; Yu, Q.; Zhang, S.X. Activation of the UPR protects against cigarette smoke-induced RPE apoptosis through up-regulation of Nrf2. J. Biol. Chem. 2015, 290, 5367–5380. [Google Scholar] [CrossRef] [PubMed]
- Sui, G.Y.; Liu, G.C.; Liu, G.Y.; Gao, Y.Y.; Deng, Y.; Wang, W.Y.; Tong, S.H.; Wang, L. Is sunlight exposure a risk factor for age-related macular degeneration? A systematic review and meta-analysis. Br. J. Ophthalmol. 2013, 97, 389–394. [Google Scholar] [CrossRef]
- Busch, E.M.; Gorgels, T.G.; van Norren, D. Temporal sequence of changes in rat retina after UV-A and blue light exposure. Vis. Res. 1999, 39, 1233–1247. [Google Scholar] [CrossRef]
- King, A.; Gottlieb, E.; Brooks, D.G.; Murphy, M.P.; Dunaief, J.L. Mitochondria-derived reactive oxygen species mediate blue light-induced death of retinal pigment epithelial cells. Photochem. Photobiol. 2004, 79, 470–475. [Google Scholar] [CrossRef]
- Lu, B.; Zhang, P.; Zhou, M.; Wang, W.; Gu, Q.; Feng, J.; Luo, X.; Sun, X.; Wang, F. Involvement of XBP1s in Blue Light-Induced A2E-Containing Retinal Pigment Epithelium Cell Death. Ophthalmic Res. 2017, 57, 252–262. [Google Scholar] [CrossRef]
- Bergen, A.A.; Arya, S.; Koster, C.; Pilgrim, M.G.; Wiatrek-Moumoulidis, D.; van der Spek, P.J.; Hauck, S.M.; Boon, C.J.F.; Emri, E.; Stewart, A.J.; et al. On the origin of proteins in human drusen: The meet, greet and stick hypothesis. Prog. Retin. Eye Res. 2019, 70, 55–84. [Google Scholar] [CrossRef]
- Jeffrey, B.G.; Weisinger, H.S.; Neuringer, M.; Mitchell, D.C. The role of docosahexaenoic acid in retinal function. Lipids 2001, 36, 859–871. [Google Scholar] [CrossRef] [PubMed]
- De La Paz, M.A.; Anderson, R.E. Lipid peroxidation in rod outer segments. Role of hydroxyl radical and lipid hydroperoxides. Investig. Ophthalmol. Vis. Sci. 1992, 33, 2091–2096. [Google Scholar]
- Wang, A.L.; Lukas, T.J.; Yuan, M.; Du, N.; Tso, M.O.; Neufeld, A.H. Autophagy and exosomes in the aged retinal pigment epithelium: Possible relevance to drusen formation and age-related macular degeneration. PLoS ONE 2009, 4, e4160. [Google Scholar] [CrossRef] [PubMed]
- Kaarniranta, K.; Sinha, D.; Blasiak, J.; Kauppinen, A.; Veréb, Z.; Salminen, A.; Boulton, M.E.; Petrovski, G. Autophagy and heterophagy dysregulation leads to retinal pigment epithelium dysfunction and development of age-related macular degeneration. Autophagy 2013, 9, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Jia, L.; Khan, N.; Lin, C.; Mitter, S.K.; Boulton, M.E.; Dunaief, J.L.; Klionsky, D.J.; Guan, J.L.; Thompson, D.A.; et al. Deletion of autophagy inducer RB1CC1 results in degeneration of the retinal pigment epithelium. Autophagy 2015, 11, 939–953. [Google Scholar] [CrossRef]
- Sparrow, J.R.; Nakanishi, K.; Parish, C.A. The lipofuscin fluorophore A2E mediates blue light-induced damage to retinal pigmented epithelial cells. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1981–1989. [Google Scholar]
- Moon, J.; Yun, J.; Yoon, Y.D.; Park, S.I.; Seo, Y.J.; Park, W.S.; Chu, H.Y.; Park, K.H.; Lee, M.Y.; Lee, C.W.; et al. Blue light effect on retinal pigment epithelial cells by display devices. Integr. Biol. 2017, 9, 436–443. [Google Scholar] [CrossRef]
- Zhang, J.; Bai, Y.; Huang, L.; Qi, Y.; Zhang, Q.; Li, S.; Wu, Y.; Li, X. Protective effect of autophagy on human retinal pigment epithelial cells against lipofuscin fluorophore A2E: Implications for age-related macular degeneration. Cell Death Dis. 2015, 6, e1972. [Google Scholar] [CrossRef]
- Parmar, V.M.; Parmar, T.; Arai, E.; Perusek, L.; Maeda, A. A2E-associated cell death and inflammation in retinal pigmented epithelial cells from human induced pluripotent stem cells. Stem Cell Res. 2018, 27, 95–104. [Google Scholar] [CrossRef]
- Iriyama, A.; Fujiki, R.; Inoue, Y.; Takahashi, H.; Tamaki, Y.; Takezawa, S.; Takeyama, K.; Jang, W.D.; Kato, S.; Yanagi, Y. A2E, a pigment of the lipofuscin of retinal pigment epithelial cells, is an endogenous ligand for retinoic acid receptor. J. Biol. Chem. 2008, 283, 11947–11953. [Google Scholar] [CrossRef]
- Terao, R.; Honjo, M.; Totsuka, K.; Miwa, Y.; Kurihara, T.; Aihara, M. The role of sphingosine 1-phosphate receptors on retinal pigment epithelial cells barrier function and angiogenic effects. Prostaglandins Other Lipid Mediat. 2019, 145, 106365. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, T.; Hata, Y.; Yoshikawa, H.; Nakagawa, K.; Sueishi, K.; Inomata, H. Expression of vascular endothelial growth factor in experimental choroidal neovascularization. Graefes Arch. Clin. Exp. Ophthalmol. 1997, 235, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Yuda, K.; Takahashi, H.; Inoue, T.; Ueta, T.; Iriyama, A.; Kadonosono, K.; Tamaki, Y.; Aburatani, H.; Nagai, R.; Yanagi, Y. Adrenomedullin inhibits choroidal neovascularization via CCL2 in the retinal pigment epithelium. Am. J. Pathol. 2012, 181, 1464–1472. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Tu, Y.; Wang, Y.; Xu, X.; Sun, X.; Xie, L.; Zhao, Q.; Guo, Y.; Gu, Y.; Du, J.; et al. Prodrug of epigallocatechin-3-gallate alleviates choroidal neovascularization via downregulating HIF-1α/VEGF/VEGFR2 pathway and M1 type macrophage/microglia polarization. Biomed. Pharmacother. 2020, 121, 109606. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Takagi, H.; Takagi, C.; Suzuma, K.; Otani, A.; Ishida, K.; Matsumura, M.; Ogura, Y.; Honda, Y. The potential angiogenic role of macrophages in the formation of choroidal neovascular membranes. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1891–1898. [Google Scholar]
- Lechner, J.; Chen, M.; Hogg, R.E.; Toth, L.; Silvestri, G.; Chakravarthy, U.; Xu, H. Peripheral blood mononuclear cells from neovascular age-related macular degeneration patients produce higher levels of chemokines CCL2 (MCP-1) and CXCL8 (IL-8). J. Neuroinflamm. 2017, 14, 42. [Google Scholar] [CrossRef]
- Jonas, J.B.; Tao, Y.; Neumaier, M.; Findeisen, P. Monocyte chemoattractant protein 1, intercellular adhesion molecule 1, and vascular cell adhesion molecule 1 in exudative age-related macular degeneration. Arch. Ophthalmol. 2010, 128, 1281–1286. [Google Scholar] [CrossRef]
- Fang, I.M.; Yang, C.H.; Yang, C.M.; Chen, M.S. Linoleic acid-induced expression of inducible nitric oxide synthase and cyclooxygenase II via p42/44 mitogen-activated protein kinase and nuclear factor-kappaB pathway in retinal pigment epithelial cells. Exp. Eye Res. 2007, 85, 667–677. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Salminen, A. NF-kappaB signaling as a putative target for omega-3 metabolites in the prevention of age-related macular degeneration (AMD). Exp. Gerontol. 2009, 44, 685–688. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, Z.; Zhang, H.; Zhang, Y.; Lin, D. The COX-2-Selective Antagonist (NS-398) Inhibits Choroidal Neovascularization and Subretinal Fibrosis. PLoS ONE 2016, 11, e0146808. [Google Scholar] [CrossRef]
- Koto, T.; Nagai, N.; Mochimaru, H.; Kurihara, T.; Izumi-Nagai, K.; Satofuka, S.; Shinoda, H.; Noda, K.; Ozawa, Y.; Inoue, M.; et al. Eicosapentaenoic acid is anti-inflammatory in preventing choroidal neovascularization in mice. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4328–4334. [Google Scholar] [CrossRef]
- Prokopiou, E.; Kolovos, P.; Kalogerou, M.; Neokleous, A.; Papagregoriou, G.; Deltas, C.; Malas, S.; Georgiou, T. Therapeutic potential of omega-3 fatty acids supplementation in a mouse model of dry macular degeneration. BMJ Open Ophthalmol. 2017, 1, e000056. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Marcheselli, V.L.; Barreiro, S.; Hu, J.; Bok, D.; Bazan, N.G. Neurotrophins enhance retinal pigment epithelial cell survival through neuroprotectin D1 signaling. Proc. Natl. Acad. Sci. USA 2007, 104, 13152–13157. [Google Scholar] [CrossRef] [PubMed]
- Sheets, K.G.; Zhou, Y.; Ertel, M.K.; Knott, E.J.; Regan, C.E.; Elison, J.R.; Gordon, W.C.; Gjorstrup, P.; Bazan, N.G. Neuroprotectin D1 attenuates laser-induced choroidal neovascularization in mouse. Mol. Vis. 2010, 16, 320–329. [Google Scholar]
- Sheets, K.G.; Jun, B.; Zhou, Y.; Zhu, M.; Petasis, N.A.; Gordon, W.C.; Bazan, N.G. Microglial ramification and redistribution concomitant with the attenuation of choroidal neovascularization by neuroprotectin D1. Mol. Vis. 2013, 19, 1747–1759. [Google Scholar]
- Tian, H.; Lu, Y.; Sherwood, A.M.; Hongqian, D.; Hong, S. Resolvins E1 and D1 in choroid-retinal endothelial cells and leukocytes: Biosynthesis and mechanisms of anti-inflammatory actions. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3613–3620. [Google Scholar] [CrossRef]
- Merle, B.M.; Delyfer, M.N.; Korobelnik, J.F.; Rougier, M.B.; Malet, F.; Féart, C.; Le Goff, M.; Peuchant, E.; Letenneur, L.; Dartigues, J.F.; et al. High concentrations of plasma n3 fatty acids are associated with decreased risk for late age-related macular degeneration. J. Nutr. 2013, 143, 505–511. [Google Scholar] [CrossRef]
- Gorusupudi, A.; Liu, A.; Hageman, G.S.; Bernstein, P.S. Associations of human retinal very long-chain polyunsaturated fatty acids with dietary lipid biomarkers. J. Lipid Res. 2016, 57, 499–508. [Google Scholar] [CrossRef]
- Liu, A.; Chang, J.; Lin, Y.; Shen, Z.; Bernstein, P.S. Long-chain and very long-chain polyunsaturated fatty acids in ocular aging and age-related macular degeneration. J. Lipid Res. 2010, 51, 3217–3229. [Google Scholar] [CrossRef]
- Rezende, F.A.; Lapalme, E.; Qian, C.X.; Smith, L.E.; SanGiovanni, J.P.; Sapieha, P. Omega-3 supplementation combined with anti-vascular endothelial growth factor lowers vitreal levels of vascular endothelial growth factor in wet age-related macular degeneration. Am. J. Ophthalmol. 2014, 158, 1071–1078. [Google Scholar] [CrossRef]
- Seddon, J.M.; Cote, J.; Rosner, B. Progression of age-related macular degeneration: Association with dietary fat, transunsaturated fat, nuts, and fish intake. Arch. Ophthalmol. 2003, 121, 1728–1737. [Google Scholar] [CrossRef] [PubMed]
- Christen, W.G.; Schaumberg, D.A.; Glynn, R.J.; Buring, J.E. Dietary ω-3 fatty acid and fish intake and incident age-related macular degeneration in women. Arch. Ophthalmol. 2011, 129, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.S.; Wang, J.J.; Flood, V.; Mitchell, P. Dietary fatty acids and the 10-year incidence of age-related macular degeneration: The Blue Mountains Eye Study. Arch. Ophthalmol. 2009, 127, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cho, E.; Giovannucci, E.L.; Rosner, B.A.; Sastry, S.M.; Willett, W.C.; Schaumberg, D.A. Dietary Intakes of Eicosapentaenoic Acid and Docosahexaenoic Acid and Risk of Age-Related Macular Degeneration. Ophthalmology 2017, 124, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Wu, Y.; Meng, Y.F.; Xing, Q.; Tao, J.J.; Lu, J. Fish Consumption and Age-Related Macular Degeneration Incidence: A Meta-Analysis and Systematic Review of Prospective Cohort Studies. Nutrients 2016, 8, 743. [Google Scholar] [CrossRef] [PubMed]
- SanGiovanni, J.P.; Chew, E.Y.; Clemons, T.E.; Davis, M.D.; Ferris, F.L.; Gensler, G.R.; Kurinij, N.; Lindblad, A.S.; Milton, R.C.; Seddon, J.M.; et al. The relationship of dietary lipid intake and age-related macular degeneration in a case-control study: AREDS Report No. 20. Arch. Ophthalmol. 2007, 125, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Chew, E.Y.; Clemons, T.E.; SanGiovanni, J.P.; Danis, R.; Ferris, F.L.; Elman, M.; Antoszyk, A.; Ruby, A.; Orth, D.; Bressler, S.; et al. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: The Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA 2013, 309, 2005–2015. [Google Scholar] [CrossRef]
- Souied, E.H.; Delcourt, C.; Querques, G.; Bassols, A.; Merle, B.; Zourdani, A.; Smith, T.; Benlian, P.; Nutritional AMD Treatment 2 Study Group. Oral docosahexaenoic acid in the prevention of exudative age-related macular degeneration: The Nutritional AMD Treatment 2 study. Ophthalmology 2013, 120, 1619–1631. [Google Scholar] [CrossRef]
- Querques, G.; Merle, B.M.; Pumariega, N.M.; Benlian, P.; Delcourt, C.; Zourdani, A.; Leisy, H.B.; Lee, M.D.; Smith, R.T.; Souied, E.H. Dynamic Drusen Remodelling in Participants of the Nutritional AMD Treatment-2 (NAT-2) Randomized Trial. PLoS ONE 2016, 11, e0149219. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).