Extra Virgin Olive Oil and Nigella sativa Oil Produced in Central Italy: A Comparison of the Nutrigenomic Effects of Two Mediterranean Oils in a Low-Grade Inflammation Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Cells Culture
2.2. Cytotoxicity Assay
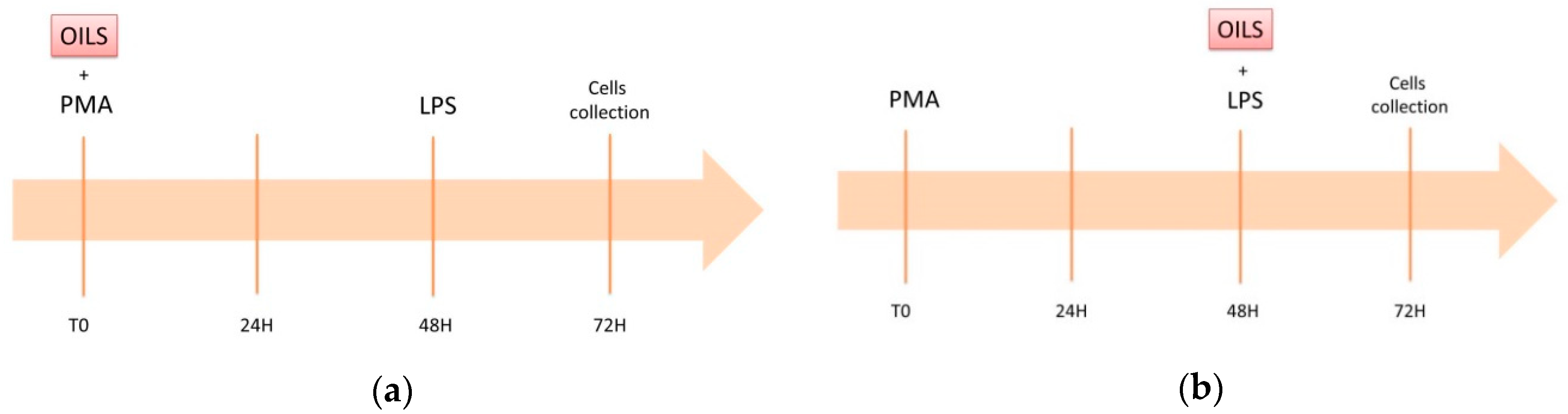
2.3. In Vitro Model of Low-Grade Inflammation
2.4. Gene Expression Assessment
2.5. Global DNA Methylation and Hydroxymethylation
2.6. MtDNA Copy Number Evaluation
2.7. Reduced Glutathione to Oxidized Glutathione (GSH/GSSG) Ratio Measurement
2.8. Membrane Fluidity
2.9. Statistical Analysis
3. Results
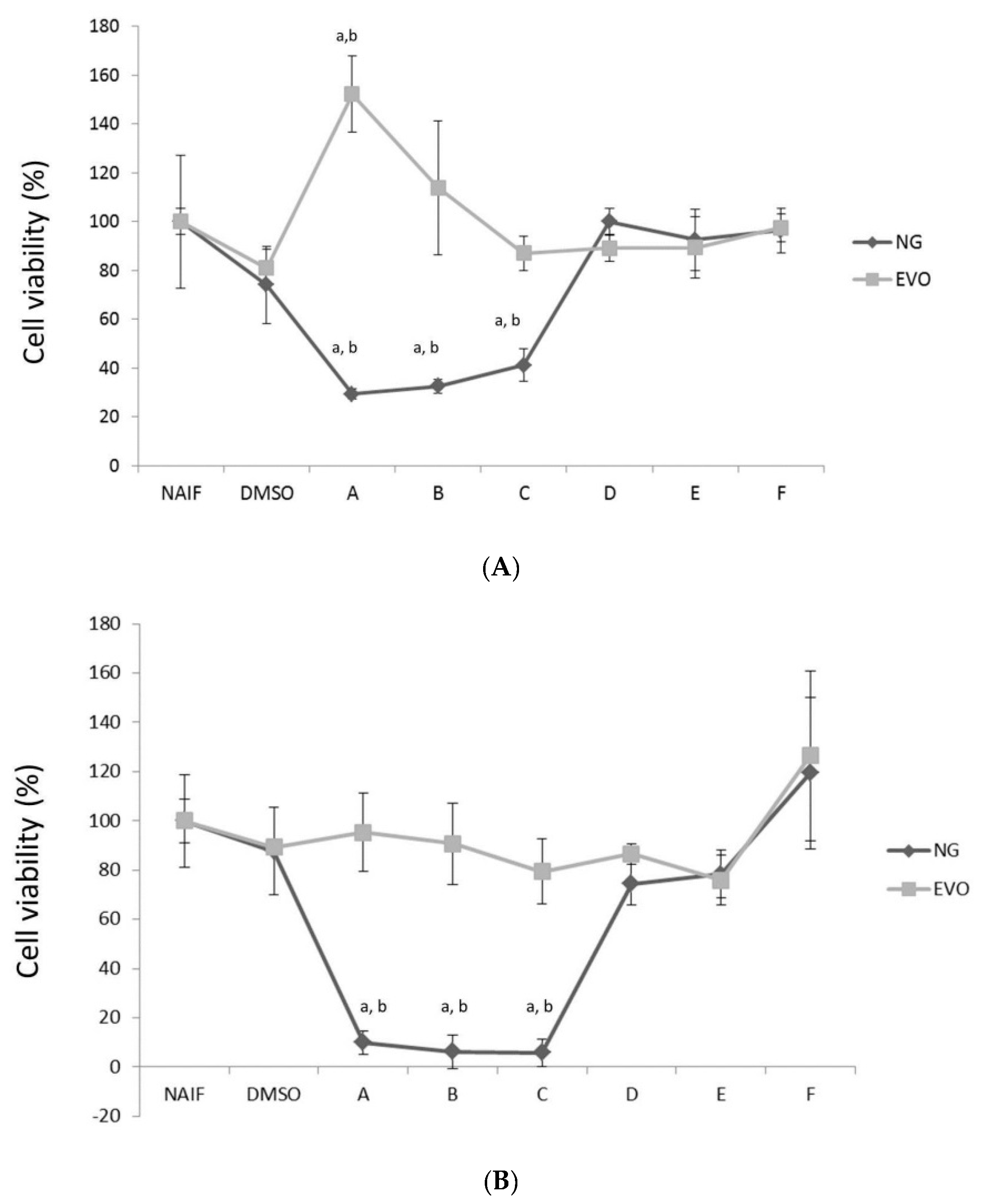
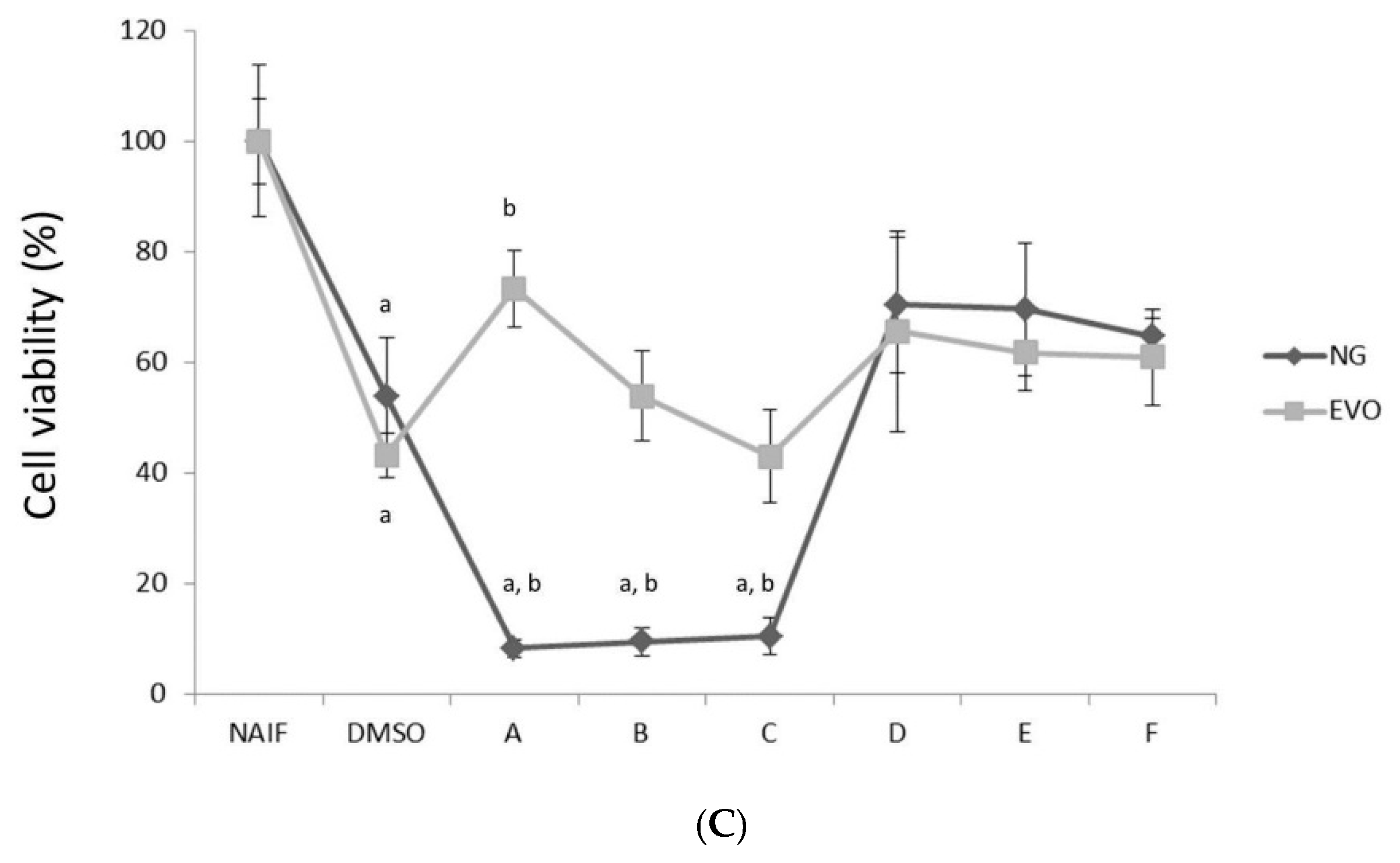
3.1. NG but Not EVO Oil Shows Cytotoxic Properties
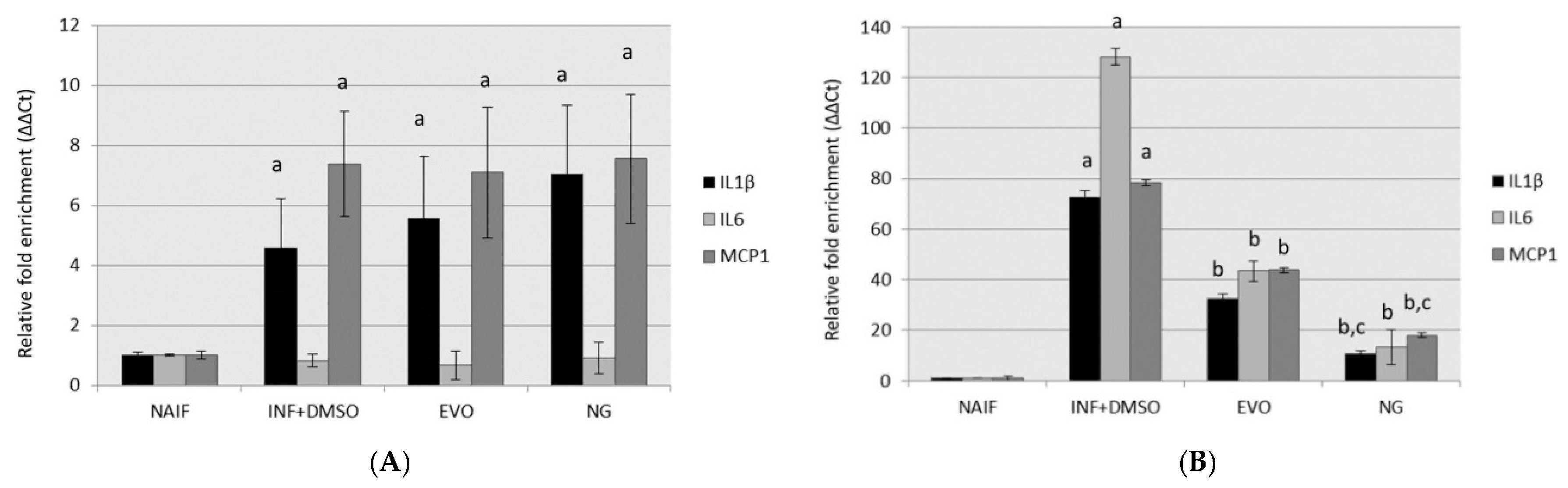
3.2. Both NG and EVO Oils Have Anti-Inflammatory Effects after 24 h of Exposure
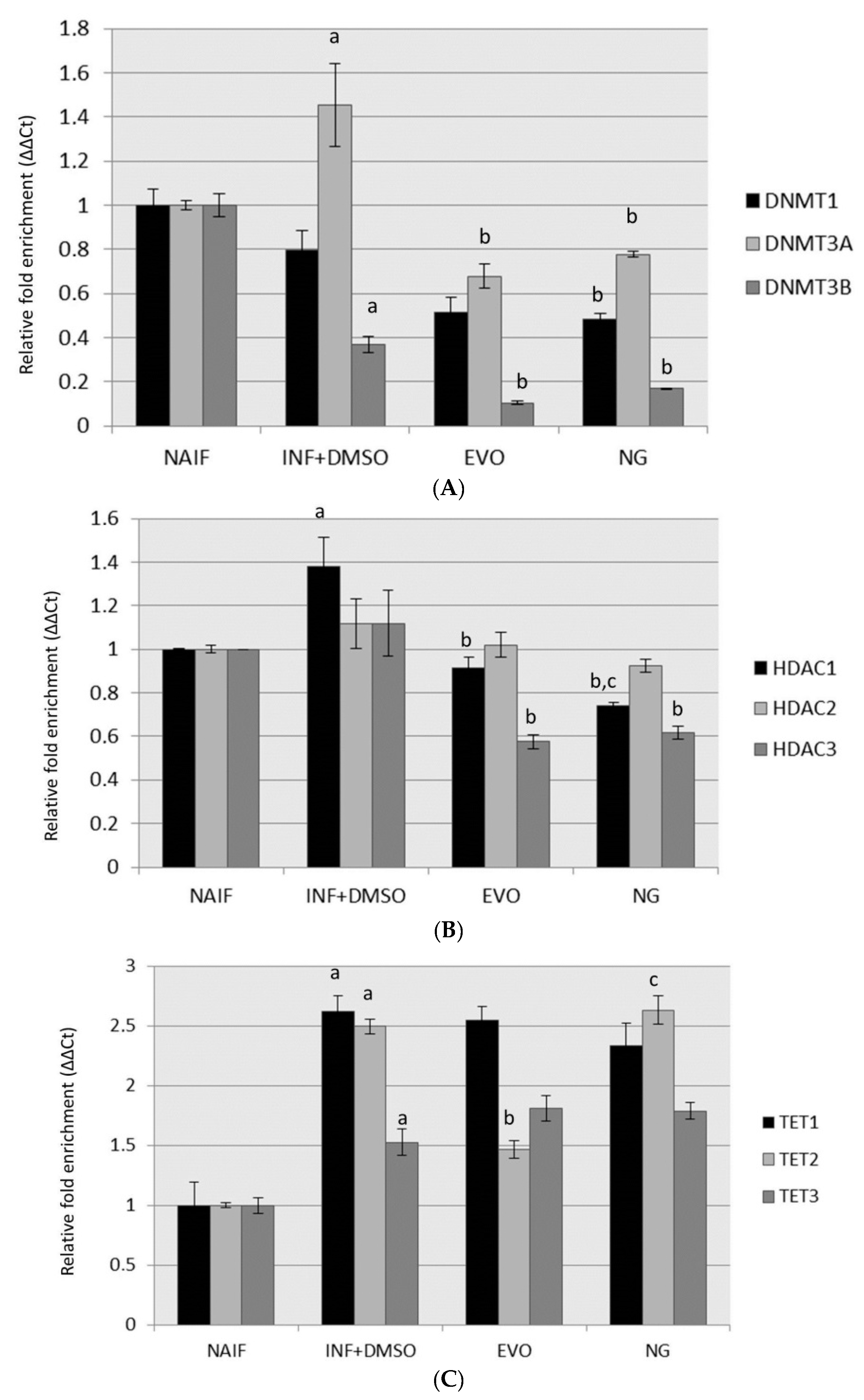
3.3. Modulation of DNMTs and HDACs
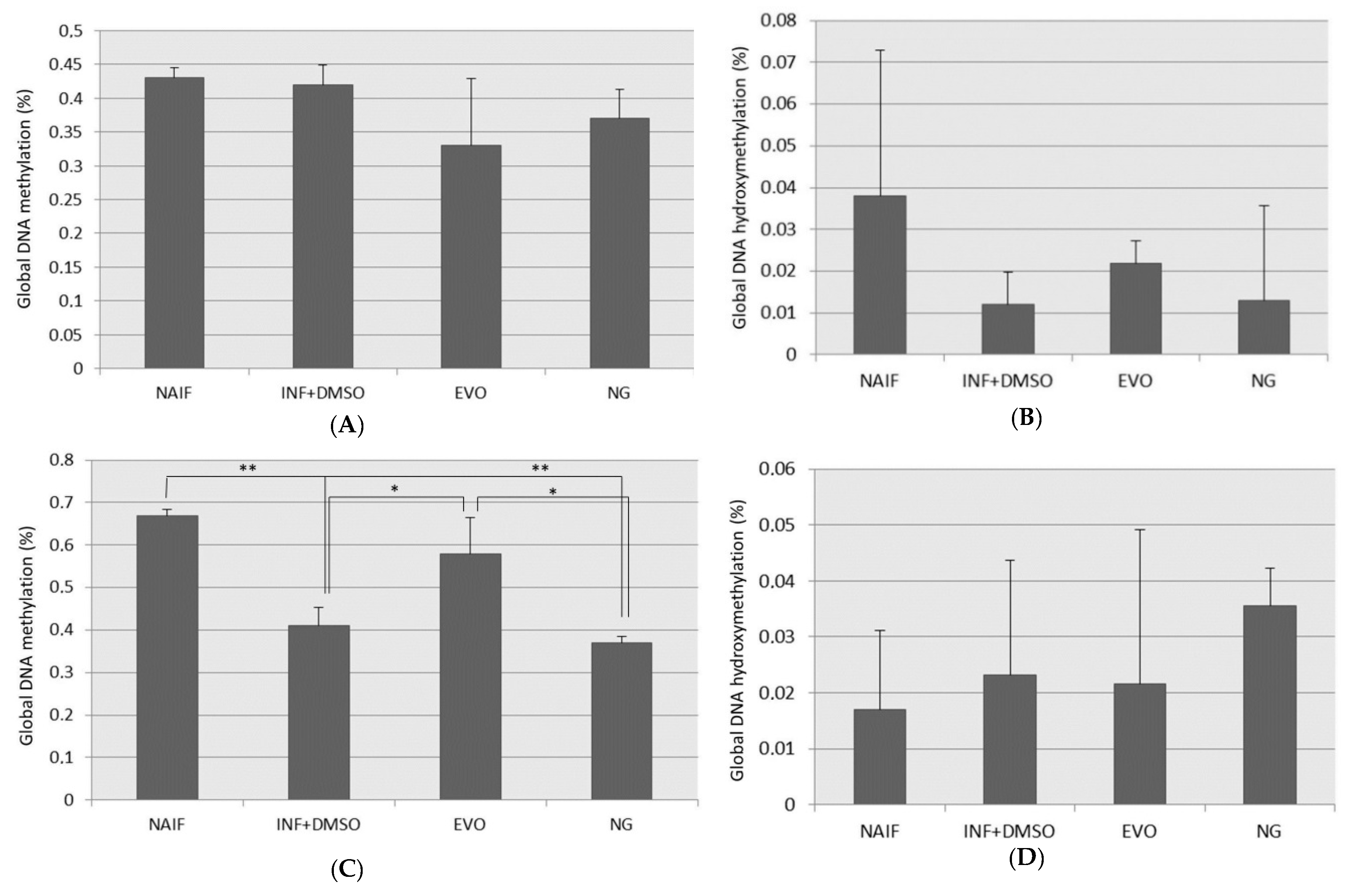
3.4. Global DNA Methylation and Hydroxymethylation Alteration
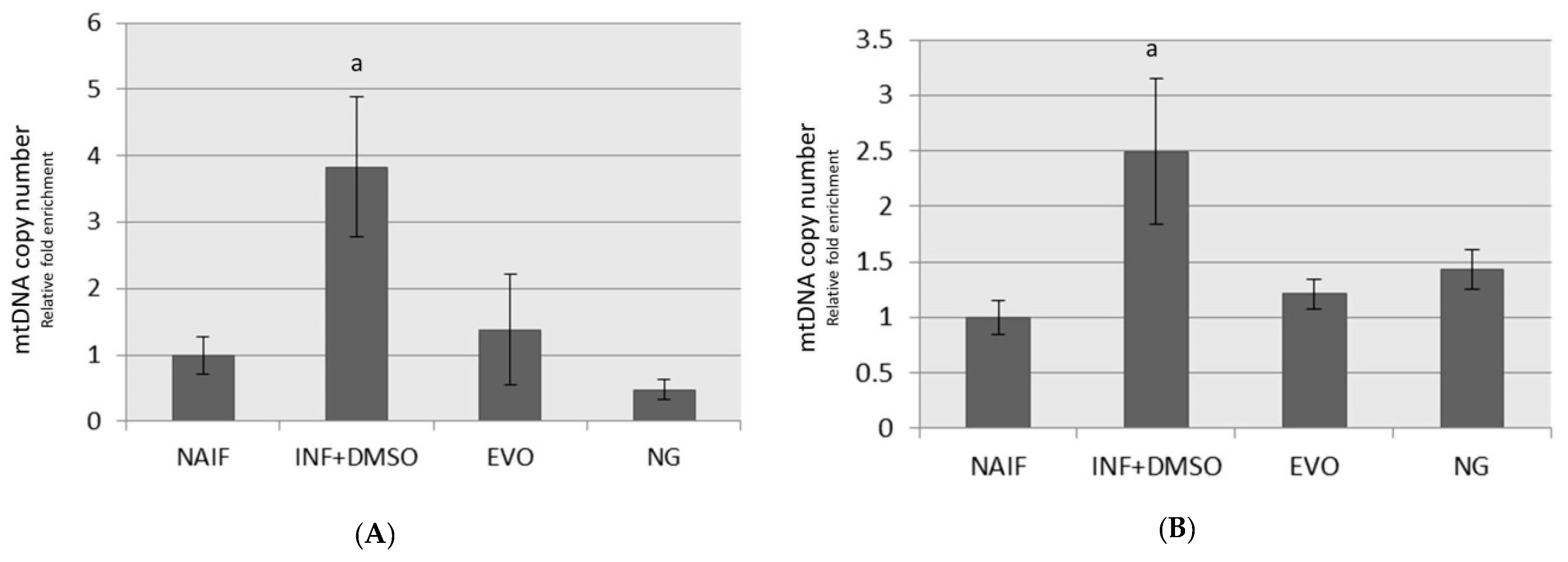
3.5. mtDNA Copy Number
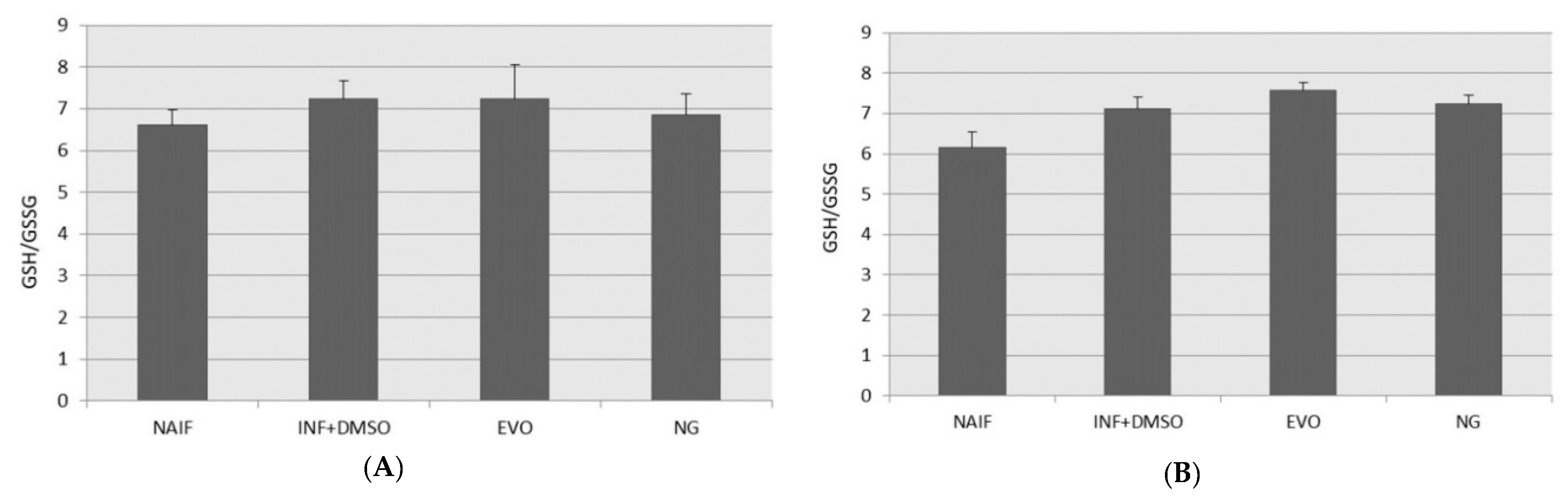
3.6. GSH/GSSG Levels
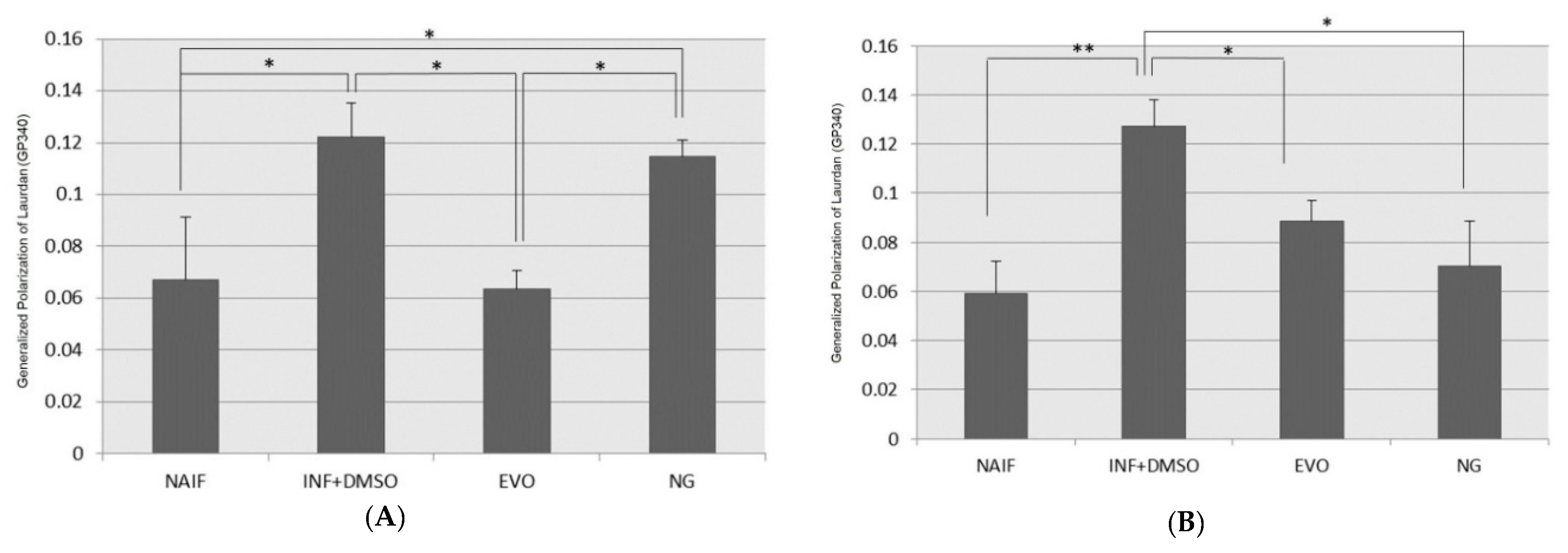
3.7. Cell Membrane Fluidity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Libby, P. Inflammation in atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef]
- Geng, S.; Chen, K.; Yuan, R.; Peng, L.; Maitra, U.; Diao, N.; Chen, C.; Zhang, Y.; Hu, Y.; Qi, C.-F.; et al. The persistence of low-grade inflammatory monocytes contributes to aggravated atherosclerosis. Nat. Commun. 2016, 7, 13436. [Google Scholar] [CrossRef]
- Candore, G.; Caruso, C.; Jirillo, E.; Magrone, T.; Vasto, E. Low Grade Inflammation as a Common Pathogenetic Denominator in Age-Related Diseases: Novel Drug Targets for Anti-Ageing Strategies and Successful Ageing Achievement. Curr. Pharm. Des. 2010, 16, 584. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef]
- Ortega-Gomez, A.; Perretti, M.; Soehnlein, O. Resolution of inflammation: An integrated view. EMBO Mol. Med. 2013, 5, 661–674. [Google Scholar] [CrossRef]
- Calder, P.C.; Albers, R.; Antoine, J.-M.; Blum, S.; Bourdet-Sicard, R.; Ferns, G.A.; Folkerts, G.; Friedmann, P.S.; Frost, G.S.; Guarner, F.; et al. Inflammatory disease processes and interactions with nutrition. Br. J. Nutr. 2009, 101 (Suppl. S1), 1–45. [Google Scholar] [CrossRef]
- Minihane, A.M.; Vinoy, S.; Russell, W.R.; Baka, A.; Roche, H.M.; Tuohy, K.M.; Teeling, J.L.; Blaak, E.E.; Fenech, M.; Vauzour, D.; et al. Low-grade inflammation, diet composition and health: Current research evidence and its translation. Br. J. Nutr. 2015, 114, 999–1012. [Google Scholar] [CrossRef]
- Miller, S.I.; Ernst, R.K.; Bader, M.W. LPS, TLR4 and infectious disease diversity. Nat. Rev. Microbiol. 2005, 3, 36–46. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Sethi, G. Role of epigenetics in inflammation-associated diseases. In Epigenetics: Development and Disease; Subcellular Biochemistry; Springer: Berlin, Germany, 2013; Volume 61, pp. 627–657. [Google Scholar] [CrossRef]
- Stylianou, E. Epigenetics of chronic inflammatory diseases. J. Inflamm. Res. 2019, 12, 1–14. [Google Scholar] [CrossRef]
- Gonzalez-Jaramillo, V.; Portilla-Fernandez, E.; Glisic, M.; Voortman, T.; Ghanbari, M.; Bramer, W.; Chowdhury, R.; Nijsten, T.; Dehghan, A.; Franco, O.H.; et al. Epigenetics and Inflammatory Markers: A Systematic Review of the Current Evidence. Int. J. Inflamm. 2019, 2019, 6273680. [Google Scholar] [CrossRef] [PubMed]
- Remely, M.; Stefanska, B.; Lovrecic, L.; Magnet, U.; Haslberger, A.G. Nutriepigenomics: The role of nutrition in epigenetic control of human diseases. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Remely, M.; Lovrecic, L.; de la Garza, A.L.; Migliore, L.; Peterlin, B.; Milagro, F.I.; Martinez, A.J.; Haslberger, A.G. Therapeutic perspectives of epigenetically active nutrients. Br. J. Pharmacol. 2015, 172, 2756–2768. [Google Scholar] [CrossRef]
- Butt, A.S.; Nisar, N.; Mughal, T.A.; Ghani, N.; Altaf, I. Anti-oxidative and anti-proliferative activities of extracted phytochemical compound thymoquinone. J. Pak. Med. Assoc. 2019, 69, 1479–1485. [Google Scholar] [CrossRef]
- Babar, Z.M.; Jaswir, I.; Tareq, A.M.; Ali Reza, A.S.M.; Azizi, W.M.; Hafidz, M.; Ahfter, F.; Hasan, M.; Farhad, S.; Uddin, M.M.R.; et al. In vivo anxiolytic and in vitro anti-inflammatory activities of water-soluble extract (WSE) of Nigella sativa (L.) seeds. Nat. Prod. Res. 2019, 1–6. [Google Scholar] [CrossRef]
- Nordin, A.; Kamal, H.; Yazid, M.D.; Saim, A.; Idrus, R. Effect of Nigella sativa and its bioactive compound on type 2 epithelial to mesenchymal transition: A systematic review. BMC Complement. Altern. Med. 2019, 19, 290. [Google Scholar] [CrossRef]
- Ijaz, H.; Tulain, U.R.; Qureshi, J.; Danish, Z.; Musayab, S.; Akhtar, M.F.; Saleem, A.; Khan, K.K.; Zaman, M.; Waheed, I.; et al. Review: Nigella sativa (Prophetic Medicine): A Review. Pak. J. Pharm. Sci. 2017, 30, 229–234. [Google Scholar]
- Kooti, W.; Hasanzadeh-Noohi, Z.; Sharafi-Ahvazi, N.; Asadi-Samani, M.; Ashtary-Larky, D. Phytochemistry, pharmacology, and therapeutic uses of black seed (Nigella sativa). Chin. J. Nat. Med. 2016, 14, 732–745. [Google Scholar] [CrossRef]
- Amin, B.; Hosseinzadeh, H. Black Cumin (Nigella sativa) and Its Active Constituent, Thymoquinone: An Overview on the Analgesic and Anti-inflammatory Effects. Planta Med. 2016, 82, 8–16. [Google Scholar] [CrossRef]
- Yubero-Serrano, E.M.; Lopez-Moreno, J.; Gomez-Delgado, F.; Lopez-Miranda, J. Extra virgin olive oil: More than a healthy fat. Eur. J. Clin. Nutr. 2019, 72, 8–17. [Google Scholar] [CrossRef]
- Oliveras-Lopez, M.-J.; Molina, J.J.M.; Mir, M.V.; Rey, E.F.; Martin, F.; de la Serrana, H.L.-G. Extra virgin olive oil (EVOO) consumption and antioxidant status in healthy institutionalized elderly humans. Arch. Gerontol. Geriatr. 2013, 57, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, C.; Vari, R.; Scazzocchio, B.; De Sanctis, P.; Giovannini, C.; D’Archivio, M.; Masella, R. Anti-inflammatory Activity of Extra Virgin Olive Oil Polyphenols: Which Role in the Prevention and Treatment of Immune-Mediated Inflammatory Diseases? Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Crespo, M.C.; Tome-Carneiro, J.; Davalos, A.; Visioli, F. Pharma-Nutritional Properties of Olive Oil Phenols. Transfer of New Findings to Human Nutrition. Foods 2018, 7, 90. [Google Scholar] [CrossRef]
- Visioli, F.; Davalos, A.; López de las Hazas, M.-C.; Crespo, M.C.; Tomé-Carneiro, J. An overview of the pharmacology of olive oil and its active ingredients. Br. J. Pharmacol. 2019. [Google Scholar] [CrossRef]
- Kabaran, S. Olive Oil: Antioxidant Compounds and Their Potential Effects over Health. IntechOpen 2018. [Google Scholar] [CrossRef]
- Covas, M.-I.; de la Torre, R.; Fito, M. Virgin olive oil: A key food for cardiovascular risk protection. Br. J. Nutr. 2015, 113 (Suppl. S2), S19–S28. [Google Scholar] [CrossRef]
- Di Francesco, A.; Falconi, A.; Di Germanio, C.; Micioni Di Bonaventura, M.V.; Costa, A.; Caramuta, S.; Del Carlo, M.; Compagnone, D.; Dainese, E.; Cifani, C.; et al. Extravirgin olive oil up-regulates CB(1) tumor suppressor gene in human colon cancer cells and in rat colon via epigenetic mechanisms. J. Nutr. Biochem. 2015, 26, 250–258. [Google Scholar] [CrossRef]
- Capurso, A.; Crepaldi, G.; Capurso, C. Epigenetics/Epigenomics of Olive Oil and the Mediterranean Diet BT-Benefits of the Mediterranean Diet in the Elderly Patient; Capurso, A., Crepaldi, G., Capurso, C., Eds.; Springer: Cham, Switzerland, 2018; pp. 115–138. ISBN 978-3-319-78084-9. [Google Scholar]
- Fiorini, D.; Boarelli, M.C.; Conti, P.; Alfei, B.; Caprioli, G.; Ricciutelli, M.; Sagratini, G.; Fedeli, D.; Gabbianelli, R.; Pacetti, D. Chemical and sensory differences between high price and low price extra virgin olive oils. Food Res. Int. 2018, 105, 65–75. [Google Scholar] [CrossRef]
- Nasuti, C.; Fedeli, D.; Bordoni, L.; Piangerelli, M.; Servili, M.; Selvaggini, R.; Gabbianelli, R. Anti-Inflammatory, Anti-Arthritic and Anti-Nociceptive Activities of Nigella sativa Oil in a Rat Model of Arthritis. Antioxidants 2019, 8, 342. [Google Scholar] [CrossRef]
- Ayuob, N.N.; Abdel-Hamid, A.A.H.M.; Helal, G.M.M.; Mubarak, W.A. Thymoquinone reverses nonalcoholic fatty liver disease (NAFLD) associated with experimental hypothyroidism. Rom. J. Morphol. Embryol. 2019, 60, 479–486. [Google Scholar]
- Imran, M.; Rauf, A.; Khan, I.A.; Shahbaz, M.; Qaisrani, T.B.; Fatmawati, S.; Abu-Izneid, T.; Imran, A.; Rahman, K.U.; Gondal, T.A. Thymoquinone: A novel strategy to combat cancer: A review. Biomed. Pharmacother. 2018, 106, 390–402. [Google Scholar] [CrossRef]
- Darakhshan, S.; Bidmeshki Pour, A.; Hosseinzadeh Colagar, A.; Sisakhtnezhad, S. Thymoquinone and its therapeutic potentials. Pharmacol. Res. 2015, 95, 138–158. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, L.; Fedeli, D.; Nasuti, C.; Maggi, F.; Papa, F.; Wabitsch, M.; De Caterina, R.; Gabbianelli, R. Antioxidant and Anti-Inflammatory Properties of Nigella sativa Oil in Human Pre-Adipocytes. Antioxidants 2019, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Park, E.K.; Jung, H.S.; Yang, H.I.; Yoo, M.C.; Kim, C.; Kim, K.S. Optimized THP-1 differentiation is required for the detection of responses to weak stimuli. Inflamm. Res. 2007, 56, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Chanput, W.; Mes, J.; Vreeburg, R.A.M.; Savelkoul, H.F.J.; Wichers, H.J. Transcription profiles of LPS-stimulated THP-1 monocytes and macrophages: A tool to study inflammation modulating effects of food-derived compounds. Food Funct. 2010, 1, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, L.; Smerilli, V.; Nasuti, C.; Gabbianelli, R. Mitochondrial DNA methylation and copy number predict body composition in a young female population. J. Transl. Med. 2019, 17, 399. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Gera, R.; Purohit, M.P.; Patnaik, S.; Ghosh, D. Fluorometric Estimation of Glutathione in Cultured Microglial Cell Lysate. Bioprotocol 2017, 7, e2304. [Google Scholar] [CrossRef]
- Parasassi, T.; De Stasio, G.; d’Ubaldo, A.; Gratton, E. Phase fluctuation in phospholipid membranes revealed by Laurdan fluorescence. Biophys. J. 1990, 57, 1179–1186. [Google Scholar] [CrossRef]
- Parasassi, T.; De Stasio, G.; Ravagnan, G.; Rusch, R.M.; Gratton, E. Quantitation of lipid phases in phospholipid vesicles by the generalized polarization of Laurdan fluorescence. Biophys. J. 1991, 60, 179–189. [Google Scholar] [CrossRef]
- Hunter, D.J.; James, L.; Hussey, B.; Wadley, A.J.; Lindley, M.R.; Mastana, S.S. Impact of aerobic exercise and fatty acid supplementation on global and gene-specific DNA methylation. Epigenetics 2019, 14, 294–309. [Google Scholar] [CrossRef]
- Bayat, S.; Mansoori Derakhshan, S.; Mansoori Derakhshan, N.; Shekari Khaniani, M.; Alivand, M.R. Downregulation of HDAC2 and HDAC3 via oleuropein as a potent prevention and therapeutic agent in MCF-7 breast cancer cells. J. Cell. Biochem. 2019, 120, 9172–9180. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Shen, N.; Yan, F.; Zhao, N.; Dou, L.; Wu, L.-C.; Seiler, C.L.; Yu, L.; Yang, K.; Bachanova, V.; et al. Thymoquinone exerts potent growth-suppressive activity on leukemia through DNA hypermethylation reversal in leukemia cells. Oncotarget 2017, 8, 34453–34467. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Tania, M.; Fu, J. Epigenetic role of thymoquinone: Impact on cellular mechanism and cancer therapeutics. Drug Discov. Today 2019, 24, 2315–2322. [Google Scholar] [CrossRef] [PubMed]
- Qadi, S.A.; Hassan, M.A.; Sheikh, R.A.; Baothman, O.A.; Zamzami, M.A.; Choudhry, H.; Al-Malki, A.L.; Albukhari, A.; Alhosin, M. Thymoquinone-Induced Reactivation of Tumor Suppressor Genes in Cancer Cells Involves Epigenetic Mechanisms. Epigenetics Insights 2019, 12, 2516865719839011. [Google Scholar] [CrossRef]
- Fiorini, D.; Boarelli, M.C.; Gabbianelli, R.; Fedeli, D.; Sagratini, G.; Caprioli, G.; Ricciutelli, M.; Giovannetti, R.; Ferraro, S.; Conti, P.; et al. Chemical compositional peculiarities and functional properties of monovarietal extra virgin olive oils from Marche Region. In Proceedings of the Congress Cibo e nutraceutici: Direzione salute Camerino, Camerino, Italy, 10 July 2018; p. 19, ISBN 978-88-6768-034-4. [Google Scholar]
- Commission Regulation (EU) 432/2012 Establishing a list of permitted health claims made on foods, other than those referring to the reduction of disease risk and to children’s development and health. Off. J. Eur. Union 2012, 136, 1–40.
- Knez, J.; Marrachelli, V.G.; Cauwenberghs, N.; Winckelmans, E.; Zhang, Z.; Thijs, L.; Brguljan-Hitij, J.; Plusquin, M.; Delles, C.; Monleon, D.; et al. Peripheral blood mitochondrial DNA content in relation to circulating metabolites and inflammatory markers: A population study. PLoS ONE 2017, 12, e0181036. [Google Scholar] [CrossRef]
- Wu, I.-C.; Lin, C.-C.; Liu, C.-S.; Hsu, C.-C.; Chen, C.-Y.; Hsiung, C.A. Interrelations Between Mitochondrial DNA Copy Number and Inflammation in Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 937–944. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bordoni, L.; Fedeli, D.; Fiorini, D.; Gabbianelli, R. Extra Virgin Olive Oil and Nigella sativa Oil Produced in Central Italy: A Comparison of the Nutrigenomic Effects of Two Mediterranean Oils in a Low-Grade Inflammation Model. Antioxidants 2020, 9, 20. https://doi.org/10.3390/antiox9010020
Bordoni L, Fedeli D, Fiorini D, Gabbianelli R. Extra Virgin Olive Oil and Nigella sativa Oil Produced in Central Italy: A Comparison of the Nutrigenomic Effects of Two Mediterranean Oils in a Low-Grade Inflammation Model. Antioxidants. 2020; 9(1):20. https://doi.org/10.3390/antiox9010020
Chicago/Turabian StyleBordoni, Laura, Donatella Fedeli, Dennis Fiorini, and Rosita Gabbianelli. 2020. "Extra Virgin Olive Oil and Nigella sativa Oil Produced in Central Italy: A Comparison of the Nutrigenomic Effects of Two Mediterranean Oils in a Low-Grade Inflammation Model" Antioxidants 9, no. 1: 20. https://doi.org/10.3390/antiox9010020
APA StyleBordoni, L., Fedeli, D., Fiorini, D., & Gabbianelli, R. (2020). Extra Virgin Olive Oil and Nigella sativa Oil Produced in Central Italy: A Comparison of the Nutrigenomic Effects of Two Mediterranean Oils in a Low-Grade Inflammation Model. Antioxidants, 9(1), 20. https://doi.org/10.3390/antiox9010020







