The Potential of Seaweeds as a Source of Functional Ingredients of Prebiotic and Antioxidant Value
Abstract
1. Macroalgae Classification
2. The Potential of Invasive Seaweeds
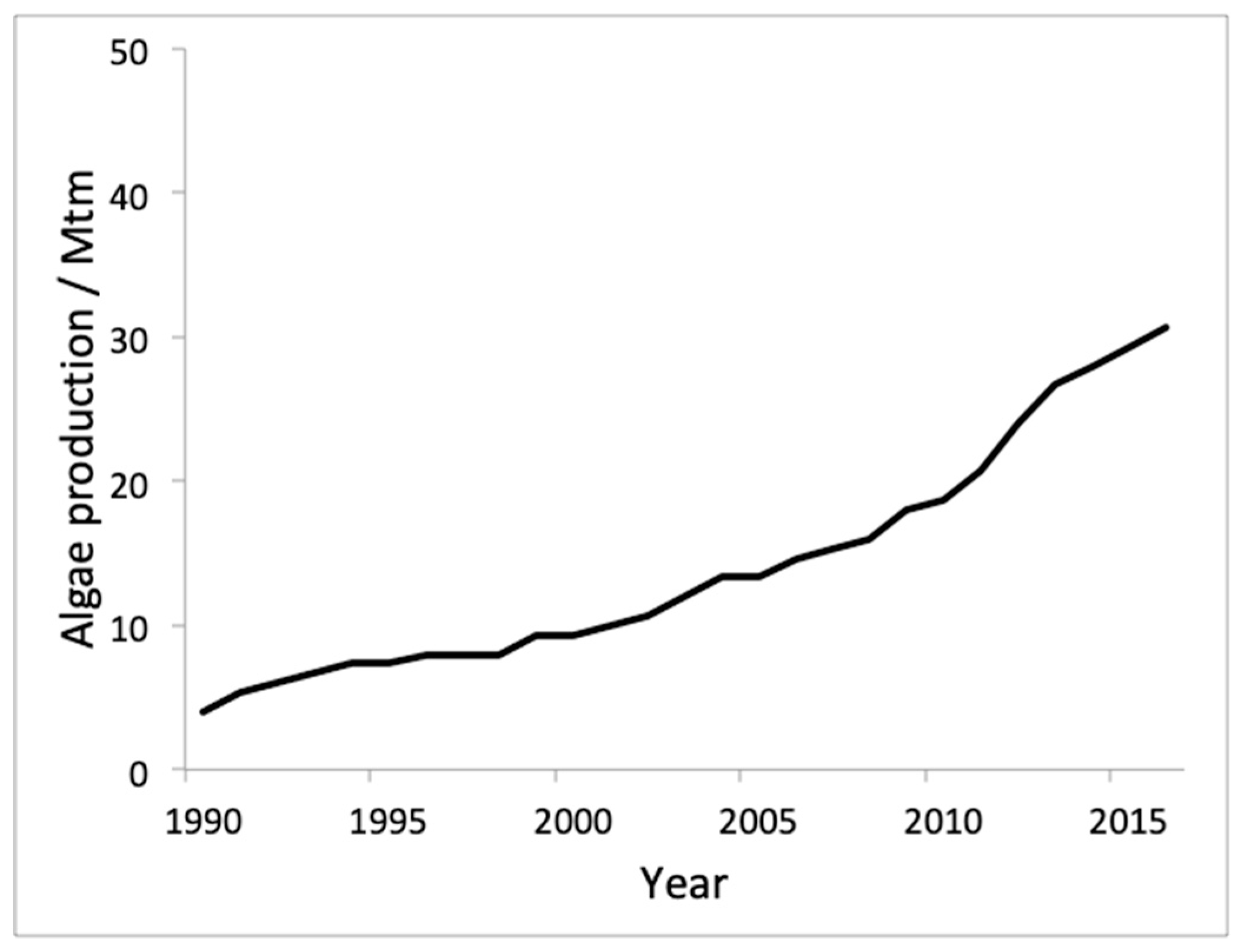
3. Algae Aquaculture
4. General Current Seaweed Industrial Applications
4.1. Anti-Biofilm Applications
4.2. Biofuel and Bioremediation Applications
4.3. Fish Feed Applications
4.4. General Food Applications
4.5. Pharmacology and/or Medical Applications
4.5.1. Contraceptive Activity Applications
4.5.2. Antibiotic, Antiviral, and Antifungal Activity
4.5.3. Anticancer Activity
4.5.4. Anticoagulant Activity
4.5.5. Anti-Inflammatory Activity
4.5.6. Antioxidant Activity
4.6. General Cosmeceuticals Applications
4.7. Other Applications
5. Food and Technical Uses of Algae
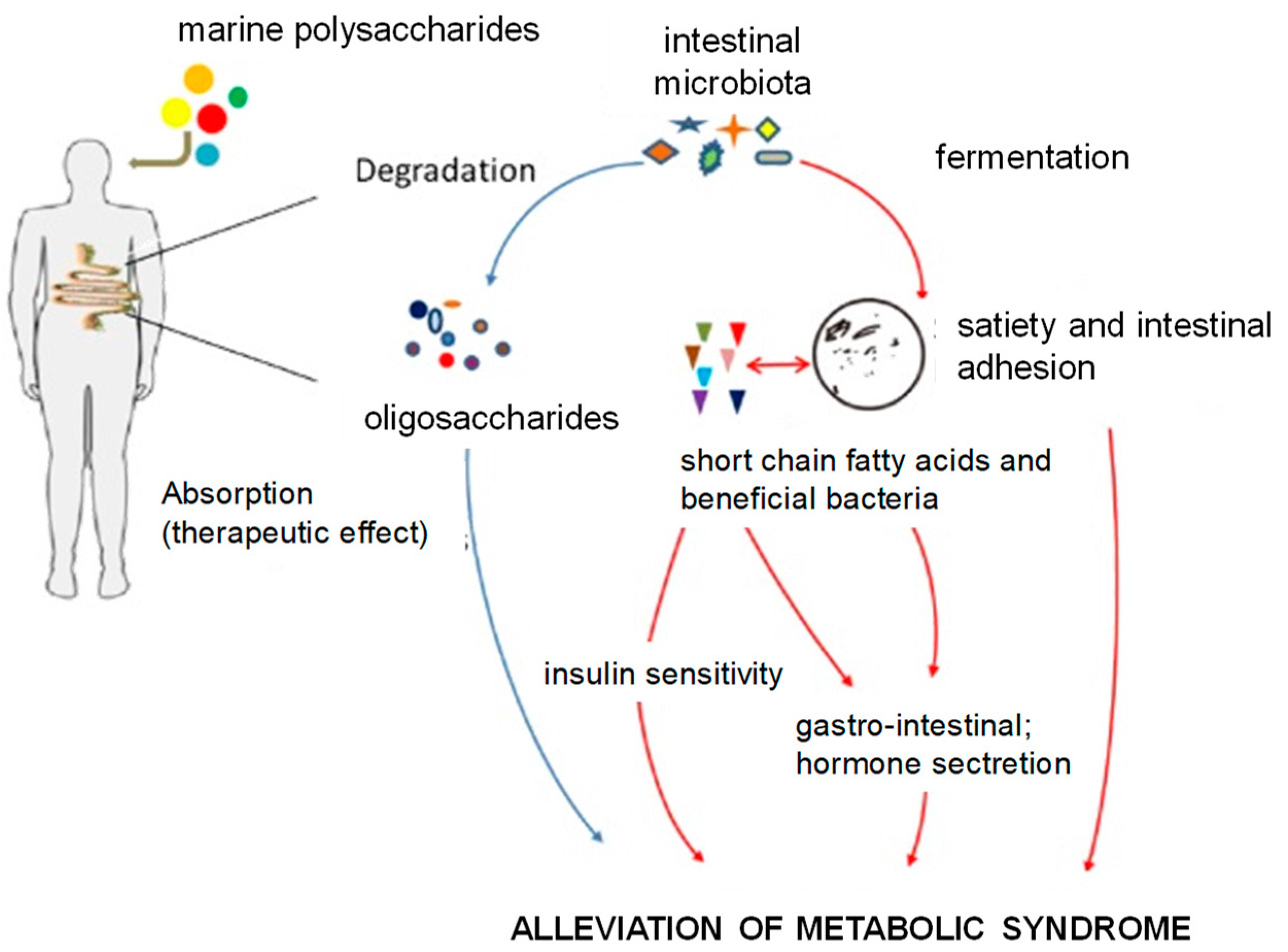
6. Prebiotics from Algae
6.1. Chemistry and Obtaining of Prebiotic Compounds from Seaweeds
6.2. Prebiotic Properties of Oligo and Polysaccharides from Seaweeds
7. Antioxidants from Algae
7.1. Carotenoid Pigments
7.2. Phycobilin Pigments
7.3. Phenolic Compounds
7.4. Vitamins and Minerals
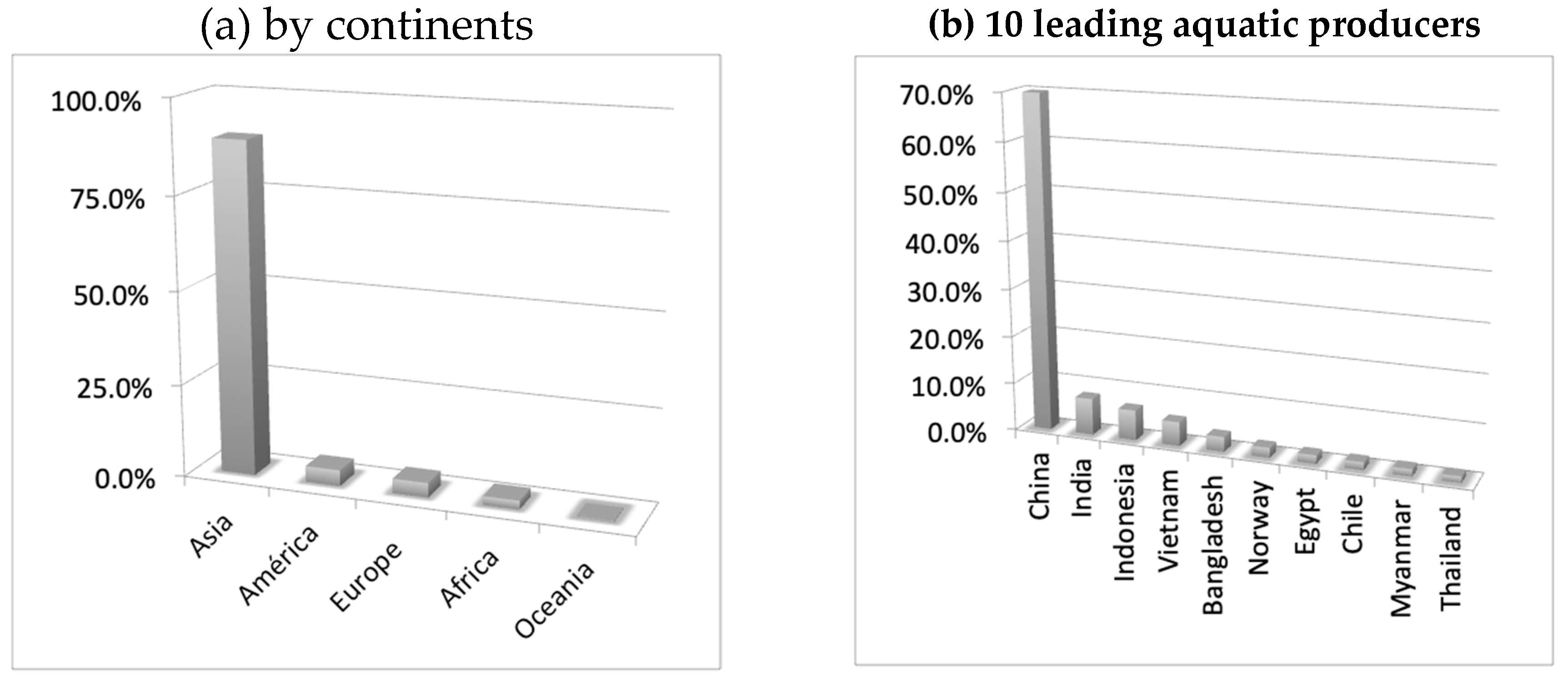
8. Production and Consumption Statistics and Future Markets
9. Conclusions on Trends and Challenges for the Sector
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sáa, C.F. Algas de Galicia, Alimento y Salud. Las Verduras del Océano Atlántico: Propiedades, Recetas, Descripción; Redondela: Algamar, Spain, 2002; ISBN 84-607-4503-1. [Google Scholar]
- Rupérez, P.; Saura-Calixto, F. Dietary fibre and physicochemical properties of edible Spanish seaweeds. Eur. Food Res. Technol. 2001, 212, 349–354. [Google Scholar] [CrossRef]
- Santos, S.A.O.; Vilela, C.; Freire, C.S.R.; Abreu, M.H.; Rocha, S.M.; Silvestre, A.J.D. Chlorophyta and Rhodophyta macroalgae: A source of health promoting phytochemicals. Food Chem. 2015, 183, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, K.; Mamat, R.; Samykano, M.; Azmi, W.H.; Ishak, W.F.W.; Yusaf, T. An overview of marine macroalgae as bioresource. Renew. Sustain. Energy Rev. 2018, 91, 165–179. [Google Scholar] [CrossRef]
- Guiry, M.D. How many species of algae are there? J. Phycol. 2012, 48, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Adl, S.M.; Simpson, A.G.B.; Farmer, M.A.; Andersen, R.A.; Anderson, R.; Barta, J.R.; Bowser, S.S.; Brugerolle, G.; Fensome, R.A.; Fredericq, S.; et al. The New Higher Level Classification of Eukaryotes with Emphasis on the Taxonomy of Protists. J. Eukaryot. Mrcrobrol. 2005, 52, 399–451. [Google Scholar] [CrossRef] [PubMed]
- Portugal, A.B.; Carvalho, F.L.; de Oliveira Soares, M.; Horta, P.A.; de Castro Nunes, J.M. Structure of macroalgal communities on tropical rocky shores inside and outside a marine protected area. Mar. Environ. Res. 2017, 130, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Draisma, S.G.A.; Prud’homme van Reine, W.F.; Herandarudewi, S.M.C.; Hoeksema, B.W. Macroalgal diversity along an inshore-offshore environmental gradient in the Jakarta Bay–Thousand Islands reef complex, Indonesia. Estuar. Coast. Shelf Sci. 2018, 200, 258–269. [Google Scholar] [CrossRef]
- Vuong, D.; Kaplan, M.; Lacey, H.J.; Crombie, A.; Lacey, E.; Piggott, A.M. A study of the chemical diversity of macroalgae from South Eastern Australia. Fitoterapia 2017, 126, 53–64. [Google Scholar] [CrossRef]
- Ross, A.B.; Jones, J.M.; Kubacki, M.L.; Bridgeman, T. Classification of macroalgae as fuel and its thermochemical behaviour. Bioresour. Technol. 2008, 99, 6494–6504. [Google Scholar] [CrossRef]
- Zhao, F.; Xu, N.; Zhou, R.; Ma, M.; Luo, H.; Wang, H. Community structure and species diversity of intertidal benthic macroalgae in Fengming Island, Dalian. Acta Ecol. Sin. 2016, 36, 77–84. [Google Scholar] [CrossRef]
- Robin, A.; Chavel, P.; Chemodanov, A.; Israel, A.; Golberg, A. Diversity of monosaccharides in marine macroalgae from the Eastern Mediterranean Sea. Algal Res. 2017, 28, 118–127. [Google Scholar] [CrossRef]
- Bonanno, G.; Orlando-Bonaca, M. Chemical elements in Mediterranean macroalgae. A review. Ecotoxicol. Environ. Saf. 2018, 148, 44–71. [Google Scholar] [CrossRef]
- Navarro-Barranco, C.; Florido, M.; Ros, M.; González-Romero, P.; Guerra-García, J.M. Impoverished mobile epifaunal assemblages associated with the invasive macroalga Asparagopsis taxiformis in the Mediterranean Sea. Mar. Environ. Res. 2018, 141, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Timme, R.E.; Bachvaroff, T.R.; Delwiche, C.F. Broad phylogenomic sampling and the sister lineage of land plants. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Werlinger, C.; Alveal, K.; Romo, H. Biología Marina y Oceanografía: Conceptos y Procesos; Consejo Nacional del Libro y la Lectura: Santiago, Chile, 2004. [Google Scholar]
- Leliaert, F.; Smith, D.R.; Moreau, H.; Herron, M.D.; Verbruggen, H.; Delwiche, C.F.; De Clerck, O. Phylogeny and Molecular Evolution of the Green Algae. CRC Crit. Rev. Plant Sci. 2012, 31, 1–46. [Google Scholar] [CrossRef]
- Hewitt, C.L.; Gollasch, S.; Minchin, D. The Vessel as a Vector–Biofouling, Ballast Water and Sediments. In Biological Invasions in Marine Ecosystems; Springer: Berlin, Germany, 2009; pp. 117–131. ISBN 0882-2786. [Google Scholar]
- Minchin, D.; Gollasch, S.; Cohen, A.N.; Hewitt, C.L.; Olenin, S. Characterizing Vectors of Marine Invasion. In Biological Invasions in Marine Ecosystems; Rilov, G., Crooks, J.A., Eds.; Springer: Berlin, Germany, 2009; pp. 109–116. ISBN 978-3-540-79236-9. [Google Scholar]
- Andreakis, N.; Schaffelke, B. Invasive Marine Seaweeds: Pest or Prize? In Investment Management and Financial Innovations; Wiencke, C., Bischof, K., Eds.; Springer: Berlin, Germany, 2012; Volume 219, pp. 235–262. ISBN 978-3-642-28450-2. [Google Scholar]
- Booth, D.; Provan, J.; Maggs, C.A. Molecular approaches to the study of invasive seaweeds. In Seaweed Invasions: A Synthesis of Ecological, Economic and Legal Imperatives; Walter de Gruyter: Berlin, Germany; New York, NY, USA, 2007; Volume 50, pp. 385–396. ISBN 9783110211344. [Google Scholar] [CrossRef]
- Forrest, B.M.; Taylor, M.D. Assessing invasion impact: Survey design considerations and implications for management of an invasive marine plant. Biol. Invasions 2002, 4, 375–386. [Google Scholar] [CrossRef]
- Nyberg, C.D.; Wallentinus, I. Can species traits be used to predict marine macroalgal introductions? Biol. Invasions 2005, 7, 265–279. [Google Scholar] [CrossRef]
- Wikström, S.A. Marine Seaweed Invasions—The Ecology of Introduced Fucus Evanescens. Ph.D. Thesis, Botaniska Institutionen, Stockholm, Sweden, 2004. [Google Scholar]
- Shea, K.; Chesson, P. Community ecology theory as a framework for biological invasions. Trends Ecol. Evol. 2002, 17, 170–176. [Google Scholar] [CrossRef]
- Pavia, H.; Toth, G. Inducible Chemical Resistance To Herbivory in the Brown Seaweed Ascophyllum Nodosum. Ecology 2000, 81, 3212–3225. [Google Scholar] [CrossRef]
- Thornber, C.S.; Kinlan, B.P.; Graham, M.H.; Stachowicz, J.J. Population ecology of the invasive kelp Undaria pinnatifida in California: Environmental and biological controls on demography. Mar. Ecol. Prog. Ser. 2004, 268, 69–80. [Google Scholar] [CrossRef]
- Aitken, D.; Bulboa, C.; Godoy-Faundez, A.; Turrion-Gomez, J.L.; Antizar-Ladislao, B. Life cycle assessment of macroalgae cultivation and processing for biofuel production. J. Clean. Prod. 2014, 75, 45–56. [Google Scholar] [CrossRef]
- Moon, S.M.; Lee, S.A.; Han, S.H.; Park, B.R.; Choi, M.S.; Kim, J.S.; Kim, S.G.; Kim, H.J.; Chun, H.S.; Kim, D.K.; et al. Aqueous extract of Codium fragile alleviates osteoarthritis through the MAPK/NF-κB pathways in IL-1β-induced rat primary chondrocytes and a rat osteoarthritis model. Biomed. Pharmacother. 2018, 97, 264–270. [Google Scholar] [CrossRef] [PubMed]
- FAO. The State of Fisheries and Aquaculture in the World 2018; FAO: Rome, Italy, 2018. [Google Scholar]
- Ridler, N.; Wowchuk, M.; Robinson, B.; Barrington, K.; Chopin, T.; Robinson, S.; Page, F.; Reid, G.; Szemerda, M.; Sewuster, J.; et al. Integrated multi-trophic aquaculture (IMTA): A potential strategic choice for farmers. Aquac. Econ. Manag. 2007, 11, 99–110. [Google Scholar] [CrossRef]
- Kim, J.K.; Kottuparambil, S.; Moh, S.H.; Lee, T.K.; Kim, Y.J.; Rhee, J.S.; Choi, E.M.; Kim, B.H.; Yu, Y.J.; Yarish, C.; et al. Potential applications of nuisance microalgae blooms. J. Appl. Phycol. 2015, 27, 1223–1234. [Google Scholar] [CrossRef]
- García-Casal, M.N.; Ramírez, J.; Leets, I.; Pereira, A.C.; Quiroga, M.F. Antioxidant capacity, polyphenol content and iron bioavailability from algae (Ulva sp., Sargassum sp. and Porphyra sp.) in human subjects. Br. J. Nutr. 2009, 101, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Abreu, M.H.; Pereira, R.; Yarish, C.; Buschmann, A.H.; Sousa-Pinto, I. IMTA with Gracilaria vermiculophylla: Productivity and nutrient removal performance of the seaweed in a land-based pilot scale system. Aquaculture 2011, 312, 77–87. [Google Scholar] [CrossRef]
- Corey, P.; Kim, J.K.; Duston, J.; Garbary, D.J. Growth and nutrient uptake by Palmaria palmata integrated with Atlantic halibut in a land-based aquaculture system. ALGAE 2014, 29, 35–45. [Google Scholar] [CrossRef]
- Kim, J.K.; Kraemer, G.P.; Yarish, C. Use of sugar kelp aquaculture in Long Island Sound and the Bronx River Estuary for nutrient extraction. Mar. Ecol. Prog. Ser. 2015. [Google Scholar] [CrossRef]
- Rose, J.M.; Deonarine, S.; Ferreira, J.G. Nutrient Bioextraction. Encycl. Sustain. Sci. Technol. 2015, 1–33. [Google Scholar] [CrossRef]
- Wu, H.; Kim, J.K.; Huo, Y.; Zhang, J.; He, P. Nutrient removal ability of seaweeds on Pyropia yezoensis aquaculture rafts in China’s radial sandbanks. Aquat. Bot. 2017, 137, 72–79. [Google Scholar] [CrossRef]
- Jun, J.Y.; Jung, M.J.; Jeong, I.H.; Yamazaki, K.; Kawai, Y.; Kim, B.M. Antimicrobial and antibiofilm activities of sulfated polysaccharides from marine algae against dental plaque bacteria. Mar. Drugs 2018, 16, 301. [Google Scholar] [CrossRef]
- Gandhi, A.D.; Vizhi, D.K.; Lavanya, K.; Kalpana, V.N.; Devi Rajeswari, V.; Babujanarthanam, R. In vitro anti- biofilm and anti-bacterial activity of Sesbania grandiflora extract against Staphylococcus aureus. Biochem. Biophys. Rep. 2017, 12, 193–197. [Google Scholar] [CrossRef]
- Janssens, J.C.A.; Steenackers, H.; Robijns, S.; Gellens, E.; Levin, J.; Zhao, H.; Hermans, K.; De Coster, D.; Verhoeven, T.L.; Marchal, K.; et al. Brominated furanones inhibit biofilm formation by Salmonella enterica serovar Typhimurium. Appl. Environ. Microbiol. 2008, 74, 6639–6648. [Google Scholar] [CrossRef]
- Baghel, R.S.; Trivedi, N.; Gupta, V.; Neori, A.; Reddy, C.R.K.; Lali, A.; Jha, B. Biorefining of marine macroalgal biomass for production of biofuel and commodity chemicals. Green Chem. 2015, 17, 2436–2443. [Google Scholar] [CrossRef]
- Milledge, J.J.; Nielsen, B.V.; Bailey, D. High-value products from macroalgae: The potential uses of the invasive brown seaweed, Sargassum muticum. Rev. Environ. Sci. Biotechnol. 2016, 15, 67–88. [Google Scholar] [CrossRef]
- Li, R.; Zhong, Z.; Jin, B.; Zheng, A. Selection of temperature for bio-oil production from pyrolysis of algae from lake blooms. Energy Fuels 2012, 26, 2996–3002. [Google Scholar] [CrossRef]
- Hu, Z.; Zheng, Y.; Yan, F.; Xiao, B.; Liu, S. Bio-oil production through pyrolysis of blue-green algae blooms (BGAB): Product distribution and bio-oil characterization. Energy 2013, 52, 119–125. [Google Scholar] [CrossRef]
- Bwapwa, J.K.; Jaiyeola, A.T.; Chetty, R. Bioremediation of acid mine drainage using algae strains: A review. S. Afr. J. Chem. Eng. 2017, 24, 62–70. [Google Scholar] [CrossRef]
- Zeraatkar, A.K.; Ahmadzadeh, H.; Talebi, A.F.; Moheimani, N.R.; McHenry, M.P. Potential use of algae for heavy metal bioremediation, a critical review. J. Environ. Manag. 2016, 181, 817–831. [Google Scholar] [CrossRef]
- Buschbaum, C.; Chapman, A.S.; Saier, B. How an introduced seaweed can affect epibiota diversity in different coastal systems. Mar. Biol. 2006, 148, 743–754. [Google Scholar] [CrossRef]
- Ratnasooriya, W.D.; Premakumara, G.A.S.; Tillekeratne, L.M.V. Post-coital contraceptive activity of crude extracts of Sri Lankan marine red algae. Contraception 1994, 50, 291–299. [Google Scholar] [CrossRef]
- de Almeida, C.L.; Falcão, H.D.S.; Lima, G.R.; Montenegro, C.D.A.; Lira, N.S.; de Athayde-Filho, P.F.; Rodrigues, L.C.; de Souza Mde, F.; Barbosa-Filho, J.M.; Batista, L.M. Bioactivities from marine algae of the genus Gracilaria. Int. J. Mol. Sci. 2011, 12, 4550–4573. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Morton, D.W. Cosmeceuticals Derived from Bioactive Substances Found in Marine Algae. J. Oceanogr. Mar. Res. 2013, 1. [Google Scholar] [CrossRef]
- Kim, S.K. Marine Cosmeceuticals: Trends and Prospects, 1st ed.; Kim, S.K., Ed.; Tayor & Francis Group: Boca Ratón, FL, USA, 2012; ISBN 9781439860281. [Google Scholar]
- Uysal, O.; Uysal, F.O.; Ekinci, K. Evaluation of Microalgae as Microbial Fertilizer. Eur. J. Sustain. Dev. 2015, 4, 77–82. [Google Scholar] [CrossRef]
- Rizvi, M.A.; Shameel, M. Pharmaceutical biology of seaweeds from the Karachi coast of Pakistan. Pharm. Biol. 2005, 43, 97–107. [Google Scholar] [CrossRef]
- Chingizova, E.A.; Skriptsova, A.V.; Anisimov, M.M.; Aminin, D.L.; Chingizova, E.A. Antimicrobial activity of marine algal extracts. Int. J. Phytomed. 2017, 9, 113–122. [Google Scholar]
- Omar, H.H.; Al-Judaibiand, A.; El-Gendy, A. Antimicrobial, antioxidant, anticancer activity and phytochemical analysis of the red alga, laurencia papillosa. Int. J. Pharmacol. 2018, 14, 572–583. [Google Scholar] [CrossRef]
- De Souza Barros, C.; Teixeira, V.L.; Paixão, I.C.N.P. Seaweeds with anti-herpes simplex virus type 1 activity. J. Appl. Phycol. 2015, 27, 1623–1637. [Google Scholar] [CrossRef]
- Smit, A.J. Medicinal and pharmaceutical uses of seaweed natural products: A review. J. Appl. Phycol. 2004, 16, 245–262. [Google Scholar] [CrossRef]
- Martínez Andrade, K.A.; Lauritano, C.; Romano, G.; Ianora, A. Marine microalgae with anti-cancer properties. Mar. Drugs 2018, 16, 165. [Google Scholar] [CrossRef]
- Palermo, J.A.; Flower, P.B.; Seldes, A.M. Chondriamides A and B, new indolic metabolites from the red alga Chondria sp. Tetrahedron Lett. 1992, 33, 3097–3100. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Cao, S.; He, X.; Qin, L.; He, M.; Yang, Y.; Hao, J.; Mao, W. Structural characteristics and anticoagulant property in vitro and in vivo of a seaweed Sulfated Rhamnan. Mar. Drugs 2018, 16, 243. [Google Scholar] [CrossRef]
- Kim, S.K.; Wijesekara, I. Anticoagulant Effect of Marine Algae, 1st ed.; Elsevier Inc.: Philadelphia, PA, USA, 2011; Volume 64, ISBN 9780123876690. [Google Scholar]
- Magalhaes, K.D.; Costa, L.S.; Fidelis, G.P.; Oliveira, R.M.; Nobre, L.T.D.B.; Dantas-Santos, N.; Camara, R.B.G.; Albuquerque, I.R.L.; Cordeiro, S.L.; Sabry, D.A.; et al. Anticoagulant, antioxidant and antitumor activities of heterofucans from the seaweed dictyopteris delicatula. Int. J. Mol. Sci. 2011, 12, 3352–3365. [Google Scholar] [CrossRef]
- Athukorala, Y.; Lee, K.W.; Kim, S.K.; Jeon, Y.J. Anticoagulant activity of marine green and brown algae collected from Jeju Island in Korea. Bioresour. Technol. 2007, 98, 1711–1716. [Google Scholar] [CrossRef]
- Lin, H.T.V.; Lu, W.J.; Tsai, G.J.; Te Chou, C.; Hsiao, H.I.; Hwang, P.A. Enhanced anti-inflammatory activity of brown seaweed Laminaria japonica by fermentation using Bacillus subtilis. Process Biochem. 2016, 51, 1945–1953. [Google Scholar] [CrossRef]
- Delgado, N.G.; Frías Vázquez, A.I.; Sánchez, H.C.; del Valle, R.M.S.; Gómez, Y.S.; Suárez Alfonso, A.M. Anti-inflammatory and antinociceptive activities of methanolic extract from red seaweed Dichotomaria obtusata. Braz. J. Pharm. Sci. 2013, 49, 65–74. [Google Scholar] [CrossRef]
- Radhika, D.; Veerabahu, C.; Prira, R. Anti-inflammatory activities of some seaweed collected from the gulf of mannar coast, tuticorin, south india. Int. J. Pharma Bio Sci. 2013, 4, 39–44. [Google Scholar]
- Surget, G.; Roberto, V.P.; Le Lann, K.; Mira, S.; Guérard, F.; Laizé, V.; Poupart, N.; Cancela, M.L.; Stiger-Pouvreau, V. Marine green macroalgae: A source of natural compounds with mineralogenic and antioxidant activities. J. Appl. Phycol. 2017, 29, 575–584. [Google Scholar] [CrossRef]
- Figueiroa, F.L.; Gil, C.; Rico, R.M.; Moriñigo, M.Á.; Gómez-Pinchetti, J.L.; Abdala Díaz, R. Biofiltración de efluentes mediante algas: Valorización de la biomasa (alimentos funcionales y biodiesel). In Las Algas Como Recurso: Valorización, Aplicaciones Industriales y Tendencias; CETMAR: Vigo, Spain, 2011; pp. 209–224. ISBN 9788461535934. [Google Scholar]
- Bourgougnon, N.; Bedoux, G.; Sangiardi, A.; Stiger-Pouvreau, V. Las algas: Potencial nutritivo y aplicaciones cosméticas. In Las Algas Como Recurso. Valorización. Aplicaciones Industriales y Tendencias Aplicaciones; Centro Tecnológico del Mar-Fundación CETMAR: Vigo, Spain, 2011. [Google Scholar]
- Cañavate Hors, J.P. Funciones de las microalgas en acuicultura. In Las Algas Como Recurso. Valorización. Aplicaciones Industriales y Tendencias Aplicaciones; CETMAR: Vigo, Spain, 2011. [Google Scholar]
- Olsen, A.I.; Olsen, Y.; Attramadal, Y.; Christie, K.; Birkbeck, T.H.; Skjermo, J.; Vadstein, O. Effects of short term feeding of microalgae on the bacterial flora associated with juvenile Artemia franciscana. Aquaculture 2000, 190, 11–25. [Google Scholar] [CrossRef]
- Shields, R.J.; Lupatsch, I. Algae for Aquaculture and Animal Feeds. Tech. Theor. Prax. 2012, 21, 23–37. [Google Scholar]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Salvador, N.; Gómez Garreta, A.; Lavelli, L.; Ribera, M.A. Antimicrobial activity of Iberian macroalgae. Sci. Mar. 2007, 71, 101–114. [Google Scholar] [CrossRef]
- Rizzo, C.; Genovese, G.; Morabito, M.; Faggio, C.; Pagano, M.; Spanò, A.; Zammuto, V.; Minicante, S.A.; Manghisi, A.; Cigala, R.M.; et al. Potential antibacterial activity of marine macroalgae against pathogens relevant for aquaculture and human health. J. Pure Appl. Microbiol. 2017, 11, 1695–1706. [Google Scholar] [CrossRef]
- Capillo, G.; Savoca, S.; Costa, R.; Sanfilippo, M.; Rizzo, C.; Giudice, A.L.; Albergamo, A.; Rando, R.; Bartolomeo, G.; Spanò, N.; et al. New insights into the culture method and antibacterial potential of gracilaria gracilis. Mar. Drugs 2018, 16, 492. [Google Scholar] [CrossRef]
- Fedorov, S.N.; Ermakova, S.P.; Zvyagintseva, T.N.; Stonik, V.A. Anticancer and cancer preventive properties of marine polysaccharides: Some results and prospects. Mar. Drugs 2013, 11, 4876. [Google Scholar] [CrossRef]
- Faggio, C.; Pagano, M.; Dottore, A.; Genovese, G.; Morabito, M. Evaluation of anticoagulant activity of two algal polysaccharides. Nat. Prod. Res. 2016, 30, 1934–1937. [Google Scholar] [CrossRef]
- Arive, P.L.C.; Inquimboy, I.H.; No, N.L.I. In Vitro Antioxidant Activity of Selected Seaweeds in the Philippines. Int. J. Theor. Appl. Sci. 2017, 9, 212–216. [Google Scholar]
- Moubayed, N.M.S.; Al Houri, H.J.; Al Khulaifi, M.M.; Al Farraj, D.A. Antimicrobial, antioxidant properties and chemical composition of seaweeds collected from Saudi Arabia (Red Sea and Arabian Gulf). Saudi J. Biol. Sci. 2017, 24, 162–169. [Google Scholar] [CrossRef]
- Lima, R.L.D.; Pires-Cavalcante, M.K.D.S.; De Alencar, D.B.; Viana, A.F.; Sampaio, A.H.; Saker-Sampaio, S. Acta Scientiarum In vitro evaluation of antioxidant activity of methanolic extracts obtained from seaweeds endemic to the coast of Ceará, Brazil. Acta Sci. Technol. 2016, 38, 247–255. [Google Scholar] [CrossRef]
- Foon, T.S.; Ai, L.A.; Kuppusamy, P.; Yusoff, M.M.; Govindan, N. Studies on in vitro antioxidant activity of marine edible seaweed from east coastal region, peninsular Malaysia using different extraction methods. Res. J. Appl. Sci. 2013, 1, 193–198. [Google Scholar]
- Lee, J.H.; Kim, G.H. Evaluation of antioxidant activity of marine algae-extracts from Korea. J. Aquat. Food Prod. Technol. 2015, 24, 227–240. [Google Scholar] [CrossRef]
- Vieira, V.; Prieto, M.A.; Barros, L.; Coutinho, J.A.P.; Ferreira, I.C.F.R.; Ferreira, O. Enhanced extraction of phenolic compounds using choline chloride based deep eutectic solvents from Juglans regia L. Ind. Crop. Prod. 2018, 115, 261–271. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Yousefi, S.; Capillo, G.; Paknejad, H.; Khalili, M.; Tabarraei, A.; Van Doan, H.; Spanò, N.; Faggio, C. Mucosal immune parameters, immune and antioxidant defence related genes expression and growth performance of zebrafish (Danio rerio) fed on Gracilaria gracilis powder. Fish Shellfish Immunol. 2018, 83, 232–237. [Google Scholar] [CrossRef]
- McReynolds, C. Invasive Marine Macroalgae and Their Current and Potential Use in Cosmetics. 2017. Available online: http://hdl.handle.net/10400.8/2653 (accessed on 12 April 2019).
- Bedoux, G.; Hardouin, K.; Burlot, A.S.; Bourgougnon, N. Bioactive Components from Seaweeds: Cosmetic Applications and Future Development; Elsevier: Amsterdam, The Netherlands, 2014; Volume 71, ISBN 9780124080621. [Google Scholar]
- Pinela, J.; Prieto, M.A.; Barros, L.; Carvalho, A.M.; Oliveira, M.B.P.P.; Saraiva, J.A.; Ferreira, I.C.F.R. Cold extraction of phenolic compounds from watercress by high hydrostatic pressure: Process modelling and optimization. Sep. Purif. Technol. 2018, 192, 501–512. [Google Scholar] [CrossRef]
- Hennequart, F. Extractos de algas como bioestimuladores del crecimiento de las plantas. In Las Algas Como Recurso: Valorización, Aplicaciones Industriales y Tendencias; CETMAR, Ed.; CETMAR: Vigo, Spain, 2011; pp. 49–62. ISBN 9788461535934. [Google Scholar]
- Sohn, C.H. Porphyra, Undaria and Hizikia Cultivation in Korea. Korean J. Phycol. 1993, 8, 207–216. [Google Scholar]
- Oren, A. A hundred years of Dunaliella research: 1905–2005. Saline Syst. 2005, 1, 2. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Giger-Reverdin, S.; Lessire, M.; Lebas, F.; Ankers, P. Seaweeds for livestock diets: A review. Anim. Feed Sci. Technol. 2016, 212, 1–17. [Google Scholar] [CrossRef]
- Gavio, B.; Fredericq, S. Grateloupia Turuturu (Halymeniaceae, Rhodophyta): The Correct Identity of the Invasive Species in the Atlantic Known as Grateloupia Doryphora as Inferred From Molecular and Morphological Evidence. J. Phycol. 2003, 38, 12. [Google Scholar] [CrossRef]
- Peinado, I.; Girón, J.; Koutsidis, G.; Ames, J.M. Chemical composition, antioxidant activity and sensory evaluation of five different species of brown edible seaweeds. Food Res. Int. 2014, 66, 36–44. [Google Scholar] [CrossRef]
- Gómez-Ordóñez, E.; Jiménez-Escrig, A.; Rupérez, P. Dietary fibre and physicochemical properties of several edible seaweeds from the northwestern Spanish coast. Food Res. Int. 2010, 43, 2289–2294. [Google Scholar] [CrossRef]
- Ramnani, P.; Chitarrari, R.; Tuohy, K.; Grant, J.; Hotchkiss, S.; Philp, K.; Campbell, R.; Gill, C.; Rowland, I. Invitro fermentation and prebiotic potential of novel low molecular weight polysaccharides derived from agar and alginate seaweeds. Anaerobe 2012, 18, 1–6. [Google Scholar] [CrossRef]
- Delattre, C.; Fenoradosoa, T.A.; Michaud, P. Galactans: An overview of their most important sourcing and applications as natural polysaccharides. Braz. Arch. Biol. Technol. 2011, 54, 1075–1092. [Google Scholar] [CrossRef]
- Gupta, S.; Abu-Ghannam, N. Bioactive potential and possible health effects of edible brown seaweeds. Trends Food Sci. Technol. 2011, 22, 315–326. [Google Scholar] [CrossRef]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important determinants for fucoidan bioactivity: A critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar. Drugs 2011, 9, 2106. [Google Scholar] [CrossRef]
- Jiménez-Escrig, A.; Sánchez-Muniz, F.J. Dietary fibre from edible seaweeds: Chemical structure, physicochemical properties and effects on cholesterol metabolism. Nutr. Res. 2000, 20, 585–598. [Google Scholar] [CrossRef]
- Alderkamp, A.C.; Buma, A.G.J.; Van Rijssel, M. The carbohydrates of Phaeocystis and their degradation in the microbial food web. In Phaeocystis, Major Link in the Biogeochemical Cycling of Climate-Relevant Elements; Springer: Berlin/Heidelberg, Germany, 2007; ISBN 9781402062131. [Google Scholar]
- Pangestuti, R.; Kim, S. An Overview of Phycocolloids: The Principal Commercial. In Marine Algae Extracts; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 319–330. [Google Scholar]
- Ammar, H.H.; Lajili, S.; Sakly, N.; Cherif, D.; Rihouey, C.; Cerf, D.L.; Bouraoui, A.; Majdoub, H. Influence of the uronic acid composition on the gastroprotective activity of alginates from three different genus of Tunisian brown algae. Food Chem. 2017. [Google Scholar] [CrossRef]
- Lee, A.W.; Lim, Y.; Leow, A.T. Biosynthesis of agar in red seaweeds: A review. Carbohydr. Polym. 2017, 164, 23–30. [Google Scholar] [CrossRef]
- Chi, Z.; Fallon, J.V.O.; Chen, S. Bicarbonate produced from carbon capture for algae culture. Trends Biotechnol. 2011, 29, 537–541. [Google Scholar] [CrossRef]
- McLachlan, J. Macroalgae (seaweeds): Industrial resources and their utilization. Plant Soil 1985, 89, 137–157. [Google Scholar] [CrossRef]
- Andrade, P.B.; Barbosa, M.; Pedro, R.; Lopes, G.; Vinholes, J.; Mouga, T.; Valentão, P. Valuable compounds in macroalgae extracts. Food Chem. 2013, 138, 1819–1828. [Google Scholar] [CrossRef]
- Menshova, R.V.; Shevchenko, N.M.; Imbs, T.I.; Zvyagintseva, T.N.; Malyarenko, O.S.; Zaporoshets, T.S.; Besednova, N.N.; Ermakova, S.P. Fucoidans from Brown Alga Fucus evanescens: Structure and Biological Activity. Front. Mar. Sci. 2016, 3, 1–9. [Google Scholar] [CrossRef]
- Yun, E.J.; Yu, S.; Kim, K.H. Current knowledge on agarolytic enzymes and the industrial potential of agar-derived sugars. Appl. Microbiol. Biotechnol. 2017, 101, 5581–5589. [Google Scholar] [CrossRef]
- Seedevi, P.; Moovendhan, M.; Viramani, S.; Shanmugam, A. Bioactive potential and structural chracterization of sulfated polysaccharide from seaweed (Gracilaria corticata). Carbohydr. Polym. 2017, 155, 516–524. [Google Scholar] [CrossRef]
- Souza, B.W.S.; Cerqueira, M.A.; Bourbon, A.I.; Pinheiro, A.C.; Martins, J.T.; Teixeira, J.A.; Coimbra, M.A.; Vicente, A.A. Chemical characterization and antioxidant activity of sulfated polysaccharide from the red seaweed Gracilaria birdiae. Food Hydrocoll. 2012, 27, 287–292. [Google Scholar] [CrossRef]
- Cheong, K.L.; Qiu, H.M.; Du, H.; Liu, Y.; Khan, B.M. Oligosaccharides derived from red seaweed: Production, properties, and potential health and cosmetic applications. Molecules 2018, 23, 2451. [Google Scholar] [CrossRef]
- De Jesus Raposo, M.F.; De Morais, A.M.M.B.; De Morais, R.M.S.C. Emergent sources of prebiotics: Seaweeds and microalgae. Mar. Drugs 2016, 14, 27. [Google Scholar] [CrossRef]
- Peso-Echarri, P.; Frontela-Saseta, C.; González-Bermúdez, C.A.; Ros-Berruezo, G.F.; Martínez-Graciá, C. Polisacáridos de algas como ingredientes funcionales en acuicultura marina: Alginato, carragenato y ulvano. Rev. Biol. Mar. Oceanogr. 2012, 47, 373–381. [Google Scholar] [CrossRef]
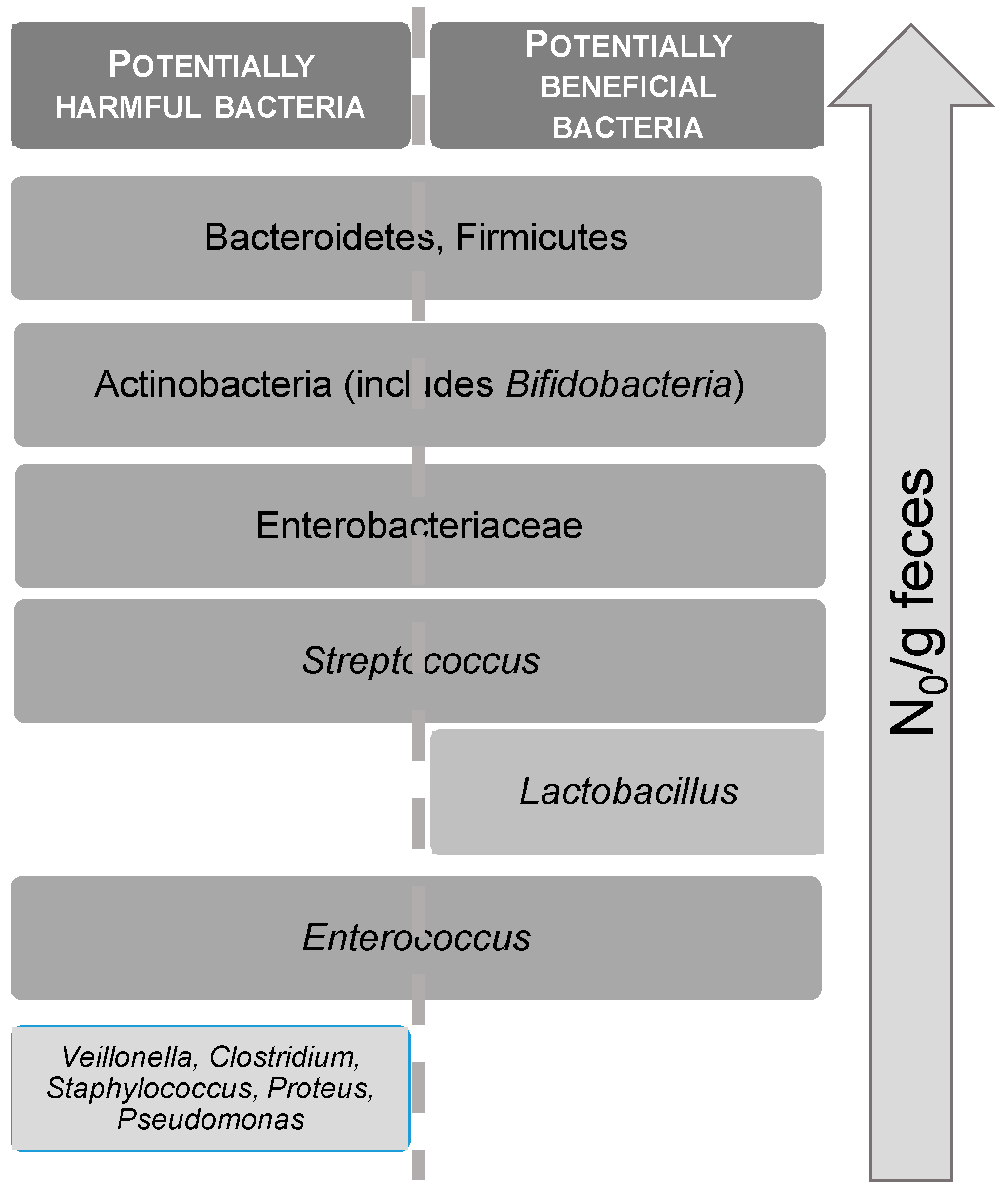
- Wang, X.; Wang, X.; Jiang, H.; Cai, C.; Li, G.; Hao, J.; Yu, G. Marine polysaccharides attenuate metabolic syndrome by fermentation products and altering gut microbiota: An overview. Carbohydr. Polym. 2018, 195, 601–612. [Google Scholar] [CrossRef]
- Chen, L.; Xu, W.; Chen, D.; Chen, G.; Liu, J.; Zeng, X.; Shao, R.; Zhu, H. Digestibility of sulfated polysaccharide from the brown seaweed Ascophyllum nodosum and its effect on the human gut microbiota in vitro. Int. J. Biol. Macromol. 2018, 112, 1055–1061. [Google Scholar] [CrossRef]
- Ale, M.T.; Meyer, A.S. Fucoidans from brown seaweeds: An update on structures, extraction techniques and use of enzymes as tools for structural elucidation. RSC Adv. 2013, 3, 8131–8141. [Google Scholar] [CrossRef]
- De Jesus Raposo, M.F.; De Morais, A.M.B.; De Morais, R.M.S.C. Marine polysaccharides from algae with potential biomedical applications. Mar. Drugs 2015, 13, 2967. [Google Scholar] [CrossRef] [PubMed]
- Zaporozhets, T.S.; Besednova, N.N.; Kuznetsova, T.A.; Zvyagintseva, T.N.; Makarenkova, I.D.; Kryzhanovsky, S.P.; Melnikov, V.G. The prebiotic potential of polysaccharides and extracts of seaweeds. Russ. J. Mar. Biol. 2014, 40, 1–9. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Courtois, J. Oligosaccharides from land plants and algae: Production and applications in therapeutics and biotechnology. Curr. Opin. Microbiol. 2009, 12, 261–273. [Google Scholar] [CrossRef]
- Klarzynski, O.; Descamps, V.; Plesse, B.; Yvin, J.C.; Kloareg, B.; Fritig, B. Sulfated Fucan Oligosaccharides Elicit Defense Responses in Tobacco and Local and Systemic Resistance Against Tobacco Mosaic Virus. Mol. Plant Microbe Interact. 2003, 16, 115–122. [Google Scholar] [CrossRef]
- Kardos, N.; Luche, J.L. Sonochemistry of carbohydrate compounds. Carbohydr. Res. 2001, 332, 115–131. [Google Scholar] [CrossRef]
- Lii, C.Y.; Chen, C.H.; Yeh, A.I.; Lai, V.M.F. Preliminary study on the degradation kinetics of agarose and carrageenans by ultrasound. Food Hydrocoll. 1999, 13, 477–481. [Google Scholar] [CrossRef]
- Sun, L.; Wang, C.; Shi, Q.; Ma, C. Preparation of different molecular weight polysaccharides from Porphyridium cruentum and their antioxidant activities. Int. J. Biol. Macromol. 2009, 45, 42–47. [Google Scholar] [CrossRef]
- Zúñiga, E.A.; Matsuhiro, B.; Mejías, E. Preparation of a low-molecular weight fraction by free radical depolymerization of the sulfated galactan from Schizymenia binderi (Gigartinales, Rhodophyta) and its anticoagulant activity. Carbohydr. Polym. 2006, 66, 208–215. [Google Scholar] [CrossRef]
- Leal, B. Obtenção de Oligossacarídeos Prebióticos a Partir da Hidrólise Fosfórica da Biomassa de Microalgas Utilizadas na Biomitigação de CO2 de Efluente Gasoso de Churrascaria. Master’s Thesis, Universidade Tecnológica Federal do Paraná, Curitiba, Brasil, 2015. [Google Scholar]
- Nardella, A.; Chaubet, F.; Boisson-Vidal, C.; Blondin, C.; Durand, P.; Jozefonvicz, J. Anticoagulant low molecular weight fucans produced by radical process and ion exchange chromatography of high molecular weight fucans extracted from the brown seaweed Ascophyllum nodosum. Carbohydr. Res. 1996, 289, 201–208. [Google Scholar] [CrossRef]
- Patel, S.; Goyal, A. The current trends and future perspectives of prebiotics research: A review. 3 Biotech 2012, 2, 115–125. [Google Scholar] [CrossRef]
- Zhou, G.; Sun, Y.P.; Xin, H.; Zhang, Y.; Li, Z.; Xu, Z. In vivo antitumor and immunomodulation activities of different molecular weight lambda-carrageenans from Chondrus ocellatus. Pharmacol. Res. 2004, 50, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, L.; Zhou, Y. Immunomodulation and antitumor activities of different-molecular-weight polysaccharides from Porphyridium cruentum. Carbohydr. Polym. 2012, 87, 1206–1210. [Google Scholar] [CrossRef]
- López, C.J.; Caleja, C.; Prieto, M.A.; Barreiro, M.F.; Barros, L.; Ferreira, I.C.F.R. Optimization and comparison of heat and ultrasound assisted extraction techniques to obtain anthocyanin compounds from Arbutus unedo L. Fruits. Food Chem. 2018, 264, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Di, T.; Chen, G.; Sun, Y.; Ou, S.; Zeng, X.; Ye, H. In vitro digestion by saliva, simulated gastric and small intestinal juices and fermentation by human fecal microbiota of sulfated polysaccharides from Gracilaria rubra. J. Funct. Foods 2018, 40, 18–27. [Google Scholar] [CrossRef]
- Sullivan, L.; Murphy, B.; McLoughlin, P.; Duggan, P.; Lawlor, P.G.; Hughes, H.; Gardiner, G.E. Prebiotics from marine macroalgae for human and animal health applications. Mar. Drugs 2010, 8, 2038. [Google Scholar] [CrossRef] [PubMed]
- Charoensiddhi, S.; Conlon, M.A.; Methacanon, P.; Franco, C.M.M.; Su, P.; Zhang, W. Gut health benefits of brown seaweed Ecklonia radiata and its polysaccharides demonstrated in vivo in a rat model. J. Funct. Foods 2017, 37, 676–684. [Google Scholar] [CrossRef]
- Wang, Y.; Han, F.; Hu, B.; Li, J.; Yu, W. In vivo prebiotic properties of alginate oligosaccharides prepared through enzymatic hydrolysis of alginate. Nutr. Res. 2006, 26, 597–603. [Google Scholar] [CrossRef]
- Hu, B.; Gong, Q.; Wang, Y.; Ma, Y.; Li, J.; Yu, W. Prebiotic effects of neoagaro-oligosaccharides prepared by enzymatic hydrolysis of agarose. Anaerobe 2006, 12, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Kuda, T.; Yano, T.; Matsuda, N.; Nishizawa, M. Inhibitory effects of laminaran and low molecular alginate against the putrefactive compounds produced by intestinal microflora in vitro and in rats. Food Chem. 2005, 91, 745–749. [Google Scholar] [CrossRef]
- Kong, Q.; Dong, S.; Gao, J.; Jiang, C. In vitro fermentation of sulfated polysaccharides from E. prolifera and L. japonica by human fecal microbiota. Int. J. Biol. Macromol. 2016, 91, 867–871. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Cao, C.; Ren, B.; Zhang, B.; Huang, Q.; Li, C. Structural characterization and in vitro fermentation of a novel polysaccharide from Sargassum thunbergii and its impact on gut microbiota. Carbohydr. Polym. 2018, 183, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Devillé, C.; Gharbi, M.; Dandrifosse, G.; Peulen, O. Study on the effects of laminarin, a polysaccharide from seaweed, on gut characteristics. J. Sci. Food Agric. 2007, 87, 1717–1725. [Google Scholar] [CrossRef]
- Holck, J.; Hjernø, K.; Lorentzen, A.; Vigsnæs, L.K.; Hemmingsen, L.; Licht, T.R.; Mikkelsen, J.D.; Meyer, A.S. Tailored enzymatic production of oligosaccharides from sugar beet pectin and evidence of differential effects of a single DP chain length difference on human faecal microbiota composition after in vitro fermentation. Process Biochem. 2011, 46, 1039–1049. [Google Scholar] [CrossRef]
- Roriz, C.L.; Barros, L.; Prieto, M.A.; Morales, P.; Ferreira, I.C.F.R. Floral parts of Gomphrena globosa L. as a novel alternative source of betacyanins: Optimization of the extraction using response surface methodology. Food Chem. 2017, 229, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, B.R.; Prieto, M.A.; Barreiro, M.F.; Rodrigues, A.; Curran, T.P.; Barros, L.; Ferreira, I.C.F.R. Catechin-based extract optimization obtained from Arbutus unedo L. fruits using maceration/microwave/ultrasound extraction techniques. Ind. Crops Prod. 2016, 95, 404–415. [Google Scholar] [CrossRef]
- Del Campo, J.A.; García-González, M.; Guerrero, M.G. Outdoor cultivation of microalgae for carotenoid production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2007, 74, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Baroli, I.; Niyogi, K.K.; Barber, J.; Heifetz, P. Molecular genetics of xanthophyll-dependent photoprotection in green algae and plants. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2000, 355, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Grünewald, K.; Hirschberg, J.; Hagen, C. Ketocarotenoid Biosynthesis Outside of Plastids in the Unicellular Green Alga Haematococcus pluvialis. J. Biol. Chem. 2001, 276, 6023–6029. [Google Scholar] [CrossRef]
- Lohr, M.; Wilhelm, C. Xanthophyll synthesis in diatoms: Quantification of putative intermediates and comparison of pigment conversion kinetics with rate constants derived from a model. Planta 2001, 212, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, P. Environmental and cultural stimulants in the production of carotenoids from microorganisms. Appl. Microbiol. Biotechnol. 2004, 63, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Raja, R.; Hemaiswarya, S.; Rengasamy, R. Exploitation of Dunaliella for β-carotene production. Appl. Microbiol. Biotechnol. 2007, 74, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Dufossé, L.; Galaup, P.; Yaron, A.; Arad, S.M.; Blanc, P.; Murthy, K.N.C.; Ravishankar, G.A. Microorganisms and microalgae as sources of pigments for food use: A scientific oddity or an industrial reality? Trends Food Sci. Technol. 2005, 16, 389–406. [Google Scholar] [CrossRef]
- Van Den Berg, H.; Faulks, R.; Granado, H.F.; Hirschberg, J.; Olmedilla, B.; Sandmann, G.; Southon, S.; Stahl, W. The potential for the improvement of carotenoid levels in foods and the likely systemic effects. J. Sci. Food Agric. 2000, 80, 880–912. [Google Scholar] [CrossRef]
- Jespersen, L.; Strømdahl, L.D.; Olsen, K.; Skibsted, L.H. Heat and light stability of three natural blue colorants for use in confectionery and beverages. Eur. Food Res. Technol. 2005, 220, 261–266. [Google Scholar] [CrossRef]
- Duan, X.J.; Zhang, W.W.; Li, X.M.; Wang, B.G. Evaluation of antioxidant property of extract and fractions obtained from a red alga, Polysiphonia urceolata. Food Chem. 2006, 95, 37–43. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Pinela, J.; Prieto, M.A.; Barreiro, M.F.; Carvalho, A.M.; Oliveira, M.B.P.P.; Vázquez, J.A.; Ferreira, I.C.F.R. Optimization of microwave-assisted extraction of hydrophilic and lipophilic antioxidants from a surplus tomato crop by response surface methodology. Food Bioprod. Process. 2016, 98, 283–298. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef]
- Pinela, J.; Prieto, M.A.; Barreiro, M.F.; Curran, T.P.; Ferreira, I.C.F.R. Valorisation of tomato wastes for development of nutrient-rich antioxidant ingredients: A sustainable approach towards the needs of the today’s society. Innov. Food Sci. Emerg. Technol. 2017, 41, 160–171. [Google Scholar] [CrossRef]
- Thomas, N.V.; Kim, S.K. Potential pharmacological applications of polyphenolic derivatives from marine brown algae. Environ. Toxicol. Pharmacol. 2011, 32, 325–335. [Google Scholar] [CrossRef]
- Wang, T.; Jónsdóttir, R.; Liu, H.; Gu, L.; Kristinsson, H.G.; Raghavan, S.; Ólafsdóttir, G. Antioxidant capacities of phlorotannins extracted from the brown algae Fucus vesiculosus. J. Agric. Food Chem. 2012, 60, 5874–5883. [Google Scholar] [CrossRef]
- Kelman, D.; Posner, E.K.; McDermid, K.J.; Tabandera, N.K.; Wright, P.R.; Wright, A.D. Antioxidant activity of Hawaiian marine algae. Mar. Drugs 2012, 10, 403. [Google Scholar] [CrossRef]
- Becker, W. Handbook of microalgal culture: Biotechnology and applied phycology. In Handbook of Microalgal Culture: Biotechnology an Applied Phycology; Richmond, A., Ed.; Blackwell Science Ltd: Hoboken, NJ, USA, 2004; ISBN 0632059532. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture 2018-Meeting the Sustainable Development Goals; FAO: Rome, Italy, 2018; Volume 35, ISBN 9789251060292. [Google Scholar]
- FAO. FAO Yearbook. Fishery and Aquaculture Statistics 2011/FAO Annuaire; FAO: Rome, Italy, 2011. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture, 2012; FAO: Rome, Italy, 2013. [Google Scholar]
- Barry, L. Seaweed, Potential as a Marine Vegetable and Other Opportunities; Rural Industries Research and Development Corporation: Canberra, Australia, 2008. [Google Scholar]
- Zemke-White, W.L.; Ohno, M. World seaweed utilisation: An end-of-century summary. J. Appl. Phycol. 1999, 11, 369–376. [Google Scholar] [CrossRef]
- Wikfors, G.H.; Ohno, M. Impact of algal research in aquaculture. J. Phycol. 2001, 37, 968–974. [Google Scholar] [CrossRef]
- FAO. World Aquaculture 2010; FAO: Rome, Italy, 2011; ISBN 9789251069974. [Google Scholar]
- McHugh, D.J. A Guide to the Seaweed Industry; FAO: Rome, Italy, 2003. [Google Scholar]
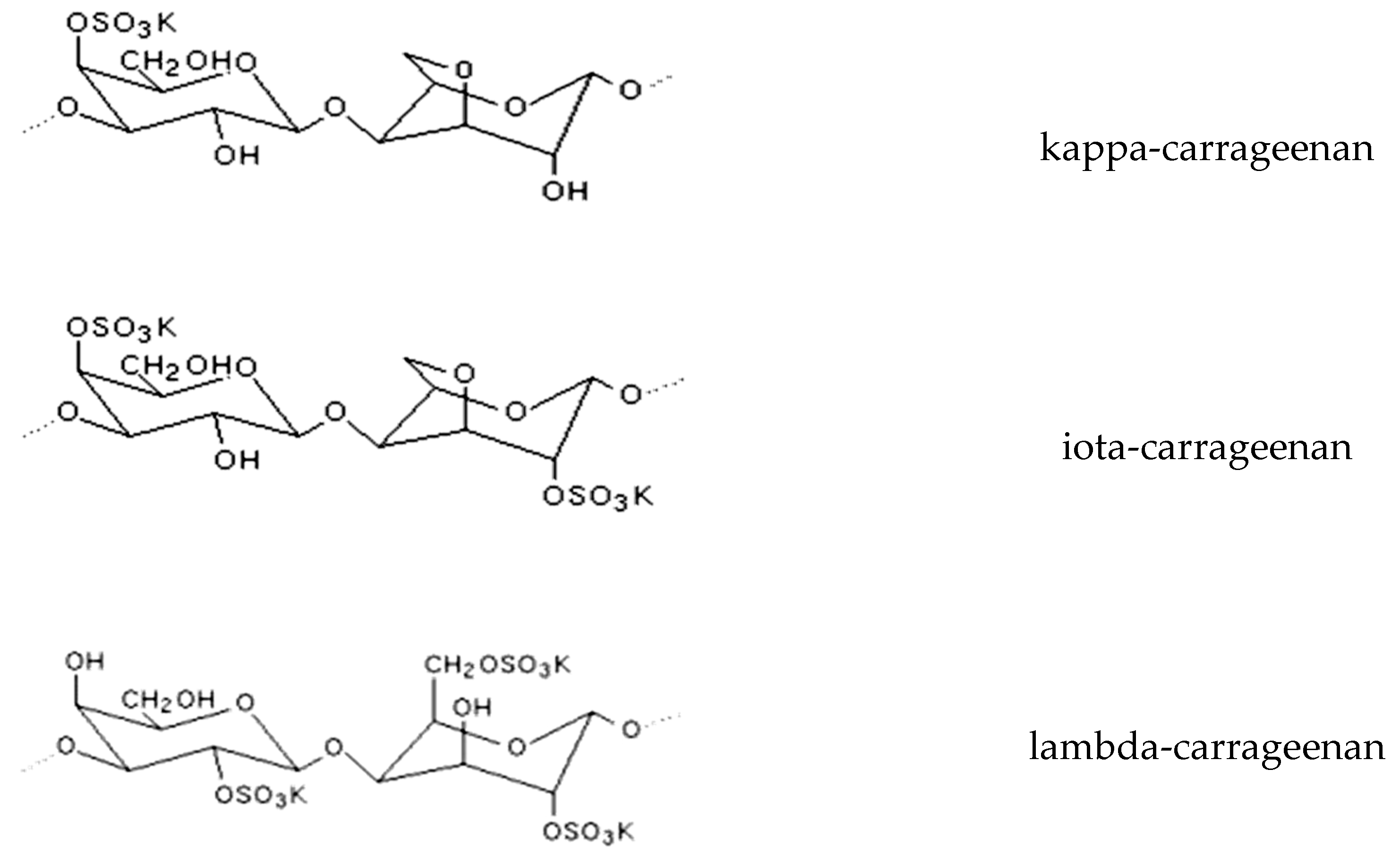
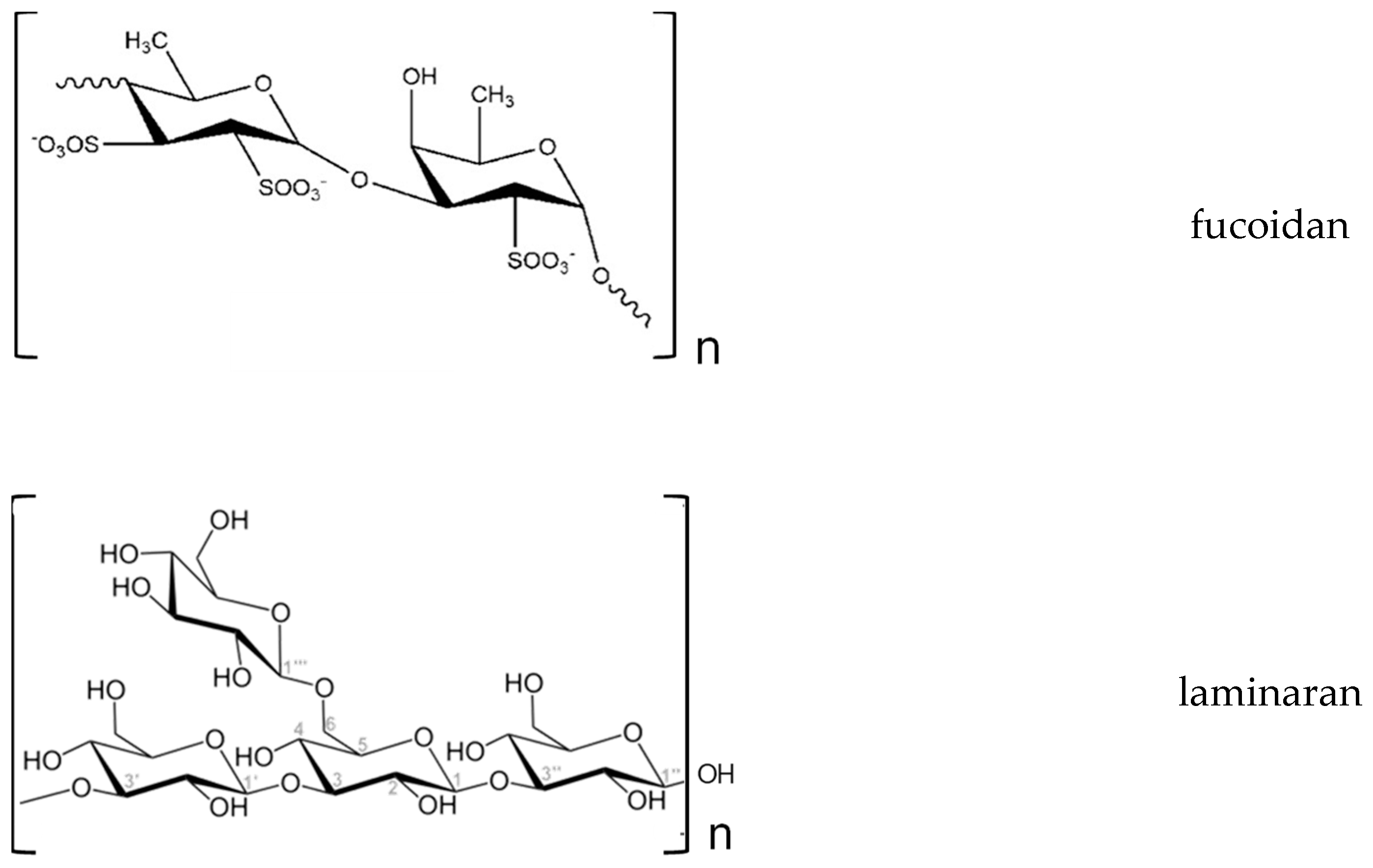
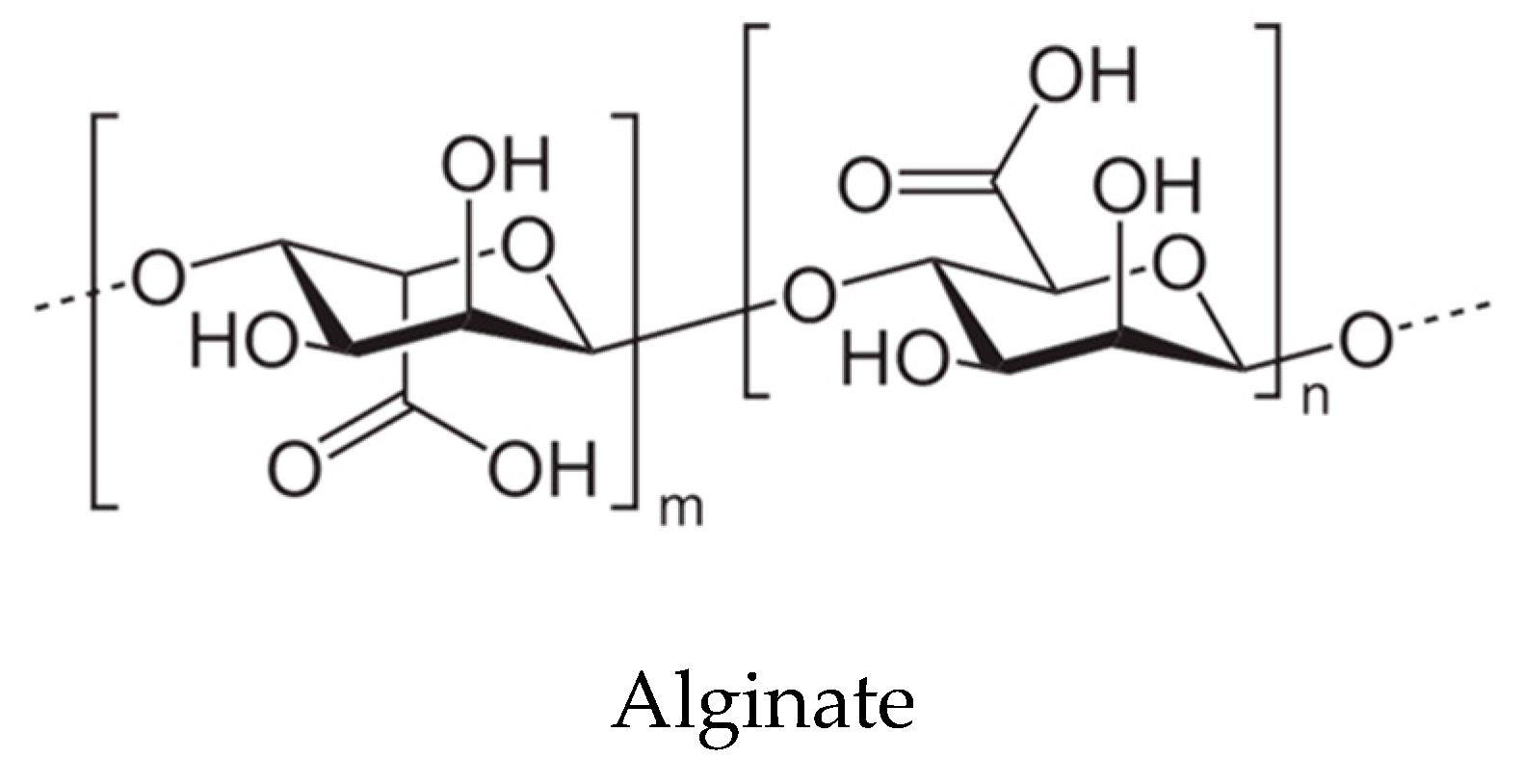
| Kingdom | Phylum/Division | Classes | Orders |
|---|---|---|---|
| Chromist | Ochrophyta | Phaeophyceae | Ascoseirales; Desmarestiales; Discosporangiales; Dictyotales Ectocarpales; Fucales; Laminariales; Nemodermatales Ralfsiales. |
| Plantae | Charophyta | Charophyceae; Chlorokybophyceae; Coleochaetophyceae; Klebsormidiophyceae; Mesostigmatophyceae; Zygnematophyceae. | |
| Chlorophyta | Ulvophyceae | Bryopsidales; Cladophorales; Dasycladales; Oltmannsiellopsidaes; Trentepohliales; Ulotrichales; Ulvales | |
| Rhodophyta | Bangiophyceae; Compsopogonophyceae; Florideophyceae; Porphyridiophyceae; Rhodellophyceae; Stylonematophyceae. |
| Pigment Class | Green Algae | Brown Algae | Red Algae | Reference |
|---|---|---|---|---|
| Chlorophylls | Chlorophyll a and b, and derivatives | Chlorophylls b and c, and derivatives | Chlorophylls a and d, and derivatives | [16] |
| Carotenoids | β-carotene, xanthophylls | Fucoxanthin and xanthophylls, β-carotene | Xanthophylls | [13,16,17] |
| Phycobiliproteins | - | - | Phycoerythrin and phycocyanin | [13,16] |
| Example |  Halimeda sp |  Fucus serratus |  Palmaria palmata |
| Applications | Specific | Authors |
|---|---|---|
| Anti-biofilm activity | [39,40,41] | |
| Biofuels | [32,42,43,44,45] | |
| Bioremediation | [46,47,48] | |
| Contraceptive activity | [49,50] | |
| Cosmeceuticals | [51,52] | |
| Fertilizer | [32,43,53] | |
| Fish feed | [32,43] | |
| Food ingredients | [32,43] | |
| Pharmacology/medical | General | [32,54] |
| Antibiotic, antiviral, antifungal activity | [55,56,57,58] | |
| Anticancer | [56,59,60] | |
| Anticoagulant | [61,62,63,64] | |
| Anti-inflammatory | [65,66,67] | |
| Antioxidants | [56,68] | |
| Other applications: | Filter | [69] |
| Mineralogenic | [43,70] |
| Name | Applications | Region/Country | References |
|---|---|---|---|
| Red Alga (Rhodophyta) | |||
| Porphyra (alga nori) | Cultivated for food | Asia | [93] |
| Saccharina japonica | Cultivated for food | Japan | [93] |
| Palmaria palmata (Dulse) | Culinary ingredient, flavor-enhancer | USA, Canada, Scotland, Ireland, Iceland | [93] |
| Gelidium sp., Gracilaria sp., Pterocladia sp., Acanthopeltis sp., Ahnfeltia sp. | Instant pie fillings, canned meats or fish, bakery icings, beer and wine clarifiers | Asia | [93] |
| Eucheuma sp., Chondria sp., Iridaea sp. | Thickening and stabilizers, imitation of creams, puddings, syrups, canned pet foods. | Philippines, Ireland, Chile, USA, Canada | [93] |
| Grateloupia sp. | As vegetable | Indo-pacific region | [94] |
| Brown Alga (Pheophyta) | |||
| Sargassum fusiforme, Sargassum dentifolium | Farmed in small quantities (poultry, improves quality of eggs) | Europe, Asia, North America | [93] |
| Ascophyllum nodosum | Animal feed (ruminant and poultry diets), human consumption | Norway, UK, Portugal, USA | [93] |
| Undaria sp., Hizikia sp. | Fried in oil, boiled in soup | Japan, Korea, China | [91] |
| Macrocystis sp., Laminaria sp. | Ice-creams, syrups, salad dressings (texturizers, emulsifiers, thickeners) | Europe, USA | [93] |
| Ascophyllum sp. | Land animal feed (i.e., ruminants) | Iceland | [93] |
| Laminaria digitata, L. hyperborea, L. latissima | Animal feed | Europe, Asia | [91] |
| Laminaria japonica | Soup, fried in oil, with soy sauce | Asia | [91] |
| Fucus vesiculosum | Pigs diet | Sweden | [93] |
| Enteromorpha prolifera | Poultry diet | Europe | [93] |
| Pelvetia canaliculata | Pigs diet, human consumption during times of famine | Scotland, Ireland | [93] |
| Green Alga (Chlorophyta) | |||
| Caulerpa sp. | Farmed in small quantities (poultry, improves quality of eggs), food (“green caviar”) | Europe, Asia, Northamerica | [93] |
| Monostroma sp. | Salads, soups, relishes, meat and fish dishes | Europe, Asia | [93] |
| Ulva lactuca | Lambs feed, soups, salads | Europe, USA, Asia, Australia, New Zealand | [93] |
| Ulva intestinalis | Rabbits feed | Egypt, Saudi Arabia | [93] |
| Chaetomorpha linum | Lambs’ feed | Tunisia | [93] |
| Prebiotic/Prebiotic Canditate | Origin/Source | Health Beneficial Effects | References |
|---|---|---|---|
| AGAROS (agaro-oligosaccharides) | Pheophyta (brown algae) | Immunomodulatory (decrease of pro-inflamatory cytokynes) antiinflammatory, carcinostatic, antioxidant, hepatoprotective, and α-glucosidase inhibitory activities | [110] |
| NAOS (neoagaro-oligosaccharides) | Gracilaria sp., Monostroma sp. | ROS scavenging, antioxidant and immunomodulatory effects, stimulation of lactobacilli and bifidobacteria populations | [110,111,112,113] |
| COS (carrageenan-oligosaccharides) | Kappaphycus sp., Porphyria sp., Gracilaria sp. | Immunomodulation, skin whitening, and moisturizing, stimulation of lactobacilli and bifidobacteria populations, repair of intestinal damage | [110,113,114,115] |
| ALGOS (alginate-oligosaccharides) | Ascophyllum sp., Fucus sp., Undaria sp., Sargassum sp., Laminaria sp. and Macrocystis sp. | Reactive oxygen species (ROS) scavenging, antioxidant and immunomodulatory effects, weight control, reduction of cholesterol, diabetes control (hypoglycemic and hypolipidemic properties), promotion of fecal microbiota metabolism, production of short chain fatty acids by the gut microbiota; decrease of putrefactive compounds and microorganisms, decrease of metabolic syndrome risk | [115,116] |
| Fucoidans (FUCOS) | Cladosiphon (aka Okinawa) Ascophyllum (nodosum), Fucus sp. Sargassum sp. | Hypocholesterolaemic, immunomodulatory, anti-obesity, anti-hyperlipidemia, attenuation of hepatic steatosis, anti-diabetes (reduction of insulin resistance), anti-hypertensive, antioxidant | [99,114,116,117] |
| Fucus evanescens | Anticoagulant, antioxidant | [109,118] | |
| Fucus vesciculosus | Anticancer, antimetastatic | [109] | |
| Galactofucans | Laminaria japonica, Sargassum sp. | Anti-lipidaemic, increases HDL, antiviral, antitumor, immunomodulator, antioxidant, neuroprotective | [114] |
| Undaria pinnatifida | Antiviral, anticoagulant, antitumor, anti-proliferative, immunomodulatory, anti-inflammatory induced osteoblastic differentiation | [119] | |
| Dictyota menstrualis | Peripheral anti-nociceptive, anti-inflammatory, antioxidant; anticoagulant, anti-proliferative | [119] | |
| Lobophora variegata | Antioxidant, anticoagulant, anti-inflammatory | [119] | |
| Adenocystis utricularis | Antiviral | [119] | |
| Xylo-galactofucans | Spatoglossum schröederi | Anti-thrombotic; Peripheral anti-nociceptive; Anti-proliferative, anti-adhesive, antioxidant | [119] |
| Arabinoxylans | Ascophyllum | Modulation of intestinal microbiota | [120] |
| Glucans | Chlorela vulgaris | Antitumor, infection preventive agent | [119] |
| Laminarin | Ascophyllum sp., Fucus sp., Laminaria sp., Saccharina sp., Undaria, Enteromorpha sp. | Antilipidemic, hypocholesterolaemic, fast decrease of blood glucose | [114,116] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomez-Zavaglia, A.; Prieto Lage, M.A.; Jimenez-Lopez, C.; Mejuto, J.C.; Simal-Gandara, J. The Potential of Seaweeds as a Source of Functional Ingredients of Prebiotic and Antioxidant Value. Antioxidants 2019, 8, 406. https://doi.org/10.3390/antiox8090406
Gomez-Zavaglia A, Prieto Lage MA, Jimenez-Lopez C, Mejuto JC, Simal-Gandara J. The Potential of Seaweeds as a Source of Functional Ingredients of Prebiotic and Antioxidant Value. Antioxidants. 2019; 8(9):406. https://doi.org/10.3390/antiox8090406
Chicago/Turabian StyleGomez-Zavaglia, Andrea, Miguel A. Prieto Lage, Cecilia Jimenez-Lopez, Juan C. Mejuto, and Jesus Simal-Gandara. 2019. "The Potential of Seaweeds as a Source of Functional Ingredients of Prebiotic and Antioxidant Value" Antioxidants 8, no. 9: 406. https://doi.org/10.3390/antiox8090406
APA StyleGomez-Zavaglia, A., Prieto Lage, M. A., Jimenez-Lopez, C., Mejuto, J. C., & Simal-Gandara, J. (2019). The Potential of Seaweeds as a Source of Functional Ingredients of Prebiotic and Antioxidant Value. Antioxidants, 8(9), 406. https://doi.org/10.3390/antiox8090406





