A Dual Perspective of the Action of Lysine on Soybean Oil Oxidation Process Obtained by Combining 1H NMR and LC–MS: Antioxidant Effect and Generation of Lysine–Aldehyde Adducts
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Oxidation Process
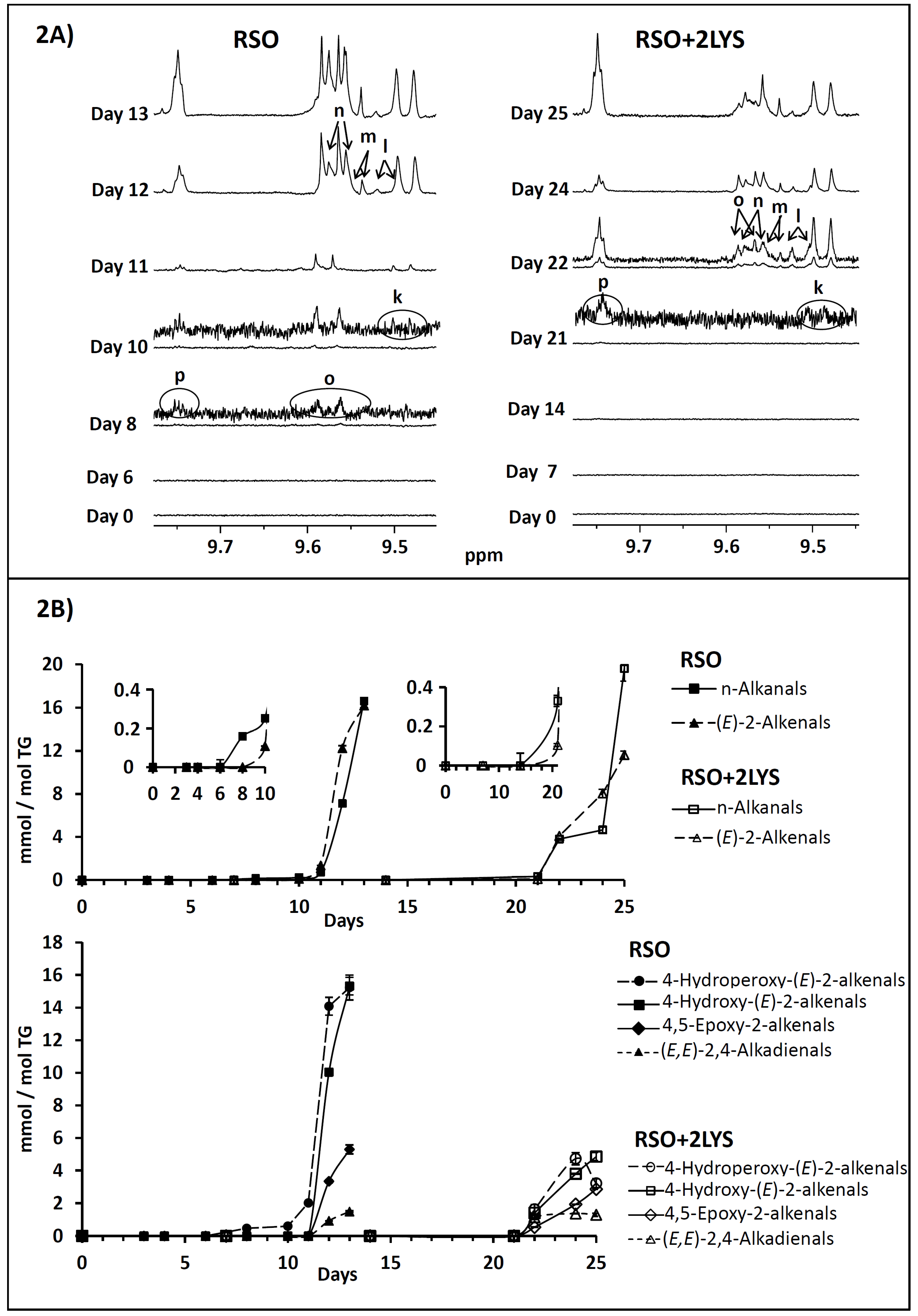
2.2.1. Monitoring by 1H NMR of the Evolution of RSO and of RSO + 2LYS Samples throughout the Oxidation Process
2.2.2. Extraction of Lysine and Some of its Derivatives from the RSO + 2LYS Sample after Being Submitted for Different Periods of Time to Oxidative Conditions
Study of the Extracts by LC-MS
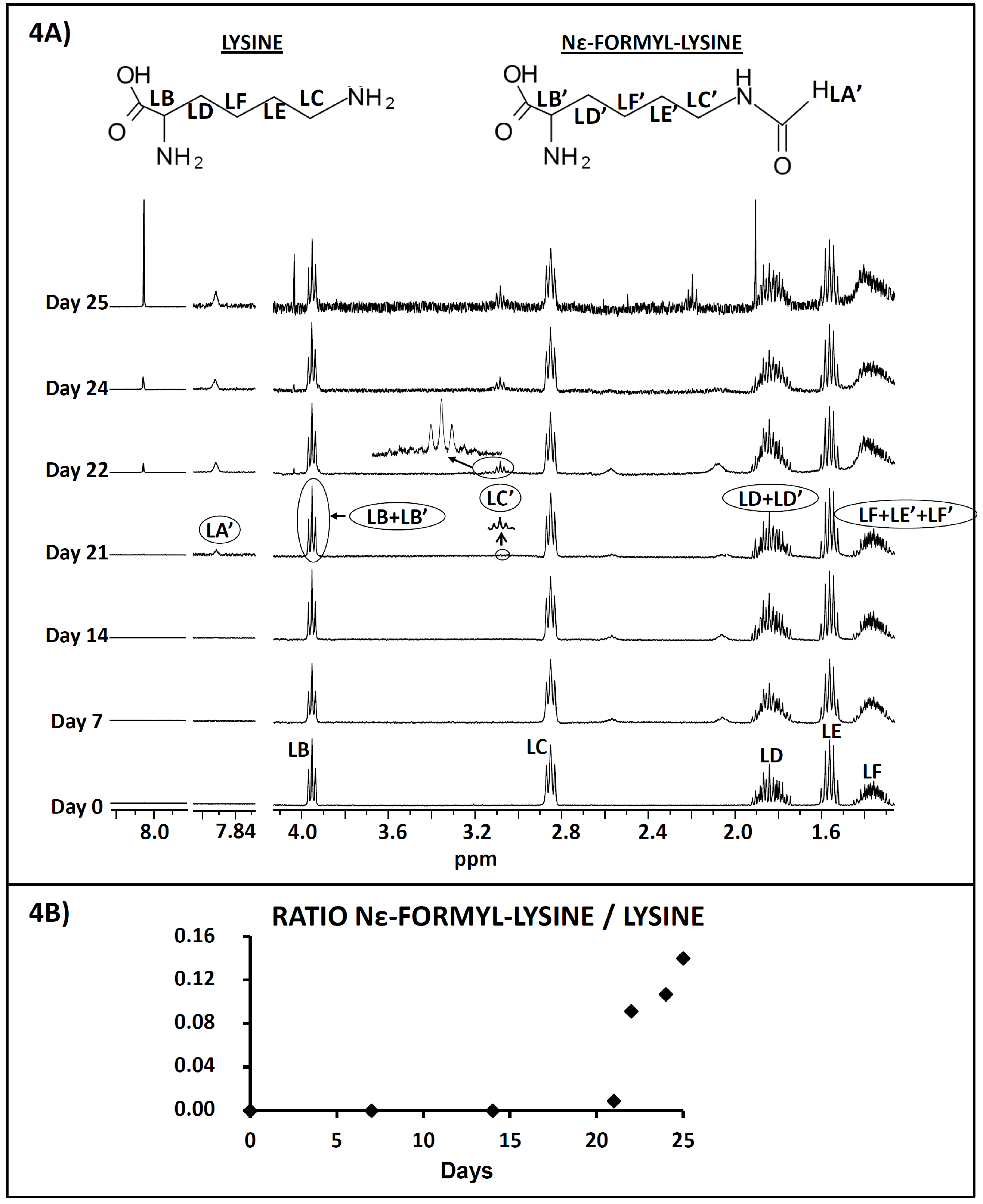
Study of the Extracts by 1H NMR
3. Results and Discussion
3.1. Effect of the Presence of Lysine on Soybean Oil Evolution. Monitoring by 1H NMR
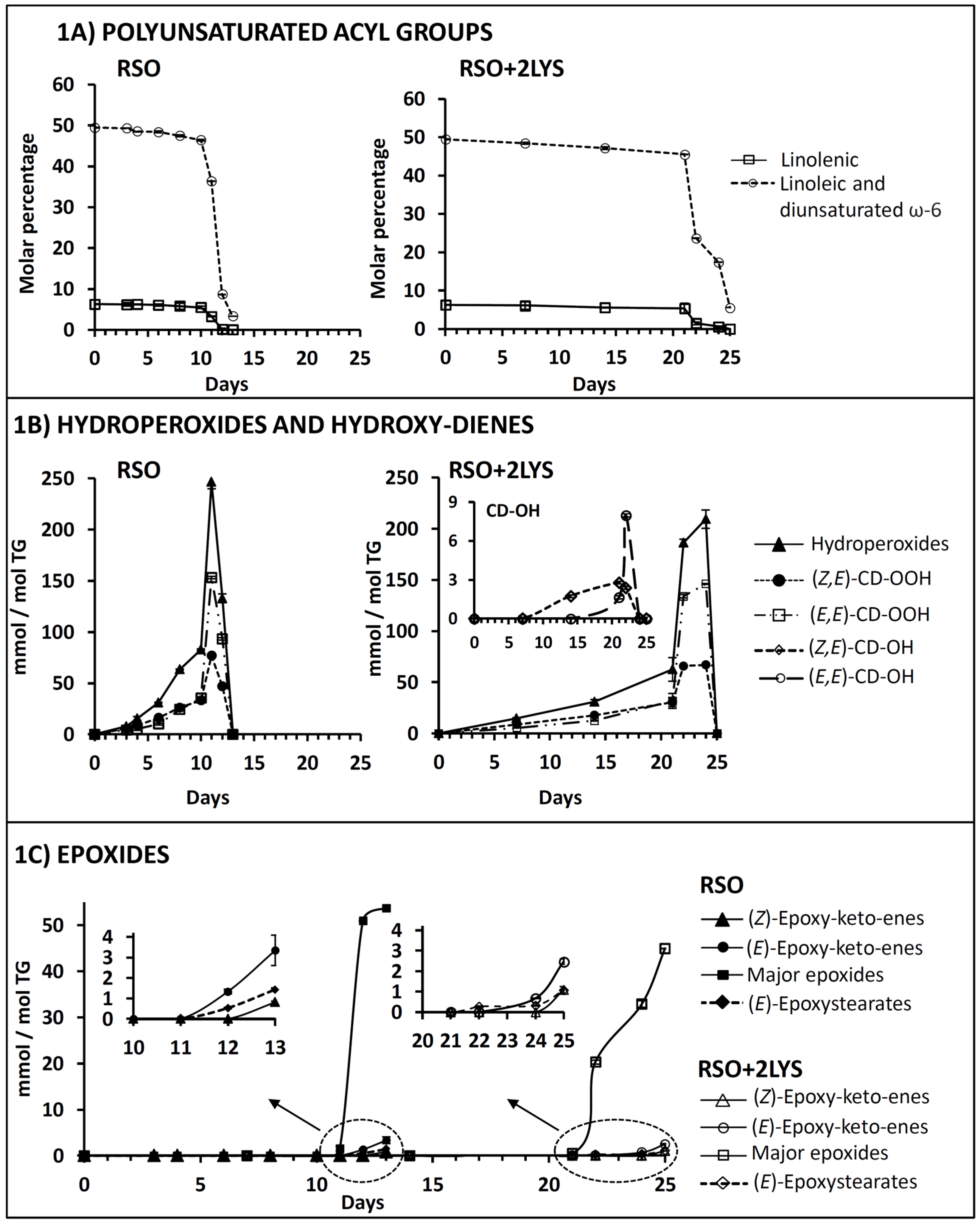
3.1.1. Effect on the Degradation Rate of Polyunsaturated Acyl Groups
3.1.2. Effect on Hydroperoxide Formation
3.1.3. Effect on Conjugated (Z,E)- and (E,E)-Hydroxy-Diene Formation
3.1.4. Effect on Epoxide Formation
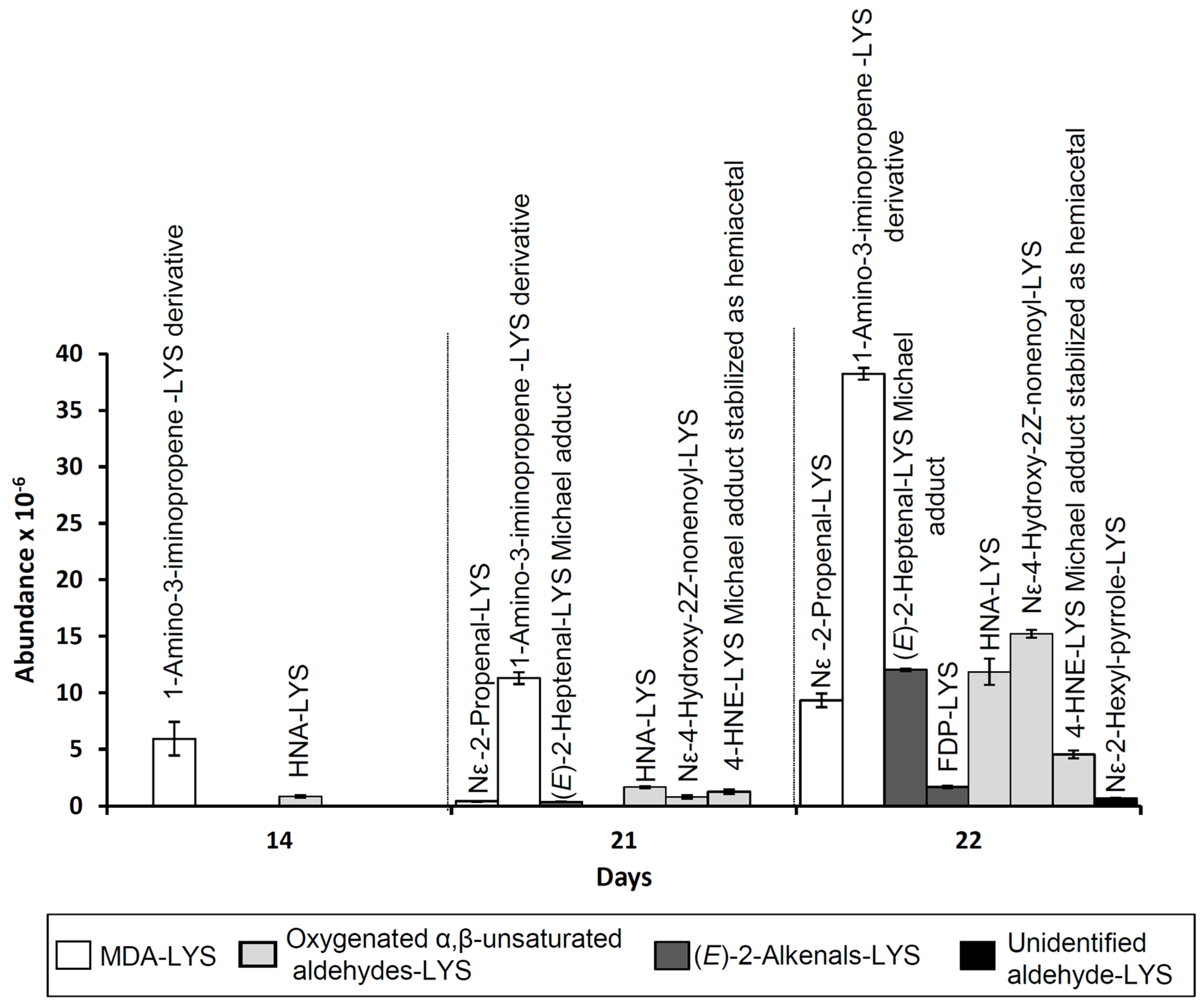
3.1.5. Effect on Aldehyde Formation and on Their Concentration Evolution
3.2. Analysis of the Changes that Lysine Might Undergo or the Reactions in Which it Could be Involved during the Oxidation Process of the RSO + 2LYS Sample
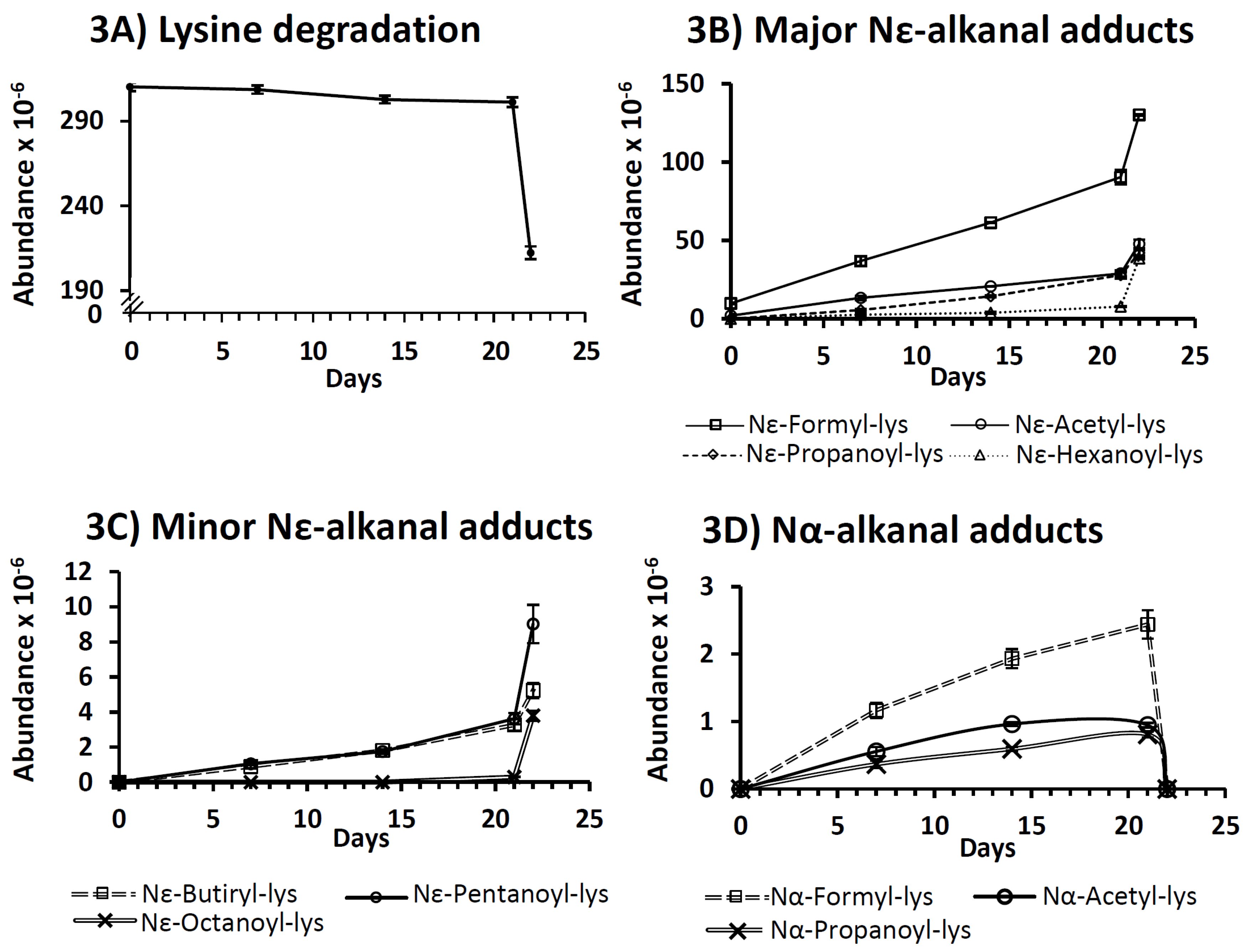
3.2.1. Decrease in Lysine Concentration
3.2.2. Metal Chelation Reactions
3.2.3. Oxidation Reactions Yielding α-Aminoadipic Semialdehyde
3.2.4. Reactions with Lipid Oxidation Products
Reaction with Hydroperoxides
Reaction with Epoxides
Reaction with Saturated Aldehydes
Reaction with More Reactive Aldehydes
Pyrrolization reactions
Polymerization Reactions
3.3. Analysis of the Potential Role of Lysine–Aldehyde Adducts on the Antioxidant Effect Observed
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Elias, R.J.; Kellerby, S.S.; Decker, E.A. Antioxidant activity of proteins and peptides. Crit. Rev. Food Sci. Nutr. 2008, 48, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Elias, R.J.; Bridgewater, J.D.; Vachet, R.W.; Waraho, T.; McClements, D.J.; Decker, E.A. Antioxidant mechanisms of enzymatic hydrolysates of β-lactoglobulin in food lipid dispersions. J. Agric. Food Chem. 2006, 54, 9565–9572. [Google Scholar] [CrossRef] [PubMed]
- Jónsdóttir, R.; Geirsdóttir, M.; Hamaguchi, P.Y.; Jamnik, P.; Kristinsson, H.G.; Undeland, I. The ability of in vitro antioxidant assays to predict the efficiency of a cod protein hydrolysate and brown seaweed extract to prevent oxidation in marine food model systems. J. Sci. Food Agric. 2016, 96, 2125–2135. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R.; Levine, R.L. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 2003, 25, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Gardner, H.W. Lipid hydroperoxide reactivity with proteins and amino acids: A review. J. Agric. Food Chem. 1979, 27, 220–229. [Google Scholar] [CrossRef]
- Uchida, K. Histidine and lysine as targets of oxidative modification. Amino Acids 2003, 25, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.M.; Al-Hakim, S.; Adel, A.; Shehata, Y. The antioxidant activity of amino acids in two vegetable oils. J. Am. Oil Chem. Soc. 1983, 60, 837–840. [Google Scholar] [CrossRef]
- Xu, P.; Zheng, Y.; Zhu, X.; Li, S.; Zhou, C. l-lysine and l-arginine inhibit the oxidation of lipids and proteins of emulsion sausage by chelating iron ion and scavenging radical. Asian Australas. J. Anim. Sci. 2018, 31, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Filippenko, T.A.; Gribova, N.Y. Antioxidant activity of amino acids during oxidation of sunflower oil in an emulsion. Pharm. Chem. J. 2011, 45, 296. [Google Scholar] [CrossRef]
- Hwang, H.S.; Winkler-Moser, J.K. Antioxidant activity of amino acids in soybean oil at frying temperature: Structural effects and synergism with tocopherols. Food Chem. 2017, 221, 1168–1177. [Google Scholar] [CrossRef] [PubMed]
- Martin-Rubio, A.S.; Sopelana, P.; Guillén, M.D. The potential of lysine to extend the shelf life of soybean oil evidenced by 1H Nuclear Magnetic Resonance. LWT-Food Sci. Technol. 2019, 105, 169–176. [Google Scholar] [CrossRef]
- Soladoye, O.P.; Juárez, M.L.; Aalhus, J.L.; Shand, P.; Estévez, M. Protein oxidation in processed meat: Mechanisms and potential implications on human health. Compr. Rev. Food Sci. Food saf. 2015, 14, 106–122. [Google Scholar] [CrossRef]
- Guillén, M.D.; Uriarte, P.S. Study by 1H NMR spectroscopy of the evolution of extra virgin olive oil composition submitted to frying temperature in an industrial fryer for a prolonged period of time. Food Chem. 2012, 134, 162–172. [Google Scholar] [CrossRef]
- O’Neil, M.J.; Heckelman, P.E.; Koch, C.B.; Roman, K.J. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 14th ed.; Merck and Co., Inc.: Whitehouse Station, NJ, USA, 2006; p. 977. [Google Scholar]
- Maskan, M. Change in colour and rheological behaviour of sunflower seed oil during frying and after adsorbent treatment of used oil. Eur. Food Res. Technol. 2003, 218, 20–25. [Google Scholar] [CrossRef]
- Schaich, K.M. Co-oxidation of proteins by oxidizing lipids. In Lipid Oxidation Pathways; Kamal-Eldin, A., Min, D.B., Eds.; AOCS Press: Urbana, IL, USA, 2008; Volume 2, pp. 183–274. [Google Scholar]
- Guillén, M.D.; Goicoechea, E. Toxic oxygenated α,β-unsaturated aldehydes and their study in foods: A review. Crit. Rev. Food Sci. Nutr. 2008, 48, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Zamora, R.; Hidalgo, F.J. Linoleic acid oxidation in the presence of amino compounds produces pyrroles by carbonyl amine reactions. Biochim. Biophys. Acta Lipids Lipid Metab. 1995, 1258, 319–327. [Google Scholar] [CrossRef]
- Sacchi, R.; Patumi, M.; Fontanazza, G.; Barone, P.; Fiordiponti, P.; Mannina, L.; Rossi, E.; Segre, A.L. A high-field 1H Nuclear Magnetic Resonance study of the minor components in virgin olive oils. J. Am. Oil Chem. Soc. 1996, 73, 747–758. [Google Scholar] [CrossRef]
- Sadler, P.J.; Tucker, A.; Viles, J.H. Involvement of a lysine residue in the N-terminal Ni2+ and Cu2+ binding site of serum albumins: Comparison with Co2+, Cd2+ and Al3+. Eur. J. Biochem. 1994, 220, 193–200. [Google Scholar] [CrossRef]
- Selvakannan, P.R.; Mandal, S.; Phadtare, S.; Pasricha, R.; Sastry, M. Capping of gold nanoparticles by the amino acid lysine renders them water-dispersible. Langmuir 2003, 19, 3545–3549. [Google Scholar] [CrossRef]
- Akagawa, M.; Sasaki, D.; Ishii, Y.; Kurota, Y.; Yotsu-Yamashita, M.; Uchida, K.; Suyama, K. New method for the quantitative determination of major protein carbonyls, α-aminoadipic and γ-glutamic semialdehydes: Investigation of the formation mechanism and chemical nature in vitro and in vivo. Chem. Res. Toxicol. 2006, 19, 1059–1065. [Google Scholar] [CrossRef]
- Requena, J.R.; Chao, C.C.; Levine, R.L.; Stadtman, E.R. Glutamic and aminoadipic semialdehydes are the main carbonyl products of metal-catalyzed oxidation of proteins. Proc. Natl. Acad. Sci. USA 2001, 98, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, F.J.; Kinsella, J.E. Changes induced in beta-lactoglobulin B following interactions with linoleic acid 13-hydroperoxide. J. Agric. Food Chem. 1989, 37, 860–866. [Google Scholar] [CrossRef]
- Wu, W.; Hou, L.; Zhang, C.; Kong, X.; Hua, Y. Structural modification of soy protein by 13-hydroperoxyoctadecadienoic acid. Eur. Food Res. Technol. 2009, 229, 771–778. [Google Scholar] [CrossRef]
- Kato, Y.; Mori, Y.; Makino, Y.; Morimitsu, Y.; Hiroi, S.; Ishikawa, T.; Osawa, T. Formation of Nε-(hexanonyl) lysine in protein exposed to lipid hydroperoxide. A plausible marker for lipid hydroperoxide-derived protein modification. J. Biol. Chem. 1999, 274, 20406–20414. [Google Scholar] [CrossRef] [PubMed]
- Ishino, K.; Shibata, T.; Ishii, T.; Liu, Y.T.; Toyokuni, S.; Zhu, X.; Sayre, L.M.; Uchida, K. Protein N-acylation: H2O2-mediated covalent modification of protein by lipid peroxidation-derived saturated aldehydes. Chem. Res. Toxicol. 2008, 21, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Pokorny, J.; Klein, S.; Koren, J. Reaction of oxidized lipids with protein. II. Reactions of albumin with epoxy derivatives. Nahrung 1966, 10, 321–325. [Google Scholar]
- Lederer, M.O. Reactivity of lysine moieties toward γ-hydroxy-α,β-unsaturated epoxides: A model study on protein-lipid oxidation product interaction. J. Agric. Food Chem. 1996, 44, 2531–2537. [Google Scholar] [CrossRef]
- Hidalgo, F.J.; Zamora, R. In vitro production of long chain pyrrole fatty esters from carbonyl-amine reactions. J. Lipid Res. 1995, 36, 725–735. [Google Scholar]
- Shibata, T.; Shimozu, Y.; Wakita, C.; Shibata, N.; Kobayashi, M.; Machida, S.; Kato, R.; Itabe, H.; Zhu, X.; Sayre, L.M.; et al. Lipid peroxidation modification of protein generates Nε-(4-oxononanoyl)lysine as a pro-inflammatory ligand. J. Biol. Chem. 2011, 286, 19943–19957. [Google Scholar] [CrossRef]
- Benedetti, A.; Comporti, M. Formation, reactions and toxicity of aldehydes produced in the course of lipid peroxidation in cellular membranes. Bioelectrochem. Bioenerg. 1987, 18, 187–202. [Google Scholar] [CrossRef]
- Zamora, R.; Alaiz, M.; Hidalgo, F.J. Contribution of pyrrole formation and polymerization to the nonenzymatic browning produced by amino-carbonyl reactions. J. Agric. Food Chem. 2000, 48, 3152–3158. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef]
- Shimozu, Y.; Hirano, K.; Shibata, T.; Shibata, N.; Uchida, K. 4-Hydroperoxy-2-nonenal is not just an intermediate but a reactive molecule that covalently modifies proteins to generate unique intramolecular oxidation products. J. Biol. Chem. 2011, 286, 29313–29324. [Google Scholar] [CrossRef] [PubMed]
- Meynier, A.; Rampon, V.; Dalgalarrondo, M.; Genot, C. Hexanal and t-2-hexenal form covalent bonds with whey proteins and sodium caseinate in aqueous solution. Int. Dairy J. 2004, 14, 681–690. [Google Scholar] [CrossRef]
- Globisch, M.; Schindler, M.; Kreßler, J.; Henle, T. Studies on the reaction of trans-2-heptenal with peanut proteins. J. Agric. Food Chem. 2014, 62, 8500–8507. [Google Scholar] [CrossRef]
- Miyashita, H.; Chikazawa, M.; Otaki, N.; Hioki, Y.; Shimozu, Y.; Nakashima, F.; Shibata, T.; Hagihara, Y.; Maruyama, S.; Matsumi, N.; et al. Lysine pyrrolation is a naturally-occurring covalent modification involved in the production of DNA mimic proteins. Sci. Rep. 2014, 4, 5343. [Google Scholar] [CrossRef] [PubMed]
- Globisch, M.; Kaden, D.; Henle, T. 4-Hydroxy-2-nonenal (4-HNE) and its lipation product 2-pentylpyrrole lysine (2-PPL) in peanuts. J. Agric. Food Chem. 2015, 63, 5273–5281. [Google Scholar] [CrossRef]
- Zamora, R.; Hidalgo, F.J. Coordinate contribution of lipid oxidation and Maillard reaction to the nonenzymatic food browning. Crit. Rev. Food Sci. Nutr. 2005, 45, 49–59. [Google Scholar] [CrossRef]
- Burton, H.S.; McWeeny, D.J.; Biltcliffe, D.O. Non-enzymic browning: The role of unsaturated carbonyl compounds as intermediates and of SO2 as an inhibitor of browning. J. Sci. Food Agric. 1963, 14, 911–920. [Google Scholar] [CrossRef]
- Alaiz, M.; Zamora, R.; Hidalgo, F.J. Antioxidative activity of pyrrole, imidazole, dihydropyridine, and pyridinium salt derivatives produced in oxidized lipid/amino acid browning reactions. J. Agric. Food Chem. 1996, 44, 686–691. [Google Scholar] [CrossRef]
- Alaiz, M.; Hidalgo, F.J.; Zamora, R. Comparative antioxidant activity of Maillard- and oxidized lipid-damaged bovine serum albumin. J. Agric. Food Chem. 1997, 45, 3250–3254. [Google Scholar] [CrossRef]
| Cone 20 V | Cone 35 V | ||||
|---|---|---|---|---|---|
| RT (min) | Compound 1 | BP 2 | Other Fragments | BP | Other Fragments 3 |
| 1:94 | Nε-octanoyl-lysine | 273 | 147 | 273 | 84, 210, 130, 147 |
| 2:26 | Nε-hexanol-lysine | 245 | 147 | 245 | 84, 182, 147, 130 |
| 2:36 | Nε-pentanoyl-lysine | 231 | 147 | 231 | 84, 168, 147, 130 |
| 2:53 | Nε-butiryl-lysine | 217 | 147 | 84 | 217, 147, 154, 130 |
| 2:71 | Nε-propanoyl-lysine | 203 | 140 | 203, 84, 130, 147 | |
| 2:91 | Nε-acetyl-lysine | 189 | 126 | 84, 189, 147, 130 | |
| 2:99 | Nε-formyl-lysine | 175 | 112 | 112 | 175, 84, 130, 147 |
| 3:45 | Nα-propanoyl-lysine | 203 | 84 | 203 | |
| 3:71 | Nα-acetyl-lysine | 189 | 84 | 189 | |
| 3:73 | Nα-formyl-lysine | 175 | 175 | 84 | |
| Cone 20 V | Cone 35 V | |||||||
|---|---|---|---|---|---|---|---|---|
| Reaction with | Detection Day | Compound | Structure | BP | Other Fragments | BP | Other Fragments | References |
| MDA | ||||||||
| 14 | N,N’-disubstituted 1-amino-3-iminopropene LYS derivative |  | 329 | 147 | 329 | 84 | [6] | |
| 21 | Nε- (2-Propenal)-LYS | 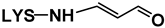 | 201 | 84 | 130, 201, 138 | [6] | ||
| (E)-2-Alkenals | ||||||||
| (E)-2-Heptenal | 21 | (E)-2-Heptenal-LYS Michael adduct | 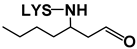 | 259 | 259 | 130, 196 | [36] | |
| Acrolein | 22 | Nε- (3-formyl-3,4-dehydropiperidino) lysine (FDP-LYS) |  | 241 | 130 | 241 | 195, 84, 96, 130 | [6] |
| Oxygenated α,β-unsaturated aldehydes | ||||||||
| 4-HPNE | 14 | Nε-4-Hydroxynonanoic acid-LYS (HNA-LYS) | 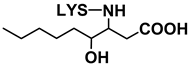 | 319 | 319 | 130 | [35] | |
| 4-HPNE | 21 | Nε-4-Hydroxy-(2Z)-nonenoyl-LYS | 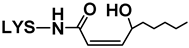 | 301 | 147, 130 | 147 | 84, 130, 301 | [35] |
| 4-HNE | 21 | 4-HNE-LYS Michael adduct stabilized as hemiacetal |  | 303 | 147,130 | 303 | 84, 130 | [6] |
| Unidentified aldehyde | 22 | Nε-2-Hexyl-pyrrole-LYS |  | 281 | 197 | 281, 84, 236, 134 | [38] | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin-Rubio, A.S.; Sopelana, P.; Nakashima, F.; Shibata, T.; Uchida, K.; Guillén, M.D. A Dual Perspective of the Action of Lysine on Soybean Oil Oxidation Process Obtained by Combining 1H NMR and LC–MS: Antioxidant Effect and Generation of Lysine–Aldehyde Adducts. Antioxidants 2019, 8, 326. https://doi.org/10.3390/antiox8090326
Martin-Rubio AS, Sopelana P, Nakashima F, Shibata T, Uchida K, Guillén MD. A Dual Perspective of the Action of Lysine on Soybean Oil Oxidation Process Obtained by Combining 1H NMR and LC–MS: Antioxidant Effect and Generation of Lysine–Aldehyde Adducts. Antioxidants. 2019; 8(9):326. https://doi.org/10.3390/antiox8090326
Chicago/Turabian StyleMartin-Rubio, Ana S., Patricia Sopelana, Fumie Nakashima, Takahiro Shibata, Koji Uchida, and María D. Guillén. 2019. "A Dual Perspective of the Action of Lysine on Soybean Oil Oxidation Process Obtained by Combining 1H NMR and LC–MS: Antioxidant Effect and Generation of Lysine–Aldehyde Adducts" Antioxidants 8, no. 9: 326. https://doi.org/10.3390/antiox8090326
APA StyleMartin-Rubio, A. S., Sopelana, P., Nakashima, F., Shibata, T., Uchida, K., & Guillén, M. D. (2019). A Dual Perspective of the Action of Lysine on Soybean Oil Oxidation Process Obtained by Combining 1H NMR and LC–MS: Antioxidant Effect and Generation of Lysine–Aldehyde Adducts. Antioxidants, 8(9), 326. https://doi.org/10.3390/antiox8090326




