Characterization of an Antioxidant and Antimicrobial Extract from Cool Climate, White Grape Marc
Abstract
1. Introduction
2. Materials and Methods
2.1. Phenolic Compound Extraction
2.2. Antioxidant Activity (Folin-Ciocalteu Total Phenolic Content)
2.3. Antioxidant Activity (DPPH Radical Scavenging)
2.4. HPLC for Monomeric Phenolic Determination
2.5. Spectroscopic Analysis
2.6. Solid-State Nuclear Magnetic Resonance (SSNMR)
2.7. Antimicrobial Activity
2.8. Rebelein Residual Sugar
2.9. Statistical Analysis
3. Results and Discussion
3.1. Extraction
3.2. Monomeric Phenolic Compounds Determined by HPLC-UV/Vis-ECD
3.3. Spectroscopic Techniques
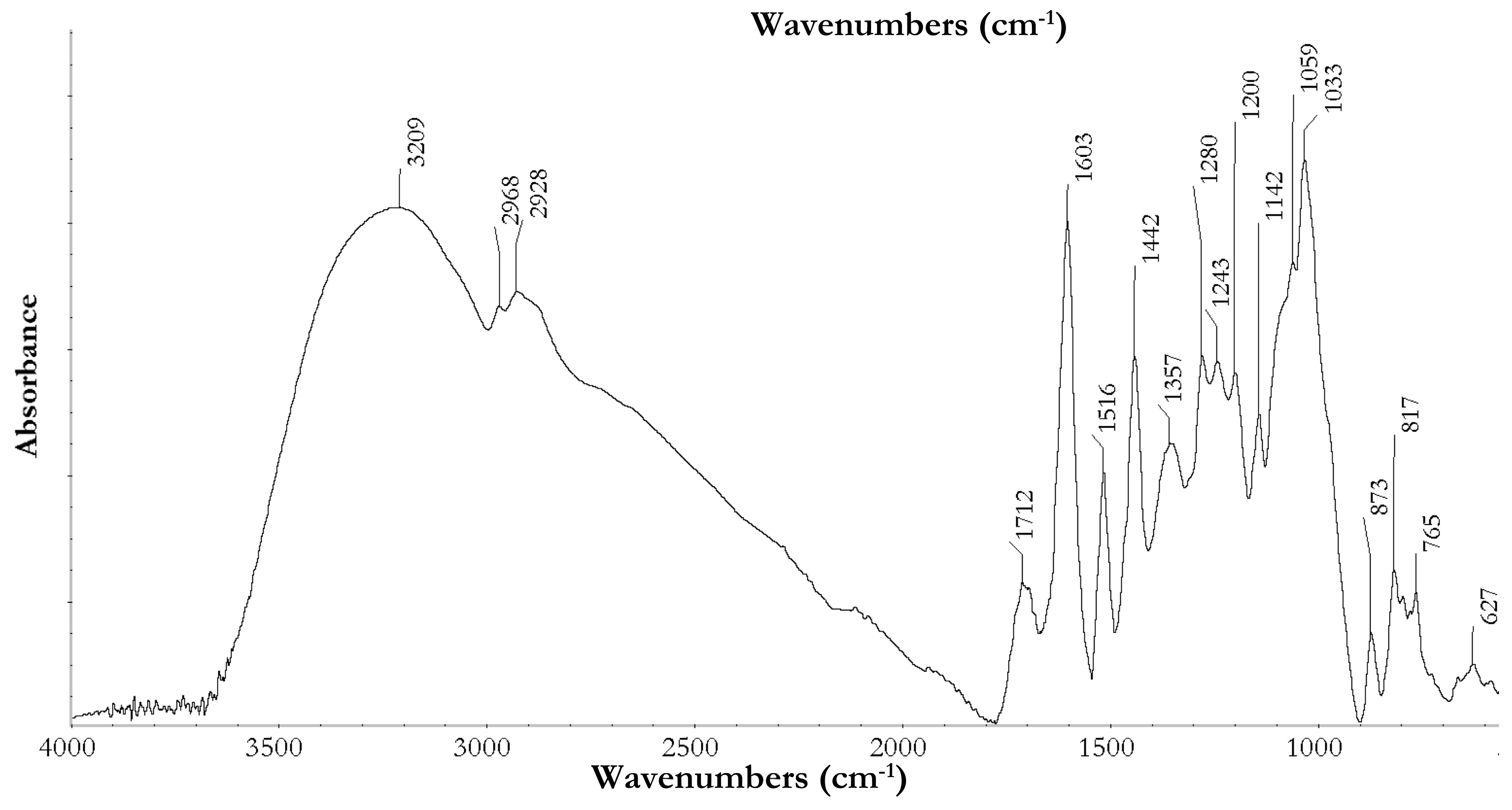
3.3.1. ATR-Fourier Transform Infrared Spectroscopy
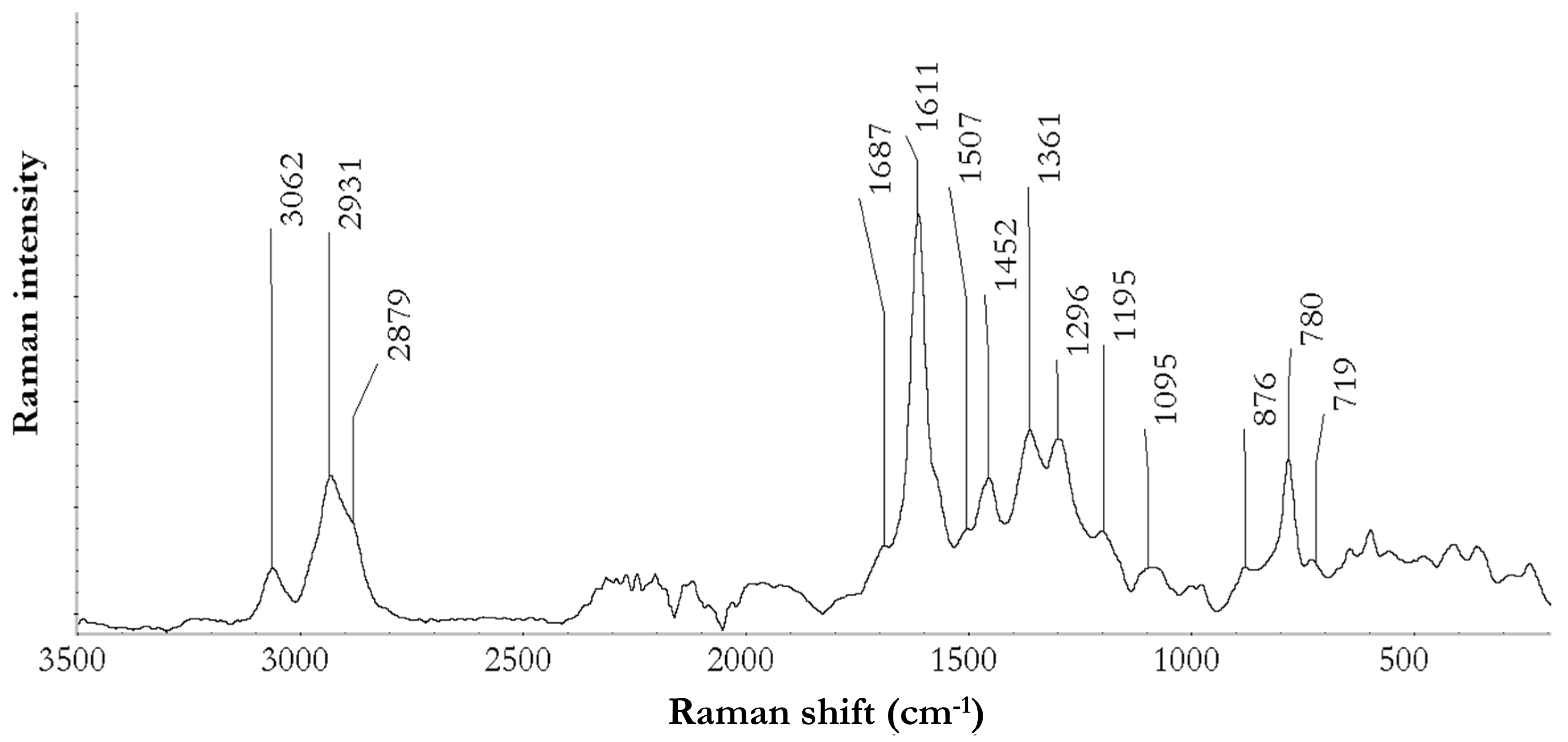
3.3.2. Fourier Transform Raman Spectroscopy
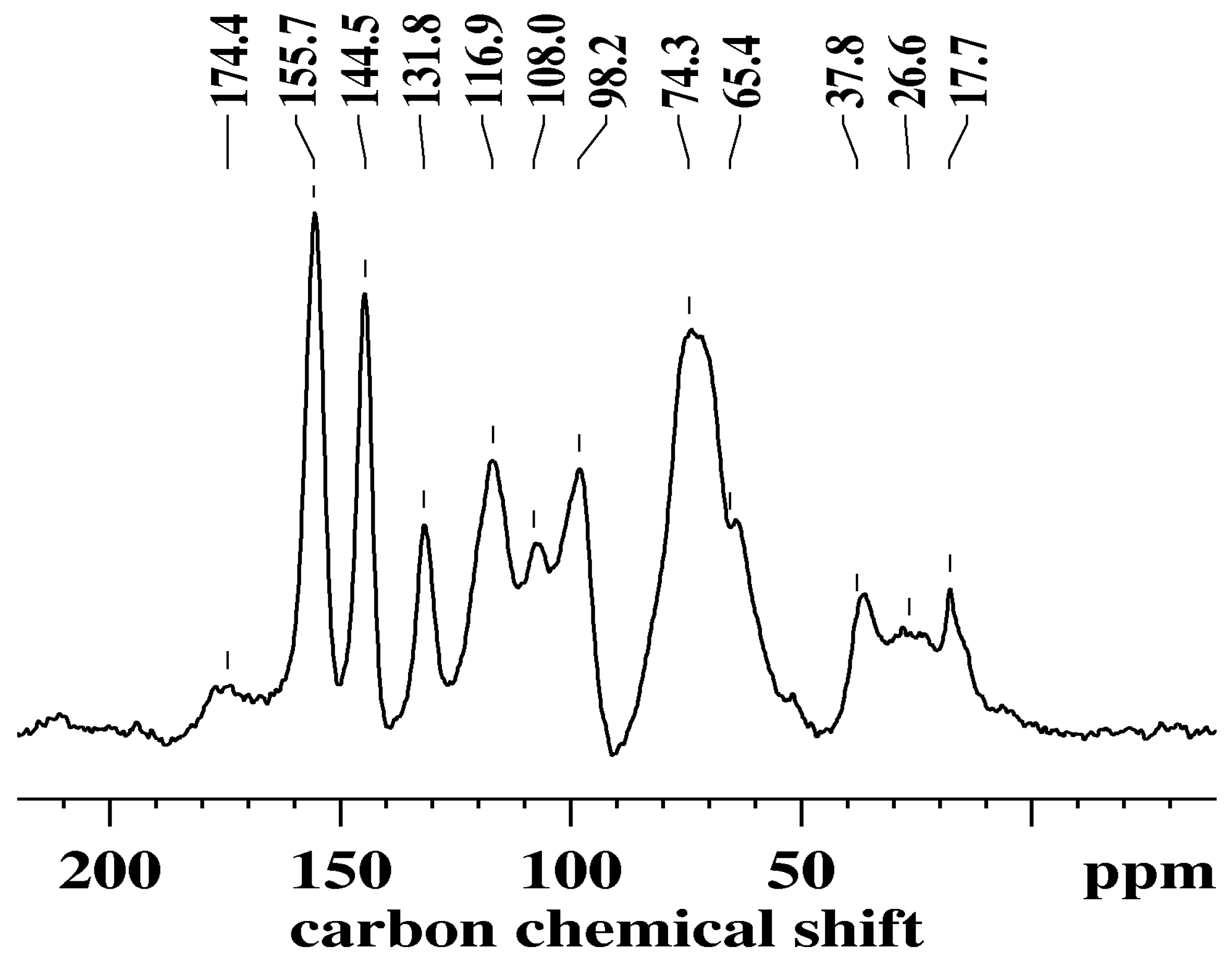
3.4. Solid-State Nuclear Magnetic Resonance
3.5. Antioxidant Activity
3.6. Antimicrobial Activity
3.7. Extract Residual Sugar
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schieber, A.; Stintzing, F.C.; Carle, R. By-products of plant food processing as a source of functional compounds—Recent developments. Trends Food Sci. Technol. 2001, 12, 401–413. [Google Scholar] [CrossRef]
- Özkan, G.; Sagdiç, O.; Göktürk Baydar, N.; Kurumahmutoglu, Z. Antibacterial activities and total phenolic contents of grape pomace extracts. J. Sci. Food Agric. 2004, 84, 1807–1811. [Google Scholar] [CrossRef]
- Waterhouse, A.L. Wine Phenolics. Ann. N. Y. Acad. Sci. 2002, 957, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Da Ricardo Silva, J.M.; Rigaud, J.; Cheynier, V.; Cheminat, A.; Moutounet, M. Procyanidin dimers and Trimers from grape seeds. Phytochemistry 1991, 30, 1259–1264. [Google Scholar] [CrossRef]
- Schofield, P.; Mbugua, D.M.; Pell, A.N. Analysis of condensed tannins: A review. Anim. Feed Sci. Technol. 2001, 91, 21–40. [Google Scholar] [CrossRef]
- McRae, J.M.; Kennedy, J.A. Wine and grape tannin interactions with salivary proteins and their impact on astringency: A review of current research. Molecules 2011, 16, 2348–2364. [Google Scholar] [CrossRef] [PubMed]
- Ali, K.; Maltese, F.; Choi, Y.H.; Verpoorte, R. Metabolic constituents of grapevine and grape-derived products. Phytochem. Rev. 2010, 9, 357–378. [Google Scholar] [CrossRef] [PubMed]
- Gharwalová, L.; Hutár, D.; Masák, J.; Kolouchová, I. Antioxidant activity and phenolic content of organic and conventional vine cane extracts. Czech J. Food Sci. 2018, 36, 289–295. [Google Scholar]
- Rodríguez Montealegre, R.; Romero Peces, R.; Chacón Vozmediano, J.L.; Martínez Gascueña, J.; García Romero, E. Phenolic compounds in skins and seeds of ten grape Vitis vinifera varieties grown in a warm climate. J. Food Compos. Anal. 2006, 19, 687–693. [Google Scholar] [CrossRef]
- Alldrick, A.J.; Flynn, J.; Rowland, I.R. Effects of plant-derived flavonoids and polyphenolic acids on the activity of mutagens from cooked food. Mutat. Res. Fundam. Mol. Mech. Mutagenes. 1986, 163, 225–232. [Google Scholar] [CrossRef]
- Bekhit, A.E.-D.A.; Cheng, V.J.; McConnell, M.; Zhao, J.H.; Sedcole, R.; Harrison, R. Antioxidant activities, sensory and anti-influenza activity of grape skin tea infusion. Food Chem. 2011, 129, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Cueva, C.; Mingo, S.; Muñoz-González, I.; Bustos, I.; Requena, T.; del Campo, R.; Martín-Álvarez, P.J.; Bartolomé, B.; Moreno-Arribas, M.V. Antibacterial activity of wine phenolic compounds and oenological extracts against potential respiratory pathogens. Lett. Appl. Microbiol. 2012, 54, 557–563. [Google Scholar] [CrossRef]
- Soobrattee, M.A.; Neergheen, V.S.; Luximon-Ramma, A.; Aruoma, O.I.; Bahorun, T. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutat. Res. Fundam. Mol. Mech. Mutagenes. 2005, 579, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Villano, D.; Fernandez-Pachon, M.S.; Moya, M.L.; Troncoso, A.M.; Garcia-Parrilla, M.C. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta 2007, 71, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Aliakbarian, B.; Fathi, A.; Perego, P.; Dehghani, F. Extraction of antioxidants from winery wastes using subcritical water. J. Supercrit. Fluids 2012, 65, 18–24. [Google Scholar] [CrossRef]
- Spigno, G.; Tramelli, L.; De Faveri, D.M. Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J. Food Eng. 2007, 81, 200–208. [Google Scholar] [CrossRef]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef]
- Bajčan, D.; Harangozo, Ĺ.; Hrabovská, D.; Bončíková, D. Optimizing conditions for spectrophotometri determination of total polyphenols in wines using Folin-Ciocalteu reagent. J. Microbiol. Biotechnol. Food Sci. 2013, 2, 1699–1708. [Google Scholar]
- Olejar, K.J.; Fedrizzi, B.; Kilmartin, P.A. Antioxidant activity and phenolic profiles of Sauvignon blanc wines made by various maceration techniques. Aust. J. Grape Wine Res. 2015, 21, 57–68. [Google Scholar] [CrossRef]
- Garcia, L.S.; Isenberg, H.D. Clinical Microbiology Procedures Handbook, 3rd ed.; 2007 update; ASM Press: Washington, DC, USA, 2010. [Google Scholar]
- Gizdavic-Nikolaidis, M.R.; Bennett, J.R.; Swift, S.; Easteal, A.J.; Ambrose, M. Broad spectrum antimicrobial activity of functionalized polyanilines. Acta Biomater. 2011, 7, 4204–4209. [Google Scholar] [CrossRef]
- Zoecklein, B.W. Carbohydrates. In Production Wine Analysis; Fugelsang, K.C., Gump, B.H., Nury, F.S., Eds.; Springer: Boston, MA, USA, 1995; pp. 114–128. [Google Scholar]
- de Campos, L.M.A.S.; Leimann, F.V.; Pedrosa, R.C.; Ferreira, S.R.S. Free radical scavenging of grape pomace extracts from Cabernet sauvingnon (Vitis vinifera). Bioresour. Technol. 2008, 99, 8413–8420. [Google Scholar] [CrossRef]
- Spigno, G.; De Faveri, D.M. Antioxidants from grape stalks and marc: Influence of extraction procedure on yield, purity and antioxidant power of the extracts. J. Food Eng. 2007, 78, 793–801. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Toledo, R.T. Major Flavonoids in Grape Seeds and Skins: Antioxidant Capacity of Catechin, Epicatechin, and Gallic Acid. J. Agric. Food Chem. 2003, 52, 255–260. [Google Scholar] [CrossRef]
- Plumb, G.W.; De Pascual-Teresa, S.; Santos-Buelga, C.; Cheynier, V.; Williamson, G. Antioxidant properties of catechins and proanthocyanidins: Effect of polymerisation, galloylation and glycosylation. Free Radic. Res. 1998, 29, 351–358. [Google Scholar] [CrossRef]
- Creasy, G.; Creasy, L. Grape-derived wine flavonoids and stilbenes. In Wine; CRC Press: Boca Raton, FL, USA, 2002; pp. 199–227. [Google Scholar]
- Santos-Buelga, C.; Francia-Aricha, E.M.; Escribano-Bailón, M.T. Comparative flavan-3-ol composition of seeds from different grape varieties. Food Chem. 1995, 53, 197–201. [Google Scholar] [CrossRef]
- Ricci, A.; Olejar, K.J.; Parpinello, G.P.; Kilmartin, P.A.; Versari, A. Application of Fourier Transform Infrared (FTIR) Spectroscopy in the Characterization of Tannins. Appl. Spectrosc. Rev. 2015, 50, 407–442. [Google Scholar] [CrossRef]
- Edwards, H.G.M.; Villar, S.E.J.; de Oliveira, L.F.C.; Hyaric, M.L. Analytical Raman spectroscopic study of cacao seeds and their chemical extracts. Anal. Chim. Acta 2005, 538, 175–180. [Google Scholar] [CrossRef]
- Buchweitz, M.; Gudi, G.; Carle, R.; Kammerer, D.R.; Schulz, H. Systematic investigations of anthocyanin—Metal interactions by Raman spectroscopy. J. Raman Spectrosc. 2012, 43, 2001–2007. [Google Scholar] [CrossRef]
- Merlin, J.C.; Cornard, J.P.; Stastoua, A.; Saidi-Idrissi, M.; Lautie, M.F.; Brouillard, R. Vibrational analysis of hydroxyflavylium derivatives by IR, Fourier transform Raman and resonance Raman spectroscopies. Spectrochim. Acta Part A Mol. Spectrosc. 1994, 50, 703–712. [Google Scholar] [CrossRef]
- Nickless, E.M.; Holroyd, S.E.; Stephens, J.M.; Gordon, K.C.; Wargent, J.J. Analytical FT-Raman spectroscopy to chemotype Leptospermum scoparium and generate predictive models for screening for dihydroxyacetone levels in floral nectar. J. Raman Spectrosc. 2014, 45, 890–894. [Google Scholar] [CrossRef]
- Himmelsbach, D.S.; Akin, D.E. Near-Infrared Fourier-Transform Raman Spectroscopy of Flax (Linum usitatissimum L.) Stems. J. Agric. Food Chem. 1998, 46, 991–998. [Google Scholar] [CrossRef]
- Newman, R.; Porter, L. Solid State 13C-NMR Studies on Condensed Tannins. In Plant Polyphenols; Hemingway, R., Laks, P., Eds.; Springer: New York, NY, USA, 1992; Volume 59, pp. 339–347. [Google Scholar]
- Navarrete, P.; Pizzi, A.; Pasch, H.; Rode, K.; Delmotte, L. MALDI-TOF and 13C NMR characterization of maritime pine industrial tannin extract. Ind. Crops Prod. 2010, 32, 105–110. [Google Scholar] [CrossRef]
- Martínez-Richa, A.; Joseph-Nathan, P. Carbon-13 CP-MAS nuclear magnetic resonance studies of teas. Solid State Nucl. Magn. 2003, 23, 119–135. [Google Scholar] [CrossRef]
- Wawer, I.; Wolniak, M.; Paradowska, K. Solid state NMR study of dietary fiber powders from aronia, bilberry, black currant and apple. Solid State Nucl. Magn. 2006, 30, 106–113. [Google Scholar] [CrossRef]
- Pizzi, A.; Stephanou, A. A comparative C13 NMR study of polyflavonoid tannin extracts for phenolic polycondensates. J. Appl. Polym. Sci. 1993, 50, 2105–2113. [Google Scholar] [CrossRef]
- Rácz, A.; Papp, N.; Balogh, E.; Fodor, M.; Héberger, K. Comparison of antioxidant capacity assays with chemometric methods. Anal. Methods 2015, 7, 4216–4224. [Google Scholar] [CrossRef]
- Apostolou, A.; Stagos, D.; Galitsiou, E.; Spyrou, A.; Haroutounian, S.; Portesis, N.; Trizoglou, I.; Wallace Hayes, A.; Tsatsakis, A.M.; Kouretas, D. Assessment of polyphenolic content, antioxidant activity, protection against ROS-induced DNA damage and anticancer activity of Vitis vinifera stem extracts. Food Chem. Toxicol. 2013, 61, 60–68. [Google Scholar] [CrossRef]
- Sagdic, O.; Ozturk, I.; Ozkan, G.; Yetim, H.; Ekici, L.; Yilmaz, M.T. RP-HPLC–DAD analysis of phenolic compounds in pomace extracts from five grape cultivars: Evaluation of their antioxidant, antiradical and antifungal activities in orange and apple juices. Food Chem. 2011, 126, 1749–1758. [Google Scholar] [CrossRef]
- Cheng, V.J.; Bekhit, A.E.-D.A.; McConnell, M.; Mros, S.; Zhao, J. Effect of extraction solvent, waste fraction and grape variety on the antimicrobial and antioxidant activities of extracts from wine residue from cool climate. Food Chem. 2012, 134, 474–482. [Google Scholar] [CrossRef]
- Delgado Adámez, J.; Gamero Samino, E.; Valdés Sánchez, E.; González-Gómez, D. In vitro estimation of the antibacterial activity and antioxidant capacity of aqueous extracts from grape-seeds (Vitis vinifera L.). Food Control 2012, 24, 136–141. [Google Scholar] [CrossRef]
- Tesaki, S.; Tanabe, S.; Moriyama, M.; Fukushi, E.; Kawabata, J.; Watanabe, M. Isolation and Identification of an Antibacterial Compound from Grape and Its Application to Foods. J. Agric. Chem. Soc. Jpn. 1999, 73, 125–128. [Google Scholar] [CrossRef]
- Friedman, M. Antibacterial, Antiviral, and Antifungal Properties of Wines and Winery Byproducts in Relation to Their Flavonoid Content. J. Agric. Food Chem. 2014, 62, 6025–6042. [Google Scholar] [CrossRef]
- Yokozawa, T.; Chen, C.P.; Dong, E.; Tanaka, T.; Nonaka, G.-I.; Nishioka, I. Study of injibitory effect of tannins and flavanols against the 1,1-Diphenyl-2-picrylhydrazyl radical. Biochem. Pharmacol. 1998, 56, 213–222. [Google Scholar] [CrossRef]
| Phenolic Compound | μg/mg of Extract |
|---|---|
| Phenolic acids | |
| Gallic acid | 2.63 ± 0.03 |
| Ferulic acid | 6.59 ± 0.13 |
| p-Coumaric acid | 2.01 ± 0.11 |
| Caffeic acid | 5.63 ± 0.32 |
| Sum of phenolic acids | 16.9 ± 0.4 |
| Flavan-3-ols | |
| (+)-Catechin | 7.01 ± 0.02 |
| (-)-Epicatechin | 16.8 ± 1.1 |
| Epicatechin gallate † | 15.4 ± 1.5 |
| Epigallocatechin gallate | 7.89 ± 0.40 |
| Sum of flavan-3-ols | 47.1 ± 1.9 |
| Flavonols | |
| Quercetin glycoside ‡ | 7.59 ± 0.08 |
| Sum of monomeric phenolics | c. 70 (i.e., 7% by weight) |
| Antioxidant Activity | Experimental Extract | Commercial Extract |
| Folin-Ciocalteu (mg GAE/g extract) | 255 ± 3 | 258 ± 4 |
| DPPH (µmol DPPH/mg extract) | 1.15 ± 0.06 | 1.01 ± 0.06 |
| DPPH (% radical scavenged) | 83.9 ± 0.8 | 81.5 ± 0.3 |
| Antimicrobial Activity (MBC) | Experimental Extract | Commercial Extract |
| Staphylococcus aureus (% w/v) | 0.125 | 0.125 |
| Escherichia coli (% w/v) | 2.0 | N.E. |
| Candida albicans (% w/v) | N.E. | N.E. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olejar, K.J.; Ricci, A.; Swift, S.; Zujovic, Z.; Gordon, K.C.; Fedrizzi, B.; Versari, A.; Kilmartin, P.A. Characterization of an Antioxidant and Antimicrobial Extract from Cool Climate, White Grape Marc. Antioxidants 2019, 8, 232. https://doi.org/10.3390/antiox8070232
Olejar KJ, Ricci A, Swift S, Zujovic Z, Gordon KC, Fedrizzi B, Versari A, Kilmartin PA. Characterization of an Antioxidant and Antimicrobial Extract from Cool Climate, White Grape Marc. Antioxidants. 2019; 8(7):232. https://doi.org/10.3390/antiox8070232
Chicago/Turabian StyleOlejar, Kenneth J., Arianna Ricci, Simon Swift, Zoran Zujovic, Keith C. Gordon, Bruno Fedrizzi, Andrea Versari, and Paul A. Kilmartin. 2019. "Characterization of an Antioxidant and Antimicrobial Extract from Cool Climate, White Grape Marc" Antioxidants 8, no. 7: 232. https://doi.org/10.3390/antiox8070232
APA StyleOlejar, K. J., Ricci, A., Swift, S., Zujovic, Z., Gordon, K. C., Fedrizzi, B., Versari, A., & Kilmartin, P. A. (2019). Characterization of an Antioxidant and Antimicrobial Extract from Cool Climate, White Grape Marc. Antioxidants, 8(7), 232. https://doi.org/10.3390/antiox8070232









