Melatonin Prevents Oxidative Stress-Induced Mitochondrial Dysfunction and Apoptosis in High Glucose-Treated Schwann Cells via Upregulation of Bcl2, NF-κB, mTOR, Wnt Signalling Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture and Treatment
2.3. Measurement of Cell Viability
2.4. Measurement of Reactive Oxygen Species (ROS)
2.5. Measurement of Mitochondrial Trans-Membrane Potential (ΔΨm)
2.6. Measurement of Cell Apoptosis
2.7. Measurement of Protein Expression
2.8. Statistical Analysis
3. Results
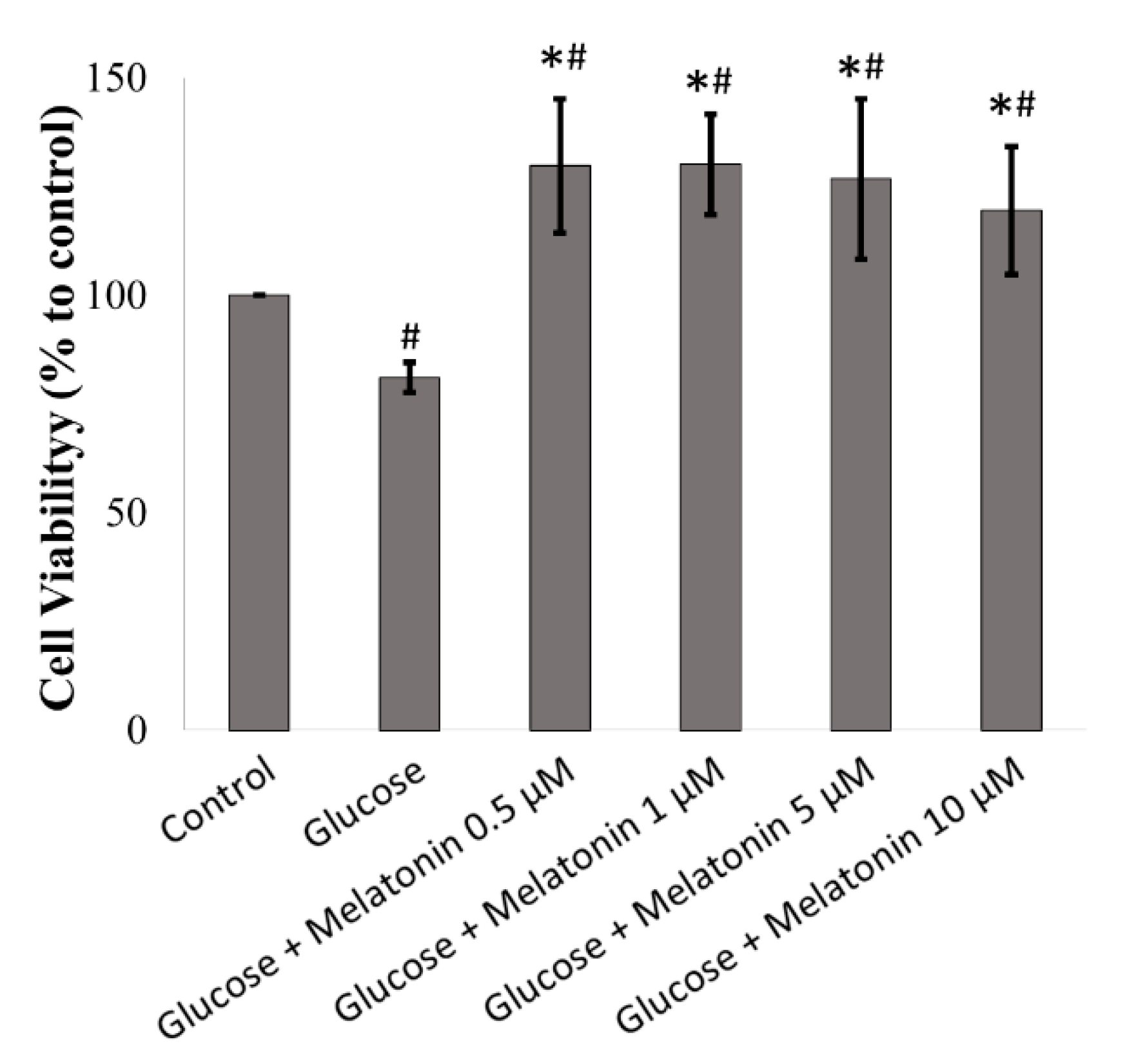
3.1. Effects of Melatonin on the Cell Viability of High Glucose-Treated Schwann Cells
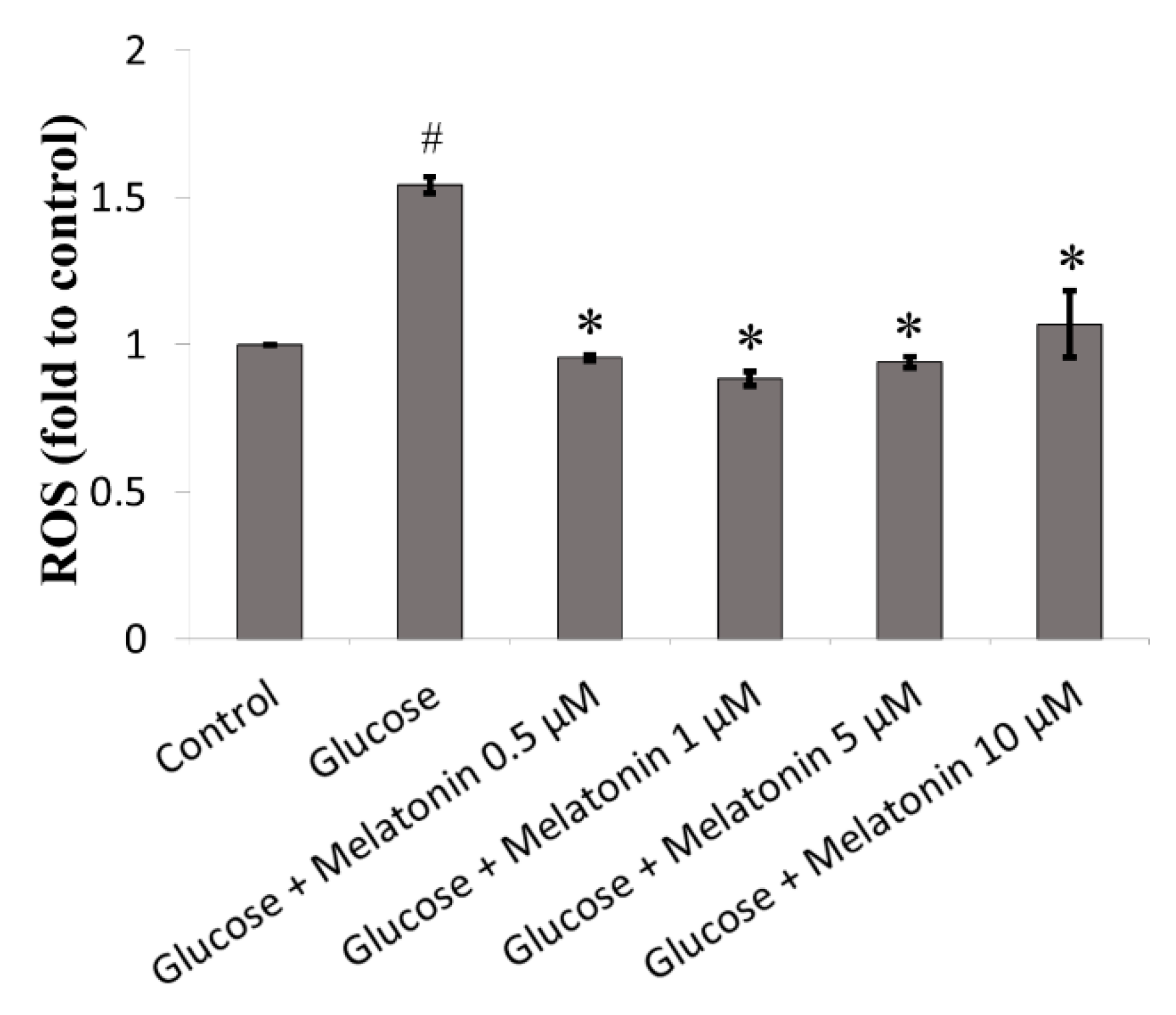
3.2. Effect of Melatonin on ROS Generation of High Glucose-Treated Schwann Cells
3.3. Effects of Melatonin on Mitochondrial Membrane Potential (ΔΨm) Changes in High Glucose-Treated Schwann Cells
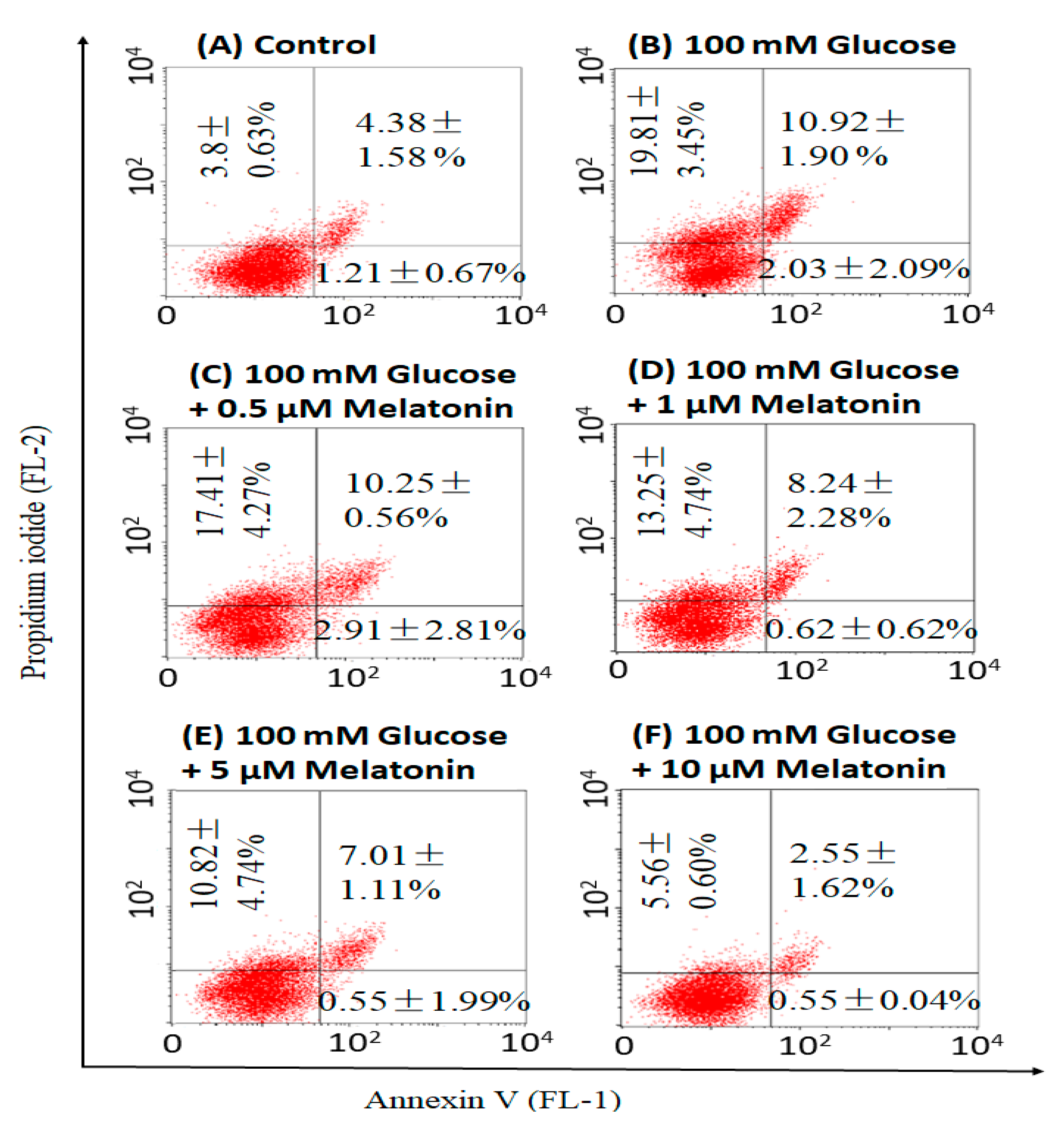
3.4. Effects of Melatonin on High Glucose-Induced Apoptosis in Schwann Cells
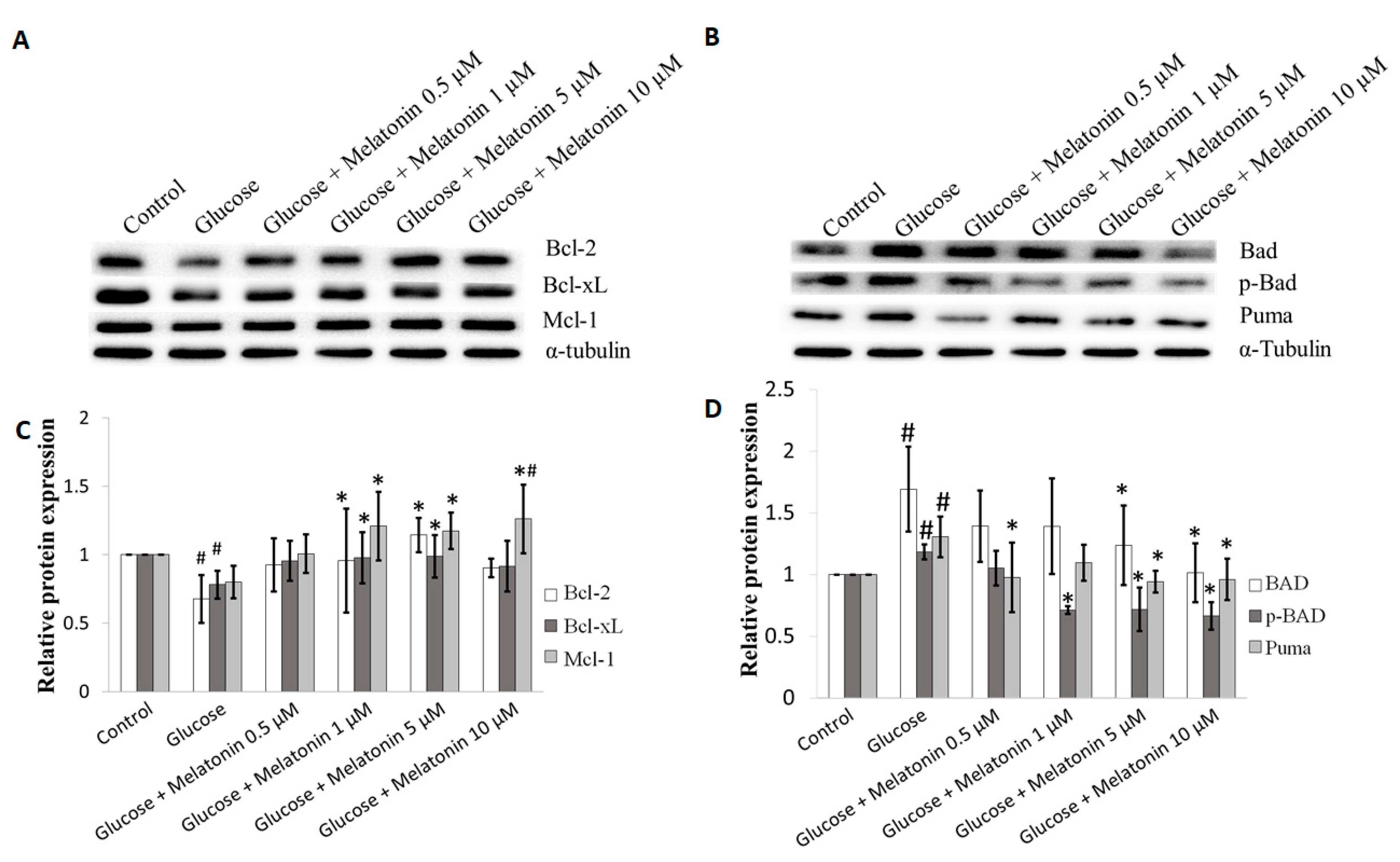
3.5. Effects of Melatonin on Bcl-2 Family Proteins Expression in High Glucose-Treated Schwann Cells
3.6. Effects of Melatonin on NF-κB Proteins Expression in High Glucose-Treated Schwann Cells
3.7. Effects of Melatonin on mTOR Family Proteins Expression in High Glucose-Treated Schwann Cells
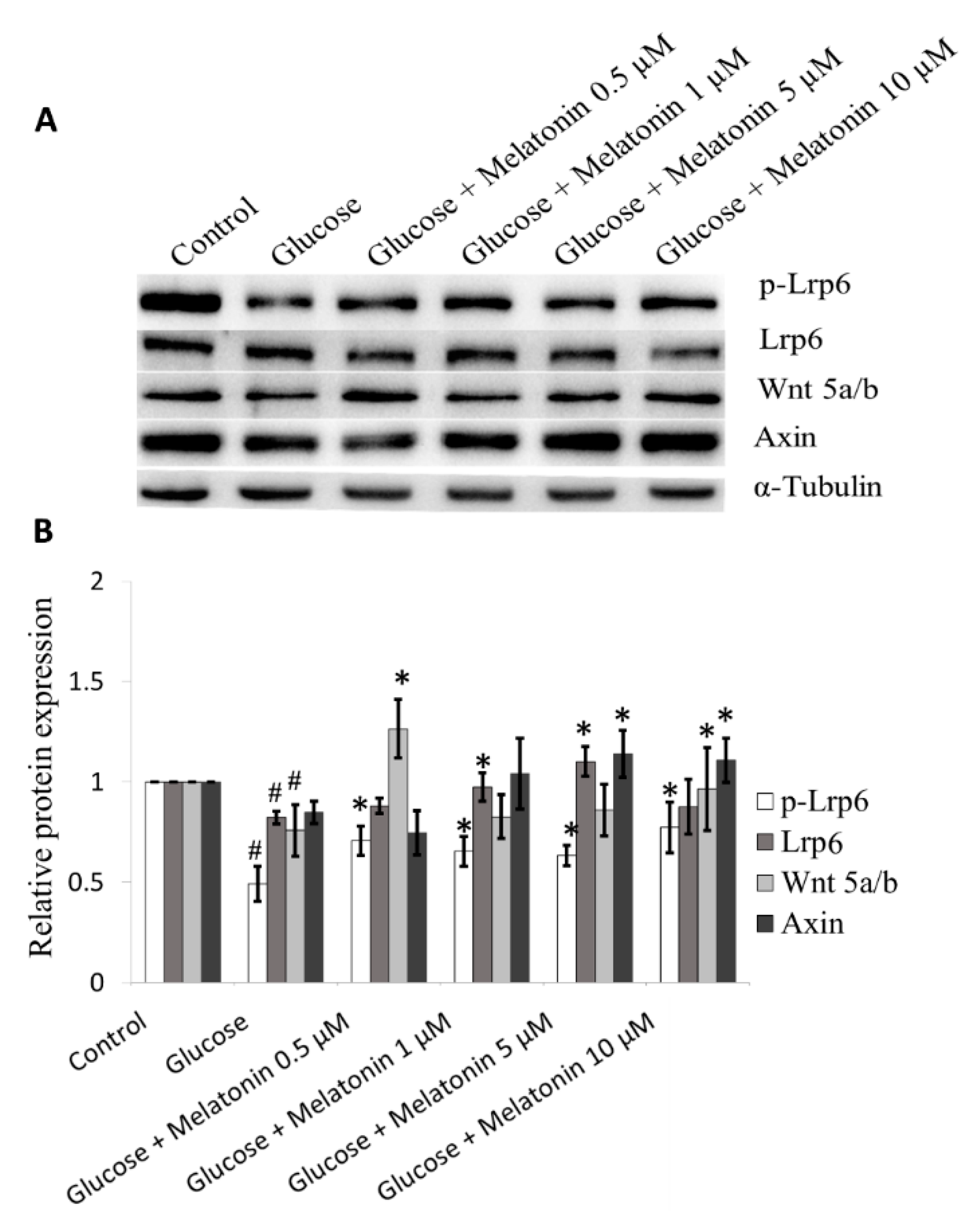
3.8. Effects of Melatonin on Wnt Family Proteins Expression in High Glucose-Treated Schwann Cells
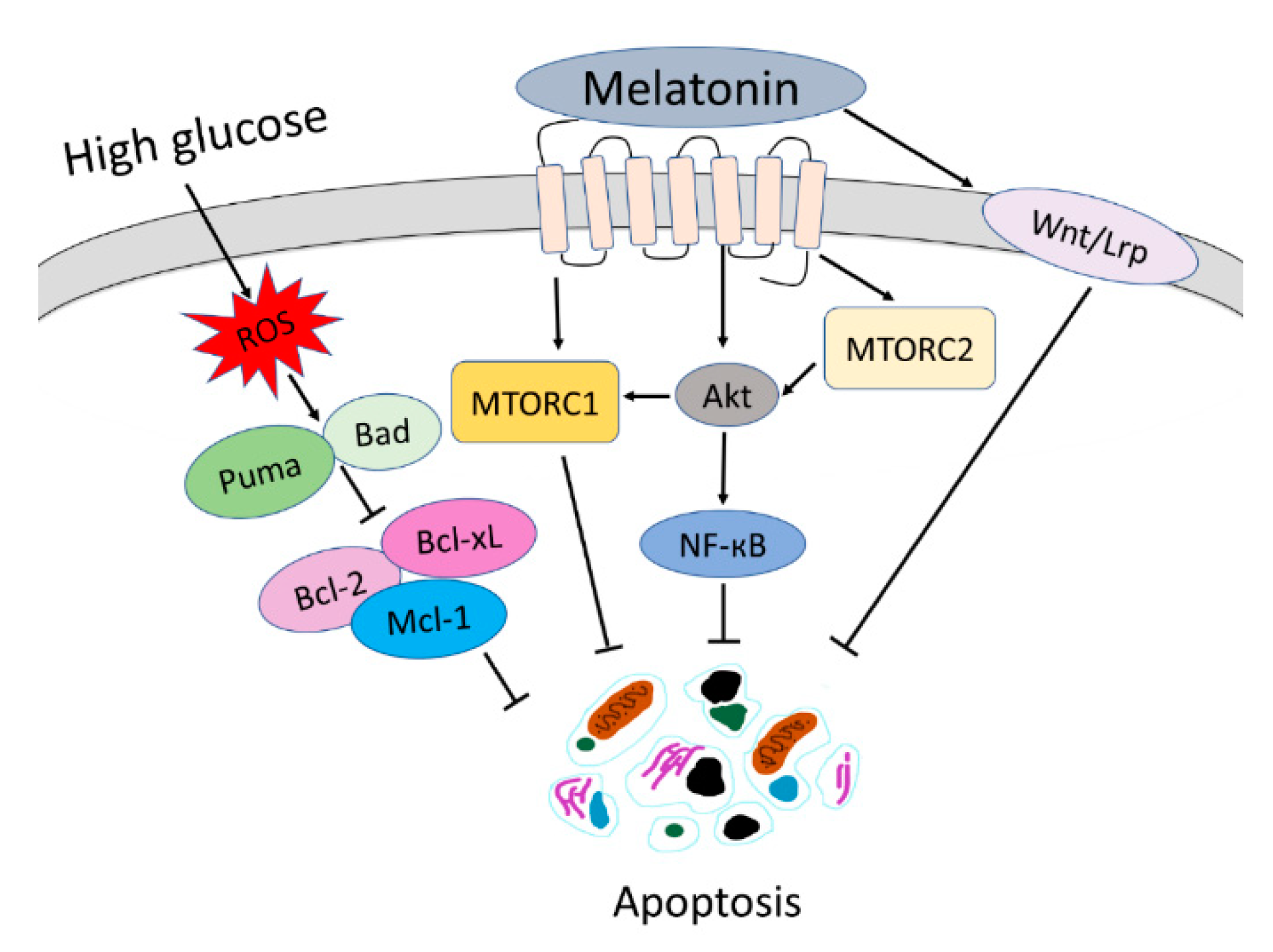
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Deli, G.; Bosnyak, E.; Pusch, A.; Komoly, S.; Feher, G. Diabetic neuropathies: Diagnosis and management. Neuroendocrinology 2013, 98, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Farmer, K.L.; Li, C.; Dobrowsky, R.T. Diabetic peripheral neuropathy: Should a chaperone accompany our therapeutic approach? Pharm. Rev. 2012, 64, 880–900. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, N.P.; Vægte, C.B.; Andersen, H.; Østergaard, L.; Calcutt, N.A.; Jensen, T.S. Schwann cell interactions with axons and microvessels in diabetic neuropathy. Neurology 2017, 13, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Assaly, R.; de Tassigny, A.D.; Paradis, S.; Jacquin, S.; Berdeau, A.; Morin, D. Oxidative stress, mitochondrial permeability transition pore opening and cell death during hypoxia-reoxygenation in adult cardiomyocytes. Eur. J. Pharmacol. 2012, 675, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Marchi, S.; Giorgi, C.; Suski, J.M.; Agnoletto, C.; Bononi, A.; Bonora, M.; De Marchi, E.; Missiroli, S.; Patergnani, S.; Poletti, F.; et al. Mitochondria-ros crosstalk in the control of cell death and aging. J. Signal Transduct. 2012, 2012, 329635. [Google Scholar] [CrossRef]
- Kuznetsov, A.V.; Margreiter, R.; Amberger, A.; Saks, V.; Grimm, M. Changes in mitochondrial redox state, membrane potential and calcium precede mitochondrial dysfunction in doxorubicin-induced cell death. Biochim. Biophys. Acta 2011, 1813, 1144–1152. [Google Scholar] [CrossRef]
- Hosseini, A.; Mohammad Abdollahi, M. Diabetic neuropathy and oxidative Stress: Therapeutic perspectives. Oxidat. Med. Cell. Longev. 2013, 2013, 168039. [Google Scholar] [CrossRef]
- Dan, H.C.; Cooper, M.J.; Cogswell, P.C.; Duncan, J.A.; Ting, J.P.; Baldwin, A.S. Akt-dependent regulation of NF-κB is controlled by mTOR and Raptor in association with IKK. Genes Dev. 2008, 22, 1490–1500. [Google Scholar] [CrossRef]
- Du, Q.; Geller, D.A. Cross-Regulation between Wnt and NF-κB Signaling Pathways. Forum Immunopathol. Dis. Ther. 2010, 1, 155–181. [Google Scholar] [CrossRef]
- Meng, X.; Li, Y.; Li, S.; Zhou, Y.; Gan, R.Y.; Xu, D.P.; Li, H.B. Dietary Sources and Bioactivities of Melatonin. Nutrients 2017, 9, 367. [Google Scholar] [CrossRef] [PubMed]
- Choi, D. Potency of melatonin in living beings. Dev. Reprod. 2013, 17, 149–177. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Dickson, E.J.; Jung, S.R.; Koh, D.S.; Hille, B. High membrane permeability for melatonin. J. Gen. Physiol. 2016, 147, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Babaei-Balderlou, F.; Zare, S.; Heidari, R.; Farrokhi, F. Effects of melatonin and vitamin E on peripheral neuropathic pain in streptozotocin-induced diabetic rats. Iran J. Basic Med. Sci. 2010, 13, 1–8. [Google Scholar]
- Brzezinski, A. Melatonin in Humans. N. Engl. J. Med. 1997, 336, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Figueroa, B.E.; Stavrovskaya, I.G.; Zhang, Y.; Sirianni, A.C.; Zhu, S.; Day, A.L.; Kristal, B.S.; Friedlander, R.M. Methazolamide and melatonin inhibit mitochondrial cytochrome C release and are neuroprotective in experimental models of ischemic injury. Stroke 2009, 40, 1877–1885. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Zhang, L.M.; Lu, S.D.; Li, X.J.; Sun, F.Y. Protective effect of melatonin on injuried cerebral neurons is associated with bcl-2 protein over-expression. Zhongguo Yao Li Xue Bao 1999, 20, 409–414. [Google Scholar] [PubMed]
- Koh, P.O. Melatonin attenuates the focal cerebral ischemic injury by inhibiting the dissociation of pBad from 14-3-3. J. Pineal Res. 2008, 44, 101–106. [Google Scholar] [CrossRef]
- Delaney, C.L.; Russell, J.W.; Cheng, H.L.; Feldman, E.L. Insulin-like growth factor-I and over-expression of Bcl-xL prevent glucose-mediated apoptosis in Schwann cells. J. Neuropathol. Exp. Neurol. 2001, 60, 147–160. [Google Scholar] [CrossRef]
- Li, Y.; Wu, H.; Liu, N.; Cao, X.; Yang, Z.; Lu, B.; Hu, R.; Wang, X.; Wen, J. Melatonin exerts an inhibitory effect on insulin gene transcription via MTNR1B and the downstream Raf-1/ERK signaling pathway. Int. J. Mol. Med. 2018, 41, 955–961. [Google Scholar] [CrossRef]
- Webster, K.A. Mitochondrial membrane permeabilization and cell death during myocardial infarction: Roles of calcium and reactive oxygen species. Future Cardiol. 2012, 8, 863–884. [Google Scholar] [CrossRef] [PubMed]
- Czabotar, P.E.; Lessene, G.; Strasser, A.; Adams, J.M. Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 2014, 15, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Mettang, M.; Reichel, S.N.; Lattke, M.; Palmer, A.; Abaei, A.; Rasche, V.; Huber-Lang, M.; Baumann, B.; Wirth, T. IKK2/NF-κB signaling protects neurons after traumatic brain injury. FASEB J. 2018, 32, 1916–1932. [Google Scholar] [CrossRef] [PubMed]
- Mincheva, S.; Garcera, A.; Gou-Fabregas, M.; Encinas, M.; Dolcet, X.; Soler, R.M. The canonical nuclear factor-κB pathway regulates cell survival in a developmental model of spinal cord motoneurons. J. Neurosci. 2011, 31, 6493–6503. [Google Scholar] [CrossRef] [PubMed]
- Imielski, Y.; Schwamborn, J.C.; Lüningschrör, P.; Heimann, P.; Holzberg, M.; Werner, H.; Leske, O.; Püschel, A.W.; Memet, S.; Heumann, R.; et al. Regrowing the adult brain: NF-κB controls functional circuit formation and tissue homeostasis in the dentate gyrus. PLoS ONE 2012, 7, e30838. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, B. Wnt/β-Catenin Signaling Pathway Governs a Full Program for Dopaminergic Neuron Survival, Neurorescue and Regeneration in the MPTP Mouse Model of Parkinson’s Disease. Int. J. Mol. Sci. 2018, 19, 3743. [Google Scholar] [CrossRef]
- Zarkou, V.; Galaras, A.; Giakountis, A.; Hatzis, P. Crosstalk mechanisms between the WNT signaling pathway and long non-coding RNAs. Noncod. RNA Res. 2018, 3, 42–53. [Google Scholar] [CrossRef]
- Victor, V.M. Mitochondria oxidative stress in diabetes. In Preedy VR. Diabetes-Oxidative Stress and Dietary Antioxidants; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 21–29. [Google Scholar]
- Asmat, U.; Abad, K.; Ismail, K. Diabetes mellitus and oxidative stress—A concise review. Saudi Pharm. J. 2016, 24, 547–553. [Google Scholar] [CrossRef]
- Volpe, C.M.O.; Villar-Delfino, P.H.; Dos Anjos, P.M.F.; Nogueira-Machado, J.A. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018, 9, 119. [Google Scholar] [CrossRef]
- Hao, W.; Tashiro, S.; Hasegawa, T.; Sato, Y.; Kobayashi, T.; Tando, T.; Katsuyama, E.; Fujie, A.; Watanabe, R.; Morita, M.; et al. Hyperglycemia promotes Schwann cell de-differentiation and de-myelination via sorbitol accumulation and Igf1 protein down-regulation. J. Biol. Chem. 2015, 290, 17106–17115. [Google Scholar] [CrossRef]
- Túnez, I.; Muñoz, M.C.; Medina, F.J.; Salcedo, M.; Feijóo, M.; Montilla, P. Comparison of melatonin, vitamin E and L-carnitine in the treatment of neuro- and hepatotoxicity induced by thioacetamide. Cell Biochem. Funct. 2007, 25, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Ramis, M.R.; Esteban, S.; Miralles, A.; Tan, D.X.; Reiter, R.J. Protective effects of melatonin and mitochondria-targeted antioxidants against oxidative Stress: A Review. Curr. Med. Chem. 2015, 22, 2690–2711. [Google Scholar] [CrossRef] [PubMed]
- Debnath, B.; Islam, W.; Min Li, M.; Sun, Y.; Lu, X.; Mitra, S.; Hussain, M.; Liu, S.; Qiu, D. Melatonin mediates enhancement of stress tolerance in plants. Int. J. Mol. Sci. 2019, 20, 1040. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.; Spence, D.W.; Pandi-Perumal, S.R.; Brown, G.M.; Cardinali, D.P. Melatonin in mitochondrial dysfunction and related disorders. Int. J. Alzheimers Dis. 2011, 2011, 326320. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.M.; Hao, H.S.; Du, W.H.; Zhao, S.J.; Wang, H.Y.; Wang, N.; Wang, D.; Liu, Y.; Qin, T.; Zhu, H.B. Melatonin inhibits apoptosis and improves the developmental potential of vitrified bovine oocytes. J. Pineal Res. 2016, 60, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Acuña Castroviejo, D.; López, L.C.; Escames, G.; López, A.; García, J.A.; Reiter, R.J. Melatonin-mitochondria interplay in health and disease. Curr. Top. Med. Chem. 2011, 11, 221–240. [Google Scholar] [CrossRef] [PubMed]
- Thorens, B.; Mueckler, M. Glucose transporters in the 21st Century. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E141–E145. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xue, B.; Li, X.; Liu, H. Puerarin prevents high glucose-induced apoptosis of Schwann cells by inhibiting oxidative stress. Neural Regen. Res. 2012, 7, 2583–2591. [Google Scholar] [PubMed]
- Li, H.; Zhang, Y.; Liu, S.; Li, F.; Wang, B.; Wang, J.; Cao, L.; Xia, T.; Yao, Q.; Chen, H.; et al. Melatonin Enhances Proliferation and Modulates Differentiation of Neural Stem Cells Via Autophagy in Hyperglycemia: Mel Protects NSCs from Autophagy in HG. Stem Cells 2019, 37, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.M.; Liu, C.H.; Hsu, W.M.; Chen, L.Y.; Wang, H.P.; Wu, T.H.; Chen, K.Y.; Ho, W.H.; Liao, W.C. Proliferative effects of melatonin on Schwann cells: Implication for nerve regeneration following peripheral nerve injury. J. Pineal Res. 2014, 56, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Ekmekcioglu, C. Melatonin receptors in humans: Biological role and clinical relevance. Biomed. Pharmacother. 2006, 60, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Uz, T.; Arslan, A.D.; Kurtuncu, M.; Imbesi, M.; Akhisaroglu, M.; Dwivedi, Y.; Pandey, G.N.; Manev, H. The regional and cellular expression profile of the melatonin receptor MT1 in the central dopaminergic system. Mol. Brain Res. 2005, 136, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Carlson, L.L.; Weaver, D.R.; Reppert, S.M. Melatonin signal transduction in hamster brain: Inhibition of adenylyl cyclase by a pertussis toxin-sensitive g protein. Endocrinology 1989, 125, 2670–2676. [Google Scholar] [CrossRef] [PubMed]
- Petit, L.; Lacroix, I.; De Coppet, P.; Strosberg, A.D.; Jockers, R. Differential signaling of human Mel1a and Mel1b melatonin receptors through the cyclic guanosine 3′-5′-monophosphate pathway. Biochem. Pharmacol. 1999, 58, 633–639. [Google Scholar] [CrossRef]
- Jockers, R.; Maurice, P.; Boutin, J.A.; Delagrange, P. Melatonin receptors, heterodimerization, signal transduction and binding sites: What’s new? Br. J. Pharmacol. 2008, 154, 1182–1195. [Google Scholar] [CrossRef] [PubMed]
- Brydon, L.; Roka, F.; Petit, L.; de Coppet, P.; Tissot, M.; Barrett, P.; Morgan, P.J.; Nanoff, C.; Strosberg, A.D.; Jockers, R. Dual signalling of human Mel 1a melatonin receptors via G(i2), g(i3) and G(q/11) proteins. Mol. Endocrinol. 1999, 13, 2025–2038. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, T.J.; Yoo, Y.M. Melatonin combined with endoplasmic reticulum stress induces cell death via the PI3K/Akt/mTOR pathway in B16F10 melanoma cells. PLoS ONE 2017, 19, e92627. [Google Scholar] [CrossRef]
- Kilic, U.; Caglayan, A.B.; Beker, M.C.; Gunal, M.Y.; Caglayan, B.; Yalcin, E.; Kelestemur, T.; Gundogdu, R.Z.; Yulug, B.; Yılmaz, B.; et al. Particular phosphorylation of PI3K/Akt on Thr308 via PDK-1 and PTEN mediates melatonin’s neuroprotective activity after focal cerebral ischemia in mice. Redox Biol. 2017, 12, 657–665. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, S. Melatonin Promotes Ubiquitination of Phosphorylated Pro-Apoptotic Protein Bcl-2-Interacting Mediator of Cell Death-Extra Long (BimEL) in Porcine Granulosa Cells. Int. J. Mol. Sci. 2018, 19, 3431. [Google Scholar] [CrossRef]
- Serasinghe, M.N.; Missert, D.J.; Asciolla, J.J.; Podgrabinska, S.; Wieder, S.Y.; Izadmehr, S.; Belbin, G.; Skobe, M.; Chipuk, J.E. Anti-apoptotic BCL-2 proteins govern cellular outcome following B-RAF(V600E) inhibition and can be targeted to reduce resistance. Oncogene 2015, 34, 857–867. [Google Scholar] [CrossRef]
- Carrington, E.M.; Zhan, Y.; Bray, J.L.; Zhang, J.G.; Sutherland, R.M.; Anstee, N.S.; Schenk, R.L.; Vikstrom, I.B.; Delconte, R.B.; Segal, D.; et al. Anti-apoptotic proteins BCL-2, MC-1 and A1 summate collectively to maintain survival of immune cell populations both in vitro and in vivo. Cell Death Differ. 2017, 24, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Ruland, J. Return to homeostasis: Downregulation of NF-κB responses. Nat. Immunol. 2011, 12, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Mariani, E.; Polidori, M.C.; Cherubini, A.; Mecocci, P. Oxidative stress in brain aging, neurodegenerative and vascular diseases: An overview. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2005, 827, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Sompol, P.; Xu, Y.; Ittarat, W.; Daosukho, C.; St Clair, D. NF-kappaB-associated MnSOD induction protects against beta-amyloid-induced neuronal apoptosis. J. Mol. Neurosci. 2006, 29, 279–288. [Google Scholar] [CrossRef]
- Ji, L.L.; Gomez-Cabrera, M.C.; Vina, J. Role of nuclear factor kappaB and mitogen-activated protein kinase signaling in exercise-induced antioxidant enzyme adaptation. Appl. Physiol. Nutr. Metab. 2007, 32, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Engelman, J.A. Targeting PI3K signalling in cancer: Opportunities, challenges and limitations. Nat. Rev. Cancer 2009, 9, 550–562. [Google Scholar] [CrossRef] [PubMed]
- Schmelzle, T.; Hall, M.N. mTOR, a central controller of cell growth. Cell 2000, 103, 253–262. [Google Scholar] [CrossRef]
- Selvaraj, S.; Sun, Y.; Sukumaran, P.; Singh, B.B. Resveratrol activates autophagic cell death in prostate cancer cells via downregulation of STIM1 and the mTOR pathway. Mol. Carcinog. 2016, 55, 818–831. [Google Scholar] [CrossRef]
- Geuna, E.; Roda, D.; Rafii, S.; Jimenez, B.; Capelan, M.; Rihawi, K.; Montemurro, F.; Yap, T.A.; Kaye, S.B.; De Bono, J.S.; et al. Complications of hyperglycemia with PI3K-AKT-mTOR inhibitors in patients with advanced solid tumours on Phase 1 clinical trials. Br. J. Cancer 2015, 113, 1541–1547. [Google Scholar] [CrossRef][Green Version]
- Crouthamel, M.C.; Kahana, J.A.; Korenchuk, S.; Zhang, S.Y.; Sundaresan, G.; Eberwein, D.J.; Brown, K.K.; Kumar, R. Mechanism and management of AKT inhibitor-induce hyperglycemia. Clin. Cancer Res. 2009, 15, 217–225. [Google Scholar] [CrossRef]
- Zhu, L.; Hao, J.; Cheng, M.; Zhang, C.; Huo, C.; Liu, Y.; Du, W.; Zhang, X. Hyperglycemia-induced Bcl-Bax-mediated apoptosis of Schwann cells via mTORC1/S6K1 inhibition in diabetic peripheral neuropathy. Exp. Cell Res. 2018, 367, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Koh, P.O. Melatonin prevent ischemic brain injury through activation of the mTOR/p70S6 kinase signaling pathway. Neurosci. Lett. 2008, 444, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.L.; Wang, J.Y.; Huang, Y.T.; Kuo, Y.H.; Surendran, K.; Wang, F.S. Wnt/β-catenin signaling modulates survival of high glucose-stressed mesangial cells. Pathophysiol. Ren. Dis. Prog. 2006, 17, 2812–2820. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Choi, M.Y.; Yu, J.; Castro, J.E.; Kipps, T.J.; Carson, D.A. Salinomycin inhibits Wnt signalig and selectively induces apoptosis in chronic lymphocytic leukaemia cells. Proc. Natl. Acad. Sci. USA 2011, 108, 13253–13257. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Hur, M.W.; Lee, S.K.; Choi, W.L.; Kwon, Y.G.; Yun, C.O. A novel sLRP6E1E2 inhibits conical Wnt signaling, epithelial-to-mesenchymal transition, and induces mitochondria-dependent apoptosis in lung cancer. PLoS ONE 2012, 7, e36520. [Google Scholar]
- Grigoryan, T.; Stein, S.; Qi, J.; Wende, H.; Garrratt, A.N.; Nave, K.A.; Birchmeier, C.; Birchmeier, W. Wnt/Rspondin/β-catenin signals control axonal sorting and lineage progression in Schwann cell development. Proc. Natl. Acad. Sci. USA 2013, 110, 18174–18179. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Zhou, Z.; Gao, S.; Guo, Y.; Gao, K.; Wang, H.; Dang, X. Melatonin inhibits neural cell apoptosis and promotes locomotor recovery via activation of Wnt/β-catenin signaling pathway after spinal cord injury. Neurochem. Res. 2017, 42, 2336–2343. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiong, Y.L.; Ng, K.Y.; Koh, R.Y.; Ponnudurai, G.; Chye, S.M. Melatonin Prevents Oxidative Stress-Induced Mitochondrial Dysfunction and Apoptosis in High Glucose-Treated Schwann Cells via Upregulation of Bcl2, NF-κB, mTOR, Wnt Signalling Pathways. Antioxidants 2019, 8, 198. https://doi.org/10.3390/antiox8070198
Tiong YL, Ng KY, Koh RY, Ponnudurai G, Chye SM. Melatonin Prevents Oxidative Stress-Induced Mitochondrial Dysfunction and Apoptosis in High Glucose-Treated Schwann Cells via Upregulation of Bcl2, NF-κB, mTOR, Wnt Signalling Pathways. Antioxidants. 2019; 8(7):198. https://doi.org/10.3390/antiox8070198
Chicago/Turabian StyleTiong, Yee Lian, Khuen Yen Ng, Rhun Yian Koh, Gnanajothy Ponnudurai, and Soi Moi Chye. 2019. "Melatonin Prevents Oxidative Stress-Induced Mitochondrial Dysfunction and Apoptosis in High Glucose-Treated Schwann Cells via Upregulation of Bcl2, NF-κB, mTOR, Wnt Signalling Pathways" Antioxidants 8, no. 7: 198. https://doi.org/10.3390/antiox8070198
APA StyleTiong, Y. L., Ng, K. Y., Koh, R. Y., Ponnudurai, G., & Chye, S. M. (2019). Melatonin Prevents Oxidative Stress-Induced Mitochondrial Dysfunction and Apoptosis in High Glucose-Treated Schwann Cells via Upregulation of Bcl2, NF-κB, mTOR, Wnt Signalling Pathways. Antioxidants, 8(7), 198. https://doi.org/10.3390/antiox8070198



