Targeting Heme Oxygenase-1 in Cardiovascular and Kidney Disease
Abstract
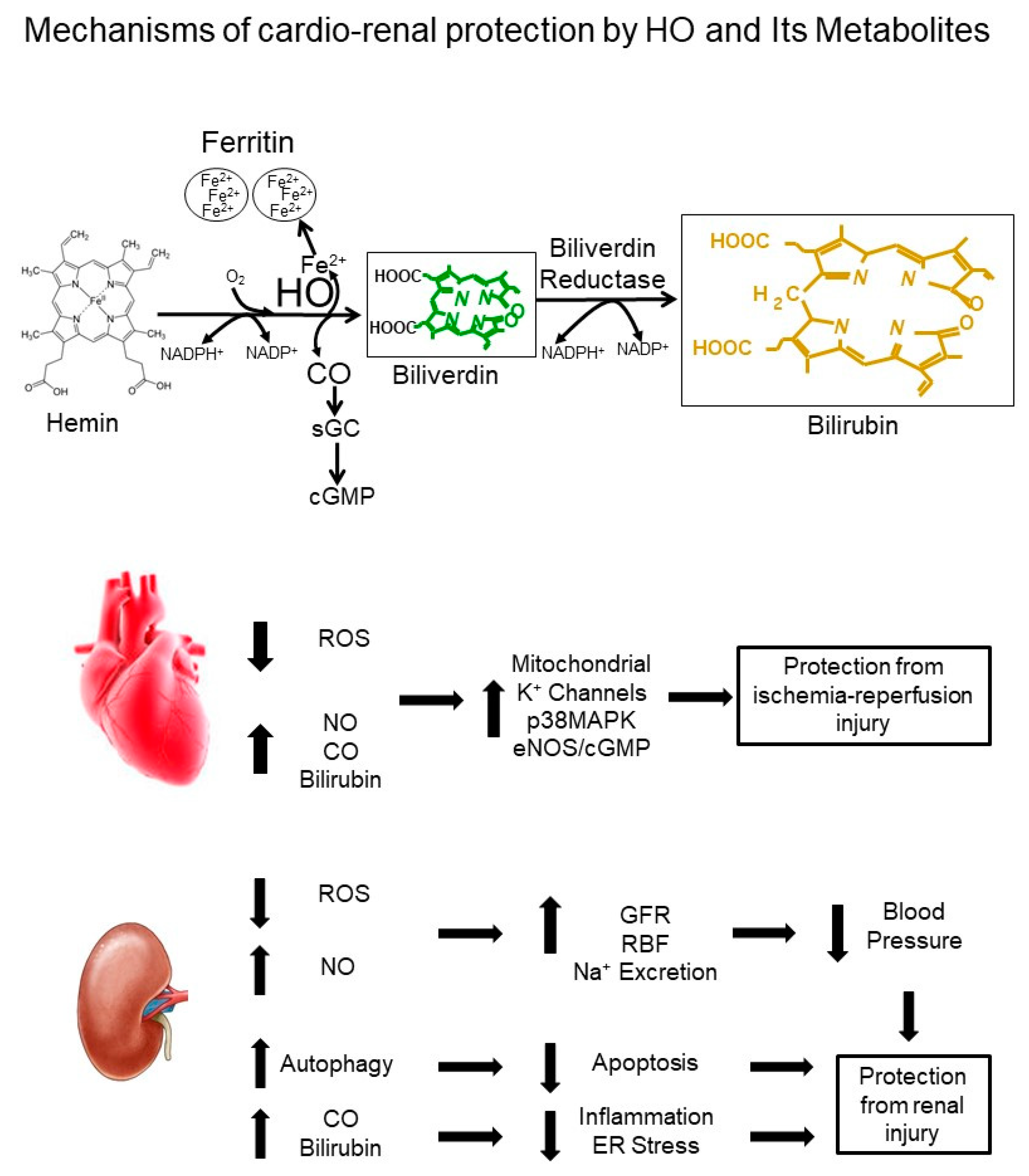
1. Introduction
2. HO and the Cardiovascular System
2.1. Role of Heme Oxygenase in the Regulation of Blood Pressure
2.2. Role of Heme Oxygenase in the Regulation of Renal Function
3. HO and Target Organ Injury
3.1. HO and the Heart
3.2. HO and the Kidney
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abraham, N.G.; Kappas, A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol. Rev. 2008, 60, 79–127. [Google Scholar] [CrossRef]
- McCoubrey, W.K., Jr.; Maines, M.D. The structure, organization and differential expression of the gene encoding rat heme oxygenase-2. Gene 1994, 139, 155–161. [Google Scholar] [CrossRef]
- Stocker, R.; Yamamoto, Y.; McDonagh, A.F.; Glazer, A.N.; Ames, B.N. Bilirubin is an antioxidant of possible physiological importance. Science 1987, 235, 1043–1046. [Google Scholar] [CrossRef]
- Stocker, R.; Peterhans, E. Antioxidant properties of conjugated bilirubin and biliverdin: Biologically relevant scavenging of hypochlorous acid. Free Radic. Res. Commun. 1989, 6, 57–66. [Google Scholar] [CrossRef]
- Lanone, S.; Bloc, S.; Foresti, R.; Almolki, A.; Taille, C.; Callebert, J.; Conti, M.; Goven, D.; Aubier, M.; Dureuil, B.; et al. Bilirubin decreases nos2 expression via inhibition of NAD(P)H oxidase: Implications for protection against endotoxic shock in rats. FASEB J. 2005, 19, 1890–1892. [Google Scholar] [CrossRef]
- Sundararaghavan, V.L.; Binepal, S.; Stec, D.E.; Sindhwani, P.; Hinds, T.D., Jr. Bilirubin, a new therapeutic for kidney transplant? Transplant. Rev. (Orlando) 2018, 32, 234–240. [Google Scholar] [CrossRef]
- Hinds, T.D., Jr.; Stec, D.E. Bilirubin, a Cardiometabolic Signaling Molecule. Hypertension 2018, 72, 788–795. [Google Scholar] [CrossRef]
- Cebova, M.; Kosutova, M.; Pechanova, O. Cardiovascular effects of gasotransmitter donors. Physiol. Res. 2016, 65, S291–S307. [Google Scholar]
- Motterlini, R.; Foresti, R. Biological signaling by carbon monoxide and carbon monoxide-releasing molecules. Am. J. Physiol. Cell Physiol. 2017, 312, C302–C313. [Google Scholar] [CrossRef]
- Scindia, Y.; Leeds, J.; Swaminathan, S. Iron Homeostasis in Healthy Kidney and its Role in Acute Kidney Injury. Semin. Nephrol. 2019, 39, 76–84. [Google Scholar] [CrossRef]
- Koorts, A.M.; Viljoen, M. Ferritin and ferritin isoforms I: Structure-function relationships, synthesis, degradation and secretion. Arch. Physiol. Biochem. 2007, 113, 30–54. [Google Scholar] [CrossRef]
- Abraham, N.G.; Kappas, A. Heme oxygenase and the cardiovascular-renal system. Free Radic. Biol. Med. 2005, 39, 1–25. [Google Scholar] [CrossRef]
- Hosick, P.A.; Stec, D.E. Heme oxygenase, a novel target for the treatment of hypertension and obesity? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R207–R214. [Google Scholar] [CrossRef]
- Raffaele, M.; Li Volti, G.; Barbagallo, I.A.; Vanella, L. Therapeutic Efficacy of Stem Cells Transplantation in Diabetes: Role of Heme Oxygenase. Front. Cell Dev. Biol. 2016, 4, 80. [Google Scholar] [CrossRef]
- Sacerdoti, D.; Escalante, B.; Abraham, N.G.; McGiff, J.C.; Levere, R.D.; Schwartzman, M.L. Treatment with tin prevents the development of hypertension in spontaneously hypertensive rats. Science 1989, 243, 388–390. [Google Scholar] [CrossRef]
- Levere, R.D.; Martasek, P.; Escalante, B.; Schwartzman, M.L.; Abraham, N.G. Effect of heme arginate administration on blood pressure in spontaneously hypertensive rats. J. Clin. Investig. 1990, 86, 213–219. [Google Scholar] [CrossRef]
- Martasek, P.; Schwartzman, M.L.; Goodman, A.I.; Solangi, K.B.; Levere, R.D.; Abraham, N.G. Hemin and L-arginine regulation of blood pressure in spontaneous hypertensive rats. J. Am. Soc. Nephrol. 1991, 2, 1078–1084. [Google Scholar]
- da Silva, J.L.; Tiefenthaler, M.; Park, E.; Escalante, B.; Schwartzman, M.L.; Levere, R.D.; Abraham, N.G. Tin-mediated heme oxygenase gene activation and cytochrome P450 arachidonate hydroxylase inhibition in spontaneously hypertensive rats. Am. J. Med. Sci. 1994, 307, 173–181. [Google Scholar] [CrossRef]
- Sabaawy, H.E.; Zhang, F.; Nguyen, X.; ElHosseiny, A.; Nasjletti, A.; Schwartzman, M.; Dennery, P.; Kappas, A.; Abraham, N.G. Human heme oxygenase-1 gene transfer lowers blood pressure and promotes growth in spontaneously hypertensive rats. Hypertension 2001, 38, 210–215. [Google Scholar] [CrossRef]
- Goodman, A.I.; Quan, S.; Yang, L.; Synghal, A.; Abraham, N.G. Functional expression of human heme oxygenase-1 gene in renal structure of spontaneously hypertensive rats. Exp. Biol. Med. (Maywood) 2003, 228, 454–458. [Google Scholar] [CrossRef]
- Botros, F.T.; Schwartzman, M.L.; Stier, C.T., Jr.; Goodman, A.I.; Abraham, N.G. Increase in heme oxygenase-1 levels ameliorates renovascular hypertension. Kidney Int. 2005, 68, 2745–2755. [Google Scholar] [CrossRef]
- Vera, T.; Yanes, L.; Reckelhoff, J.F.; Stec, D.E. Heme Oxygenase-1 Induction Prevents the Increase in Oxidative Stress in the Kidney of Angiotensin II Hypertensive Mice. Hypertension 2005, 46, 858A. [Google Scholar]
- Jadhav, A.; Torlakovic, E.; Ndisang, J.F. Hemin therapy attenuates kidney injury in deoxycorticosterone acetate-salt hypertensive rats. Am. J. Physiol. Ren. Physiol. 2009, 296, F521–F534. [Google Scholar] [CrossRef][Green Version]
- Jadhav, A.; Ndisang, J.F. Heme arginate suppresses cardiac lesions and hypertrophy in deoxycorticosterone acetate-salt hypertension. Exp. Biol. Med. (Maywood) 2009, 234, 764–778. [Google Scholar] [CrossRef]
- Nath, K.A.; d’Uscio, L.V.; Juncos, J.P.; Croatt, A.J.; Manriquez, M.C.; Pittock, S.T.; Katusic, Z.S. An analysis of the DOCA-salt model of hypertension in HO-1-/- mice and the Gunn rat. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H333–H342. [Google Scholar] [CrossRef]
- Yang, L.; Quan, S.; Nasjletti, A.; Laniado-Schwartzman, M.; Abraham, N.G. Heme oxygenase-1 gene expression modulates angiotensin II-induced increase in blood pressure. Hypertension 2004, 43, 1221–1226. [Google Scholar] [CrossRef]
- Vera, T.; Kelsen, S.; Stec, D.E. Kidney-specific induction of heme oxygenase-1 prevents angiotensin II hypertension. Hypertension 2008, 52, 660–665. [Google Scholar] [CrossRef]
- Stec, D.E.; Drummond, H.A.; Gousette, M.U.; Storm, M.V.; Abraham, N.G.; Csongradi, E. Expression of heme oxygenase-1 in thick ascending loop of henle attenuates angiotensin II-dependent hypertension. J. Am. Soc. Nephrol. 2012, 23, 834–841. [Google Scholar] [CrossRef]
- Vera, T.; Kelsen, S.; Yanes, L.L.; Reckelhoff, J.F.; Stec, D.E. HO-1 induction lowers blood pressure and superoxide production in the renal medulla of angiotensin II hypertensive mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R1472–R1478. [Google Scholar] [CrossRef]
- Stec, D.E.; Juncos, L.A.; Granger, J.P. Renal intramedullary infusion of tempol normalizes the blood pressure response to intrarenal blockade of heme oxygenase-1 in angiotensin II-dependent hypertension. J. Am. Soc. Hypertens. 2016, 10, 346–351. [Google Scholar] [CrossRef][Green Version]
- Quan, S.; Yang, L.; Shnouda, S.; Schwartzman, M.L.; Nasjletti, A.; Goodman, A.I.; Abraham, N.G. Expression of human heme oxygenase-1 in the thick ascending limb attenuates angiotensin II-mediated increase in oxidative injury. Kidney Int. 2004, 65, 1628–1639. [Google Scholar] [CrossRef]
- Kelsen, S.; Patel, B.J.; Parker, L.B.; Vera, T.; Rimoldi, J.M.; Gadepalli, R.S.; Drummond, H.A.; Stec, D.E. Heme oxygenase attenuates angiotensin II-mediated superoxide production in cultured mouse thick ascending loop of Henle cells. Am. J. Physiol. Ren. Physiol. 2008, 295, F1158–F1165. [Google Scholar] [CrossRef]
- Li, N.; Yi, F.; Dos Santos, E.A.; Donley, D.K.; Li, P.L. Role of renal medullary heme oxygenase in the regulation of pressure natriuresis and arterial blood pressure. Hypertension 2007, 49, 148–154. [Google Scholar] [CrossRef]
- Stec, D.E.; Vera, T.; Storm, M.V.; McLemore, G.R., Jr.; Ryan, M.J. Blood pressure and renal blow flow responses in heme oxygenase-2 knockout mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R1822–R1828. [Google Scholar] [CrossRef]
- Stout, J.M.; Gousset, M.U.; Drummond, H.A.; Gray, W., 3rd; Pruett, B.E.; Stec, D.E. Sex-specific effects of heme oxygenase-2 deficiency on renovascular hypertension. J. Am. Soc. Hypertens. 2013, 7, 328–335. [Google Scholar] [CrossRef]
- Li Volti, G.; Sacerdoti, D.; Di Giacomo, C.; Barcellona, M.L.; Scacco, A.; Murabito, P.; Biondi, A.; Basile, F.; Gazzolo, D.; Abella, R.; et al. Natural heme oxygenase-1 inducers in hepatobiliary function. World J. Gastroenterol. 2008, 14, 6122–6132. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, W.; Li, M.; Ren, H.; Chen, C.; Wang, J.; Wang, W.E.; Yang, J.; Zeng, C. Curcumin Exerts its Anti-hypertensive Effect by Down-regulating the AT1 Receptor in Vascular Smooth Muscle Cells. Sci. Rep. 2016, 6, 25579. [Google Scholar] [CrossRef]
- Lan, C.; Chen, X.; Zhang, Y.; Wang, W.; Wang, W.E.; Liu, Y.; Cai, Y.; Ren, H.; Zheng, S.; Zhou, L.; et al. Curcumin prevents strokes in stroke-prone spontaneously hypertensive rats by improving vascular endothelial function. BMC Cardiovasc. Disord. 2018, 18, 43. [Google Scholar] [CrossRef]
- Campbell, N.K.; Fitzgerald, H.K.; Malara, A.; Hambly, R.; Sweeney, C.M.; Kirby, B.; Fletcher, J.M.; Dunne, A. Naturally derived Heme-Oxygenase 1 inducers attenuate inflammatory responses in human dendritic cells and T cells: Relevance for psoriasis treatment. Sci. Rep. 2018, 8, 10287. [Google Scholar] [CrossRef]
- Maaliki, D.; Shaito, A.A.; Pintus, G.; El-Yazbi, A.; Eid, A.H. Flavonoids in hypertension: A brief review of the underlying mechanisms. Curr. Opin. Pharmacol. 2019, 45, 57–65. [Google Scholar] [CrossRef]
- Cho, B.O.; Yin, H.H.; Park, S.H.; Byun, E.B.; Ha, H.Y.; Jang, S.I. Anti-inflammatory activity of myricetin from Diospyros lotus through suppression of NF-kappaB and STAT1 activation and Nrf2-mediated HO-1 induction in lipopolysaccharide-stimulated RAW264.7 macrophages. Biosci. Biotechnol. Biochem. 2016, 80, 1520–1530. [Google Scholar] [CrossRef]
- Jin, L.; Beswick, R.A.; Yamamoto, T.; Palmer, T.; Taylor, T.A.; Pollock, J.S.; Pollock, D.M.; Brands, M.W.; Webb, R.C. Increased reactive oxygen species contributes to kidney injury in mineralocorticoid hypertensive rats. J. Physiol. Pharmacol. 2006, 57, 343–357. [Google Scholar]
- Taylor, N.E.; Glocka, P.; Liang, M.; Cowley, A.W., Jr. NADPH oxidase in the renal medulla causes oxidative stress and contributes to salt-sensitive hypertension in Dahl S rats. Hypertension 2006, 47, 692–698. [Google Scholar] [CrossRef]
- Wang, R.; Shamloul, R.; Wang, X.; Meng, Q.; Wu, L. Sustained normalization of high blood pressure in spontaneously hypertensive rats by implanted hemin pump. Hypertension 2006, 48, 685–692. [Google Scholar] [CrossRef]
- Zou, A.P.; Billington, H.; Su, N.; Cowley, A.W., Jr. Expression and actions of heme oxygenase in the renal medulla of rats. Hypertension 2000, 35, 342–347. [Google Scholar] [CrossRef]
- Hu, Y.; Ma, N.; Yang, M.; Semba, R. Expression and distribution of heme oxygenase-2 mRNA and protein in rat kidney. J. Histochem. Cytochem. 1998, 46, 249–256. [Google Scholar] [CrossRef]
- Johnson, R.A.; Lavesa, M.; Askari, B.; Abraham, N.G.; Nasjletti, A. A heme oxygenase product, presumably carbon monoxide, mediates a vasodepressor function in rats. Hypertension 1995, 25, 166–169. [Google Scholar] [CrossRef]
- Kozma, F.; Johnson, R.A.; Nasjletti, A. Role of carbon monoxide in heme-induced vasodilation. Eur. J. Pharmacol. 1997, 323, R1–R2. [Google Scholar] [CrossRef]
- Kaide, J.I.; Zhang, F.; Wei, Y.; Jiang, H.; Yu, C.; Wang, W.H.; Balazy, M.; Abraham, N.G.; Nasjletti, A. Carbon monoxide of vascular origin attenuates the sensitivity of renal arterial vessels to vasoconstrictors. J. Clin. Investig. 2001, 107, 1163–1171. [Google Scholar] [CrossRef]
- Zhang, F.; Kaide, J.; Wei, Y.; Jiang, H.; Yu, C.; Balazy, M.; Abraham, N.G.; Wang, W.; Nasjletti, A. Carbon monoxide produced by isolated arterioles attenuates pressure-induced vasoconstriction. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, H350–H358. [Google Scholar] [CrossRef]
- Wang, H.; Garvin, J.L.; D’Ambrosio, M.A.; Falck, J.R.; Leung, P.; Liu, R.; Ren, Y.; Carretero, O.A. Heme oxygenase metabolites inhibit tubuloglomerular feedback in vivo. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H1320–H1326. [Google Scholar] [CrossRef] [PubMed]
- Lamon, B.D.; Zhang, F.F.; Puri, N.; Brodsky, S.V.; Goligorsky, M.S.; Nasjletti, A. Dual pathways of carbon monoxide-mediated vasoregulation: Modulation by redox mechanisms. Circ. Res. 2009, 105, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, F.; Lamon, B.D.; Gong, W.; Kemp, R.; Nasjletti, A. Nitric oxide synthesis inhibition promotes renal production of carbon monoxide. Hypertension 2004, 43, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, F.; Zhang, F.; Dinocca, S.; Nasjletti, A. Nitric oxide synthesis influences the renal vascular response to heme oxygenase inhibition. Am. J. Physiol. Ren. Physiol. 2003, 284, F1255–F1262. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Morita, T.; Shindo, T.; Nagai, R.; Yazaki, Y.; Kurihara, H.; Suematsu, M.; Katayama, S. Vascular smooth muscle cell-directed overexpression of heme oxygenase-1 elevates blood pressure through attenuation of nitric oxide-induced vasodilation in mice. Circ. Res. 2001, 89, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Vera, T.; Granger, J.P.; Stec, D.E. Inhibition of bilirubin metabolism induces moderate hyperbilirubinemia and attenuates ANG II-dependent hypertension in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R738–R743. [Google Scholar] [CrossRef] [PubMed]
- Vera, T.; Stec, D.E. Moderate hyperbilirubinemia improves renal hemodynamics in ANG II-dependent hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R1044–R1049. [Google Scholar] [CrossRef] [PubMed]
- Stec, D.E.; Hosick, P.A.; Granger, J.P. Bilirubin, renal hemodynamics, and blood pressure. Front. Pharmacol. 2012, 3, 18. [Google Scholar] [CrossRef]
- Mazzone, G.L.; Rigato, I.; Tiribelli, C. Unconjugated bilirubin modulates nitric oxide production via iNOS regulation. Biosci. Trends 2010, 4, 244–248. [Google Scholar]
- Fujii, M.; Inoguchi, T.; Sasaki, S.; Maeda, Y.; Zheng, J.; Kobayashi, K.; Takayanagi, R. Bilirubin and biliverdin protect rodents against diabetic nephropathy by downregulating NAD(P)H oxidase. Kidney Int. 2010, 78, 905–919. [Google Scholar] [CrossRef]
- Kwak, J.Y.; Takeshige, K.; Cheung, B.S.; Minakami, S. Bilirubin inhibits the activation of superoxide-producing NADPH oxidase in a neutrophil cell-free system. Biochim. Biophys. Acta 1991, 1076, 369–373. [Google Scholar] [CrossRef]
- Stocker, R.; Glazer, A.N.; Ames, B.N. Antioxidant activity of albumin-bound bilirubin. Proc. Natl. Acad. Sci. USA 1987, 84, 5918–5922. [Google Scholar] [CrossRef]
- Guyton, A.C. Roles of the kidneys and fluid volumes in arterial pressure regulation and hypertension. Chin. J. Physiol. 1989, 32, 49–57. [Google Scholar]
- Guyton, A.C. The surprising kidney-fluid mechanism for pressure control—Its infinite gain! Hypertension 1990, 16, 725–730. [Google Scholar] [CrossRef]
- Botros, F.T.; Dobrowolski, L.; Navar, L.G. Renal heme oxygenase-1 induction with hemin augments renal hemodynamics, renal autoregulation, and excretory function. Int. J. Hypertens. 2012, 2012, 189512. [Google Scholar] [CrossRef]
- Rodriguez, F.; Kemp, R.; Balazy, M.; Nasjletti, A. Effects of exogenous heme on renal function: Role of heme oxygenase and cyclooxygenase. Hypertension 2003, 42, 680–684. [Google Scholar] [CrossRef]
- Liu, H.; Mount, D.B.; Nasjletti, A.; Wang, W. Carbon monoxide stimulates the apical 70-pS K+ channel of the rat thick ascending limb. J. Clin. Investig. 1999, 103, 963–970. [Google Scholar] [CrossRef]
- Wang, S.; Publicover, S.; Gu, Y. An oxygen-sensitive mechanism in regulation of epithelial sodium channel. Proc. Natl. Acad. Sci. USA 2009, 106, 2957–2962. [Google Scholar] [CrossRef]
- Tongers, J.; Fiedler, B.; Konig, D.; Kempf, T.; Klein, G.; Heineke, J.; Kraft, T.; Gambaryan, S.; Lohmann, S.M.; Drexler, H.; et al. Heme oxygenase-1 inhibition of MAP kinases, calcineurin/NFAT signaling, and hypertrophy in cardiac myocytes. Cardiovasc. Res. 2004, 63, 545–552. [Google Scholar] [CrossRef]
- Hu, C.M.; Chen, Y.H.; Chiang, M.T.; Chau, L.Y. Heme oxygenase-1 inhibits angiotensin II-induced cardiac hypertrophy in vitro and in vivo. Circulation 2004, 110, 309–316. [Google Scholar] [CrossRef]
- Foo, R.S.; Siow, R.C.; Brown, M.J.; Bennett, M.R. Heme oxygenase-1 gene transfer inhibits angiotensin II-mediated rat cardiac myocyte apoptosis but not hypertrophy. J. Cell. Physiol. 2006, 209, 1–7. [Google Scholar] [CrossRef]
- Kobayashi, A.; Ishikawa, K.; Matsumoto, H.; Kimura, S.; Kamiyama, Y.; Maruyama, Y. Synergetic antioxidant and vasodilatory action of carbon monoxide in angiotensin II—induced cardiac hypertrophy. Hypertension 2007, 50, 1040–1048. [Google Scholar] [CrossRef]
- Raju, V.S.; Maines, M.D. Renal ischemia/reperfusion up-regulates heme oxygenase-1 (HSP32) expression and increases cGMP in rat heart. J. Pharmacol. Exp. Ther. 1996, 277, 1814–1822. [Google Scholar]
- Csonka, C.; Varga, E.; Kovacs, P.; Ferdinandy, P.; Blasig, I.E.; Szilvassy, Z.; Tosaki, A. Heme oxygenase and cardiac function in ischemic/reperfused rat hearts. Free Radic. Biol. Med. 1999, 27, 119–126. [Google Scholar] [CrossRef]
- Yoshida, T.; Maulik, N.; Ho, Y.S.; Alam, J.; Das, D.K. H(mox-1) constitutes an adaptive response to effect antioxidant cardioprotection: A study with transgenic mice heterozygous for targeted disruption of the Heme oxygenase-1 gene. Circulation 2001, 103, 1695–1701. [Google Scholar] [CrossRef]
- Yet, S.F.; Tian, R.; Layne, M.D.; Wang, Z.Y.; Maemura, K.; Solovyeva, M.; Ith, B.; Melo, L.G.; Zhang, L.; Ingwall, J.S.; et al. Cardiac-specific expression of heme oxygenase-1 protects against ischemia and reperfusion injury in transgenic mice. Circ. Res. 2001, 89, 168–173. [Google Scholar] [CrossRef]
- Clark, J.E.; Foresti, R.; Sarathchandra, P.; Kaur, H.; Green, C.J.; Motterlini, R. Heme oxygenase-1-derived bilirubin ameliorates postischemic myocardial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H643–H651. [Google Scholar] [CrossRef]
- Bakrania, B.; Du Toit, E.F.; Ashton, K.J.; Kiessling, C.J.; Wagner, K.H.; Headrick, J.P.; Bulmer, A.C. Hyperbilirubinemia modulates myocardial function, aortic ejection, and ischemic stress resistance in the Gunn rat. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H1142–H1149. [Google Scholar] [CrossRef][Green Version]
- Bakrania, B.; Du Toit, E.F.; Ashton, K.J.; Wagner, K.H.; Headrick, J.P.; Bulmer, A.C. Chronically elevated bilirubin protects from cardiac reperfusion injury in the male Gunn rat. Acta Physiol. 2017, 220, 461–470. [Google Scholar] [CrossRef]
- Tang, Y.L.; Qian, K.; Zhang, Y.C.; Shen, L.; Phillips, M.I. A vigilant, hypoxia-regulated heme oxygenase-1 gene vector in the heart limits cardiac injury after ischemia-reperfusion in vivo. J. Cardiovasc. Pharmacol. Ther. 2005, 10, 251–263. [Google Scholar] [CrossRef]
- Tang, Y.L.; Tang, Y.; Zhang, Y.C.; Qian, K.; Shen, L.; Phillips, M.I. Protection from ischemic heart injury by a vigilant heme oxygenase-1 plasmid system. Hypertension 2004, 43, 746–751. [Google Scholar] [CrossRef]
- Clark, J.E.; Naughton, P.; Shurey, S.; Green, C.J.; Johnson, T.R.; Mann, B.E.; Foresti, R.; Motterlini, R. Cardioprotective actions by a water-soluble carbon monoxide-releasing molecule. Circ. Res. 2003, 93, e2–e8. [Google Scholar] [CrossRef]
- Fujimoto, H.; Ohno, M.; Ayabe, S.; Kobayashi, H.; Ishizaka, N.; Kimura, H.; Yoshida, K.; Nagai, R. Carbon monoxide protects against cardiac ischemia--reperfusion injury in vivo via MAPK and Akt--eNOS pathways. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1848–1853. [Google Scholar] [CrossRef]
- Guo, Y.; Stein, A.B.; Wu, W.J.; Tan, W.; Zhu, X.; Li, Q.H.; Dawn, B.; Motterlini, R.; Bolli, R. Administration of a CO-releasing molecule at the time of reperfusion reduces infarct size in vivo. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H1649–H1653. [Google Scholar] [CrossRef]
- Stein, A.B.; Guo, Y.; Tan, W.; Wu, W.J.; Zhu, X.; Li, Q.; Luo, C.; Dawn, B.; Johnson, T.R.; Motterlini, R.; et al. Administration of a CO-releasing molecule induces late preconditioning against myocardial infarction. J. Mol. Cell. Cardiol. 2005, 38, 127–134. [Google Scholar] [CrossRef]
- Yeom, H.J.; Koo, O.J.; Yang, J.; Cho, B.; Hwang, J.I.; Park, S.J.; Hurh, S.; Kim, H.; Lee, E.M.; Ro, H.; et al. Generation and characterization of human heme oxygenase-1 transgenic pigs. PLoS ONE 2012, 7, e46646. [Google Scholar] [CrossRef]
- Hinkel, R.; Lange, P.; Petersen, B.; Gottlieb, E.; Ng, J.K.; Finger, S.; Horstkotte, J.; Lee, S.; Thormann, M.; Knorr, M.; et al. Heme Oxygenase-1 Gene Therapy Provides Cardioprotection Via Control of Post-Ischemic Inflammation: An Experimental Study in a Pre-Clinical Pig Model. J. Am. Coll. Cardiol. 2015, 66, 154–165. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, L.; Ding, H.; Huang, S.; He, M.; Zhang, X.; Cheng, L.; Wang, D.; Hu, F.B.; Wu, T. Short (GT) (n) repeats in heme oxygenase-1 gene promoter are associated with lower risk of coronary heart disease in subjects with high levels of oxidative stress. Cell Stress Chaperones 2012, 17, 329–338. [Google Scholar] [CrossRef]
- Ono, K.; Goto, Y.; Takagi, S.; Baba, S.; Tago, N.; Nonogi, H.; Iwai, N. A promoter variant of the heme oxygenase-1 gene may reduce the incidence of ischemic heart disease in Japanese. Atherosclerosis 2004, 173, 315–319. [Google Scholar] [CrossRef]
- Ullrich, R.; Exner, M.; Schillinger, M.; Zuckermann, A.; Raith, M.; Dunkler, D.; Horvat, R.; Grimm, M.; Wagner, O. Microsatellite polymorphism in the heme oxygenase-1 gene promoter and cardiac allograft vasculopathy. J. Heart Lung Transplant. 2005, 24, 1600–1605. [Google Scholar] [CrossRef]
- Yachie, A.; Niida, Y.; Wada, T.; Igarashi, N.; Kaneda, H.; Toma, T.; Ohta, K.; Kasahara, Y.; Koizumi, S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J. Clin. Investig. 1999, 103, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, N.; Yadav, S.P.; Sachdeva, A.; Pruthi, P.K.; Sawhney, S.; Piplani, T.; Wada, T.; Yachie, A. Human heme oxygenase-1 deficiency presenting with hemolysis, nephritis, and asplenia. J. Pediatr. Hematol. Oncol. 2011, 33, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Demirogullari, B.; Ekingen, G.; Guz, G.; Bukan, N.; Erdem, O.; Ozen, I.O.; Memis, L.; Sert, S. A comparative study of the effects of hemin and bilirubin on bilateral renal ischemia reperfusion injury. Nephron Exp. Nephrol. 2006, 103, e1–e5. [Google Scholar] [CrossRef] [PubMed]
- Toda, N.; Takahashi, T.; Mizobuchi, S.; Fujii, H.; Nakahira, K.; Takahashi, S.; Yamashita, M.; Morita, K.; Hirakawa, M.; Akagi, R. Tin chloride pretreatment prevents renal injury in rats with ischemic acute renal failure. Crit. Care Med. 2002, 30, 1512–1522. [Google Scholar] [CrossRef] [PubMed]
- Nath, K.A.; Balla, G.; Vercellotti, G.M.; Balla, J.; Jacob, H.S.; Levitt, M.D.; Rosenberg, M.E. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J. Clin. Investig. 1992, 90, 267–270. [Google Scholar] [CrossRef]
- Vogt, B.A.; Alam, J.; Croatt, A.J.; Vercellotti, G.M.; Nath, K.A. Acquired resistance to acute oxidative stress. Possible role of heme oxygenase and ferritin. Lab. Investig. 1995, 72, 474–483. [Google Scholar] [PubMed]
- Agarwal, A.; Balla, J.; Alam, J.; Croatt, A.J.; Nath, K.A. Induction of heme oxygenase in toxic renal injury: A protective role in cisplatin nephrotoxicity in the rat. Kidney Int. 1995, 48, 1298–1307. [Google Scholar] [CrossRef]
- Shiraishi, F.; Curtis, L.M.; Truong, L.; Poss, K.; Visner, G.A.; Madsen, K.; Nick, H.S.; Agarwal, A. Heme oxygenase-1 gene ablation or expression modulates cisplatin-induced renal tubular apoptosis. Am. J. Physiol. Ren. Physiol. 2000, 278, F726–F736. [Google Scholar] [CrossRef]
- Bolisetty, S.; Traylor, A.; Joseph, R.; Zarjou, A.; Agarwal, A. Proximal tubule-targeted heme oxygenase-1 in cisplatin-induced acute kidney injury. Am. J. Physiol. Ren. Physiol. 2016, 310, F385–F394. [Google Scholar] [CrossRef]
- Bolisetty, S.; Traylor, A.M.; Kim, J.; Joseph, R.; Ricart, K.; Landar, A.; Agarwal, A. Heme oxygenase-1 inhibits renal tubular macroautophagy in acute kidney injury. J. Am. Soc. Nephrol. 2010, 21, 1702–1712. [Google Scholar] [CrossRef]
- Leaf, D.E.; Body, S.C.; Muehlschlegel, J.D.; McMahon, G.M.; Lichtner, P.; Collard, C.D.; Shernan, S.K.; Fox, A.A.; Waikar, S.S. Length Polymorphisms in Heme Oxygenase-1 and AKI after Cardiac Surgery. J. Am. Soc. Nephrol. 2016, 27, 3291–3297. [Google Scholar] [CrossRef] [PubMed]
- Zager, R.A.; Johnson, A.C.; Becker, K. Plasma and urinary heme oxygenase-1 in AKI. J. Am. Soc. Nephrol. 2012, 23, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Adin, C.A.; Croker, B.P.; Agarwal, A. Protective effects of exogenous bilirubin on ischemia-reperfusion injury in the isolated, perfused rat kidney. Am. J. Physiol. Ren. Physiol. 2005, 288, F778–F784. [Google Scholar] [CrossRef] [PubMed]
- Kirkby, K.; Baylis, C.; Agarwal, A.; Croker, B.; Archer, L.; Adin, C. Intravenous bilirubin provides incomplete protection against renal ischemia-reperfusion injury in vivo. Am. J. Physiol. Ren. Physiol. 2007, 292, F888–F894. [Google Scholar] [CrossRef] [PubMed]
- Han, S.S.; Na, K.Y.; Chae, D.W.; Kim, Y.S.; Kim, S.; Chin, H.J. High serum bilirubin is associated with the reduced risk of diabetes mellitus and diabetic nephropathy. Tohoku J. Exp. Med. 2010, 221, 133–140. [Google Scholar] [CrossRef]
- Stec, D.E.; John, K.; Trabbic, C.J.; Luniwal, A.; Hankins, M.W.; Baum, J.; Hinds, T.D., Jr. Bilirubin Binding to PPARalpha Inhibits Lipid Accumulation. PLoS ONE 2016, 11, e0153427. [Google Scholar] [CrossRef]
- Goebel, U.; Siepe, M.; Schwer, C.I.; Schibilsky, D.; Foerster, K.; Neumann, J.; Wiech, T.; Priebe, H.J.; Schlensak, C.; Loop, T. Inhaled carbon monoxide prevents acute kidney injury in pigs after cardiopulmonary bypass by inducing a heat shock response. Anesth. Analg. 2010, 111, 29–37. [Google Scholar] [CrossRef]
- Neto, J.S.; Nakao, A.; Kimizuka, K.; Romanosky, A.J.; Stolz, D.B.; Uchiyama, T.; Nalesnik, M.A.; Otterbein, L.E.; Murase, N. Protection of transplant-induced renal ischemia-reperfusion injury with carbon monoxide. Am. J. Physiol. Ren. Physiol. 2004, 287, F979–F989. [Google Scholar] [CrossRef]
- Vera, T.; Henegar, J.R.; Drummond, H.A.; Rimoldi, J.M.; Stec, D.E. Protective effect of carbon monoxide-releasing compounds in ischemia-induced acute renal failure. J. Am. Soc. Nephrol. 2005, 16, 950–958. [Google Scholar] [CrossRef]
- Tayem, Y.; Johnson, T.R.; Mann, B.E.; Green, C.J.; Motterlini, R. Protection against cisplatin-induced nephrotoxicity by a carbon monoxide-releasing molecule. Am. J. Physiol. Ren. Physiol. 2006, 290, F789–F794. [Google Scholar] [CrossRef]
- Stec, D.E.; Bishop, C.; Rimoldi, J.M.; Poreddy, S.R.; Vera, T.; Salahudeen, A.K. Carbon monoxide (CO) protects renal tubular epithelial cells against cold-rewarm apoptosis. Ren. Fail. 2007, 29, 543–548. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Neto, J.S.; Nakao, A.; Toyokawa, H.; Nalesnik, M.A.; Romanosky, A.J.; Kimizuka, K.; Kaizu, T.; Hashimoto, N.; Azhipa, O.; Stolz, D.B.; et al. Low-dose carbon monoxide inhalation prevents development of chronic allograft nephropathy. Am. J. Physiol. Ren. Physiol. 2006, 290, F324–F334. [Google Scholar] [CrossRef] [PubMed]
- Nakao, A.; Faleo, G.; Nalesnik, M.A.; Seda-Neto, J.; Kohmoto, J.; Murase, N. Low-dose carbon monoxide inhibits progressive chronic allograft nephropathy and restores renal allograft function. Am. J. Physiol. Ren. Physiol. 2009, 297, F19–F26. [Google Scholar] [CrossRef] [PubMed]
- Bagul, A.; Hosgood, S.A.; Kaushik, M.; Nicholson, M.L. Carbon monoxide protects against ischemia-reperfusion injury in an experimental model of controlled nonheartbeating donor kidney. Transplantation 2008, 85, 576–581. [Google Scholar] [CrossRef]
- Faleo, G.; Neto, J.S.; Kohmoto, J.; Tomiyama, K.; Shimizu, H.; Takahashi, T.; Wang, Y.; Sugimoto, R.; Choi, A.M.; Stolz, D.B.; et al. Carbon monoxide ameliorates renal cold ischemia-reperfusion injury with an upregulation of vascular endothelial growth factor by activation of hypoxia-inducible factor. Transplantation 2008, 85, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Wang, L.; Zhao, Y.; Yao, Y.; Chen, S.; Li, J.; Guo, H.; Ming, C.; Chen, S.; Gong, F.; et al. Carbon monoxide potently prevents ischemia-induced high-mobility group box 1 translocation and release and protects against lethal renal ischemia-reperfusion injury. Kidney Int. 2014, 86, 525–537. [Google Scholar] [CrossRef]
- Zheng, M.; Zhang, Q.; Joe, Y.; Kim, S.K.; Uddin, M.J.; Rhew, H.; Kim, T.; Ryter, S.W.; Chung, H.T. Carbon monoxide-releasing molecules reverse leptin resistance induced by endoplasmic reticulum stress. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E780–E788. [Google Scholar] [CrossRef]
- Joe, Y.; Kim, S.; Kim, H.J.; Park, J.; Chen, Y.; Park, H.J.; Jekal, S.J.; Ryter, S.W.; Kim, U.H.; Chung, H.T. FGF21 induced by carbon monoxide mediates metabolic homeostasis via the PERK/ATF4 pathway. FASEB J. 2018, 32, 2630–2643. [Google Scholar] [CrossRef]
- Correa-Costa, M.; Gallo, D.; Csizmadia, E.; Gomperts, E.; Lieberum, J.L.; Hauser, C.J.; Ji, X.; Wang, B.; Camara, N.O.S.; Robson, S.C.; et al. Carbon monoxide protects the kidney through the central circadian clock and CD39. Proc. Natl. Acad. Sci. USA 2018, 115, E2302–E2310. [Google Scholar] [CrossRef]
- Yoshida, J.; Ozaki, K.S.; Nalesnik, M.A.; Ueki, S.; Castillo-Rama, M.; Faleo, G.; Ezzelarab, M.; Nakao, A.; Ekser, B.; Echeverri, G.J.; et al. Ex vivo application of carbon monoxide in UW solution prevents transplant-induced renal ischemia/reperfusion injury in pigs. Am. J. Transplant. 2010, 10, 763–772. [Google Scholar] [CrossRef]
- Shin, D.H.; Park, H.M.; Jung, K.A.; Choi, H.G.; Kim, J.A.; Kim, D.D.; Kim, S.G.; Kang, K.W.; Ku, S.K.; Kensler, T.W.; et al. The NRF2-heme oxygenase-1 system modulates cyclosporin A-induced epithelial-mesenchymal transition and renal fibrosis. Free Radic. Biol. Med. 2010, 48, 1051–1063. [Google Scholar] [CrossRef]
- Sahin, K.; Tuzcu, M.; Gencoglu, H.; Dogukan, A.; Timurkan, M.; Sahin, N.; Aslan, A.; Kucuk, O. Epigallocatechin-3-gallate activates Nrf2/HO-1 signaling pathway in cisplatin-induced nephrotoxicity in rats. Life Sci. 2010, 87, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Tong, F.; Zhou, X. The Nrf2/HO-1 Pathway Mediates the Antagonist Effect of L-Arginine ON Renal Ischemia/Reperfusion Injury in Rats. Kidney Blood Press Res. 2017, 42, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Huang, Y.S.; Chen, J.S.; Huang, C.F.; Su, S.L.; Lu, K.C.; Lin, Y.F.; Chu, P.; Lin, S.H.; Sytwu, H.K. Resveratrol ameliorates renal damage, increases expression of heme oxygenase-1, and has anti-complement, anti-oxidative, and anti-apoptotic effects in a murine model of membranous nephropathy. PLoS ONE 2015, 10, e0125726. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.B.; Shi, J.; Zhang, Y.; Gong, L.R.; Dong, S.A.; Cao, X.S.; Wu, L.L.; Wu, L.N. Electroacupuncture Ameliorates Acute Renal Injury in Lipopolysaccharide-Stimulated Rabbits via Induction of HO-1 through the PI3K/Akt/Nrf2 Pathways. PLoS ONE 2015, 10, e0141622. [Google Scholar] [CrossRef] [PubMed]
- Vlahakis, J.Z.; Kinobe, R.T.; Bowers, R.J.; Brien, J.F.; Nakatsu, K.; Szarek, W.A. Imidazole-dioxolane compounds as isozyme-selective heme oxygenase inhibitors. J. Med. Chem. 2006, 49, 4437–4441. [Google Scholar] [CrossRef] [PubMed]
- Kinobe, R.T.; Ji, Y.; Vlahakis, J.Z.; Motterlini, R.; Brien, J.F.; Szarek, W.A.; Nakatsu, K. Effectiveness of novel imidazole-dioxolane heme oxygenase inhibitors in renal proximal tubule epithelial cells. J. Pharmacol. Exp. Ther. 2007, 323, 763–770. [Google Scholar] [CrossRef]
- Csongradi, E.; Vera, T.; Rimoldi, J.M.; Gadepalli, R.S.; Stec, D.E. In vivo inhibition of renal heme oxygenase with an imidazole-dioxolane inhibitor. Pharmacol. Res. 2010, 61, 525–530. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drummond, H.A.; Mitchell, Z.L.; Abraham, N.G.; Stec, D.E. Targeting Heme Oxygenase-1 in Cardiovascular and Kidney Disease. Antioxidants 2019, 8, 181. https://doi.org/10.3390/antiox8060181
Drummond HA, Mitchell ZL, Abraham NG, Stec DE. Targeting Heme Oxygenase-1 in Cardiovascular and Kidney Disease. Antioxidants. 2019; 8(6):181. https://doi.org/10.3390/antiox8060181
Chicago/Turabian StyleDrummond, Heather A., Zachary L. Mitchell, Nader G. Abraham, and David E. Stec. 2019. "Targeting Heme Oxygenase-1 in Cardiovascular and Kidney Disease" Antioxidants 8, no. 6: 181. https://doi.org/10.3390/antiox8060181
APA StyleDrummond, H. A., Mitchell, Z. L., Abraham, N. G., & Stec, D. E. (2019). Targeting Heme Oxygenase-1 in Cardiovascular and Kidney Disease. Antioxidants, 8(6), 181. https://doi.org/10.3390/antiox8060181





