Carpinus turczaninowii Extract May Alleviate High Glucose-Induced Arterial Damage and Inflammation
Abstract
1. Introduction
2. Materials and Methods
2.1. C. turczaninowii Extract Preparation
2.2. Cell Culture and Treatment Condition
2.3. Quantitative Real Time-PCR
2.4. Immunocytochemistry
2.5. UPLC-Q-TOF MS: Identification of Phenolic Compounds from C. turczaninowii Extract
2.6. Statistical Analysis
3. Results
3.1. C. turczaninowii Extract Suppresses the mRNA Expression of Inflammatory Cytokines and the ER Stress Marker in hASMCs under High Glucose Conditions In Vitro
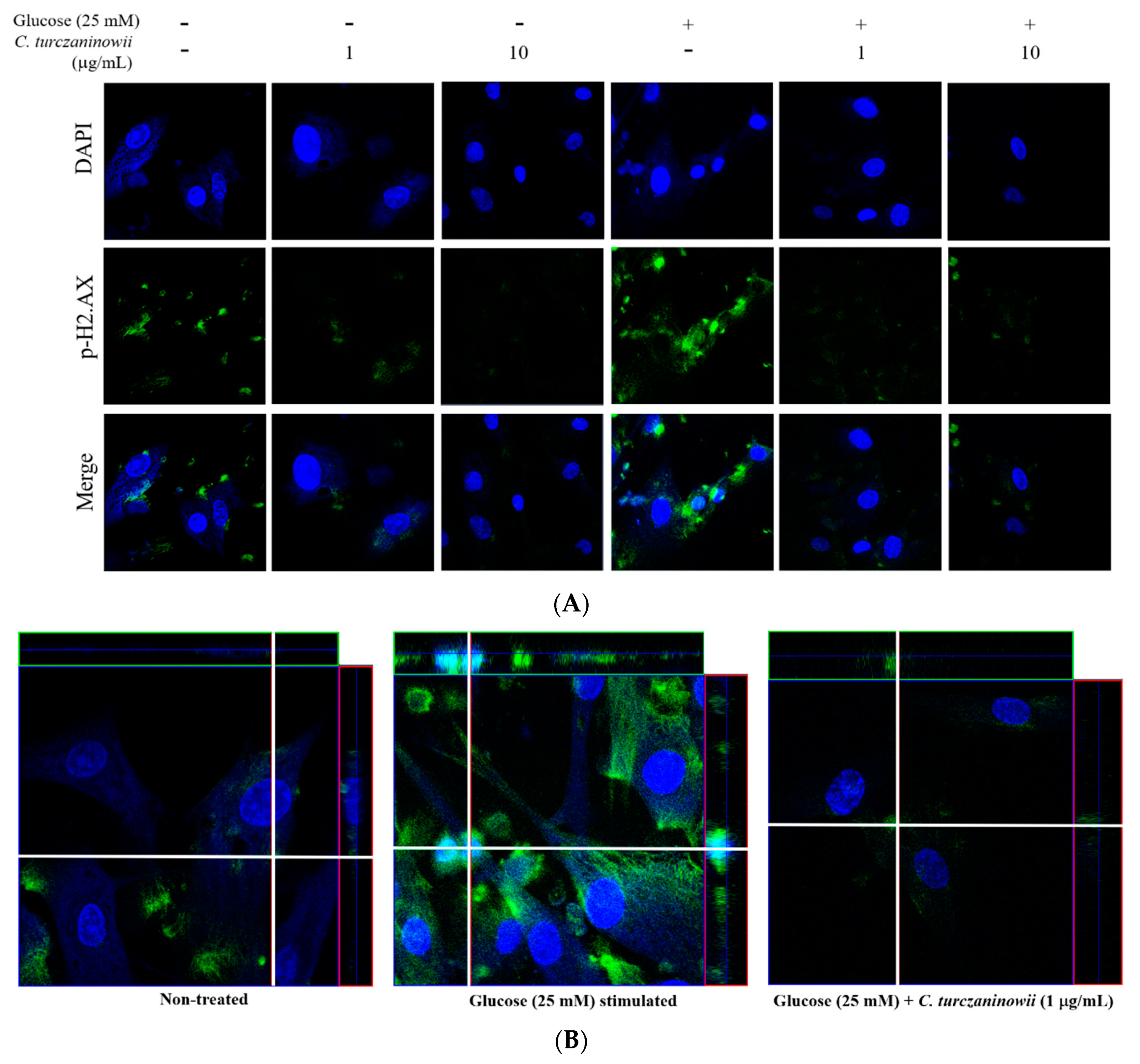
3.2. C. turczaninowii Extract Reduces the Phosphorylation of H2.AX in hASMCs under High Glucose Conditions In Vitro
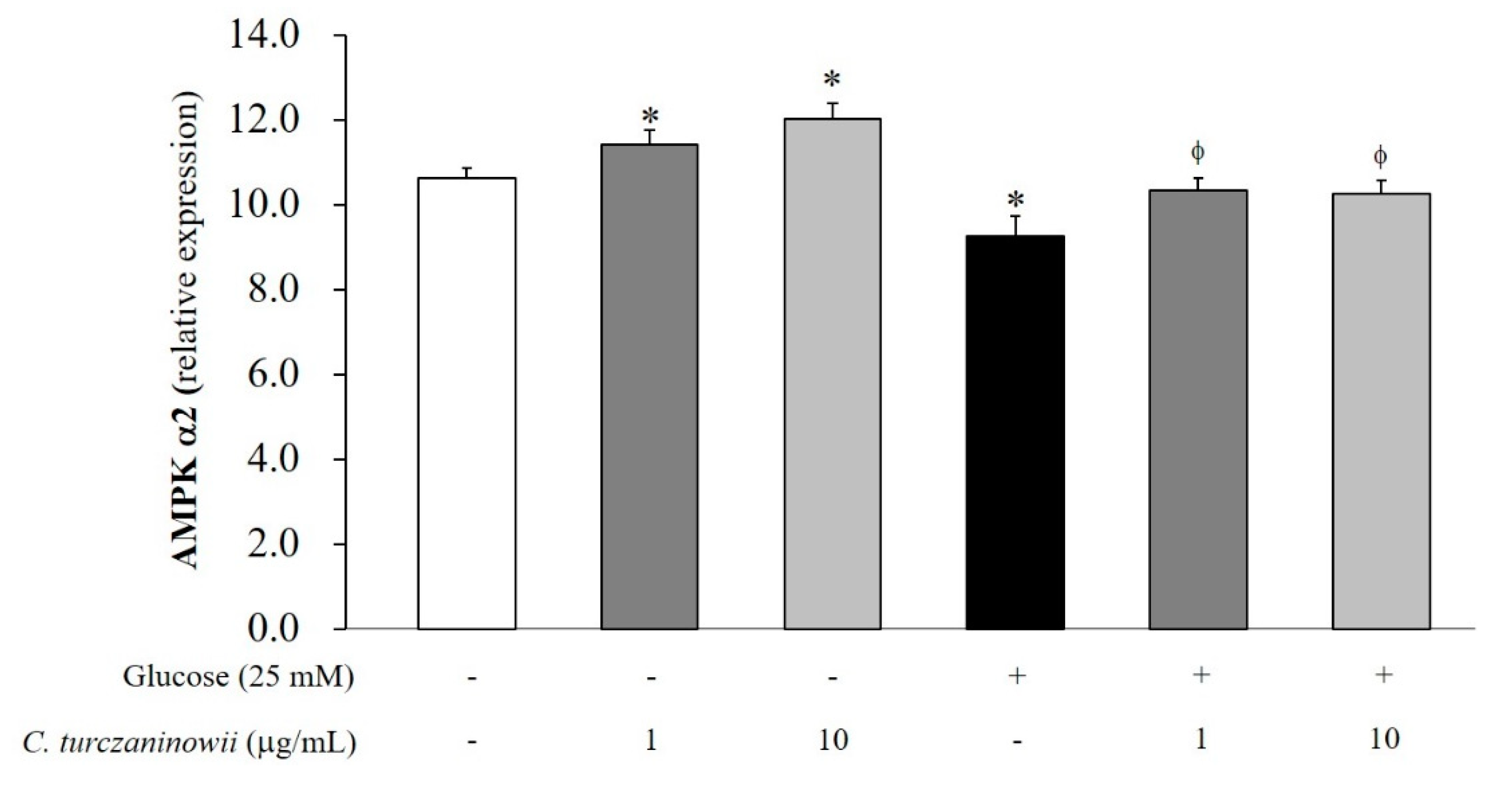
3.3. C. turczaninowii Extract Reduces the Expression of AMPK α2 in hASMCs under High Glucose Conditions In Vitro
3.4. UPLC-Q-TOF MS Analysis of C. turczaninowii Extract: Phenolic Components with Anti-Oxidant and Anti-Inflammation Capabilities
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Haffner, S.M.; Lehto, S.; Ronnemaa, T.; Pyorala, K.; Laakso, M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N. Engl. J. Med. 1998, 339, 229–234. [Google Scholar] [CrossRef] [PubMed]
- The Emerging Risk Factors Collaboration; Sarwar, N.; Gao, P.; Seshasai, S.R.; Gobin, R.; Kaptoge, S.; Di Angelantonio, E.; Ingelsson, E.; Lawlor, D.A.; Selvin, E.; et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375, 2215–2222. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, H.; Sun, G.; Li, S.; Xu, X.; Ye, C.; Li, X.; Wang, S. High glucose induces dysfunction and apoptosis in endothelial cells: Is the effect of high glucose persistence more important than concentration? Exp. Clin. Endocrinol. Diabetes Off. J. Ger. Soc. Endocrinol. Ger. Diabetes Assoc. 2011, 119, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Ying, J.; Pimentel, D.R.; Trucillo, M.; Adachi, T.; Cohen, R.A. High glucose oxidizes SERCA cysteine-674 and prevents inhibition by nitric oxide of smooth muscle cell migration. J. Mol. Cell. Cardiol. 2008, 44, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Creager, M.A.; Luscher, T.F.; Cosentino, F.; Beckman, J.A. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part I. Circulation 2003, 108, 1527–1532. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Triggle, C.R. Endothelial cell dysfunction and the vascular complications associated with type 2 diabetes: Assessing the health of the endothelium. Vasc. Health Risk Manag. 2005, 1, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Katakami, N. Mechanism of Development of Atherosclerosis and Cardiovascular Disease in Diabetes Mellitus. J. Atheroscler. Thromb. 2018, 25, 27–39. [Google Scholar] [CrossRef]
- Giugliano, D.; Ceriello, A.; Paolisso, G. Oxidative stress and diabetic vascular complications. Diabetes Care 1996, 19, 257–267. [Google Scholar] [CrossRef]
- Nishikawa, T.; Edelstein, D.; Du, X.L.; Yamagishi, S.; Matsumura, T.; Kaneda, Y.; Yorek, M.A.; Beebe, D.; Oates, P.J.; Hammes, H.P.; et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000, 404, 787–790. [Google Scholar] [CrossRef]
- Baynes, J.W.; Thorpe, S.R. Role of oxidative stress in diabetic complications: A new perspective on an old paradigm. Diabetes 1999, 48, 1–9. [Google Scholar] [CrossRef]
- Bornfeldt, K.E.; Tabas, I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 2011, 14, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Wolff, S.P. Diabetes mellitus and free radicals. Free radicals, transition metals and oxidative stress in the aetiology of diabetes mellitus and complications. Br. Med. Bull. 1993, 49, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Orasanu, G.; Plutzky, J. The pathologic continuum of diabetic vascular disease. J. Am. Coll. Cardiol. 2009, 53, S35–S42. [Google Scholar] [CrossRef] [PubMed]
- Steinert, J.R.; Wyatt, A.W.; Jacob, R.; Mann, G.E. Redox modulation of Ca2+ signaling in human endothelial and smooth muscle cells in pre-eclampsia. Antioxid. Redox Signal. 2009, 11, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- Matough, F.A.; Budin, S.B.; Hamid, Z.A.; Alwahaibi, N.; Mohamed, J. The role of oxidative stress and antioxidants in diabetic complications. Sultan Qaboos Univ. Med. J. 2012, 12, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, C.; Ruiz, E.; Gussinye, M.; Carrascosa, A. Oxidative stress at onset and in early stages of type 1 diabetes in children and adolescents. Diabetes Care 1998, 21, 1736–1742. [Google Scholar] [CrossRef]
- Sano, T.; Umeda, F.; Hashimoto, T.; Nawata, H.; Utsumi, H. Oxidative stress measurement by in vivo electron spin resonance spectroscopy in rats with streptozotocin-induced diabetes. Diabetologia 1998, 41, 1355–1360. [Google Scholar] [CrossRef]
- Marazza, J.A.; Garro, M.S.; de Giori, G.S. Aglycone production by Lactobacillus rhamnosus CRL981 during soymilk fermentation. Food Microbiol. 2009, 26, 333–339. [Google Scholar] [CrossRef]
- Jin, T.; Kim, O.Y.; Shin, M.J.; Choi, E.Y.; Lee, S.S.; Han, Y.S.; Chung, J.H. Fisetin up-regulates the expression of adiponectin in 3T3-L1 adipocytes via the activation of silent mating type information regulation 2 homologue 1 (SIRT1)-deacetylase and peroxisome proliferator-activated receptors (PPARs). J. Agric. Food Chem. 2014, 62, 10468–10474. [Google Scholar] [CrossRef]
- Moon, J.; Do, H.J.; Kim, O.Y.; Shin, M.J. Antiobesity effects of quercetin-rich onion peel extract on the differentiation of 3T3-L1 preadipocytes and the adipogenesis in high fat-fed rats. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013, 58, 347–354. [Google Scholar] [CrossRef]
- Yang, E.J.; Yim, E.Y.; Song, G.; Kim, G.O.; Hyun, C.G. Inhibition of nitric oxide production in lipopolysaccharide-activated RAW 264.7 macrophages by Jeju plant extracts. Interdiscip. Toxicol. 2009, 2, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.N.; Oh, T.H.; Baik, J.S.; Hyun, C.G.; Kim, S.S.; Lee, N.H. Anti-inflammatory activities for the extracts and Carpinontriols from branches of Caripinus Turczaninowii. Int. J. Pharmacol. 2013, 9, 157–163. [Google Scholar]
- Ko, H.N.; Kim, J.M.; Bu, H.J.; Lee, H.H. Chemical constituents from the branches of Carpinus turczaninowii with antioxidative activities. J. Korean Chem. Soc. 2013, 57, 520–522. [Google Scholar] [CrossRef]
- Kang, J.M. Identification of Anti-Oxidative, Skin Whitening, Anti-Inflammatory and Anti-Bacterial Constituents from the Leaves of Carpinus turczaninowii Hance. Master’s Thesis, Jeju National University, Jeju, Korea, 2015. [Google Scholar]
- Jeon, J.I.; Jang, J.S. Foliar flavonoids of genus Carpinus in eastern Asia primarily based on native taxa to Korea. Korean J. Plant. Tax. 2000, 30, 139–153. [Google Scholar] [CrossRef]
- Chang, C.S.; Chang, K.S. Typification of Corylopsis coreana (Hamamelidaceae) and Carpinus laxiflora var. longispica (Betulaceae). J. Jpn. Bot. 2010, 85, 270–276. [Google Scholar]
- Yeo, J.H.; Son, Y.K.; Bang, W.Y.; Kim, O.Y. Carpinus truczaninowii extract showed anti-inflammatory response on human aortic vascular smooth muscle cells. Planta Med. 2016, 82, S1–S381. [Google Scholar] [CrossRef]
- Gray, K.; Kumar, S.; Figg, N.; Harrison, J.; Baker, L.; Mercer, J.; Littlewood, T.; Bennett, M. Effects of DNA damage in smooth muscle cells in atherosclerosis. Circ. Res. 2015, 116, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Bao, L.; Ding, Y.; Dai, X.; Zhang, Z.; Li, Y. Quercetin alleviates cell apoptosis and inflammation via the ER stress pathway in vascular endothelial cells cultured in high concentrations of glucosamine. Mol. Med. Rep. 2017, 15, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.R.; Du, Y.J.; Chen, L.; Liu, Z.G.; Pan, Y.H.; Liu, J.F.; Liu, B. Quercetin protects against high glucose-induced damage in bone marrow-derived endothelial progenitor cells. Int. J. Mol. Med. 2014, 34, 1025–1031. [Google Scholar] [CrossRef]
- Nathan, D.M.; Lachin, J.; Cleary, P.; Orchard, T.; Brillon, D.J.; Backlund, J.Y.; O’Leary, D.H.; Genuth, S.; Diabetes Control and Complications Trial; Epidemiology of Diabetes Interventions and Complications Research Group. Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N. Engl. J. Med. 2003, 348, 2294–2303. [Google Scholar]
- Popov DConstantinescu, E. Arterial smooth muscle cells dysfunction in hyperglycaemia and hyperglycaemia associated with hyperlipidaemia: From causes to effects. Archiv. Physiol. Biochem. 2008, 114, 150–160. [Google Scholar] [CrossRef]
- Rizzoni, D.; Rosei, E.A. Small artery remodeling in diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Jie, W.; Wang, X.; Zhang, Y.; Guo, J.; Kuang, D.; Zhu, P.; Wang, G.; Ao, Q. SDF-1alpha/CXCR4 axis is involved in glucose-potentiated proliferation and chemotaxis in rat vascular smooth muscle cells. Int. J. Exp. Pathol. 2010, 91, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Timimi, F.K.; Ting, H.H.; Haley, E.A.; Roddy, M.A.; Ganz, P.; Creager, M.A. Vitamin C improves endothelium-dependent vasodilation in patients with insulin-dependent diabetes mellitus. J. Am. Coll. Cardiol. 1998, 31, 552–557. [Google Scholar] [CrossRef]
- Teede, H.J.; Giannopoulos, D.; Dalais, F.S.; Hodgson, J.; McGrath, B.P. Randomised, controlled, cross-over trial of soy protein with isoflavones on blood pressure and arterial function in hypertensive subjects. J. Am. Coll. Nutr. 2006, 25, 533–540. [Google Scholar] [CrossRef]
- Reshef, N.; Hayari, Y.; Goren, C.; Boaz, M.; Madar, Z.; Knobler, H. Antihypertensive effect of sweetie fruit in patients with stage I hypertension. Am. J. Hypertens. 2005, 18, 1360–1363. [Google Scholar] [CrossRef] [PubMed]
- Safi, S.Z.; Qvist, R.; Yan, G.O.; Ismail, I.S. Differential expression and role of hyperglycemia induced oxidative stress in epigenetic regulation of beta1, beta2 and beta3-adrenergic receptors in retinal endothelial cells. BMC Med. Genom. 2014, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Cafueri, G.; Parodi, F.; Pistorio, A.; Bertolotto, M.; Ventura, F.; Gambini, C.; Bianco, P.; Dallegri, F.; Pistoia, V.; Pezzolo, A.; et al. Endothelial and smooth muscle cells from abdominal aortic aneurysm have increased oxidative stress and telomere attrition. PLoS ONE 2012, 7, e35312. [Google Scholar] [CrossRef]
- Rogakou, E.P.; Boon, C.; Redon, C.; Bonner, W.M. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J. Cell. Biol. 1999, 146, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K.; Nigro, P.; Berk, B.C. Oxidative stress and vascular smooth muscle cell growth: A mechanistic linkage by cyclophilin A. Antioxid. Redox Signal. 2010, 12, 675–682. [Google Scholar] [CrossRef]
- Jung, E.J.; Kim, C.W.; Kim, D.R. Cytosolic accumulation of γH2AX is associated with tropomyosin-related kinase A-induced cell death in U2OS cell. Exp. Mol. Med. 2008, 40, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Okamura, H.; Yoshida, K.; Amorim, B.R.; Haneji, T. Histone H1.2 is translocated to mitochondria and associates with bak in bleomycin-induced apoptotic cells. J. Cell. Biochem. 2008, 103, 1488–1496. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F. Tumour necrosis factor and cancer. Nat. Rev. Cancer 2009, 9, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Beg, A.A.; Baltimore, D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science 1996, 274, 782–784. [Google Scholar] [CrossRef] [PubMed]
- Prins, J.B.; Ledgerwood, E.C.; Ameloot, P.; Vandenabeele, P.; Faraco, P.R.; Bright, N.A.; O’Rahilly, S.; Bradley, J.R. Tumor necrosis factor-induced cytotoxicity is not related to rates of mitochondrial morphological abnormalities or autophagy-changes that can be mediated by TNFR-I or TNFR-II. Biosci. Rep. 1998, 18, 329–340. [Google Scholar] [CrossRef]
- Rakesh, K.; Agrawal, D.K. Cytokines and growth factors involved in apoptosis and proliferation of vascular smooth muscle cells. Int. Immunopharmacol. 2005, 5, 1506. [Google Scholar] [CrossRef]
- McKellar, G.E.; McCarey, D.W.; Sattar, N.; McInnes, I.B. Role for TNF in atherosclerosis? Lessons from autoimmune disease. Nat. Rev. Cardiol. 2009, 6, 410–417. [Google Scholar] [CrossRef]
- Sprague, A.H.; Khalil, R.A. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem. Pharmacol. 2009, 78, 539–552. [Google Scholar] [CrossRef]
- Selzman, C.H.; Shames, B.D.; McIntyre, R.C., Jr.; Banerjee, A.; Harken, A.H. The NFkappaB inhibitory peptide, IkappaBalpha, prevents human vascular smooth muscle proliferation. Ann. Thorac. Surg. 1999, 67, 1227–1231; discussion 1231–1232. [Google Scholar] [CrossRef]
- Zhang, H.; Park, Y.; Wu, J.; Chen, X.; Lee, S.; Yang, J.; Dellsperger, K.C.; Zhang, C. Role of TNF-alpha in vascular dysfunction. Clin. Sci. 2009, 116, 219–230. [Google Scholar] [CrossRef]
- Geng, Y.J.; Wu, Q.; Muszynski, M.; Hansson, G.K.; Libby, P. Apoptosis of vascular smooth muscle cells induced by in vitro stimulation with interferon-gamma, tumor necrosis factor-alpha, and interleukin-1 beta. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, U.; Ikeda, M.; Oohara, T.; Oguchi, A.; Kamitani, T.; Tsuruya, Y.; Kano, S. Interleukin 6 stimulates growth of vascular smooth muscle cells in a PDGF-dependent manner. Am. J. Physiol. 1991, 260, H1713–H1717. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Newman, W.H. Smooth muscle cell migration stimulated by interleukin 6 is associated with cytoskeletal reorganization. J. Surg. Res. 2003, 111, 261–266. [Google Scholar] [CrossRef]
- Khan, M.I.; Pichna, B.A.; Shi, Y.; Bowes, A.J.; Werstuck, G.H. Evidence supporting a role for endoplasmic reticulum stress in the development of atherosclerosis in a hyperglycaemic mouse model. Antioxid. Redox Signal. 2009, 11, 2289–2298. [Google Scholar] [CrossRef] [PubMed]
- Werstuck, G.H.; Khan, M.I.; Femia, G.; Kim, A.J.; Tedesco, V.; Trigatti, B.; Shi, Y. Glucosamine-induced endoplasmic reticulum dysfunction is associated with accelerated atherosclerosis in a hyperglycemic mouse model. Diabetes 2006, 55, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Werstuck, G.H.; Lhotak, S.; de Koning, A.B.; Sood, S.K.; Hossain, G.S.; Moller, J.; Ritskes-Hoitinga, M.; Falk, E.; Dayal, S.; et al. Association of multiple cellular stress pathways with accelerated atherosclerosis in hyperhomocysteinemic apolipoprotein E-deficient mice. Circulation 2004, 110, 207–213. [Google Scholar] [CrossRef]
- Bennett, M.R.; Sinha, S.; Owens, G.K. Vascular Smooth Muscle Cells in Atherosclerosis. Circ. Res. 2016, 118, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Lee-Young, R.S.; Griffee, S.R.; Lynes, S.E.; Bracy, D.P.; Ayala, J.E.; McGuinness, O.P.; Wasserman, D.H. Skeletal muscle AMP-activated protein kinase is essential for the metabolic response to exercise in vivo. J. Biol. Chem. 2009, 284, 23925–23934. [Google Scholar] [CrossRef]
- Kemp, B.E.; Stapleton, D.; Campbell, D.J.; Chen, Z.P.; Murthy, S.; Walter, M.; Gupta, A.; Adams, J.J.; Katsis, F.; van Denderen, B.; et al. AMP-activated protein kinase, super metabolic regulator. Biochem. Soc. Trans. 2003, 31, 162–168. [Google Scholar] [CrossRef]
- Hawley, S.A.; Selbert, M.A.; Goldstein, E.G.; Edelman, A.M.; Carling, D.; Hardie, D.G. 5′-AMP activates the AMP-activated protein kinase cascade, and Ca2+/calmodulin activates the calmodulin-dependent protein kinase I cascade, via three independent mechanisms. J. Biol. Chem. 1995, 270, 27186–27191. [Google Scholar] [CrossRef]
- Shaw, R.J.; Kosmatka, M.; Bardeesy, N.; Hurley, R.L.; Witters, L.A.; DePinho, R.A.; Cantley, L.C. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl. Acad. Sci. USA 2004, 101, 3329–3335. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhang, M.; Wang, S.; Liang, B.; Zhao, Z.; Liu, C.; Wu, M.; Choi, H.C.; Lyons, T.J.; Zou, M.H. Activation of AMP-activated protein kinase inhibits oxidized LDL-triggered endoplasmic reticulum stress in vivo. Diabetes 2010, 59, 1386–1396. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhang, M.; Liang, B.; Xie, Z.; Zhao, Z.; Asfa, S.; Choi, H.C.; Zou, M.H. Reduction of AMP-activated protein kinase alpha2 increases endoplasmic reticulum stress and atherosclerosis in vivo. Circulation 2010, 121, 792–803. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. The AMP-activated protein kinase pathway--new players upstream and downstream. J. Cell Sci. 2004, 117, 5479–5487. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.D.; Narine, A.; Shaver, P.R.; Fox, J.C.; Vuncannon, J.R.; Tulis, D.A. AMP-activated protein kinase inhibits vascular smooth muscle cell proliferation and migration and vascular remodeling following injury. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H369–H381. [Google Scholar] [CrossRef] [PubMed]
- Winder, W.W.; Hardie, D.G. AMP-activated protein kinase, a metabolic master switch: Possible roles in type 2 diabetes. Am. J. Physiol. 1999, 277, E1–E10. [Google Scholar] [CrossRef]
- Song, P.; Wang, S.; He, C.; Wang, S.; Liang, B.; Viollet, B.; Zou, M.H. AMPKalpha2 deletion exacerbates neointima formation by upregulating Skp2 in vascular smooth muscle cells. Circ. Res. 2011, 109, 1230–1239. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, M.; Liang, B.; Xu, J.; Xie, Z.; Liu, C.; Viollet, B.; Yan, D.; Zou, M.H. AMPKalpha2 deletion causes aberrant expression and activation of NAD(P)H oxidase and consequent endothelial dysfunction in vivo: Role of 26S proteasomes. Circ. Res. 2010, 106, 1117–1128. [Google Scholar] [CrossRef]
- Mirouse, V.; Swick, L.L.; Kazgan, N.; St Johnston, D.; Brenman, J.E. LKB1 and AMPK maintain epithelial cell polarity under energetic stress. J. Cell Biol. 2007, 177, 387–392. [Google Scholar] [CrossRef]
- Jansen, M.; Ten Klooster, J.P.; Offerhaus, G.J.; Clevers, H. LKB1 and AMPK family signaling: The intimate link between cell polarity and energy metabolism. Physiol. Rev. 2009, 89, 777–798. [Google Scholar] [CrossRef]
- Barre, B.; Perkins, N.D. The Skp2 promoter integrates signaling through the NF-kappaB, p53, and Akt/GSK3beta pathways to regulate autophagy and apoptosis. Mol. Cell 2010, 38, 524–538. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Swarts, S.G.; Yin, L.; Liu, C.; Tian, Y.; Cao, Y.; Swarts, M.; Yang, S.; Zhang, S.B.; Zhang, K.; et al. Antioxidant properties of quercetin. Adv. Exp. Med. Biol. 2011, 701, 283–289. [Google Scholar] [PubMed]
- Boots, A.W.; Wilms, L.C.; Swennen, E.L.; Kleinjans, J.C.; Bast, A.; Haenen, G.R. In vitro and ex vivo anti-inflammatory activity of quercetin in healthy volunteers. Nutrition 2008, 24, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.M.; Murphy, E.A.; McClellan, J.L.; Carmichael, M.D.; Gangemi, J.D. Quercetin reduces susceptibility to influenza infection following stressful exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R505–R509. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Spagnuolo, C.; Tedesco, I.; Bilotto, S.; Russo, G.L. The flavonoid quercetin in disease prevention and therapy: Facts and fancies. Biochem. Pharmacol. 2012, 83, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, S.; Sudhakaran, P.R.; Helen, A. Quercetin attenuates atherosclerotic inflammation and adhesion molecule expression by modulating TLR-NF-kappaB signaling pathway. Cell. Immunol. 2016, 310, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Meotti, F.C.; Senthilmohan, R.; Harwood, D.T.; Missau, F.C.; Pizzolatti, M.G.; Kettle, A.J. Myricitrin as a substrate and inhibitor of myeloperoxidase: Implications for the pharmacological effects of flavonoids. Free Radic. Biol. Med. 2008, 44, 109–120. [Google Scholar] [CrossRef]
- Yokomizo, A.; Moriwaki, M. Myricitrin degraded by simulated digestion inhibits oxidation of human low-density lipoprotein. Biosci. Biotechnol. Biochem. 2005, 69, 693–699. [Google Scholar] [CrossRef]
- Yan, L.J.; Yang, H.T.; Duan, H.Y.; Wu, J.T.; Qian, P.; Fan, X.W.; Wang, S. Myricitrin inhibits vascular adhesion molecule expression in TNFalphastimulated vascular smooth muscle cells. Mol. Med. Rep. 2017, 16, 6354–6359. [Google Scholar] [CrossRef]
- Qin, M.; Luo, Y.; Meng, X.B.; Wang, M.; Wang, H.W.; Song, S.Y.; Ye, J.X.; Pan, R.L.; Yao, F.; Wu, P.; et al. Myricitrin attenuates endothelial cell apoptosis to prevent atherosclerosis: An insight into PI3K/Akt activation and STAT3 signaling pathways. Vasc. Pharmacol. 2015, 70, 23–34. [Google Scholar] [CrossRef]
- Ahangarpour, A.; Oroojan, A.A.; Khorsandi, L.; Kouchak, M.; Badavi, M. Antioxidant effect of myricitrin on hyperglycemia-induced oxidative stress in C2C12 cell. Cell Stress Chaperones 2018, 23, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.J.; Teuber, S.S.; Gobeille, A.; Cremin, P.; Waterhouse, A.L.; Steinberg, F.M. Walnut polyphenolics inhibit in vitro human plasma and LDL oxidation. J. Nutr. 2001, 131, 2837–2842. [Google Scholar] [CrossRef] [PubMed]
- Wada, L.; Ou, B. Antioxidant activity and phenolic content of Oregon caneberries. J. Agric. Food Chem. 2002, 50, 3495–3500. [Google Scholar] [CrossRef] [PubMed]
- Iino, T.; Nakahara, K.; Miki, W.; Kiso, Y.; Ogawa, Y.; Kato, S.; Takeuchi, K. Less damaging effect of whisky in rat stomachs in comparison with pure ethanol. Role of ellagic acid, the nonalcoholic component. Digestion 2001, 64, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Ayhanci, A.; Cengiz, M.; Mehtap Kutlu, H.; Vejselova, D. Protective effects of ellagic acid in D-galactosamine-induced kidney damage in rats. Cytotechnology 2016, 68, 1763–1770. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Iino, T.; Tashima, K.; Umeda, M.; Ogawa, Y.; Takeeda, M.; Takata, K.; Takeuchi, K. Effect of ellagic acid on gastric damage induced in ischemic rat stomachs following ammonia or reperfusion. Life Sci. 2002, 70, 1139–1150. [Google Scholar] [CrossRef]
- Lei, F.; Xing, D.M.; Xiang, L.; Zhao, Y.N.; Wang, W.; Zhang, L.J.; Du, L.J. Pharmacokinetic study of ellagic acid in rat after oral administration of pomegranate leaf extract. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2003, 796, 189–194. [Google Scholar] [CrossRef]
- Priyadarsini, K.I.; Khopde, S.M.; Kumar, S.S.; Mohan, H. Free radical studies of ellagic acid, a natural phenolic antioxidant. J. Agric. Food Chem. 2002, 50, 2200–2206. [Google Scholar] [CrossRef]

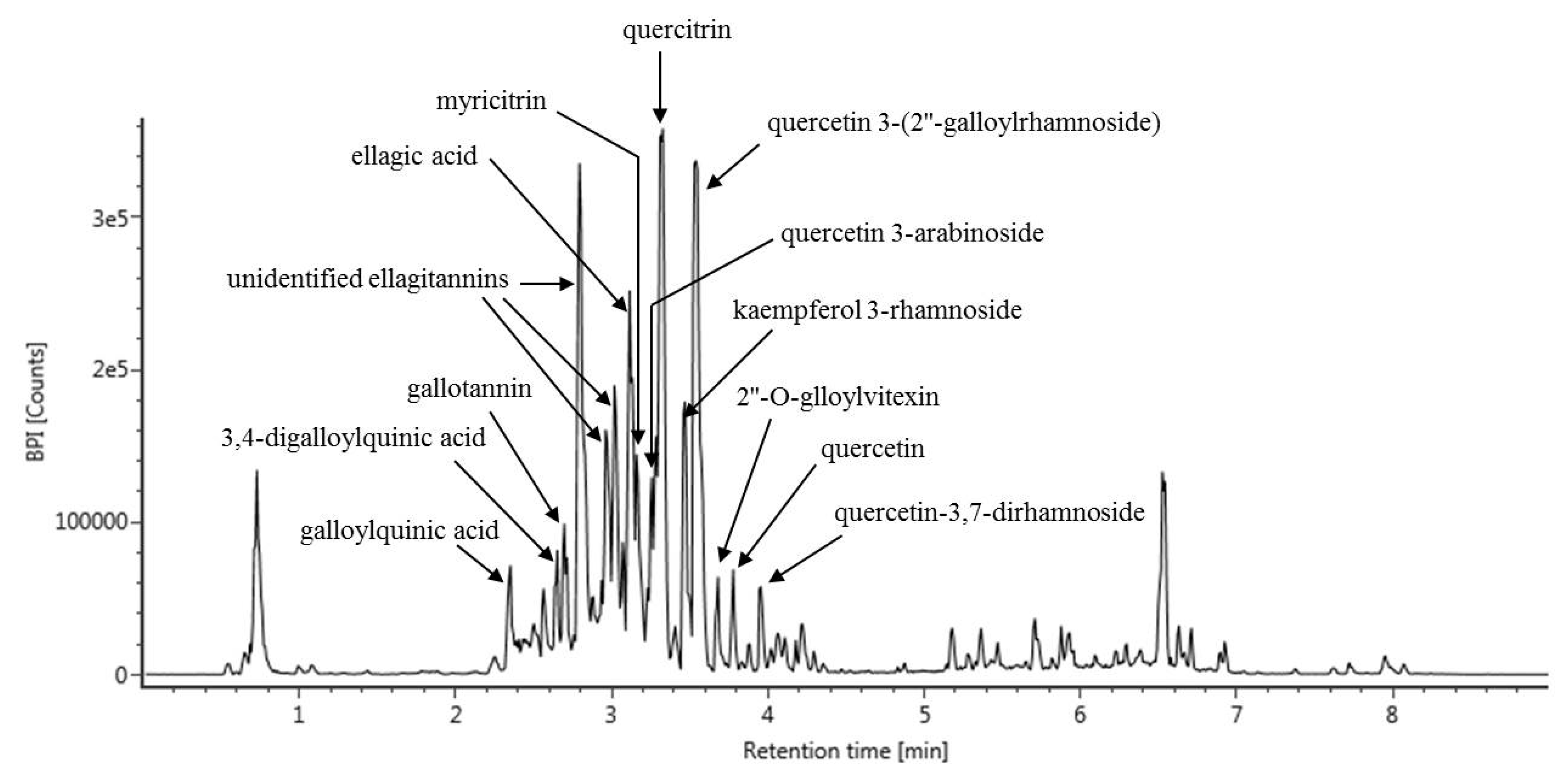
| Retention Time (min) | Compound | m/z [M − H] | MS Fragments |
|---|---|---|---|
| 2.35 | Galloylquinic acid | 343.0674 | 191, 169, 125 |
| 2.65 | 3,4-digalloylquinic acid | 495.0782 | 343, 191, 169, 125 |
| 2.7 | Gallotannin | 633.0726 | 407, 301 |
| 2.79 | Unidentified ellagitannin | 951.0701 | 615, 301, 273, 245 |
| 2.96 | Unidentified ellagitannin | 953.0868 | 615, 301, 273, 246 |
| 3.02 | Unidentified ellagitannin | 965.0850 | 615, 301, 273, 247 |
| 3.12 | Ellagic acid | 300.9992 | 282, 271, 257 |
| 3.16 | Myricitrin | 463.0885 | 301, 300, 151 |
| 3.26 | Quercetin 3-arabinoside | 433.0779 | 301, 300, 271, 255 |
| 3.32 | Quercitrin | 447.0937 | 301, 300, 151 |
| 3.47 | Kaempferol 3-rhamnoside | 431.0985 | 327, 285, 284, 227, 255 |
| 3.54 | Quercetin 3-(2″-galloylrhamnoside) | 599.1037 | 429, 301, 178, 151 |
| 3.68 | 2″-O-glloylvitexin | 583.1090 | 431, 345, 285, 125 |
| 3.78 | Quercetin | 301.0350 | 179, 151, 121 |
| 3.96 | Quercetin-3,7-dirahamnoside | 593.1293 | 447, 301, 300, 151 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.; Yoon, S.R.; Son, Y.K.; Bang, W.Y.; Bae, C.-H.; Yeo, J.-H.; Kim, H.-J.; Kim, O.Y. Carpinus turczaninowii Extract May Alleviate High Glucose-Induced Arterial Damage and Inflammation. Antioxidants 2019, 8, 172. https://doi.org/10.3390/antiox8060172
Song J, Yoon SR, Son YK, Bang WY, Bae C-H, Yeo J-H, Kim H-J, Kim OY. Carpinus turczaninowii Extract May Alleviate High Glucose-Induced Arterial Damage and Inflammation. Antioxidants. 2019; 8(6):172. https://doi.org/10.3390/antiox8060172
Chicago/Turabian StyleSong, Juhyun, So Ra Yoon, Youn Kyoung Son, Woo Young Bang, Chang-Hwan Bae, Joo-Hong Yeo, Hyun-Jin Kim, and Oh Yoen Kim. 2019. "Carpinus turczaninowii Extract May Alleviate High Glucose-Induced Arterial Damage and Inflammation" Antioxidants 8, no. 6: 172. https://doi.org/10.3390/antiox8060172
APA StyleSong, J., Yoon, S. R., Son, Y. K., Bang, W. Y., Bae, C.-H., Yeo, J.-H., Kim, H.-J., & Kim, O. Y. (2019). Carpinus turczaninowii Extract May Alleviate High Glucose-Induced Arterial Damage and Inflammation. Antioxidants, 8(6), 172. https://doi.org/10.3390/antiox8060172





