Mitochondria-Targeted Antioxidants as Potential Therapy for the Treatment of Traumatic Brain Injury
Abstract
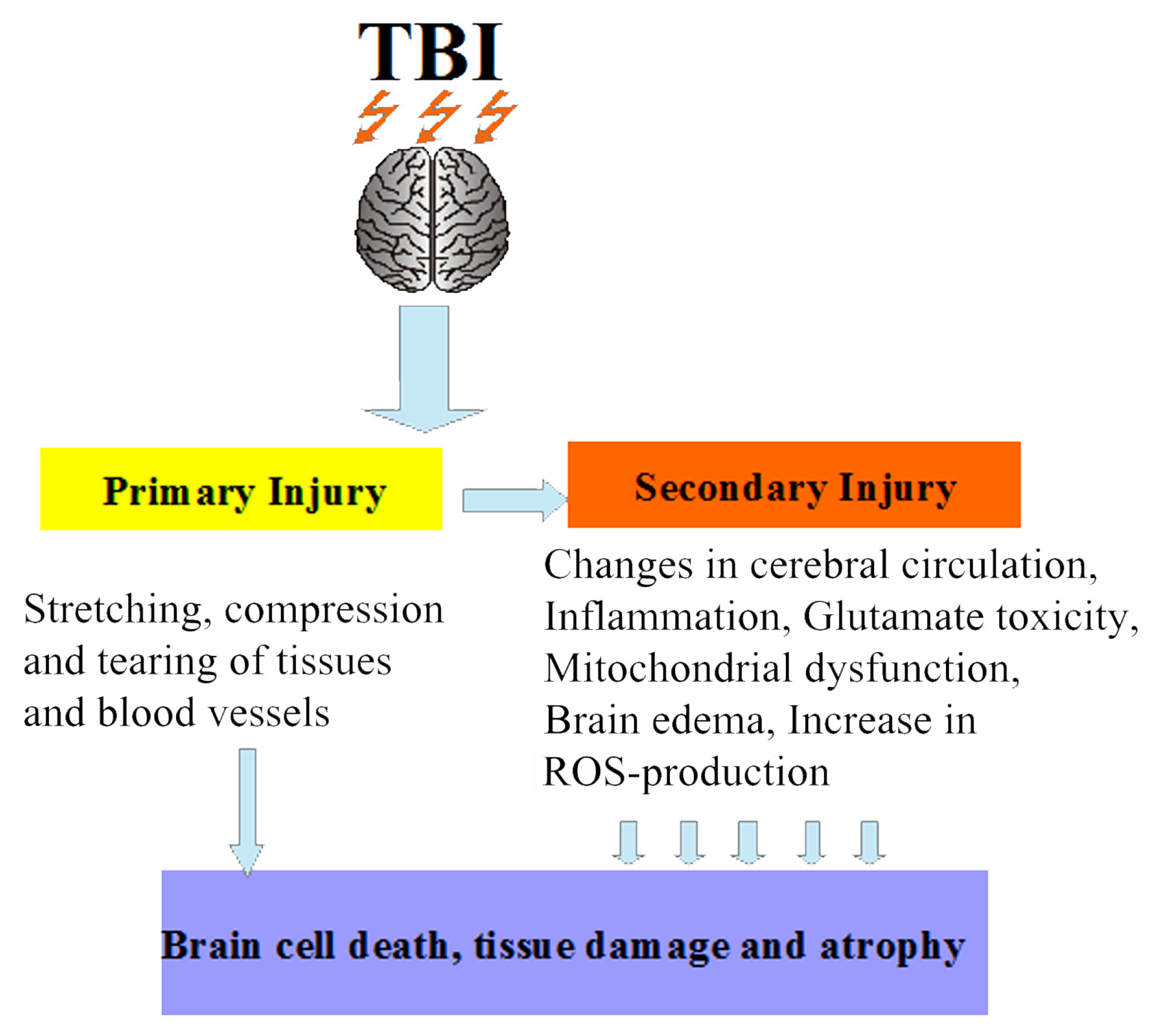
1. Introduction
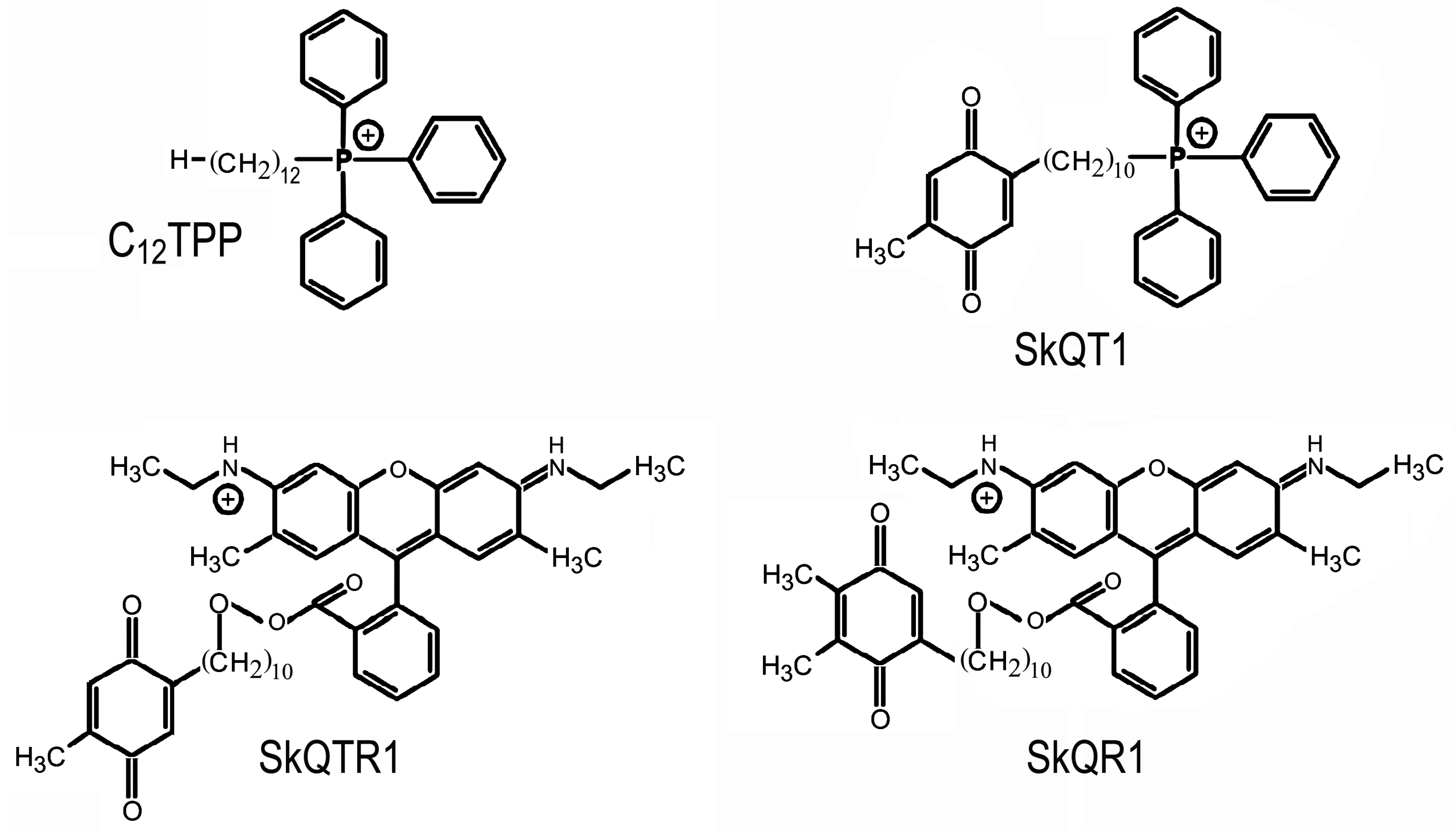
2. Mitochondria-Targeted Antioxidant Therapy in Traumatic Brain Injury
3. Conclusions
Funding
Conflicts of Interest
Abbreviations
| TBI | Traumatic brain injury |
| ROS | Reactive oxygen species |
| Nrf2 | NF-E2-related factor 2 |
| ARE | Antioxidant response element |
| EPO | Erythropoietin |
References
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 2018, 1, 1–18. [Google Scholar] [CrossRef]
- Jourdan, C.; Azouvi, P.; Genêt, F.; Selly, N.; Josseran, L.; Schnitzler, A. Disability and Health Consequences of Traumatic Brain Injury: National Prevalence. Am. J. Phys. Med. Rehabil. 2018, 97, 323–331. [Google Scholar] [CrossRef]
- Shively, S.; Scher, A.I.; Perl, D.P.; Diaz-Arrastia, R. Dementia resulting from traumatic brain injury: What is the pathology? Arch. Neurol. 2012, 69, 1245–1251. [Google Scholar] [CrossRef]
- Gupta, R.; Sen, N. Traumatic brain injury: A risk factor for neurodegenerative diseases. Rev. Neurosci. 2016, 27, 93–100. [Google Scholar] [CrossRef]
- Fernandez-Gajardo, R.; Matamala, J.M.; Carrasco, R.; Gutierrez, R.; Melo, R.; Rodrigo, R. Novel therapeutic strategies for traumatic brain injury: Acute antioxidant reinforcement. CNS Drugs 2014, 28, 229–248. [Google Scholar] [CrossRef]
- Isaev, N.K.; Stelmashook, E.V.; Genrikhs, E.E.; Korshunova, G.A.; Sumbatyan, N.V.; Kapkaeva, M.R.; Skulachev, V.P. Neuroprotective properties of mitochondria-targeted antioxidants of the SkQ-type. Rev. Neurosci. 2016, 27, 849–855. [Google Scholar] [CrossRef]
- Xiong, Y.; Mahmood, A.; Chopp, M. Animal models of traumatic brain injury. Nat. Rev. Neurosci. 2013, 14, 128–142. [Google Scholar] [CrossRef]
- Nissanka, N.; Moraes, C.T. Mitochondrial DNA damage and reactive oxygen species in neurodegenerative disease. FEBS Lett. 2018, 592, 728–742. [Google Scholar] [CrossRef]
- Van Houten, B.; Woshner, V.; Santos, J.H. Role of mitochondrial DNA in toxic responses to oxidative stress. DNA Repair (Amst.) 2006, 5, 145–152. [Google Scholar] [CrossRef]
- Pointer, C.B.; Klegeris, A. Cardiolipin in Central Nervous System Physiology and Pathology. Cell Mol. Neurobiol. 2017, 37, 1161–1172. [Google Scholar] [CrossRef]
- Calabrese, V.; Lodi, R.; Tonon, C.; D'Agata, V.; Sapienza, M.; Scapagnini, G.; Mangiameli, A.; Pennisi, G.; Stella, A.M.; Butterfield, D.A. Oxidative stress, mitochondrial dysfunction and cellular stress response in Friedreich’s ataxia. J. Neurol. Sci. 2005, 233, 145–162. [Google Scholar] [CrossRef]
- Niizuma, K.; Yoshioka, H.; Chen, H.; Kim, G.S.; Jung, J.E.; Katsu, M.; Okami, N.; Chan, P.H. Mitochondrial and apoptotic neuronal death signaling pathways in cerebral ischemia. Biochim. Biophys. Acta. 2010, 1802, 92–99. [Google Scholar] [CrossRef]
- Shen, Q.; Hiebert, J.B.; Hartwell, J.; Thimmesch, A.R.; Pierce, J.D. Systematic review of traumatic brain injury and the impact of antioxidant therapy on clinical outcomes. Worldviews Evid. Based Nurs. 2016, 13, 380–389. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative stress: A key modulator in neurodegenerative diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Cho, J.; Won, K.; Wu, D.; Soong, Y.; Liu, S.; Szeto, H.H.; Hong, M.K. Potent mitochondria-targeted peptides reduce myocardial infarction in rats. Coron. Artery Dis. 2007, 18, 215–220. [Google Scholar] [CrossRef]
- Bakeeva, L.E.; Barskov, I.V.; Egorov, M.V.; Isaev, N.K.; Kapelko, V.I.; Kazachenko, A.V.; Kirpatovsky, V.I.; Kozlovsky, S.V.; Lakomkin, V.L.; Levina, S.B.; et al. Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 2. Treatment of some ROS- and age-related diseases (heart arrhythmia, heart infarctions, kidney ischemia, and stroke). Biochemistry (Mosc.) 2008, 73, 1288–1299. [Google Scholar] [CrossRef]
- Calkins, M.J.; Manczak, M.; Reddy, P.H. Mitochondria-targeted antioxidant ss31 prevents amyloid β-induced mitochondrial abnormalities and synaptic degeneration in Alzheimer’s disease. Pharmaceuticals 2012, 5, 1103–1119. [Google Scholar] [CrossRef]
- Yao, X.; Carlson, D.; Sun, Y.; Ma, L.; Wolf, S.E.; Minei, J.P.; Zang, Q.S. Mitochondrial ROS Induces Cardiac Inflammation via a Pathway through mtDNA Damage in a Pneumonia-Related Sepsis Model. PLoS ONE 2015, 10, e0139416. [Google Scholar] [CrossRef]
- Liberman, E.A.; Topaly, V.P.; Tsofina, L.M.; Jasaitis, A.A.; Skulachev, V.P. Mechanism of coupling of oxidative phosphorylation and the membrane potential of mitochondria. Nature 1969, 65, 1076–1078. [Google Scholar] [CrossRef]
- Skulachev, V.P. A biochemical approach to the problem of aging: “Megaproject” on membrane-penetrating ions. The first results and prospects. Biochemistry (Mosc.) 2007, 72, 1385–1396. [Google Scholar] [CrossRef]
- Antonenko, Y.N.; Avetisyan, A.V.; Bakeeva, L.E.; Chernyak, B.V.; Chertkov, V.A.; Domnina, L.V.; Ivanova, O.Y.; Izyumov, D.S.; Khailova, L.S.; Klishin, S.S.; et al. Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 1. Cationic plastoquinone derivatives: synthesis and in vitro studies. Biochemistry (Mosc.) 2008, 73, 1273–1287. [Google Scholar] [CrossRef]
- Antonenko, Y.N.; Roginsky, V.A.; Pashkovskaya, A.A.; Rokitskaya, T.I.; Kotova, E.A.; Zaspa, A.A.; Chernyak, B.V.; Skulachev, V.P. Protective effects of mitochondriatargeted antioxidant SkQ in aqueous and lipid membrane environments. J. Membr. Biol. 2008, 222, 141–149. [Google Scholar] [CrossRef]
- Skulachev, M.V.; Antonenko, Y.N.; Anisimov, V.N.; Chernyak, B.V.; Cherepanov, D.A.; Chistyakov, V.A.; Egorov, M.V.; Kolosova, N.G.; Korshunova, G.A.; Lyamzaev, K.G.; et al. Mitochondrial-targeted plastoquinone derivatives. Effect on senescence and acute age-related pathologies. Curr. Drug Targets 2011, 12, 800–826. [Google Scholar] [CrossRef]
- Ma, T.; Hoeffer, C.A.; Wong, H.; Massaad, C.A.; Zhou, P.; Iadecola, C.; Murphy, M.P.; Pautler, R.G.; Klann, E. Amyloid β-induced impairments in hippocampal synaptic plasticity are rescued by decreasing mitochondrial superoxide. J. Neurosci. 2011, 31, 5589–5595. [Google Scholar] [CrossRef]
- Ji, J.; Kline, A.E.; Amoscato, A.; Samhan-Arias, A.K.; Sparvero, L.J.; Tyurin, V.A.; Tyurina, Y.Y.; Fink, B.; Manole, M.D.; Puccio, A.M.; et al. Lipidomics identifies cardiolipin oxidation as a mitochondrial target for redox therapy of brain injury. Nat. Neurosci. 2012, 15, 1407–1413. [Google Scholar] [CrossRef]
- Genrikhs, E.E.; Stelmashook, E.V.; Alexandrova, O.P.; Novikova, S.V.; Voronkov, D.N.; Glibka, Y.A.; Skulachev, V.P.; Isaev, N.K. The single intravenous administration of mitochondria-targeted antioxidant SkQR1 after traumatic brain injury attenuates neurological deficit in rats. Brain Res. Bull. 2019, 148, 100–108. [Google Scholar] [CrossRef]
- Isaev, N.K.; Novikova, S.V.; Stelmashook, E.V.; Barskov, I.V.; Silachev, D.N.; Khaspekov, L.G.; Skulachev, V.P.; Zorov, D.B. Mitochondria-targeted plastoquinone antioxidant SkQR1 decreases trauma-induced neurological deficit in rat. Biochemistry (Mosc.) 2012, 77, 996–999. [Google Scholar] [CrossRef]
- Kapay, N.A.; Popova, O.V.; Isaev, N.K.; Stelmashook, E.V.; Kondratenko, R.V.; Zorov, D.B.; Skrebitsky, V.G.; Skulachev, V.P. Mitochondria-targeted plastoquinone antioxidant SkQ1 prevents amyloid-β-induced impairment of long-term potentiation in rat hippocampal slices. J. Alzheimers Dis. 2013, 36, 377–383. [Google Scholar] [CrossRef]
- Stefanova, N.A.; Muraleva, N.A.; Skulachev, V.P.; Kolosova, N.G. Alzheimer’s disease-like pathology in senescence-accelerated OXYS rats can be partially retarded with mitochondria-targeted antioxidant SkQ1. J. Alzheimers Dis. 2014, 38, 681–694. [Google Scholar] [CrossRef]
- Genrikhs, E.E.; Stelmashook, E.V.; Popova, O.V.; Kapay, N.A.; Korshunova, G.A.; Sumbatyan, N.V.; Skrebitsky, V.G.; Skulachev, V.P.; Isaev, N.K. Mitochondria-targeted antioxidant SkQT1 decreases trauma-induced neurological deficit in rat and prevents amyloid-β-induced impairment of long-term potentiation in rat hippocampal slices. J. Drug Target. 2015, 23, 347–352. [Google Scholar] [CrossRef]
- Szarka, N.; Pabbidi, M.R.; Amrein, K.; Czeiter, E.; Berta, G.; Pohoczky, K.; Helyes, Z.; Ungvari, Z.; Koller, A.; Buki, A.; Toth, P. Traumatic Brain Injury Impairs Myogenic Constriction of Cerebral Arteries: Role of Mitochondria-Derived H(2)O(2) and TRPV4-Dependent Activation of BK(ca) Channels. J. Neurotrauma 2018, 35, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, H.; Fang, J.; Dai, W.; Zhou, J.; Wang, X.; Zhou, M. SS-31 Provides Neuroprotection by Reversing Mitochondrial Dysfunction after Traumatic Brain Injury. Oxid. Med. Cell. Longev. 2018, 2018, 4783602. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, H.; Shen, R.; Fang, J.; Yang, Y.; Dai, W.; Zhu, Y.; Zhou, M. Mitochondrial-targeted antioxidant MitoQ provides neuroprotection and reduces neuronal apoptosis in experimental traumatic brain injury possibly via the Nrf2-ARE pathway. Am. J. Transl. Res. 2018, 10, 1887–1899. [Google Scholar]
- Cho, H.Y.; Reddy, S.P.; Debiase, A.; Yamamoto, M.; Kleeberger, S.R. Gene expression profiling of NRF2-mediated protection against oxidative injury. Free Radic. Biol. Med. 2005, 38, 325–343. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.Y.; Reddy, S.P.; Kleeberger, S.R. Nrf2 defends the lung from oxidative stress. Antioxid. Redox Signal 2006, 8, 76–87. [Google Scholar] [CrossRef] [PubMed]
- De Vries, H.E.; Witte, M.; Hondius, D.; Rozemuller, A.J.; Drukarch, B.; Hoozemans, J.; van Horssen, J. Nrf2-induced antioxidant protection: A promising target to counteract ROS-mediated damage in neurodegenerative disease? Free Radic. Biol. Med. 2008, 45, 1375–1383. [Google Scholar] [CrossRef]
- Ma, Q. Role of Nrf2 in oxidative stress and toxicity. Annu Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Silachev, D.N.; Isaev, N.K.; Pevzner, I.B.; Zorova, L.D.; Stelmashook, E.V.; Novikova, S.V.; Plotnikov, E.Y.; Skulachev, V.P.; Zorov, D.B. The mitochondria-targeted antioxidants and remote kidney preconditioning ameliorate brain damage through kidney-to-brain cross-talk. PLoS ONE 2012, 7, e51553. [Google Scholar] [CrossRef]
- Nie, X.; Wang, W.; Wang, Q.; Zhu, D.; Song, H. Intranasal erythropoietin ameliorates neurological function impairments and neural pathology in mice with chronic alcoholism by regulating autophagy-related Nrf2 degradation. Mol. Med. Rep. 2019, 19, 1139–1149. [Google Scholar] [CrossRef]
- Zhou, Z.W.; Li, F.; Zheng, Z.T.; Li, Y.D.; Chen, T.H.; Gao, W.W.; Chen, J.L.; Zhang, J.N. Erythropoietin regulates immune/inflammatory reaction and improves neurological function outcomes in traumatic brain injury. Brain Behav. 2017, 7, e00827. [Google Scholar] [CrossRef]
- Chelombitko, M.A.; Averina, O.A.; Vasil'eva, T.V.; Dvorianinova, E.E.; Egorov, M.V.; Pletjushkina, O.Y.; Popova, E.N.; Fedorov, A.V.; Romashchenko, V.P.; Ilyinskaya, O.P. Comparison of the Effects of Mitochondria-Targeted Antioxidant 10-(6'-Plastoquinonyl)Decyltriphenylphosphonium Bromide (SkQ1) and a Fragment of its Molecule Dodecyltriphenylphosphonium on Carrageenan-Induced Acute Inflammation in Mouse Model of Subcuteneous Air Pouch. Bull. Exp. Biol. Med. 2017, 162, 730–733. [Google Scholar] [CrossRef]
- Liao, Y.; Liu, P.; Guo, F.; Zhang, Z.Y.; Zhang, Z. Oxidative burst of circulating neutrophils following traumatic brain injury in human. PLoS ONE 2013, 24, e68963. [Google Scholar] [CrossRef]
- Tao, L.; Li, D.; Liu, H.; Jiang, F.; Xu, Y.; Cao, Y.; Gao, R.; Chen, G. Neuroprotective effects of metformin on traumatic brain injury in rats associated with NF-κB and MAPK signaling pathway. Brain Res. Bull. 2018, 140, 154–161. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stelmashook, E.V.; Isaev, N.K.; Genrikhs, E.E.; Novikova, S.V. Mitochondria-Targeted Antioxidants as Potential Therapy for the Treatment of Traumatic Brain Injury. Antioxidants 2019, 8, 124. https://doi.org/10.3390/antiox8050124
Stelmashook EV, Isaev NK, Genrikhs EE, Novikova SV. Mitochondria-Targeted Antioxidants as Potential Therapy for the Treatment of Traumatic Brain Injury. Antioxidants. 2019; 8(5):124. https://doi.org/10.3390/antiox8050124
Chicago/Turabian StyleStelmashook, Elena V., Nickolay K. Isaev, Elisaveta E. Genrikhs, and Svetlana V. Novikova. 2019. "Mitochondria-Targeted Antioxidants as Potential Therapy for the Treatment of Traumatic Brain Injury" Antioxidants 8, no. 5: 124. https://doi.org/10.3390/antiox8050124
APA StyleStelmashook, E. V., Isaev, N. K., Genrikhs, E. E., & Novikova, S. V. (2019). Mitochondria-Targeted Antioxidants as Potential Therapy for the Treatment of Traumatic Brain Injury. Antioxidants, 8(5), 124. https://doi.org/10.3390/antiox8050124



