Polyphenols in Almond Skins after Blanching Modulate Plasma Biomarkers of Oxidative Stress in Healthy Humans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of ASP
2.3. Subjects
2.4. Study Design
2.5. Sample Collection and Storage
2.6. Analysis of Plasma Flavonoids
2.7. Biomarkers of Antioxidant Capacity and Lipid Peroxidation
2.8. Statistical Analysis
3. Results
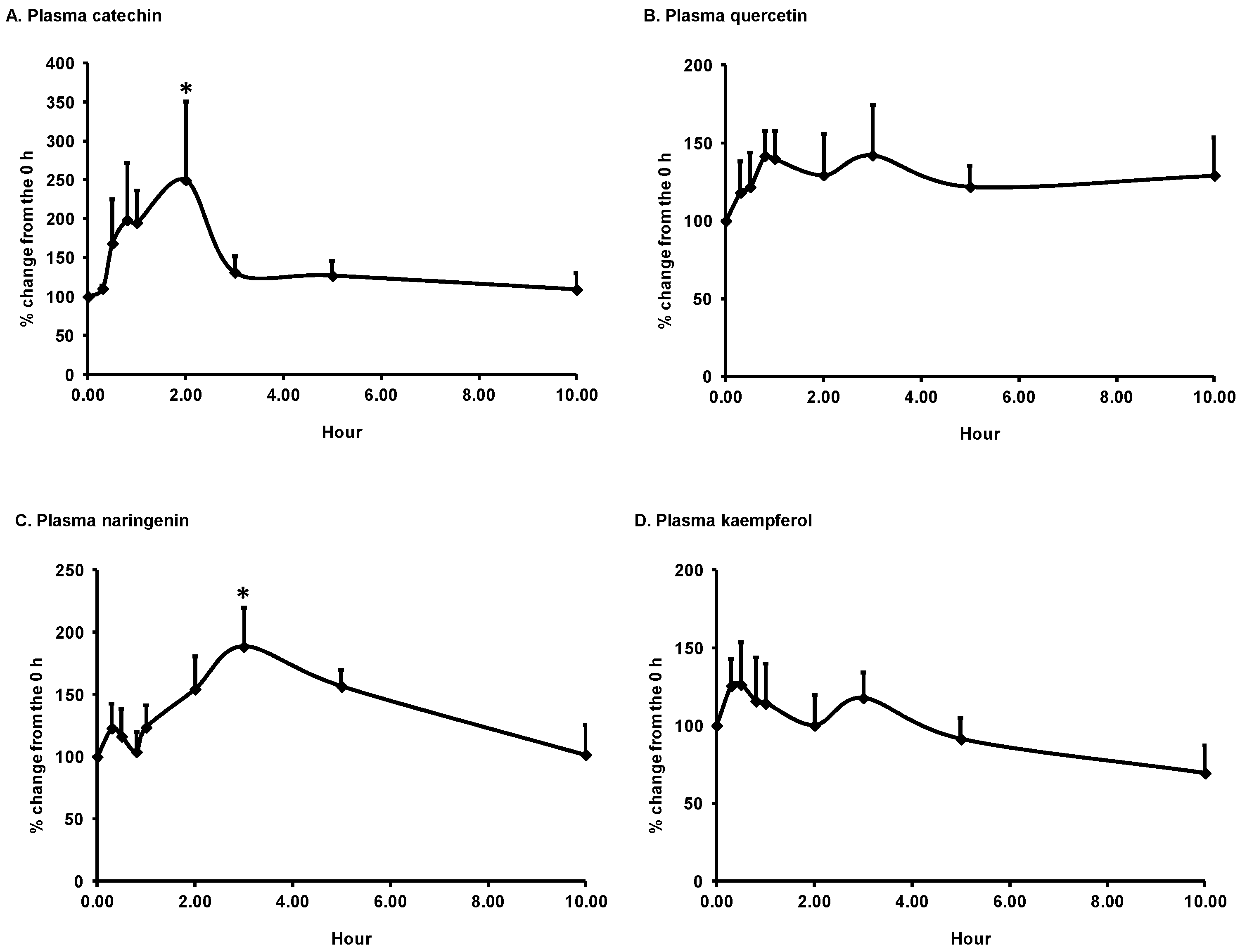
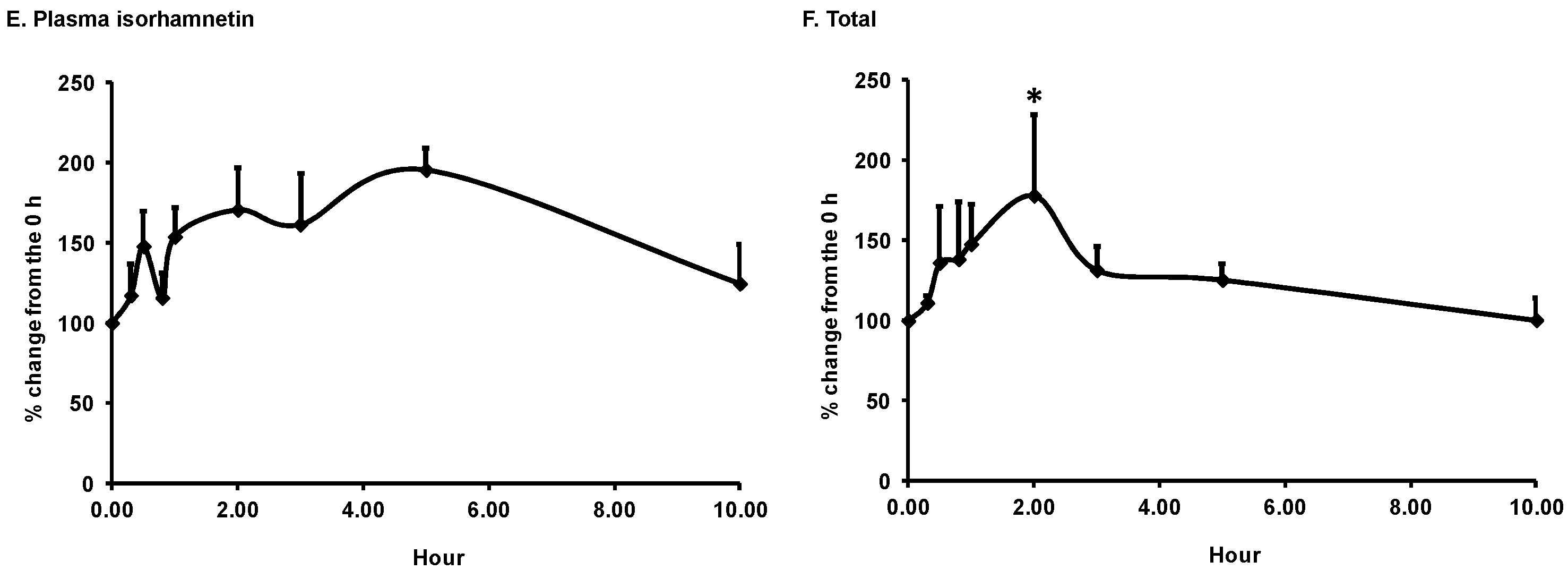
3.1. Bioavailability of Flavonoids
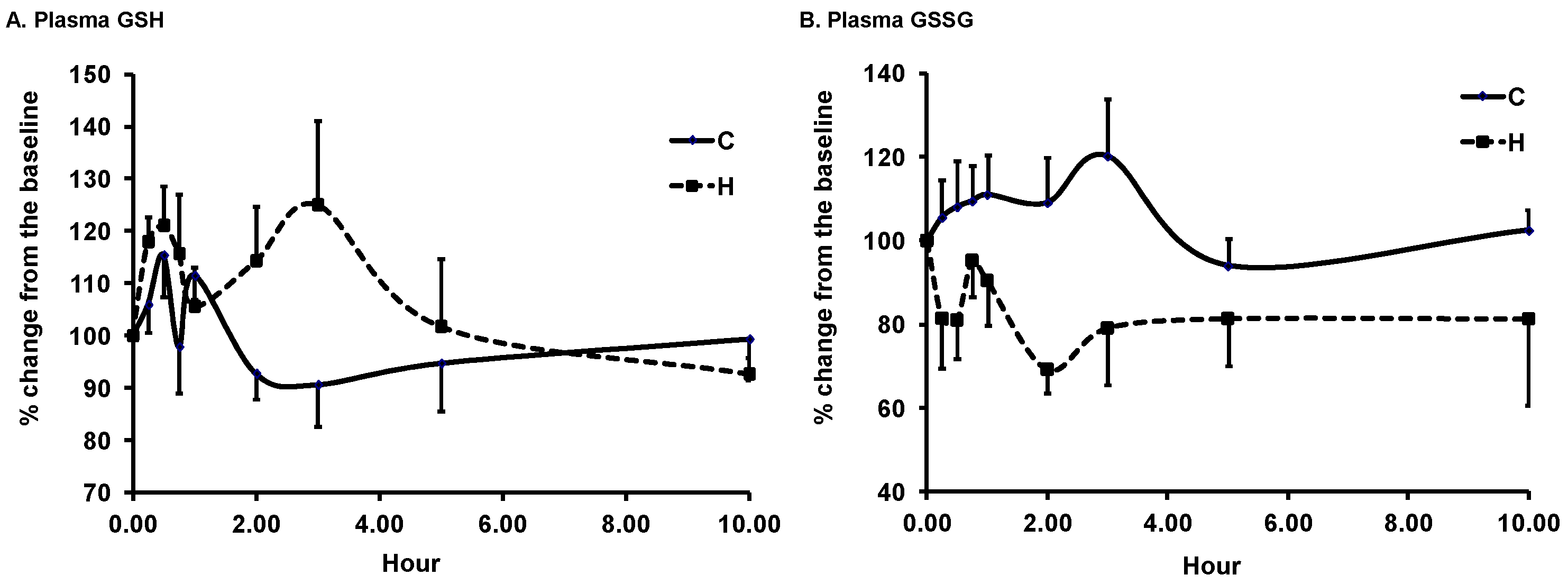
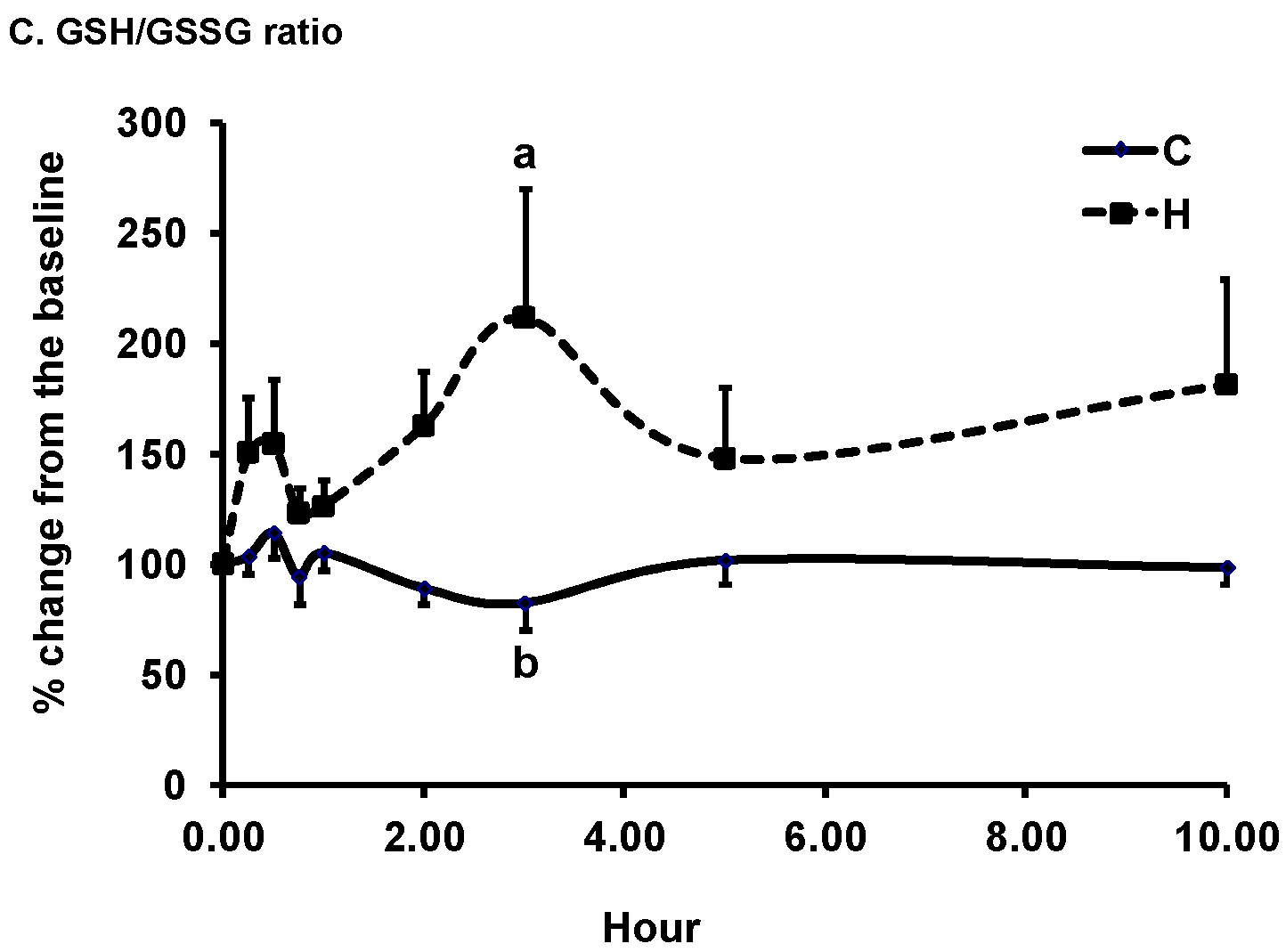
3.2. Changes in Plasma Biomarkers of Antioxidant Defense
3.3. Changes in LDL Resistance to Oxidation
3.4. Changes in Plasma Biomarkers of Oxidative Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kamil, A.; Chen, C.Y. Health benefits of almonds beyond cholesterol reduction. J. Agric. Food Chem. 2012, 60, 6694–6702. [Google Scholar] [CrossRef] [PubMed]
- Morgillo, S.; Hill, A.M.; Coates, A.M. The effects of nut consumption on vascular function. Nutrients 2019, 11, 116. [Google Scholar] [CrossRef] [PubMed]
- Neale, E.P.; Tapsell, L.C.; Guan, V.; Batterham, M.J. The effect of nut consumption on markers of inflammation and endothelial function: A systematic review and meta-analysis of randomised controlled trials. BMJ Open 2017, 7, e016863. [Google Scholar] [CrossRef]
- Chen, G.C.; Zhang, R.; Martinez-Gonzalez, M.A.; Zhang, Z.L.; Bonaccio, M.; van Dam, R.M.; Qin, L.Q. Nut consumption in relation to all-cause and cause-specific mortality: A meta-analysis 18 prospective studies. Food Funct. 2017, 8, 3893–3905. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Nut consumption and risk of cardiovascular disease, total cancer, all-cause and cause-specific mortality: A systematic review and dose-response meta-analysis of prospective studies. BMC Med. 2016, 14, 207. [Google Scholar] [CrossRef] [PubMed]
- International Nuts & Dried Fruits Council. Nuts & Dried Fruits. Statistical Yearbook 2017/2018. Available online: https://www.nutfruit.org/consumers/news/detail/inc-2017-2018-statistical-yearbook (accessed on 7 May 2018).
- Grasser, L.A.; Fadel, J.G.; Garnett, I.; DePeters, E.J. Quantity and economic importance of nine selected by-products used in California dairy rations. J. Dairy Sci. 1995, 78, 962–971. [Google Scholar] [CrossRef]
- González, J.F.; Gañán, J.; Ramiro, A.; González-García, C.M.; Encinar, J.M.; Sabio, E.; Román, S. Almond residues gasification plant for generation of electric power. Preliminary study. Fuel Process. Technol. 2006, 87, 149–155. [Google Scholar] [CrossRef]
- Varzakas, T.; Zakynthinos, G.; Verpoort, F. Plant food residues as a source of nutraceuticals and functional foods. Foods 2016, 5. [Google Scholar] [CrossRef]
- Kumar, K.; Yadav, A.N.; Kumar, V.; Vyas, P.; Dhaliwal, H.S. Food waste: A potential bioresource for extraction of nutraceuticals and bioactive compounds. Bioresour. Bioprocess. 2017, 4, 18. [Google Scholar] [CrossRef]
- Babbar, N.; Oberoi, H.S.; Sandhu, S.K. Therapeutic and nutraceutical potential of bioactive compounds extracted from fruit residues. Crit. Rev. Food Sci. Nutr. 2015, 55, 319–337. [Google Scholar] [CrossRef]
- Esfahlan, A.J.; Jamei, R.; Esfahlan, R.J. The importance of almond (Prunus amygdalus L.) and its by-products. Food Chem. 2010, 120, 349–360. [Google Scholar] [CrossRef]
- Monagas, M.; Garrido, I.; Lebron-Aguilar, R.; Bartolome, B.; Gomez-Cordoves, C. Almond (Prunus dulcis (Mill.) D.A. Webb) skins as a potential source of bioactive polyphenols. J. Agric. Food. Chem. 2007, 55, 8498–8507. [Google Scholar] [CrossRef]
- Mandalari, G.; Faulks, R.M.; Bisignano, C.; Waldron, K.W.; Narbad, A.; Wickham, M.S. In vitro evaluation of the prebiotic properties of almond skins (Amygdalus communis L.). FEMS Microbiol. Lett. 2010, 304, 116–122. [Google Scholar] [CrossRef]
- Mandalari, G. Potential health benefits of almond skin. J. Bioprocess. Biotech. 2012, 2, e110. [Google Scholar] [CrossRef]
- Bolling, B.W.; Dolnikowski, G.; Blumberg, J.B.; Oliver Chen, C.Y. Quantification of almond skin polyphenols by liquid chromatography-mass spectrometry. J. Food Sci. 2009, 74, C326–C332. [Google Scholar] [CrossRef] [PubMed]
- Milbury, P.E.; Chen, C.Y.; Dolnikowski, G.G.; Blumberg, J.B. Determination of flavonoids and phenolics and their distribution in almonds. J. Agric. Food Chem. 2006, 54, 5027–5033. [Google Scholar] [CrossRef]
- Ann Lila, M. The nature-versus-nurture debate on bioactive phytochemicals: The genome versus terroir. J. Sci. Food Agric. 2006, 86, 2510–2515. [Google Scholar] [CrossRef]
- Hooper, L.; Kroon, P.A.; Rimm, E.B.; Cohn, J.S.; Harvey, I.; Le Cornu, K.A.; Ryder, J.J.; Hall, W.L.; Cassidy, A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2008, 88, 38–50. [Google Scholar] [CrossRef]
- Kim, Y.; Je, Y. Flavonoid intake and mortality from cardiovascular disease and all causes: A meta-analysis of prospective cohort studies. Clin. Nutr. ESPEN 2017, 20, 68–77. [Google Scholar] [CrossRef]
- Grosso, G.; Micek, A.; Godos, J.; Pajak, A.; Sciacca, S.; Galvano, F.; Giovannucci, E.L. Dietary flavonoid and lignan intake and mortality in prospective cohort studies: Systematic review and dose-response meta-analysis. Am. J. Epidemiol. 2017, 185, 1304–1316. [Google Scholar] [CrossRef]
- Bolling, B.W.; Chen, C.Y.; McKay, D.L.; Blumberg, J.B. Tree nut phytochemicals: Composition, antioxidant capacity, bioactivity, impact factors. A systematic review of almonds, Brazils, cashews, hazelnuts, macadamias, pecans, pine nuts, pistachios and walnuts. Nutr. Res. Rev. 2011, 24, 244–275. [Google Scholar] [CrossRef]
- Mandalari, G.; Bisignano, C.; D’Arrigo, M.; Ginestra, G.; Arena, A.; Tomaino, A.; Wickham, M.S. Antimicrobial potential of polyphenols extracted from almond skins. Lett. Appl. Microbiol. 2010, 51, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Milbury, P.E.; Lapsley, K.; Blumberg, J.B. Flavonoids from almond skins are bioavailable and act synergistically with vitamins C and E to enhance hamster and human LDL resistance to oxidation. J. Nutr. 2005, 135, 1366–1373. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Milbury, P.E.; Chung, S.K.; Blumberg, J.B. Effect of almond skin polyphenolics and quercetin on human LDL and apolipoprotein B-100 oxidation and conformation. J. Nutr. Biochem. 2007, 18, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Blumberg, J.B. In vitro activity of almond skin polyphenols for scavenging free radicals and inducing quinone reductase. J. Agric. Food Chem. 2008, 56, 4427–4434. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Ravent, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Chen, C.Y.; Milbury, P.E.; Collins, F.W.; Blumberg, J.B. Avenanthramides are bioavailable and have antioxidant activity in humans after acute consumption of an enriched mixture from oats. J. Nutr. 2007, 137, 1375–1382. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Cao, G.; Alessio, H.M.; Cutler, R.G. Oxygen-radical absorbance capacity assay for antioxidants. Free Radic. Biol. Med. 1993, 14, 303–311. [Google Scholar] [CrossRef]
- Pleban, P.A.; Munyani, A.; Beachum, J. Determination of selenium concentration and glutathione peroxidase activity in plasma and erythrocytes. Clin. Chem. 1982, 28, 311–316. [Google Scholar]
- Volpi, N.; Tarugi, P. Improvement in the high-performance liquid chromatography malondialdehyde level determination in normal human plasma. J. Chromatogr. Biomed. Sci. Appl. 1998, 713, 433–437. [Google Scholar] [CrossRef]
- Walter, M.F.; Blumberg, J.B.; Dolnikowski, G.G.; Handelman, G.J. Streamlined F2-isoprostane analysis in plasma and urine with high-performance liquid chromatography and gas chromatography/mass spectroscopy. Anal. Biochem. 2000, 280, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, I.L.; Chee, W.S.; Poulsen, L.; Offord-Cavin, E.; Rasmussen, S.E.; Frederiksen, H.; Enslen, M.; Barron, D.; Horcajada, M.N.; Williamson, G. Bioavailability is improved by enzymatic modification of the citrus flavonoid hesperidin in humans: A randomized, double-blind, crossover trial. J. Nutr. 2006, 136, 404–408. [Google Scholar] [CrossRef] [PubMed]
- FAO. Global Food Losses and Food Waste—Extent, Causes and Prevention; FAO: Rome, Italy, 2011. [Google Scholar]
- Baiano, A. Recovery of biomolecules from food wastes—A review. Molecules 2014, 19, 14821–14842. [Google Scholar] [CrossRef] [PubMed]
- Rudra, S.G.; Nishad, J.; Jakhar, N.; Kaur, C. Food industry waste: Mine of nutraceuticals. Int. J. Sci. Environ. Technol. 2015, 4, 205–229. [Google Scholar]
- Bisignano, C.; Mandalari, G.; Smeriglio, A.; Trombetta, D.; Pizzo, M.M.; Pennisi, R.; Sciortino, M.T. Almond skin extracts abrogate HSV-1 replication by blocking virus binding to the cell. Viruses 2017, 9, 178. [Google Scholar] [CrossRef] [PubMed]
- Bisignano, C.; Filocamo, A.; La Camera, E.; Zummo, S.; Fera, M.T.; Mandalari, G. Antibacterial activities of almond skins on cagA-positive and-negative clinical isolates of Helicobacter pylori. BMC Microbiol. 2013, 13, 103. [Google Scholar] [CrossRef]
- Mandalari, G.; Bisignano, C.; Genovese, T.; Mazzon, E.; Wickham, M.S.; Paterniti, I.; Cuzzocrea, S. Natural almond skin reduced oxidative stress and inflammation in an experimental model of inflammatory bowel disease. Int. J. Immunopharmacol. 2011, 11, 915–924. [Google Scholar] [CrossRef]
- Mandalari, G.; Genovese, T.; Bisignano, C.; Mazzon, E.; Wickham, M.S.; Di Paola, R.; Bisignano, G.; Cuzzocrea, S. Neuroprotective effects of almond skins in experimental spinal cord injury. Clin. Nutr. 2011, 30, 221–233. [Google Scholar] [CrossRef]
- Arena, A.; Bisignano, C.; Stassi, G.; Mandalari, G.; Wickham, M.S.; Bisignano, G. Immunomodulatory and antiviral activity of almond skins. Immunol. Lett. 2010, 132, 18–23. [Google Scholar] [CrossRef]
- Mandalari, G.; Tomaino, A.; Rich, G.T.; Lo Curto, R.B.; Arcoraci, T.; Martorana, M.; Bisignano, C.; Saija, A.; Parker, M.L.; Waldron, K.; et al. Polyphenol and nutrient release from skin of almonds during simulated human digestion. Food Chem. 2010, 122, 1083–1088. [Google Scholar] [CrossRef]
- Bartolome, B.; Monagas, M.; Garrido, I.; Gomez-Cordoves, C.; Martin-Alvarez, P.J.; Lebron-Aguilar, R.; Urpi-Sarda, M.; Llorach, R.; Andres-Lacueva, C. Almond (Prunus dulcis (Mill.) D.A. Webb) polyphenols: From chemical characterization to targeted analysis of phenolic metabolites in humans. Arch. Biochem. Biophys. 2010, 501, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Schulz, H.U.; Schurer, M.; Bassler, D.; Weiser, D. Investigation of pharmacokinetic data of hypericin, pseudohypericin, hyperforin and the flavonoids quercetin and isorhamnetin revealed from single and multiple oral dose studies with a hypericum extract containing tablet in healthy male volunteers. Arzneimittelforschung 2005, 55, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Garrido, I.; Urpi-Sarda, M.; Monagas, M.; Gomez-Cordoves, C.; Martin-Alvarez, P.J.; Llorach, R.; Bartolome, B.; Andres-Lacueva, C. Targeted analysis of conjugated and microbial-derived phenolic metabolites in human urine after consumption of an almond skin phenolic extract. J. Nutr. 2010, 140, 1799–1807. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230s–242s. [Google Scholar] [CrossRef]
- Williamson, G.; Manach, C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005, 81, 243s–255s. [Google Scholar] [CrossRef] [PubMed]
- Llorach, R.; Garrido, I.; Monagas, M.; Urpi-Sarda, M.; Tulipani, S.; Bartolome, B.; Andres-Lacueva, C. Metabolomics study of human urinary metabolome modifications after intake of almond (Prunus dulcis (Mill.) D.A. Webb) skin polyphenols. J. Proteome Res. 2010, 9, 5859–5867. [Google Scholar] [CrossRef]
- Hollman, P.C.; Cassidy, A.; Comte, B.; Heinonen, M.; Richelle, M.; Richling, E.; Serafini, M.; Scalbert, A.; Sies, H.; Vidry, S. The biological relevance of direct antioxidant effects of polyphenols for cardiovascular health in humans is not established. J. Nutr. 2011, 141, 989s–1009s. [Google Scholar] [CrossRef]
- Moon, Y.J.; Wang, X.; Morris, M.E. Dietary flavonoids: Effects on xenobiotic and carcinogen metabolism. Toxicol. In Vitro 2006, 20, 187–210. [Google Scholar] [CrossRef]
- Myhrstad, M.C.; Carlsen, H.; Nordstrom, O.; Blomhoff, R.; Moskaug, J.O. Flavonoids increase the intracellular glutathione level by transactivation of the gamma-glutamylcysteine synthetase catalytical subunit promoter. Free Radic. Biol. Med. 2002, 32, 386–393. [Google Scholar] [CrossRef]
- Milner, J.A.; McDonald, S.S.; Anderson, D.E.; Greenwald, P. Molecular targets for nutrients involved with cancer prevention. Nutr. Cancer 2001, 41, 1–16. [Google Scholar] [PubMed]
- Moskaug, J.O.; Carlsen, H.; Myhrstad, M.C.; Blomhoff, R. Polyphenols and glutathione synthesis regulation. Am. J. Clin. Nutr. 2005, 81, 277s–283s. [Google Scholar] [CrossRef] [PubMed]
- Hassimotto, N.M.; Pinto Mda, S.; Lajolo, F.M. Antioxidant status in humans after consumption of blackberry (Rubus fruticosus L.) juices with and without defatted milk. J. Agric. Food Chem. 2008, 56, 11727–11733. [Google Scholar] [CrossRef] [PubMed]
- Duthie, S.J.; Jenkinson, A.M.; Crozier, A.; Mullen, W.; Pirie, L.; Kyle, J.; Yap, L.S.; Christen, P.; Duthie, G.G. The effects of cranberry juice consumption on antioxidant status and biomarkers relating to heart disease and cancer in healthy human volunteers. Eur. J. Nutr. 2006, 45, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Garrido, I.; Monagas, M.; Gomez-Cordoves, C.; Bartolome, B. Polyphenols and antioxidant properties of almond skins: Influence of industrial processing. J. Food Sci. 2008, 73, C106–C115. [Google Scholar] [CrossRef] [PubMed]
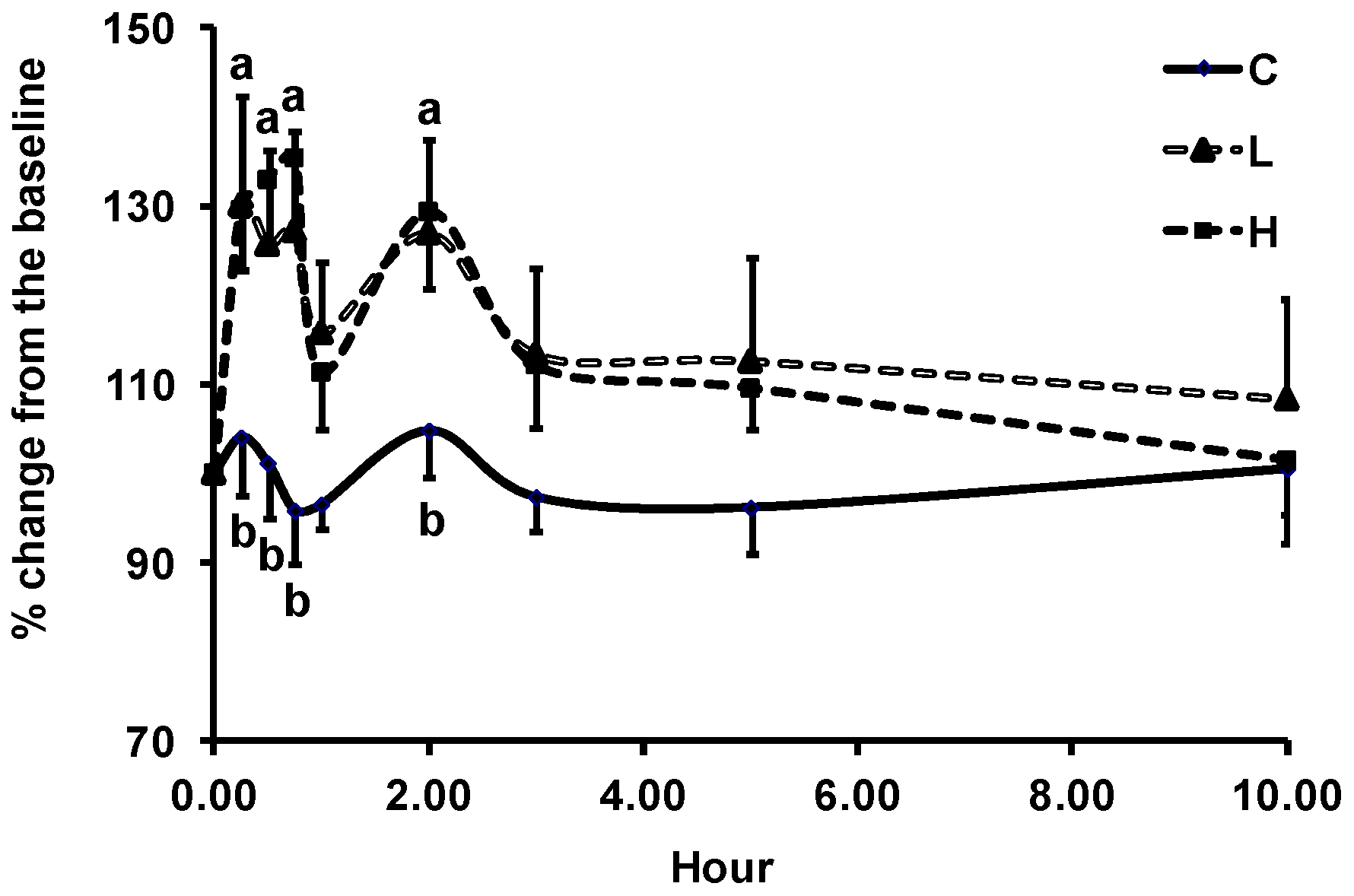
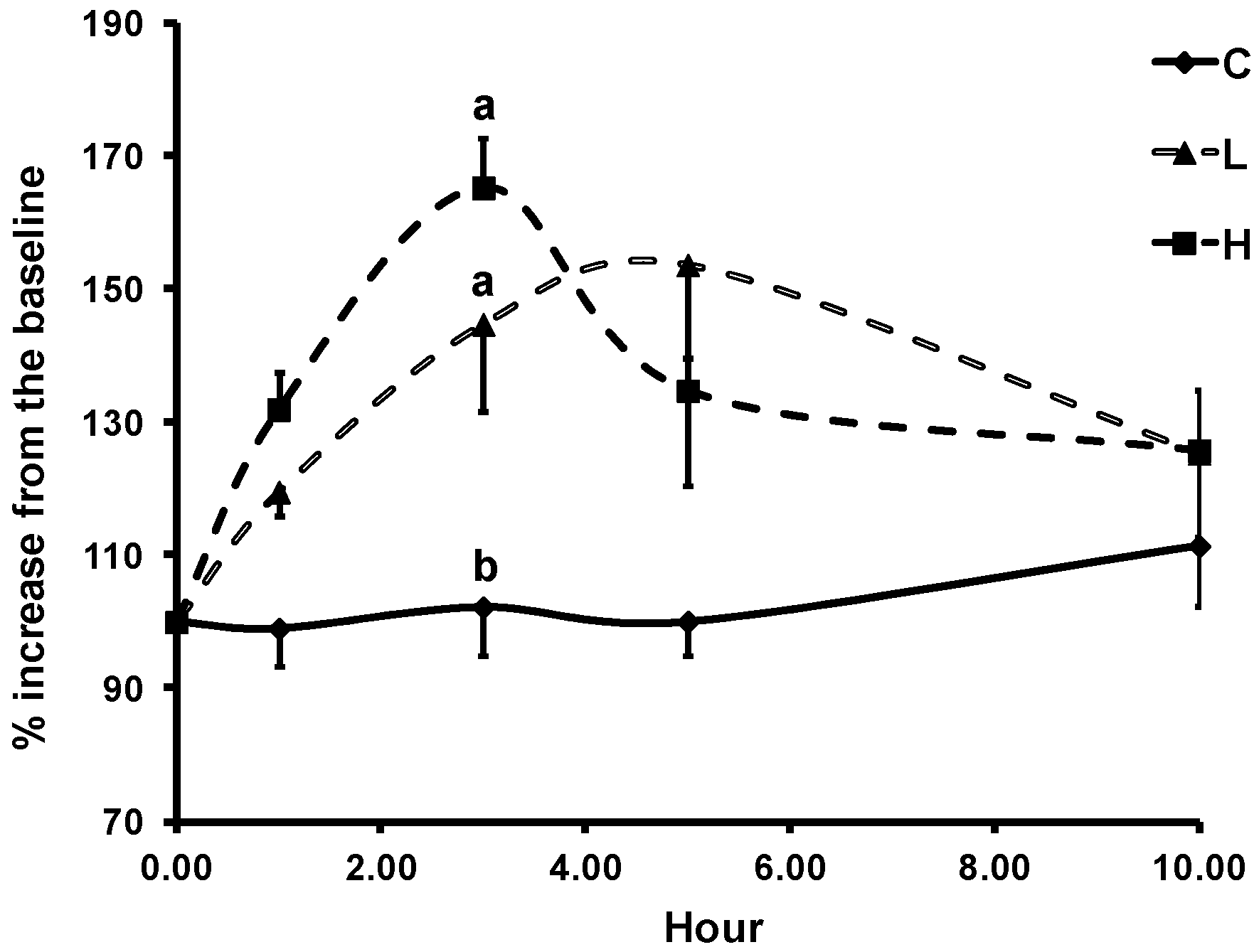
| ASP | GSH | GSSG | GSH/GSSG | GPx | ORACpca 2 |
|---|---|---|---|---|---|
| AUC (%∙h) 3 | |||||
| C | 971 ± 64 | 1037 ± 55 | 972 ± 63 a | 987 ± 43 | 1114 ± 50 |
| L | - | - | - | 1142 ± 101 | 1026 ± 51 |
| H | 1057 ± 67 | 810 ± 106 | 1649 ± 300 b | 1116 ± 47 | 1108 ± 33 |
| ASP | Plasma MDA | Plasma ISOPROSTANES | Lag Time of LDL Oxidation | Lag Time of LDL Oxidation with α-Tocopherol 2 |
|---|---|---|---|---|
| AUC (%∙h) 3 | ||||
| C | 954 ± 56 | 948 ± 125 | 973 ± 18 | 1031 ± 44 a |
| L | - | - | 1010 ± 26 | 1369 ± 152 b |
| H | 993 ± 70 | 1154 ± 198 | 990 ± 18 | 1364 ± 108 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-Y.O.; Milbury, P.E.; Blumberg, J.B. Polyphenols in Almond Skins after Blanching Modulate Plasma Biomarkers of Oxidative Stress in Healthy Humans. Antioxidants 2019, 8, 95. https://doi.org/10.3390/antiox8040095
Chen C-YO, Milbury PE, Blumberg JB. Polyphenols in Almond Skins after Blanching Modulate Plasma Biomarkers of Oxidative Stress in Healthy Humans. Antioxidants. 2019; 8(4):95. https://doi.org/10.3390/antiox8040095
Chicago/Turabian StyleChen, C.-Y. Oliver, Paul E. Milbury, and Jeffrey B. Blumberg. 2019. "Polyphenols in Almond Skins after Blanching Modulate Plasma Biomarkers of Oxidative Stress in Healthy Humans" Antioxidants 8, no. 4: 95. https://doi.org/10.3390/antiox8040095
APA StyleChen, C.-Y. O., Milbury, P. E., & Blumberg, J. B. (2019). Polyphenols in Almond Skins after Blanching Modulate Plasma Biomarkers of Oxidative Stress in Healthy Humans. Antioxidants, 8(4), 95. https://doi.org/10.3390/antiox8040095






