Melatonin Plus Folic Acid Treatment Ameliorates Reserpine-Induced Fibromyalgia: An Evaluation of Pain, Oxidative Stress, and Inflammation
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Induction of Experimental Fibromyalgia
2.3. Experimental Groups
2.4. Von Frey Hair Test
2.5. Hot Plate Test
2.6. The Tail-Flick Warm Water Test
2.7. Forced Swimming Test (FST)
2.8. Estimation of Lipid Peroxidation
2.9. Estimation of Non Protein Thiols
2.10. Estimation of Superoxide Dismutase
2.11. Estimation of Catalase
2.12. Mast Cells Evaluation
2.13. TNF-α and IL-1β ELISA
2.14. Western Blot Analysis
2.15. Immunohistochemical Analysis
2.16. Immunofluorescence Analysis
2.17. Materials
2.18. Statistical Evaluation
3. Results
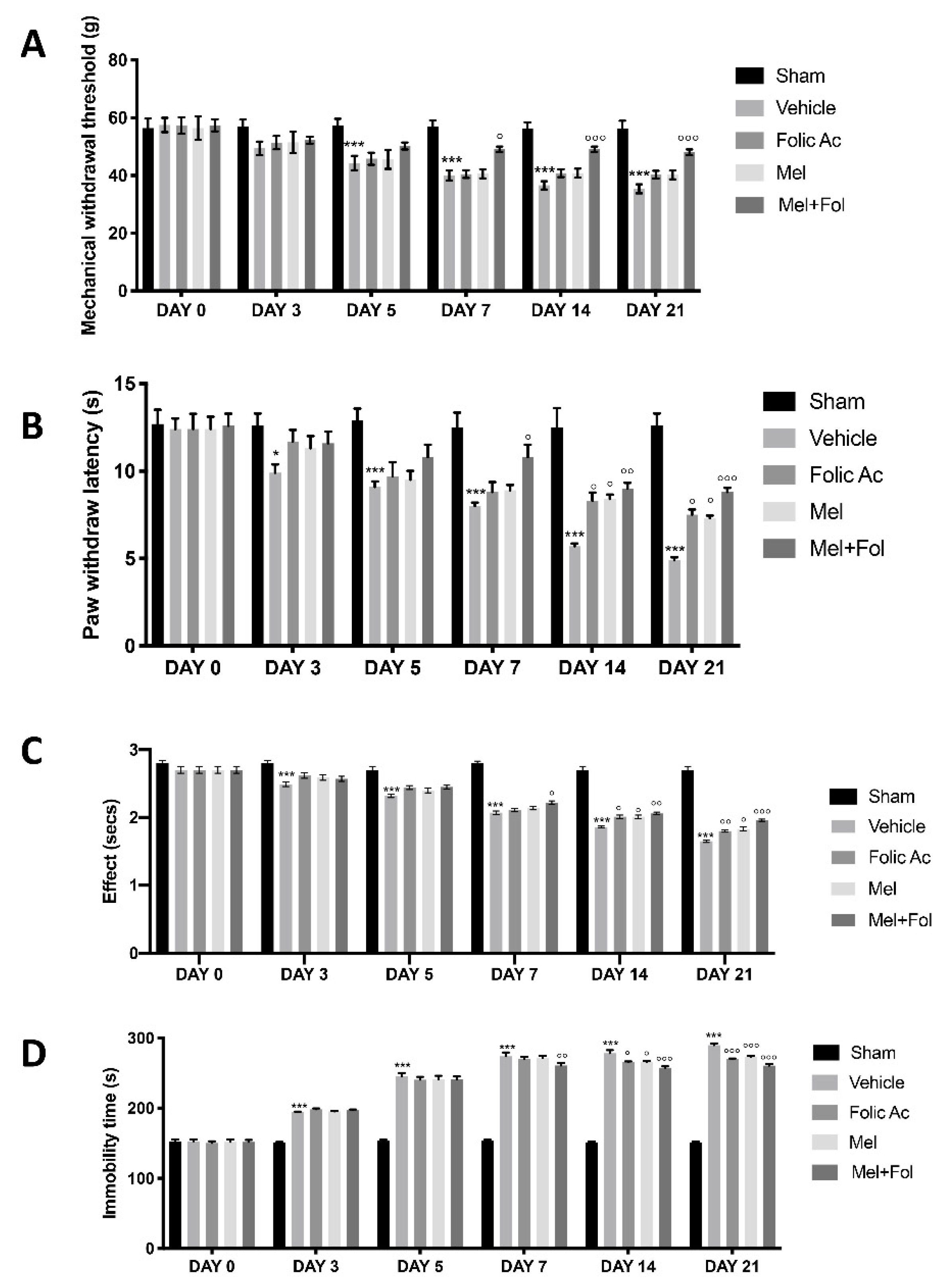
3.1. Effect of Folic Acid and Melatonin Treatment on Behavioral Defects Induced by Reserpine Injection
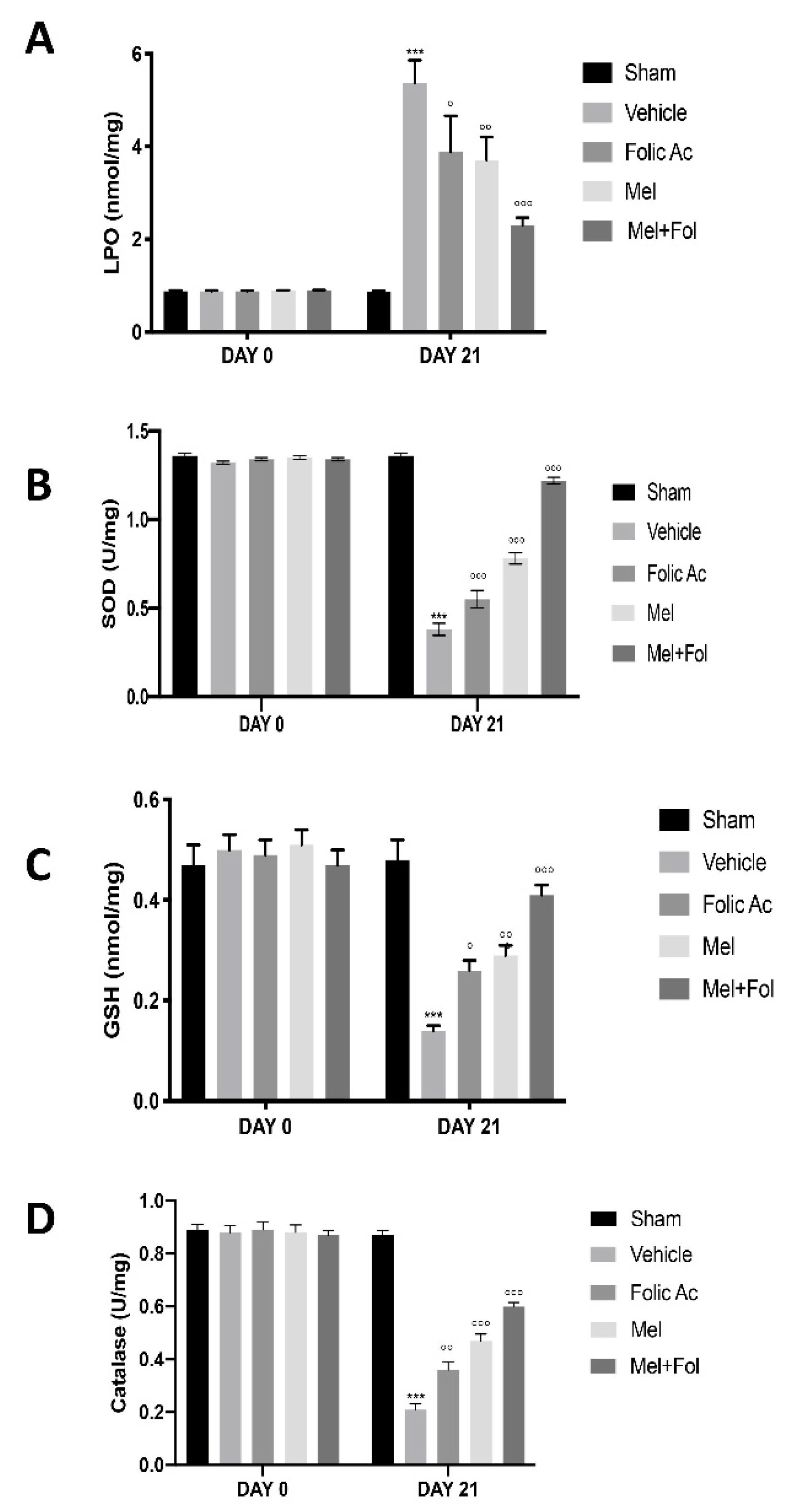
3.2. Effect of Folic Acid and Melatonin Treatment on Lipid Peroxidation and Anti-Oxidant Profile Induced by Reserpine Injection
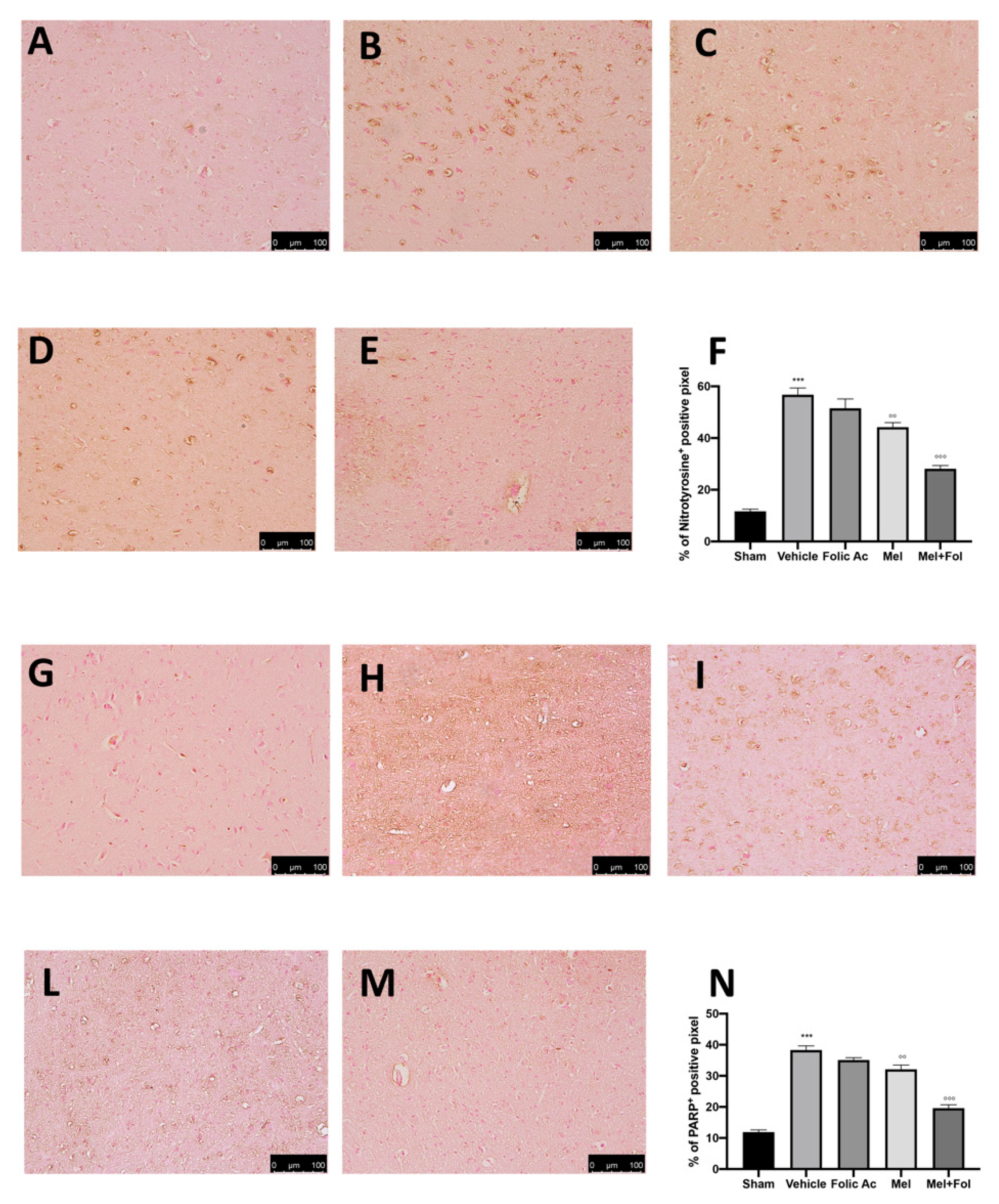
3.3. Effect of Folic Acid and Melatonin Treatment on Nitrosative Stress and PARP Expression Induced by Reserpine Injection
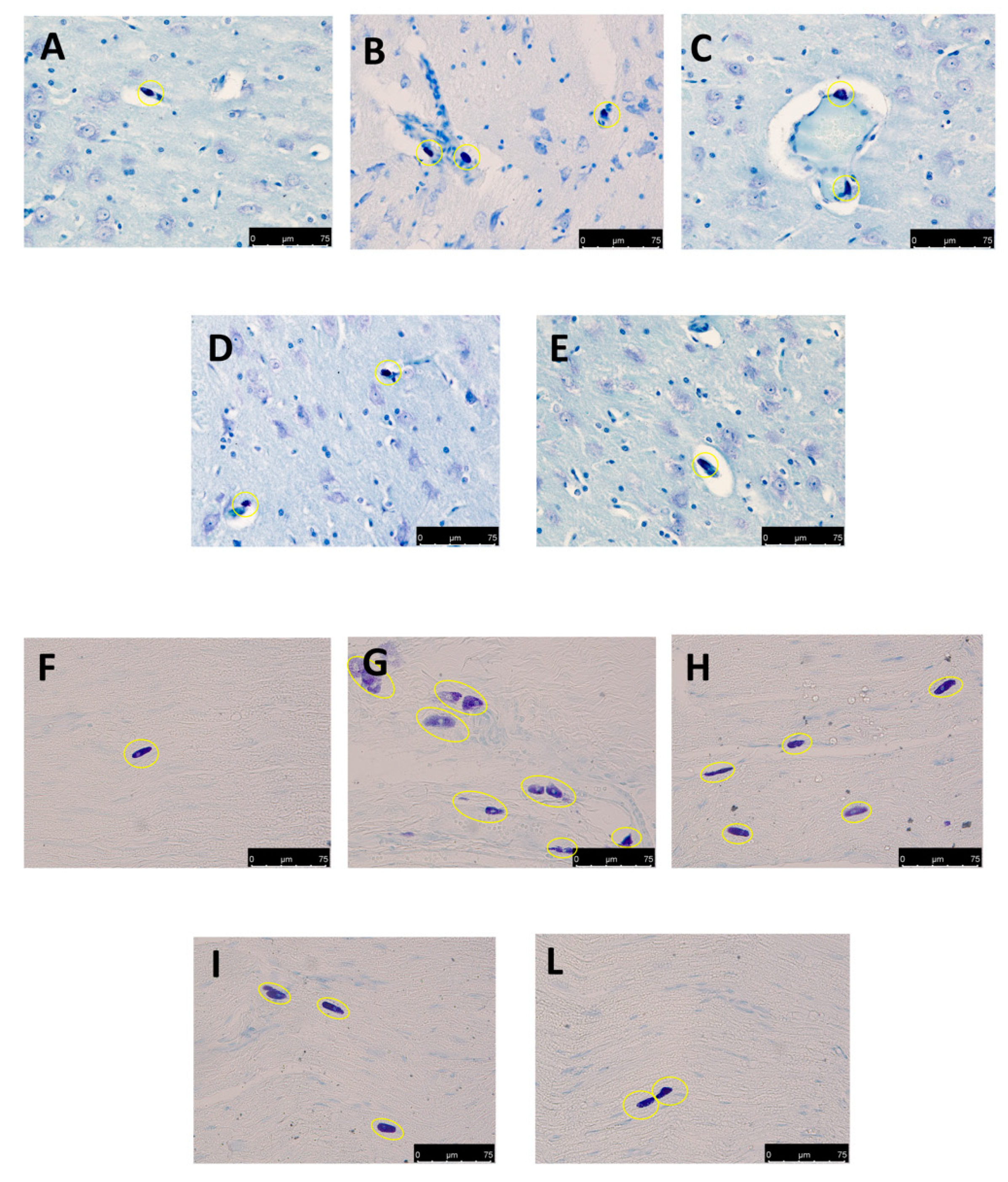
3.4. Effect of Folic Acid and Melatonin Treatment on Mast Cells Infiltration induced by Reserpine Injection
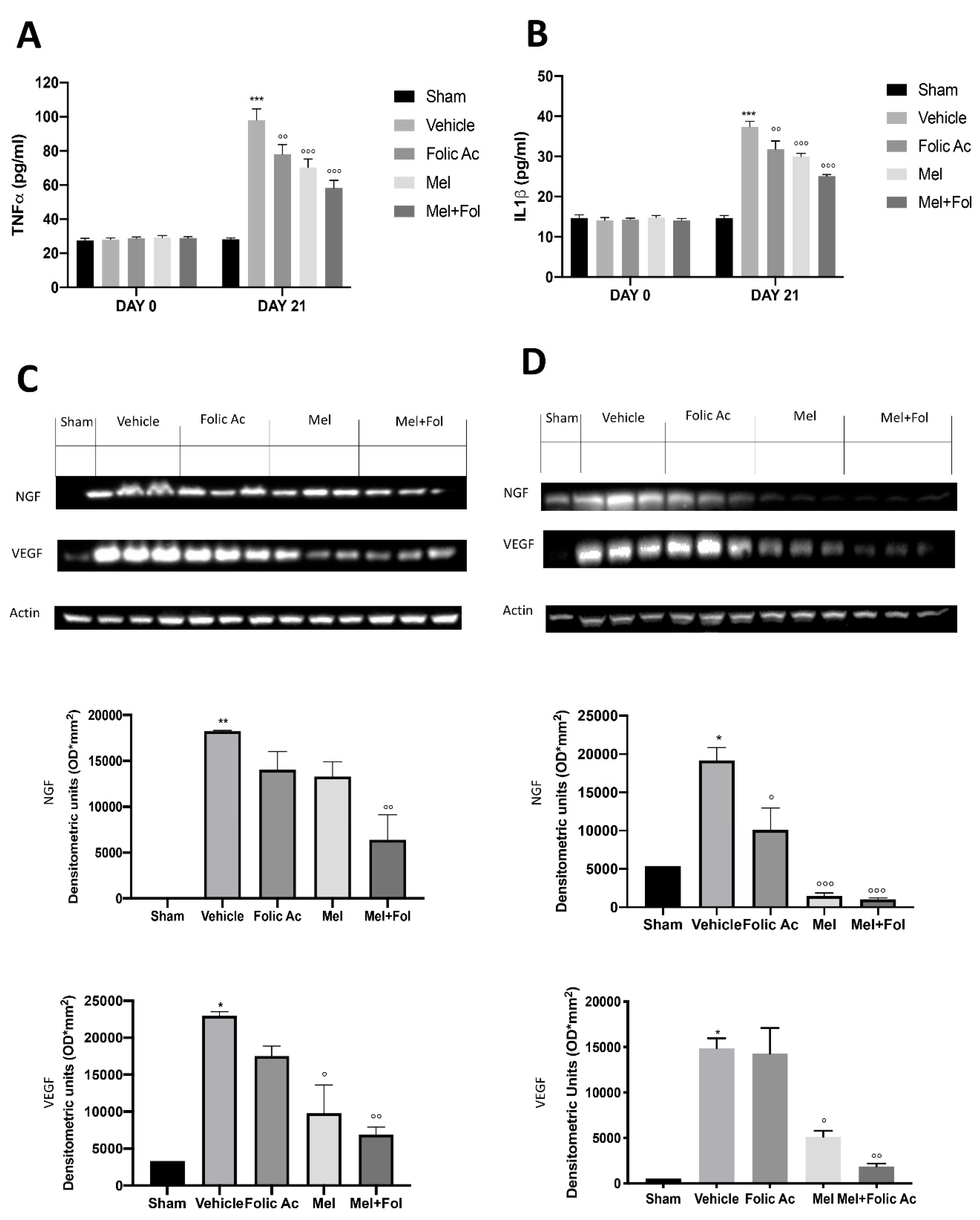
3.5. Effect of Folic acid and Melatonin Treatment on Changes in Pro-Inflammatory, Vasoactive and Neuro-Sensitizing Mediators Induced by Reserpine Injection
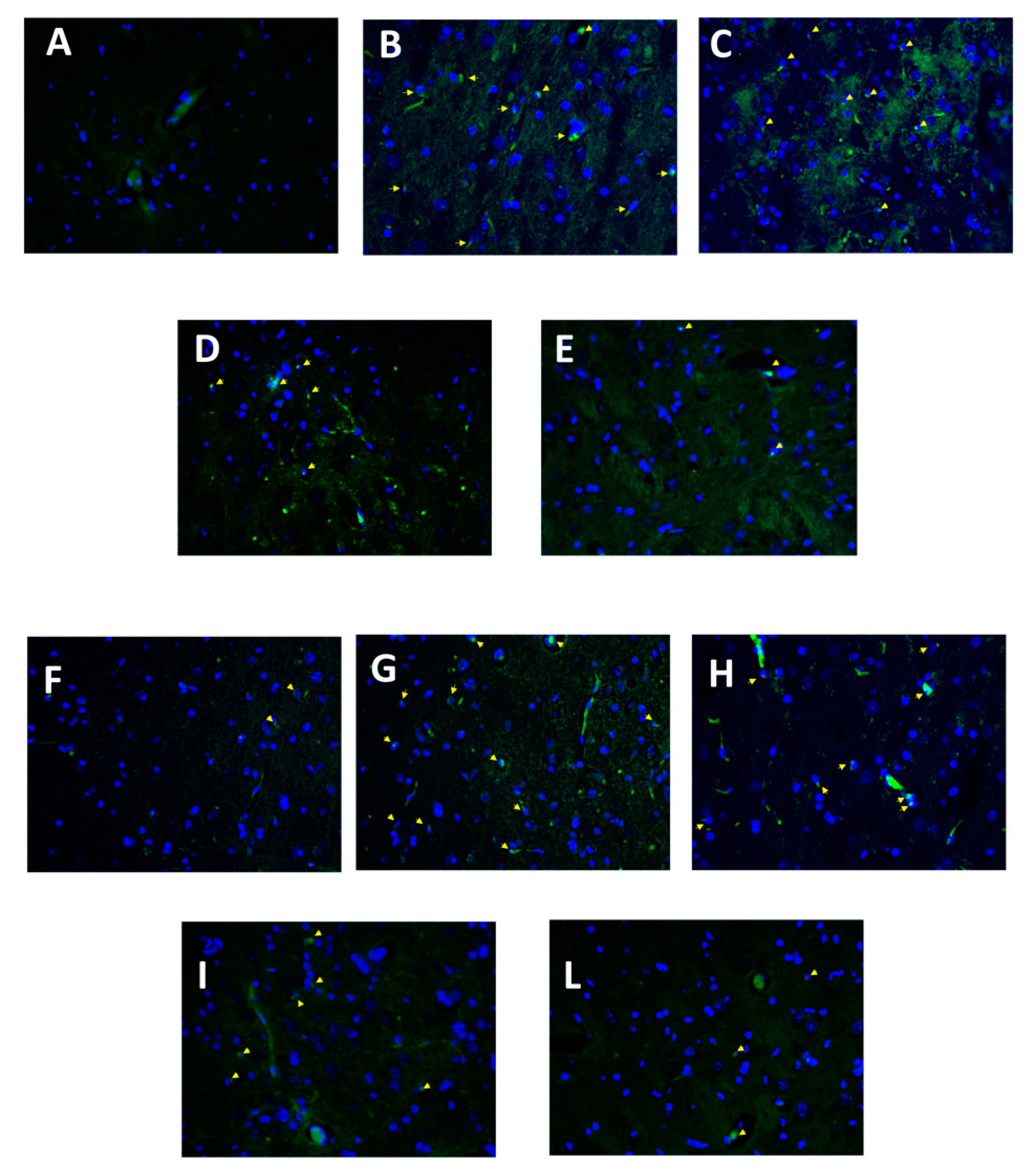
3.6. Effect of Folic Acid and Melatonin Treatment on Microglia Activation Induced by Reserpine Injection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wolfe, F.; Smythe, H.A.; Yunus, M.B.; Bennett, R.M.; Bombardier, C.; Goldenberg, D.L.; Tugwell, P.; Campbell, S.M.; Abeles, M.; Clark, P.; et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum 1990, 33, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Arnold, L.M.; Clauw, D.J.; McCarberg, B.H. FibroCollaborative, Improving the recognition and diagnosis of fibromyalgia. Mayo Clin. Proc. 2011, 86, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Eich, W.; Häuser, W.; Arnold, B.; Jäckel, W.; Offenbächer, M.; Petzke, F.; Schiltenwolf, M.; Settan, M.; Sommer, C.; Tölle, T. Das Fibromyalgiesyndrom. Der Schmerz 2012, 26, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Hauser, W.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: A modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J. Rheumatol. 2011, 38, 1113–1122. [Google Scholar] [CrossRef]
- Yunus, M.B. Central sensitivity syndromes: A new paradigm and group nosology for fibromyalgia and overlapping conditions, and the related issue of disease versus illness. Semin. Arthritis Rheum. 2008, 37, 339–352. [Google Scholar] [CrossRef]
- Glass, J.M. Fibromyalgia and cognition. J. Clin. Psychiatry 2008, 69 (Suppl. 2), 20–24. [Google Scholar]
- Menzies, V.; Lyon, D.E.; Elswick, R.K., Jr.; Montpetit, A.J.; McCain, N.L. Psychoneuroimmunological relationships in women with fibromyalgia. Biol. Res. Nurs. 2013, 15, 219–225. [Google Scholar] [CrossRef]
- Bradley, L.A. Pathophysiology of fibromyalgia. Am. J. Med. 2009, 122 (Suppl. 12), S22–S30. [Google Scholar] [CrossRef]
- Tak, L.M.; Cleare, A.J.; Ormel, J.; Manoharan, A.; Kok, I.C.; Wessely, S.; Rosmalen, J.G. Meta-analysis and meta-regression of hypothalamic-pituitary-adrenal axis activity in functional somatic disorders. Biol. Psychol. 2011, 87, 183–194. [Google Scholar] [CrossRef]
- Garcia, J.J.; Cidoncha, A.; Bote, M.E.; Hinchado, M.D.; Ortega, E. Altered profile of chemokines in fibromyalgia patients. Ann. Clin. Biochem. 2014, 51 Pt 5, 576–581. [Google Scholar] [CrossRef]
- Behm, F.G.; Gavin, I.M.; Karpenko, O.; Lindgren, V.; Gaitonde, S.; Gashkoff, P.A.; Gillis, B.S. Unique immunologic patterns in fibromyalgia. BMC Clin. Pathol. 2012, 12, 25. [Google Scholar] [CrossRef]
- Uceyler, N.; Hauser, W.; Sommer, C. Systematic review with meta-analysis: Cytokines in fibromyalgia syndrome. BMC Musculoskelet. Disord. 2011, 12, 245. [Google Scholar] [CrossRef]
- Rodriguez-Pinto, I.; Agmon-Levin, N.; Howard, A.; Shoenfeld, Y. Fibromyalgia and cytokines. Immunol. Lett. 2014, 161, 200–203. [Google Scholar] [CrossRef]
- Toker, A.; Kucuksen, S.; Kucuk, A.; Cicekler, H. Serum ischemia-modified albumin and malondialdehyde levels and superoxide dismutase activity in patients with fibromyalgia. Clin. Lab. 2014, 60, 1609–1615. [Google Scholar] [CrossRef]
- Akbas, A.; Inanir, A.; Benli, I.; Onder, Y.; Aydogan, L. Evaluation of some antioxidant enzyme activities (SOD and GPX) and their polymorphisms (MnSOD2 Ala9Val, GPX1 Pro198Leu) in fibromyalgia. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 1199–1203. [Google Scholar]
- Bagis, S.; Tamer, L.; Sahin, G.; Bilgin, R.; Guler, H.; Ercan, B.; Erdogan, C. Free radicals and antioxidants in primary fibromyalgia: An oxidative stress disorder? Rheumatol. Int. 2005, 25, 188–190. [Google Scholar] [CrossRef]
- Martinez-Lavin, M. Fibromyalgia: When Distress Becomes (Un) sympathetic Pain. Pain Res. Treat. 2012, 2012, 981565. [Google Scholar] [CrossRef]
- Littlejohn, G. Neuroinflammation in fibromyalgia and CRPS: Top-down or bottomup? Nat. Rev. Rheumatol. 2016, 12, 242. [Google Scholar] [CrossRef]
- Cassisi, G.; Sarzi-Puttini, P.; Casale, R.; Cazzola, M.; Boccassini, L.; Atzeni, F.; Stisi, S. Pain in fibromyalgia and related conditions. Reumatismo 2014, 66, 72–86. [Google Scholar] [CrossRef][Green Version]
- Sumpton, J.E.; Moulin, D.E. Fibromyalgia. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 119, pp. 513–527. [Google Scholar]
- Choy, E.H. The role of sleep in pain and fibromyalgia. Nat. Rev. Rheumatol. 2015, 11, 513–520. [Google Scholar] [CrossRef]
- Sanchez-Dominguez, B.; Bullon, P.; Roman-Malo, L.; Marin-Aguilar, F.; Alcocer-Gomez, E.; Carrion, A.M.; Sanchez-Alcazar, J.A.; Cordero, M.D. Oxidative stress, mitochondrial dysfunction and, inflammation common events in skin of patients with Fibromyalgia. Mitochondrion 2015, 21, 69–75. [Google Scholar] [CrossRef]
- Staud, R. Brain imaging in fibromyalgia syndrome. Clin. Exp. Rheumatol. 2011, 29 (Suppl. 69), S109–S117. [Google Scholar]
- Blumenthal, D.E.; Malemud, C.J. Recent strategies for drug development in fibromyalgia syndrome. Expert Rev. Neurother. 2016, 16, 1407–1411. [Google Scholar] [CrossRef]
- Danilov, A.; Kurganova, J. Melatonin in Chronic Pain Syndromes. Pain Ther. 2016, 5, 1–17. [Google Scholar] [CrossRef]
- Pernambuco, A.P.; Schetino, L.P.; Viana, R.S.; Carvalho, L.S.; d’Avila Reis, D. The involvement of melatonin in the clinical status of patients with fibromyalgia syndrome. Clin. Exp. Rheumatol. 2015, 33 (Suppl. 88), S14–S19. [Google Scholar]
- Johnston, J.D.; Skene, D.J. 60 YEARS OF NEUROENDOCRINOLOGY: Regulation of mammalian neuroendocrine physiology and rhythms by melatonin. J. Endocrinol. 2015, 226, T187–T198. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Galano, A. Melatonin: Exceeding expectations. Physiology 2014, 29, 325–333. [Google Scholar] [CrossRef]
- Manchester, L.C.; Coto-Montes, A.; Boga, J.A.; Andersen, L.P.; Zhou, Z.; Galano, A.; Vriend, J.; Tan, D.X.; Reiter, R.J. Melatonin: An ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 2015, 59, 403–419. [Google Scholar] [CrossRef]
- Ambriz-Tututi, M.; Granados-Soto, V. Oral and spinal melatonin reduces tactile allodynia in rats via activation of MT2 and opioid receptors. Pain 2007, 132, 273–280. [Google Scholar] [CrossRef]
- Andersen, L.P. The analgesic effects of exogenous melatonin in humans. Acta Anaesthesiol. Scand. 2016, 60, 1024–1025. [Google Scholar] [CrossRef]
- Ozgocmen, S.; Ozyurt, H.; Sogut, S.; Akyol, O.; Ardicoglu, O.; Yildizhan, H. Antioxidant status, lipid peroxidation and nitric oxide in fibromyalgia: Etiologic and therapeutic concerns. Rheumatol. Int. 2006, 26, 598–603. [Google Scholar] [CrossRef]
- Stanhewicz, A.E.; Kenney, W.L. Role of folic acid in nitric oxide bioavailability and vascular endothelial function. Nutr. Rev. 2017, 75, 61–70. [Google Scholar] [CrossRef]
- Nakano, E.; Higgins, J.A.; Powers, H.J. Folate protects against oxidative modification of human LDL. Br. J. Nutr. 2001, 86, 637–639. [Google Scholar] [CrossRef]
- Joshi, R.; Adhikari, S.; Patro, B.S.; Chattopadhyay, S.; Mukherjee, T. Free radical scavenging behavior of folic acid: Evidence for possible antioxidant activity. Free Radic. Biol. Med. 2001, 30, 1390–1399. [Google Scholar] [CrossRef]
- Henning, S.M.; Swendseid, M.E.; Ivandic, B.T.; Liao, F. Vitamins C, E and A and heme oxygenase in rats fed methyl/folate-deficient diets. Free Radic. Biol. Med. 1997, 23, 936–942. [Google Scholar] [CrossRef]
- Durand, P.; Prost, M.; Blache, D. Pro-thrombotic effects of a folic acid deficient diet in rat platelets and macrophages related to elevated homocysteine and decreased n-3 polyunsaturated fatty acids. Atherosclerosis 1996, 121, 231–243. [Google Scholar] [CrossRef]
- Nagakura, Y.; Oe, T.; Aoki, T.; Matsuoka, N. Biogenic amine depletion causes chronic muscular pain and tactile allodynia accompanied by depression: A putative animal model of fibromyalgia. Pain 2009, 146, 26–33. [Google Scholar] [CrossRef]
- Achon, M.; Alonso-Aperte, E.; Reyes, L.; Ubeda, N.; Varela-Moreiras, G. High-dose folic acid supplementation in rats: Effects on gestation and the methionine cycle. Br. J. Nutr. 2000, 83, 177–183. [Google Scholar] [CrossRef]
- Favero, G.; Trapletti, V.; Bonomini, F.; Stacchiotti, A.; Lavazza, A.; Rodella, L.F.; Rezzani, R. Oral Supplementation of Melatonin Protects against Fibromyalgia-Related Skeletal Muscle Alterations in Reserpine-Induced Myalgia Rats. Int. J. Mol. Sci. 2017, 18, 1389. [Google Scholar] [CrossRef]
- Bhalala, O.G.; de Morree, A. Assessment of Mechanical Allodynia in Rats Using the Electronic Von Frey Test. J. Neurosci. 2016. [Google Scholar] [CrossRef]
- Di Paola, R.; Fusco, R.; Gugliandolo, E.; Crupi, R.; Evangelista, M.; Granese, R.; Cuzzocrea, S. Co-micronized palmitoylethanolamide/polydatin treatment causes endometriotic lesion regression in a rodent model of surgically induced endometriosis. Front. Pharmacol. 2016, 7, 382. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Anton, G.; Blavet, N.; Jalfre, M. Behavioural despair in rats: A new model sensitive to antidepressant treatments. Eur. J. Pharmacol. 1978, 47, 379–391. [Google Scholar] [CrossRef]
- Detke, M.J.; Lucki, I. Detection of serotonergic and noradrenergic antidepressants in the rat forced swimming test: The effects of water depth. Behav. Brain Res. 1996, 73, 43–46. [Google Scholar] [CrossRef]
- Wills, E.D. Mechanisms of Lipid Peroxide Formation in Tissues. Role of Metals and Haematin Proteins in the Catalysis of the Oxidation Unsaturated Fatty Acids. Biochim. Biophys. Acta 1965, 98, 238–251. [Google Scholar] [CrossRef]
- Jollow, D.J.; Mitchell, J.R.; Zampaglione, N.; Gillette, J.R. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 1974, 11, 151–169. [Google Scholar] [CrossRef]
- Kono, Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch. Biochem. Biophys. 1978, 186, 189–195. [Google Scholar] [CrossRef]
- Claiborne, A. Handbook of Methods for Oxygen Radical Research; CRC Press: Boca Raton, FL, USA, 1985. [Google Scholar]
- Arora, V.; Kuhad, A.; Tiwari, V.; Chopra, K. Curcumin ameliorates reserpine-induced pain-depression dyad: Behavioural, biochemical, neurochemical and molecular evidences. Psychoneuroendocrinology 2011, 36, 1570–1581. [Google Scholar] [CrossRef]
- Fusco, R.; Cirmi, S.; Gugliandolo, E.; Di Paola, R.; Cuzzocrea, S.; Navarra, M. Anti-oxidant and anti-inflammatory effects of a flavonoid-rich extract from orange juice in experimental colitis. Free Radic. Biol. Med. 2017, 108, S37. [Google Scholar] [CrossRef]
- Di Paola, R.; Fusco, R.; Gugliandolo, E.; D’Amico, R.; Cordaro, M.; Impellizzeri, D.; Perretti, M.; Cuzzocrea, S. Formyl peptide receptor 1 signalling promotes experimental colitis in mice. Pharmacol. Res. 2019, 141, 591–601. [Google Scholar] [CrossRef]
- Jensen, K.B.; Loitoile, R.; Kosek, E.; Petzke, F.; Carville, S.; Fransson, P.; Marcus, H.; Williams, S.C.; Choy, E.; Mainguy, Y.; et al. Patients with fibromyalgia display less functional connectivity in the brain’s pain inhibitory network. Mol. Pain 2012, 8, 32. [Google Scholar] [CrossRef]
- Fatima, G.; Das, S.K.; Mahdi, A.A. Some oxidative and antioxidative parameters and their relationship with clinical symptoms in women with fibromyalgia syndrome. Int. J. Rheum. Dis. 2017, 20, 39–45. [Google Scholar] [CrossRef]
- Fatima, G.; Das, S.; Mahdi, A. Oxidative stress and antioxidative parameters and metal ion content in patients with fibromyalgia syndrome: Implications in the pathogenesis of the disease. Clin. Exp. Rheumatol. 2013, 31 (Suppl. 79), S128–S133. [Google Scholar]
- La Rubia, M.; Rus, A.; Molina, F.; Del Moral, M.L. Is fibromyalgia-related oxidative stress implicated in the decline of physical and mental health status. Clin. Exp. Rheumatol. 2013, 31 (Suppl. 79), S121–S127. [Google Scholar]
- Meeus, M.; Nijs, J.; Hermans, L.; Goubert, D.; Calders, P. The role of mitochondrial dysfunctions due to oxidative and nitrosative stress in the chronic pain or chronic fatigue syndromes and fibromyalgia patients: Peripheral and central mechanisms as therapeutic targets? Expert Opin. Ther. Targets 2013, 17, 1081–1089. [Google Scholar] [CrossRef]
- Saito, H. Mast cells. Nihon Rinsho 2005, 63 (Suppl. 4), 166–170. [Google Scholar]
- Jennings, S.; Russell, N.; Jennings, B.; Slee, V.; Sterling, L.; Castells, M.; Valent, P.; Akin, C. The Mastocytosis Society survey on mast cell disorders: Patient experiences and perceptions. J. Allergy Clin. Immunol. Pract. 2014, 2, 70–76. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Valent, P.; Akin, C. Mast Cells, Mastocytosis, and Related Disorders. N. Engl. J. Med. 2015, 373, 1885–1886. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Tsilioni, I.; Bawazeer, M. Mast Cells, Neuroinflammation and Pain in Fibromyalgia Syndrome. Front. Cell. Neurosci. 2019, 13, 353. [Google Scholar] [CrossRef]
- Lucas, H.J.; Brauch, C.M.; Settas, L.; Theoharides, T.C. Fibromyalgia—New concepts of pathogenesis and treatment. Int. J. Immunopathol. Pharmacol. 2006, 19, 5–10. [Google Scholar] [CrossRef]
- Pollack, S. Mast cells in fibromyalgia. Clin. Exp. Rheumatol. 2015, 33 (Suppl. 88), S140. [Google Scholar]
- Theoharides, T.C. Atopic conditions in search of pathogenesis and therapy. Clin. Ther. 2013, 35, 544–547. [Google Scholar] [CrossRef]
- Skaper, S.D.; Facci, L.; Zusso, M.; Giusti, P. Neuroinflammation, Mast Cells, and Glia: Dangerous Liaisons. Neuroscientist 2017, 23, 478–498. [Google Scholar] [CrossRef]
- Heron, A.; Dubayle, D. A focus on mast cells and pain. J. Neuroimmunol. 2013, 264, 1–7. [Google Scholar] [CrossRef]
- Chatterjea, D.; Martinov, T. Mast cells: Versatile gatekeepers of pain. Mol. Immunol. 2015, 63, 38–44. [Google Scholar] [CrossRef]
- Aich, A.; Afrin, L.B.; Gupta, K. Mast Cell-Mediated Mechanisms of Nociception. Int. J. Mol. Sci. 2015, 16, 29069–29092. [Google Scholar] [CrossRef]
- Edvinsson, L.; Owman, C.; Sjoberg, N.O. Autonomic nerves, mast cells, and amine receptors in human brain vessels. A histochemical and pharmacological study. Brain Res. 1976, 115, 377–393. [Google Scholar] [CrossRef]
- Lambracht-Hall, M.; Dimitriadou, V.; Theoharides, T.C. Migration of mast cells in the developing rat brain. Brain Res. Dev. Brain Res. 1990, 56, 151–159. [Google Scholar] [CrossRef]
- Galli, S.J.; Tsai, M. Mast cells: Versatile regulators of inflammation, tissue remodeling, host defense and homeostasis. J. Dermatol. Sci. 2008, 49, 7–19. [Google Scholar] [CrossRef]
- Huang, Q.-J.; Jiang, H.; Hao, X.-L.; Minor, T.R. Brain IL-1beta was involved in reserpine-induced behavioral depression in rats. Acta Pharmacol. Sin. 2004, 25, 293–296. [Google Scholar]
- Szelenyi, J.; Kiss, J.P.; Puskas, E.; Szelenyi, M.; Vizi, E.S. Contribution of differently localized alpha (2)- and beta-adrenoceptors in the modulation of TNF-alpha and IL-10 production in endotoxemic. Ann. N. Y. Acad. Sci. 2000, 917, 145–153. [Google Scholar] [CrossRef]
- Maren, S. Synapse-Specific Encoding of Fear Memory in the Amygdala. Neuron 2017, 95, 988–990. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Majid, R.M.; Marshall, J.S. Prostaglandin E-2 induces degranulation-independent production of vascular endothelial growth factor by human mast cells. J. Immunol. 2004, 172, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Chelombitko, M.A.; Fedorov, A.V.; Ilyinskaya, O.P.; Zinovkin, R.A.; Chernyak, B.V. Role of reactive oxygen species in mast cell degranulation. Biochemistry 2016, 81, 1564–1577. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.J.; Allen, S.J.; Dawbarn, D. Targeting Nerve Growth Factor in Pain What is the Therapeutic Potential. Biodrugs 2008, 22, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Vincent, L.; Vang, D.; Nguyen, J.; Gupta, M.; Luk, K.; Ericson, M.E.; Simone, D.A.; Gupta, K. Mast cell activation contributes to sickle cell pathobiology and pain in mice. Blood 2013, 122, 1853–1862. [Google Scholar] [CrossRef]
- Kainz, V.; Levy, D.; Strassman, A.M. Mast cell degranulation activates a pain pathway underlying migraine headache. Cephalalgia 2007, 27, 598. [Google Scholar]
- Taiwo, O.B.; Kovacs, K.J.; Sun, Y.X.; Larson, A.A. Unilateral spinal nerve ligation leads to an asymmetrical distribution of mast cells in the thalamus of female but not male mice. Pain 2005, 114, 131–140. [Google Scholar] [CrossRef]
- Skaper, S.D.; Giusti, P.; Facci, L. Microglia and mast cells: Two tracks on the road to neuroinflammation. Faseb J. 2012, 26, 3103–3117. [Google Scholar] [CrossRef]
- Skaper, S.D.; Facci, L.; Giusti, P. Neuroinflammation, Microglia and Mast Cells in the Pathophysiology of Neurocognitive Disorders: A Review. CNS Neurol. Disord. Drug Targets 2014, 13, 1654–1666. [Google Scholar] [CrossRef]
- Banati, R.B. Brain plasticity and microglia: Is transsynaptic glial activation in the thalamus after limb denervation linked to cortical plasticity and central sensitisation? J. Physiol. Paris 2002, 96, 289–299. [Google Scholar] [CrossRef]
- Hansson, E. Long-term pain, neuroinflammation and glial activation. Scand. J. Pain 2010, 1, 67–72. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fusco, R.; Siracusa, R.; D’Amico, R.; Peritore, A.F.; Cordaro, M.; Gugliandolo, E.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. Melatonin Plus Folic Acid Treatment Ameliorates Reserpine-Induced Fibromyalgia: An Evaluation of Pain, Oxidative Stress, and Inflammation. Antioxidants 2019, 8, 628. https://doi.org/10.3390/antiox8120628
Fusco R, Siracusa R, D’Amico R, Peritore AF, Cordaro M, Gugliandolo E, Crupi R, Impellizzeri D, Cuzzocrea S, Di Paola R. Melatonin Plus Folic Acid Treatment Ameliorates Reserpine-Induced Fibromyalgia: An Evaluation of Pain, Oxidative Stress, and Inflammation. Antioxidants. 2019; 8(12):628. https://doi.org/10.3390/antiox8120628
Chicago/Turabian StyleFusco, Roberta, Rosalba Siracusa, Ramona D’Amico, Alessio Filippo Peritore, Marika Cordaro, Enrico Gugliandolo, Rosalia Crupi, Daniela Impellizzeri, Salvatore Cuzzocrea, and Rosanna Di Paola. 2019. "Melatonin Plus Folic Acid Treatment Ameliorates Reserpine-Induced Fibromyalgia: An Evaluation of Pain, Oxidative Stress, and Inflammation" Antioxidants 8, no. 12: 628. https://doi.org/10.3390/antiox8120628
APA StyleFusco, R., Siracusa, R., D’Amico, R., Peritore, A. F., Cordaro, M., Gugliandolo, E., Crupi, R., Impellizzeri, D., Cuzzocrea, S., & Di Paola, R. (2019). Melatonin Plus Folic Acid Treatment Ameliorates Reserpine-Induced Fibromyalgia: An Evaluation of Pain, Oxidative Stress, and Inflammation. Antioxidants, 8(12), 628. https://doi.org/10.3390/antiox8120628










