Anti-Oxidized LDL Antibodies and Coronary Artery Disease: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
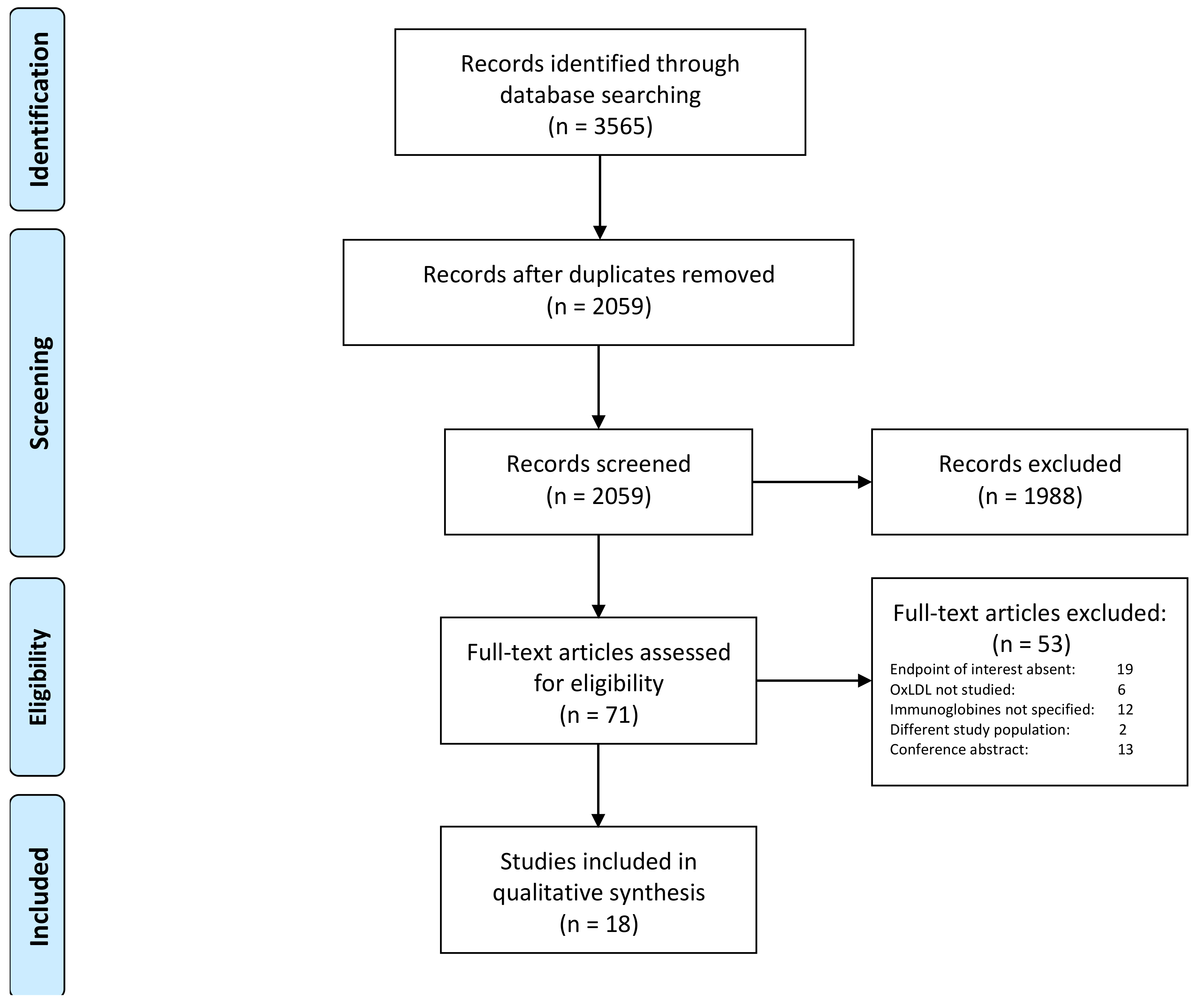
2.2. Review Method and Selection Criteria
2.3. Data Extraction
3. Results
3.1. Autoantibodies against oxLDL and Severity of CAD
3.2. Autoantibodies against oxLDL and Cardiovascular Events in Patients without Established CAD
3.3. Autoantibodies against oxLDL and Cardiovascular Events in Patients with Established CAD
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. The Top 10 Causes of Death; World Health Organization: Genewa, Switzerland, 24 May 2018. [Google Scholar]
- Hartley, A.; Haskard, D.; Khamis, R. Oxidized ldl and anti-oxidized ldl antibodies in atherosclerosis–novel insights and future directions in diagnosis and therapy. Trends Cardiovasc. Med. 2019, 29, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Fefer, P.; Tsimikas, S.; Segev, A.; Sparkes, J.; Otsuma, F.; Kolodgie, F.; Virmani, R.; Juliano, J.; Charron, T.; Strauss, B.H. The role of oxidized phospholipids, lipoprotein (a) and biomarkers of oxidized lipoproteins in chronically occluded coronary arteries in sudden cardiac death and following successful percutaneous revascularization. Cardiovasc. Revascularization Med. 2012, 13, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, R.A.; Kolodgie, F.; Ravandi, A.; Leibundgut, G.; Hu, P.P.; Prasad, A.; Mahmud, E.; Dennis, E.; Curtiss, L.K.; Witztum, J.L. Differential expression of oxidation-specific epitopes and apolipoprotein (a) in progressing and ruptured human coronary and carotid atherosclerotic lesions. J. Lipid Res. 2012, 53, 2773–2790. [Google Scholar] [CrossRef] [PubMed]
- Bertoia, M.L.; Pai, J.K.; Lee, J.-H.; Taleb, A.; Joosten, M.M.; Mittleman, M.A.; Yang, X.; Witztum, J.L.; Rimm, E.B.; Tsimikas, S. Oxidation-specific biomarkers and risk of peripheral artery disease. J. Am. Coll. Cardiol. 2013, 61, 2169–2179. [Google Scholar] [CrossRef]
- Byun, Y.S.; Yang, X.; Bao, W.; DeMicco, D.; Laskey, R.; Witztum, J.L.; Tsimikas, S.; Investigators, S.T. Oxidized phospholipids on apolipoprotein b-100 and recurrent ischemic events following stroke or transient ischemic attack. J. Am. Coll. Cardiol. 2017, 69, 147–158. [Google Scholar] [CrossRef]
- Gao, S.; Zhao, D.; Wang, M.; Zhao, F.; Han, X.; Qi, Y.; Liu, J. Association between circulating oxidized ldl and atherosclerotic cardiovascular disease: A meta-analysis of observational studies. Can. J. Cardiol. 2017, 33, 1624–1632. [Google Scholar] [CrossRef]
- Holvoet, P.; Vanhaecke, J.; Janssens, S.; Van de Werf, F.; Collen, D. Oxidized ldl and malondialdehyde-modified ldl in patients with acute coronary syndromes and stable coronary artery disease. Circulation 1998, 98, 1487–1494. [Google Scholar] [CrossRef]
- Nishi, K.; Itabe, H.; Uno, M.; Kitazato, K.T.; Horiguchi, H.; Shinno, K.; Nagahiro, S. Oxidized ldl in carotid plaques and plasma associates with plaque instability. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1649–1654. [Google Scholar] [CrossRef]
- Tsimikas, S.; Willeit, P.; Willeit, J.; Santer, P.; Mayr, M.; Xu, Q.; Mayr, A.; Witztum, J.L.; Kiechl, S. Oxidation-specific biomarkers, prospective 15-year cardiovascular and stroke outcomes, and net reclassification of cardiovascular events. J. Am. Coll. Cardiol. 2012, 60, 2218–2229. [Google Scholar] [CrossRef]
- Uno, M.; Harada, M.; Takimoto, O.; Kitazato, K.T.; Suzue, A.; Yoneda, K.; Morita, N.; Itabe, H.; Nagahiro, S. Elevation of plasma oxidized ldl in acute stroke patients is associated with ischemic lesions depicted by dwi and predictive of infarct enlargement. Neurol. Res. 2005, 27, 94–102. [Google Scholar] [CrossRef]
- Wang, A.; Yang, Y.; Su, Z.; Yue, W.; Hao, H.; Ren, L.; Wang, Y.; Cao, Y.; Wang, Y. Association of oxidized low-density lipoprotein with prognosis of stroke and stroke subtypes. Stroke 2017, 48, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Stang, A. Critical evaluation of the newcastle-ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Björkbacka, H.; Alm, R.; Persson, M.; Hedblad, B.; Nilsson, J.; Fredrikson, G.N. Low levels of apolipoprotein b-100 autoantibodies are associated with increased risk of coronary events. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Maiolino, G.; Pedon, L.; Cesari, M.; Frigo, A.C.; Barisa, M.; Rossitto, G.; Seccia, T.M.; Zanchetta, M.; Rossi, G.P. Antibodies to malondialdehyde oxidized low-density lipoproteins predict long term cardiovascular mortality in high risk patients. Int. J. Cardiol. 2013, 168, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Meeuwsen, J.A.L.; Van Duijvenvoorde, A.; Gohar, A.; Kozma, M.O.; Van de Weg, S.M.; Gijsberts, C.M.; Haitjema, S.; Björkbacka, H.; Fredrikson, G.N.; De Borst, G.J. High levels of (un) switched memory b cells are associated with better outcome in patients with advanced atherosclerotic disease. J. Am. Heart Assoc. 2017, 6, e005747. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Clopton, P.; Ayers, C.; Khera, A.; De Lemos, J.A.; Witztum, J.L.; Tsimikas, S. Relationship of autoantibodies to mda-ldl and apob-immune complexes to sex, ethnicity, subclinical atherosclerosis, and cardiovascular events. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Tsimikas, S.; Brilakis, E.S.; Lennon, R.J.; Miller, E.R.; Witztum, J.L.; McConnell, J.P.; Kornman, K.S.; Berger, P.B. Relationship of igg and igm autoantibodies to oxidized low density lipoprotein with coronary artery disease and cardiovascular events. J. Lipid Res. 2007, 48, 425–433. [Google Scholar] [CrossRef]
- Wilson, P.W.F.; Ben-Yehuda, O.; McNamara, J.; Massaro, J.; Witztum, J.; Reaven, P.D. Autoantibodies to oxidized ldl and cardiovascular risk: The framingham offspring study. Atherosclerosis 2006, 189, 364–368. [Google Scholar] [CrossRef]
- Khamis, R.Y.; Hughes, A.D.; Caga-Anan, M.; Chang, C.L.; Boyle, J.J.; Kojima, C.; Welsh, P.; Sattar, N.; Johns, M.; Sever, P. High serum immunoglobulin g and m levels predict freedom from adverse cardiovascular events in hypertension: A nested case-control substudy of the anglo-scandinavian cardiac outcomes trial. Ebiomedicine 2016, 9, 372–380. [Google Scholar] [CrossRef]
- Ravandi, A.; Boekholdt, S.M.; Mallat, Z.; Talmud, P.J.; Kastelein, J.J.P.; Wareham, N.J.; Miller, E.R.; Benessiano, J.; Tedgui, A.; Witztum, J.L. Relationship of igg and igm autoantibodies and immune complexes to oxidized ldl with markers of oxidation and inflammation and cardiovascular events: Results from the epic-norfolk study. J. Lipid Res. 2011, 52, 1829–1836. [Google Scholar] [CrossRef]
- Van den Berg, V.J.; Haskard, D.O.; Fedorowski, A.; Hartley, A.; Kardys, I.; Caga-Anan, M.; Akkerhuis, K.M.; Oemrawsingh, R.M.; Van Geuns, R.J.; De Jaegere, P. Igm anti-malondialdehyde low density lipoprotein antibody levels indicate coronary heart disease and necrotic core characteristics in the nordic diltiazem (nordil) study and the integrated imaging and biomarker study 3 (ibis-3). Ebiomedicine 2018, 36, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Bilgen, D.; Sönmez, H.; Ekmekçi, H.; Ulutin, T.; Öztürk, Z.; Kökoğlu, E.; Bayram, Ç.; Soner, A.; Domaniç, N. The relationship of tfpi, lp (a), and oxidized ldl antibody levels in patients with coronary artery disease. Clin. Biochem. 2005, 38, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Che, J.; Li, G.; Wang, W.; Li, Q.; Liu, H.; Chen, K.; Liu, T. Serum autoantibodies against human oxidized low-density lipoproteins are inversely associated with severity of coronary stenotic lesions calculated by gensini score. Cardiol. J. 2011, 18, 364–370. [Google Scholar] [PubMed]
- Chen, Q.; Reis, S.E.; Kammerer, C.; Craig, W.; McNamara, D.M.; Holubkov, R.; Sharaf, B.L.; Sopko, G.; Pauly, D.F.; Merz, C.N.B. Association of anti-oxidized ldl and candidate genes with severity of coronary stenosis in the women’s ischemia syndrome evaluation study. J. Lipid Res. 2011, 52, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Sanchez, L.; Chinchurreta, P.; Garcia-Fuentes, E.; Mora, M.; Tinahones, F.J. A higher level of igm anti-oxidized ldl antibodies is associated with a lower severity of coronary atherosclerosis in patients on statins. Int. J. Cardiol. 2010, 145, 263–264. [Google Scholar] [CrossRef]
- Gruzdeva, O.; Uchasova, E.; Dyleva, Y.; Belik, E.; Karetnikova, V.; Shilov, A.; Barbarash, O. Multivessel coronary artery disease, free fatty acids, oxidized ldl and its antibody in myocardial infarction. Lipids Health Dis. 2014, 13, 111. [Google Scholar] [CrossRef]
- Moohebati, M.; Kabirirad, V.; Ghayour-Mobarhan, M.; Esmaily, H.; Tavallaie, S.; Akhavan Rezayat, A.; Pourghadamyari, H.; Sahebkar, A. Investigation of serum oxidized low-density lipoprotein igg levels in patients with angiographically defined coronary artery disease. Int. J. Vasc. Med. 2014, 2014, 845960. [Google Scholar] [CrossRef]
- Rossi, G.P.; Cesari, M.; De Toni, R.; Zanchetta, M.; Maiolino, G.; Pedon, L.; Ganzaroli, C.; Maiolino, P.; Pessina, A.C. Antibodies to oxidized low-density lipoproteins and angiographically assessed coronary artery disease in white patients. Circulation 2003, 108, 2467–2472. [Google Scholar] [CrossRef]
- Soto, Y.; Conde, H.; Aroche, R.; Brito, V.; Luaces, P.; Nasiff, A.; Obregón, Á.; Vázquez López, A.M. Autoantibodies to oxidized low density lipoprotein in relation with coronary artery disease. Hum. Antibodies 2009, 18, 109–117. [Google Scholar] [CrossRef]
- Cheng, J.M.; Garcia-Garcia, H.M.; De Boer, S.P.M.; Kardys, I.; Heo, J.H.; Akkerhuis, K.M.; Oemrawsingh, R.M.; Van Domburg, R.T.; Ligthart, J.; Witberg, K.T. In vivo detection of high-risk coronary plaques by radiofrequency intravascular ultrasound and cardiovascular outcome: Results of the atheroremo-ivus study. Eur. Heart J. 2013, 35, 639–647. [Google Scholar] [CrossRef]
- Resch, U.; Tatzber, F.; Budinsky, A.; Sinzinger, H. Reduction of oxidative stress and modulation of autoantibodies against modified low-density lipoprotein after rosuvastatin therapy. Br. J. Clin. Pharmacol. 2006, 61, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Que, X.; Hung, M.-Y.; Yeang, C.; Gonen, A.; Prohaska, T.A.; Sun, X.; Diehl, C.; Määttä, A.; Gaddis, D.E.; Bowden, K. Oxidized phospholipids are proinflammatory and proatherogenic in hypercholesterolaemic mice. Nature 2018, 558, 301. [Google Scholar] [CrossRef] [PubMed]
| Author | Year | Population (n) | Investigated Biomarkers | Sample Size | Age in Years Mean (SD) | Male Gender (%) | DM (%) | HTN (%) | Current Smoking (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CAD | no CAD | CAD | no CAD | CAD | no CAD | CAD | no CAD | CAD | no CAD | CAD | no CAD | ||||
| Cohort studies | |||||||||||||||
| Björkbacka [14] | 2016 | Population-based prospective cohort (5393) | anti-p45 IgG, anti-p45 IgM, anti-p210 IgG, anti-p210 IgM | 398 | 4995 | 61.1 [57.2–64.7] * | 57.6 [52.2–62.5] * | 61.6 | 39.8 | 19.8 | 7.4 | 81.2 | 62.5 | 33.8 | 26.1 |
| Maiolino [15] | 2012 | CAG patients (733) b | IgG anti-MDA-LDL | 733 | 63.3 | 78.4 | NR | NR | 7.3 | ||||||
| Meeuwsen [16] | 2017 | 168 endarterectomy patients | IgG anti-oxLDL, IgM anti-oxLDL | 168 | 70.1 (9.6) | 62.8 | 23.8 | 86.6 | 35.7 | ||||||
| Prasad [17] | 2017 | Population-based prospective cohort (3509) | IgG anti-MDA-LDL, IgM anti-MDA-LDL | 190 | 2914 | 43.7 (10.1) † | 44.1† | 11.6† | 34.4† | 29.3† | |||||
| Tsimikas [18] | 2007 | CAG patients (504) a | IgG anti-MDA-LDL, IgM anti-MDA-LDL | 504 | 60.1 | 61.7 | NR | 46.0 | 7.9 | ||||||
| Tsimikas [10] | 2012 | Population-based prospective cohort (765) | IgG anti-cu-oxLDL, IgM anti-MDA-LDL | 138 | 627 | 68.8 (10.5) | 61.4 (10.9) | 48.5 | 59.4 | 13.8 | 6.9 | 75.4 | 66.0 | 17.4 | 20.4 |
| Wilson [19] | 2006 | Population-based prospective cohort (2619) | IgG anti-MDA-LDL | 151 | 2468 | 49.52 † | NR | NR | NR | NR | NR | NR | NR | NR | |
| Case-control studies | |||||||||||||||
| Khamisc [20] | 2016 | Hypertensive patients receiving blood pressure-lowering treatment (1852) | IgG anti-MDA-LDL, IgM anti-MDA-LDL | 485 | 1367 | 65.3 (7.8) | 65.3 (7.6) | 84.5 | 84.9 | 30.9 | 26.3 | NR | NR | 7.8 | 7.6 |
| Ravandi [21] | 2011 | Population-based prospective cohort (2471) | IgG anti-MDA-LDL, IgM anti-MDA-LDL | 748 | 1723 | 65.4 (7.8) | 65.4 (7.8) | 62.8 | 61.6 | NR | NR | NR | NR | 15.5 | 8.6 |
| van den Berg d [22] | 2018 | 87 subjects with CHD, 227 subjects free of CHD | IgG anti-MDA-LDL, IgM anti-MDA-LDL | 87 | 227 | 60.4 (6.3) | 59.8 (6.4) | 67.8 | 64.8 | 14.9 | 7.9 | 100 | 100 | 8 | 6.6 |
| Cross-sectional studies | |||||||||||||||
| Bilgen [23] | 2005 | CAD patients (136). healthy controls (31) | IgG anti-oxLDL | 136 | 31 | 57.6 (11.3) | 53.5 (10.2) | 70.5 | 67.8 | 13.2 | 0 | 30.9 | 0 | 47.8 | 0 |
| Che [24] | 2011 | CAG patients (154) | IgG anti-oxLDL | 117 | 37 | 63.7 (10.6) | 62.0 (11.5) | 63.4 | 64.9 | 24.8 | 16.2 | 64.1 | 70.3 | 43.6 | 24.3 |
| Chen [25] | 2011 | CAG patients (558) | IgG anti-MDA-LDL, IgM anti-MDA-LDL | 334 | 224 | 60.7 | 53.2 | 0 | 0 | 31.4 | 17.9 | 63.2 | 47.3 | 21.3 | 16.5 |
| Garrido-Sanchez [26] | 2009 | CAG patients (236) | IgG anti-oxLDL, IgM anti-oxLDL | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Gruzdeva [27] | 2014 | STEMI patients (400), 33 healthy controls | IgG anti-oxLDL | 400 | NR | 60.3 (1.1) | NR | 67.5 | NR | NR | NR | 69.8 | NR | 42 | NR |
| Maiolino [15] | 2012 | CAG patients (733) b | IgG anti-MDA-LDL | 733 | 63.3 | 78.4 | NR | NR | 7.3 | ||||||
| Moohebati [28] | 2013 | CAG patients (63), healthy controls (24) | IgG anti-oxLDL | 31 | 56 | 59.4 (10.1) | 58.3 | 38.7 | 58.9 | 41.9 | 10.7 | 64.5 | 44.6 | 51.6 | 25 |
| Rossi [29] | 2003 | CAG patients (529) | IgG anti-MDA-LDL | 445 | 84 | 63 | 62 | NR | NR | NR | NR | NR | NR | NR | NR |
| Soto [30] | 2009 | CAG patients (20), healthy controls (10) | IgG anti-oxLDL, IgM anti-oxLDL | 13 | 17 | 59 | 36.5 | 100 | 52.9 | 23.1 | 0 | 69.2 | 11.8 | 46.2 | 11.8 |
| Tsimikas [18] | 2007 | CAG patients (504) a | IgG anti-MDA-LDL, IgM anti-MDA-LDL | 504 | 60.1 | 61.7 | NR | 46.0 | 7.9 | ||||||
| van den Berg d [22] | 2018 | 87 subjects with CHD, 227 subjects free of CHD | IgG anti-MDA-LDL, IgM anti-MDA-LDL | 87 | 227 | 60.4 (6.3) | 59.8 (6.4) | 67.8 | 64.8 | 14.9 | 7.9 | 100 | 100 | 8 | 6.6 |
| Author | Index Group (n) | Reference Group (n) | Statistical Method | Confounders Adjusted for in Statistical Analysis | Outcome | IgG Anti-oxLDL | IgM Anti-oxLDL |
|---|---|---|---|---|---|---|---|
| Bilgen [23] | CAG patients (136) | Healthy controls (36) | ANOVA with post-hoc Tukey | Diseased coronary arteries (n) | NS | ||
| Che [24] | CAG patients (154) | Multiple linear stepwise regression | hs-CRP, fasting glucose, serum albumin | ln(Gensini score + 1) | Beta −0.020 (−0.033, −0.006) | ||
| Chen [25] | Female CAG patients (558) | ANOVA | Age, smoking, and total and LDL cholesterol | <20% stenosis vs >20% stenosis | NS | ↓ | |
| Garrido-Sanchez [26] | CAG patiens (236) | NR | Diseased coronary arteries (n) | NS | ↓ | ||
| Gruzdeva [27] | STEMI patients (400) | Healthy controls (33) | Kruskal-Wallis, followed by Mann-Whitney with Bonferonni correction | Diseased coronary arteries (>75%) (n) | ↑ | ||
| Maiolino [15] | CAG patients (733) | ANOVA | Duke CAD score | NS | |||
| Moohebati [28] | CAG patients (63) | Healthy controls (24) | ANOVA | 1 or more stenosis (>50%) vs no stenosis vs healthy control | NS | ||
| Rossi [29] | CAG patients (529) | ANOVA | Diseased coronary arteries (>50%) (n) | NS | |||
| Soto [30] | CAG patients (20) | Healthy controls (10) | ANOVA | 1 or more stenosis (>50%) vs no stenosis vs healthy control | unclear | ↓ | |
| Tsimikas [18] | CAG patients (504) | Logistic regression | Age, gender, LDL-C, smoking, HDL, hypertension | obstructive CAD (>50%) vs no obstructive CAD | NS, OR not given | NS, OR not given | |
| van den Berg [22] | CAG patients (143) | Linear regression, trend test among four quartiles | Age, gender, diabetes, smoking, previous statin use, LDL and HDL cholesterol | IVUS determined plaque characteristics in a non-culprit vessel, NIRS determined LCBI score in a non-culprit vessel | NS | ↓ |
| Author | Study | Follow-up Period, Years | Statistical Method | Matching Variables | Confounders Adjusted for in Statistical Analysis | Endpoint | IgG Anti-oxLDL | IgM Anti-oxLDL |
|---|---|---|---|---|---|---|---|---|
| Björkbacka [14] | Cohort study | >15 | Multivariable Cox regression | Age, gender, LDL, HDL, SBP, triglycerides, hs-CRP, smoking, anti-hypertensive treatment, diabetes | Fatal and non-fatal MI, ischemic heart disease | Adjusted HR between tertiles: IgG anti-MDA-LDL p45: 1.00 vs 0.85 (0.66–1.11) vs 0.89 (0.68–1.16) p trend:0.89; IgG anti-MDA-LDL p210: 1.00 vs 0.81 (0.63–1.06) vs 0.96 (0.74–1.23) p trend: 0.29 | Adjusted HR between tertiles: IgM anti-MDA-LDL p45: 1.00 vs 0.79 (0.t62–0.99) vs 0.59 (0.46–0.76) p trend: 0.001; IgM anti-MDA-LDL p210: 1.00 vs 0.81 (0.63–1.06) vs 0.96 (0.74–1.23) p trend: 0.29 | |
| Khamis [20] | Nested case-control study | 5.5 (median) | Conditional logistic regression | Age, Gender | Smoking, diabetes, SBP, total cholesterol, HDL, creatinine, BMI, family history of CAD, anti-hypertensive and statin treatment, CRP, NTproBNP | Fatal coronary heart disease, symptomatic non-fatal MI, coronary revascularization, fatal and non-fatal stroke | Adjusted OR per SD increase: IgG anti-MDA-LDL 0.83 (0.72–0.95) | Adjusted OR per SD increase: IgM anti-MDA-LDL 0.90 (0.78–1.03) |
| Prasad [17] | Cohort study | 10.5 (median) | Multivariabel Cox regression | Age, gender, hypertension, diabetes, smoking, BMI, LDL, HDL, triglycerides, ethnicity | Cardiac death, non-fatal MI, stroke/TIA, unstable angina requiring hospitalization and arterial vascularization that included CABG, PCI, carotid endarterectomy, carotid stenting and peripheral artery revascularization | Adjusted HR 4th quartile vs first quartile: IgG anti-MDA-LDL: 1.97 (1.30–2.99) | Adjusted HR 4th quartile vs first quartile: IgM anti-MDA-LDL: 0.96 (0.63–1.45) | |
| Ravandi [21] | Nested case-control study | 6 (mean) | Conditional logistic regression | Age, gender, time of enrollment | Diabetes, smoking, SBP, LDL, HDL | cardiac death, hospital admission with CAD | Adjusted OR between tertiles: IgG anti-MDA-LDL 1.00 vs 0.80 (0.64–1.00) vs 0.94 (0.75–1.18), ptrend: 0.4 | Adjusted OR between tertiles: IgM anti-MDA-LDL: 1.00 vs 1.01 (0.81–1.26) vs 0.91 (0.72–1.15), p trend: 0.6 |
| Tsimikas [10] | Cohort study | > 15 | Multivariabel Cox regression | Age, Gender, previous CVD, SBP, smoking, diabetes, ferritin, LDL, HDL, alcohol consumption, social status, sport activity, CRP | Stroke, MI, new-onset unstable angina, acute coronary interventions and cardiac death | Adjusted HR: IgG anti-cu-oxLDL: 1.18 (1.03–1.37) | Adjusted HR: IgM anti-MDA-LDL: 0.69 (0.50–0.95) | |
| Van den Berg [22] | Nested case-control study | 4.5 (mean) | Conditional logistic regression | Age, gender, time of enrollment | Smoking, diabetes, baseline HDL, blood pressure treatments, either total IgG or total IgM. | fatal MI, non-fatal MI Q-wave criterium, non-fatal MI T-wave criterium, sudden death, new-onset ischemic heart disease or new-onset congestive heart failure | Adjusted OR between tertiles per SD increase in loge IgG anti-MDA-LDL: 1.00 vs 0.65(0.33–1.28) vs 0.93 (0.45–1.92) ptrend: 0.82 | Adjusted OR between tertiles per SD increase in loge IgM anti-MDA-LDL: 1.00 vs 0.90 (0.45–1.80) vs 0.29 (0.11–0.76) ptrend: 0.016 |
| Wilson [19] | Cohort study | > 8 | Multivariabel Cox regression | Age, total cholesterol, HDL, smoking, SBP | Angina pectoris, unstable anginga pectoris, MI, cardiac death, TIA and stroke | Adjusted HR males and females per 1000 units: IgG anti-MDA-LDL: 1.00 (1-1); IgG anti-MDA-LDL: 1.00 (1-1) |
| Author | Index Group (n) | Reference Group (n) | Follow-up Period, Years | Statistical Method | Matching Variables | Confounders Adjusted for in Statistical Analysis | Outcome | IgG Anti-oxLDL | IgM Anti-oxLDL |
|---|---|---|---|---|---|---|---|---|---|
| Maiolino [15] | CAG patients from highest IgG anti-MDA-LDL quartile (136) | CAG patients from the lower three IgG anti-MDA-LDL quartiles matched based on propensity score | 7.2 (median) | Kaplan-Meier | Gender, Age, BMI, LDL- and HDL-cholesterol, triglycerides, serum creatinine, homocysteine, glycemia, serum sodium concentration, heart rate arterial hypertension, smoking habit, LVEF, the Duke Prognostic Index of coronary athersosclerotic burden, length of follow-up, history and treatment variables | Cardiac death, composite of non-fatal MI, non-fatal stroke, and cardiac death | Event-free survival; 1-3rd Q vs. 4th Q: 77.7% vs. 69.2% | ||
| Meeuwsen [16] | Carotid endarterectomy patients (168) | 3 | Cox regression | Composite of cardiac death, stroke, non-fatal MI, coronary intervention, and peripheral intervention (including amputation) | HR 1.01 (0.98–1.03) | ||||
| Tsimikas [18] | CAG patients (504) | 4 (median) | NR | Composite of non-fatal MI, non-fatal stroke, and cardiac death | NS | NS |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
van den Berg, V.J.; Vroegindewey, M.M.; Kardys, I.; Boersma, E.; Haskard, D.; Hartley, A.; Khamis, R. Anti-Oxidized LDL Antibodies and Coronary Artery Disease: A Systematic Review. Antioxidants 2019, 8, 484. https://doi.org/10.3390/antiox8100484
van den Berg VJ, Vroegindewey MM, Kardys I, Boersma E, Haskard D, Hartley A, Khamis R. Anti-Oxidized LDL Antibodies and Coronary Artery Disease: A Systematic Review. Antioxidants. 2019; 8(10):484. https://doi.org/10.3390/antiox8100484
Chicago/Turabian Stylevan den Berg, Victor J., Maxime M. Vroegindewey, Isabella Kardys, Eric Boersma, Dorian Haskard, Adam Hartley, and Ramzi Khamis. 2019. "Anti-Oxidized LDL Antibodies and Coronary Artery Disease: A Systematic Review" Antioxidants 8, no. 10: 484. https://doi.org/10.3390/antiox8100484
APA Stylevan den Berg, V. J., Vroegindewey, M. M., Kardys, I., Boersma, E., Haskard, D., Hartley, A., & Khamis, R. (2019). Anti-Oxidized LDL Antibodies and Coronary Artery Disease: A Systematic Review. Antioxidants, 8(10), 484. https://doi.org/10.3390/antiox8100484





