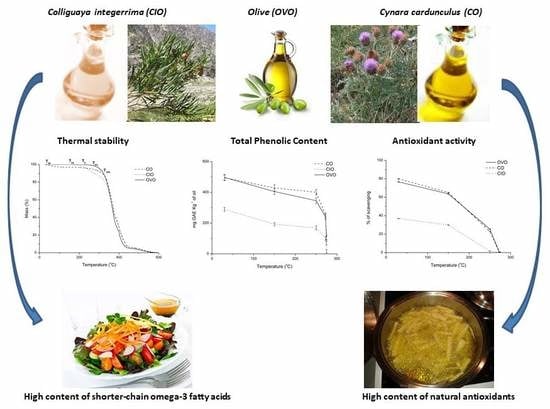
Comparison of the Oxidative Stability and Antioxidant Activity of Extra-Virgin Olive Oil and Oils Extracted from Seeds of Colliguaya integerrima and Cynara cardunculus under Normal Conditions and After Thermal Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Colliguaya integerrima and Cynara cardunculus Seeds
2.3. Vegetable Oil Extraction
2.4. Oil Characterization
2.4.1. Specific Extinction Coefficient (K270 and K232) of Extra-Virgin Olive Oil
2.4.2. Acid Value
2.4.3. Iodine Value
2.4.4. Peroxide Value
2.4.5. Saponification Number
2.4.6. Fatty Acid Composition
2.4.7. Calculated Oxidizability Value
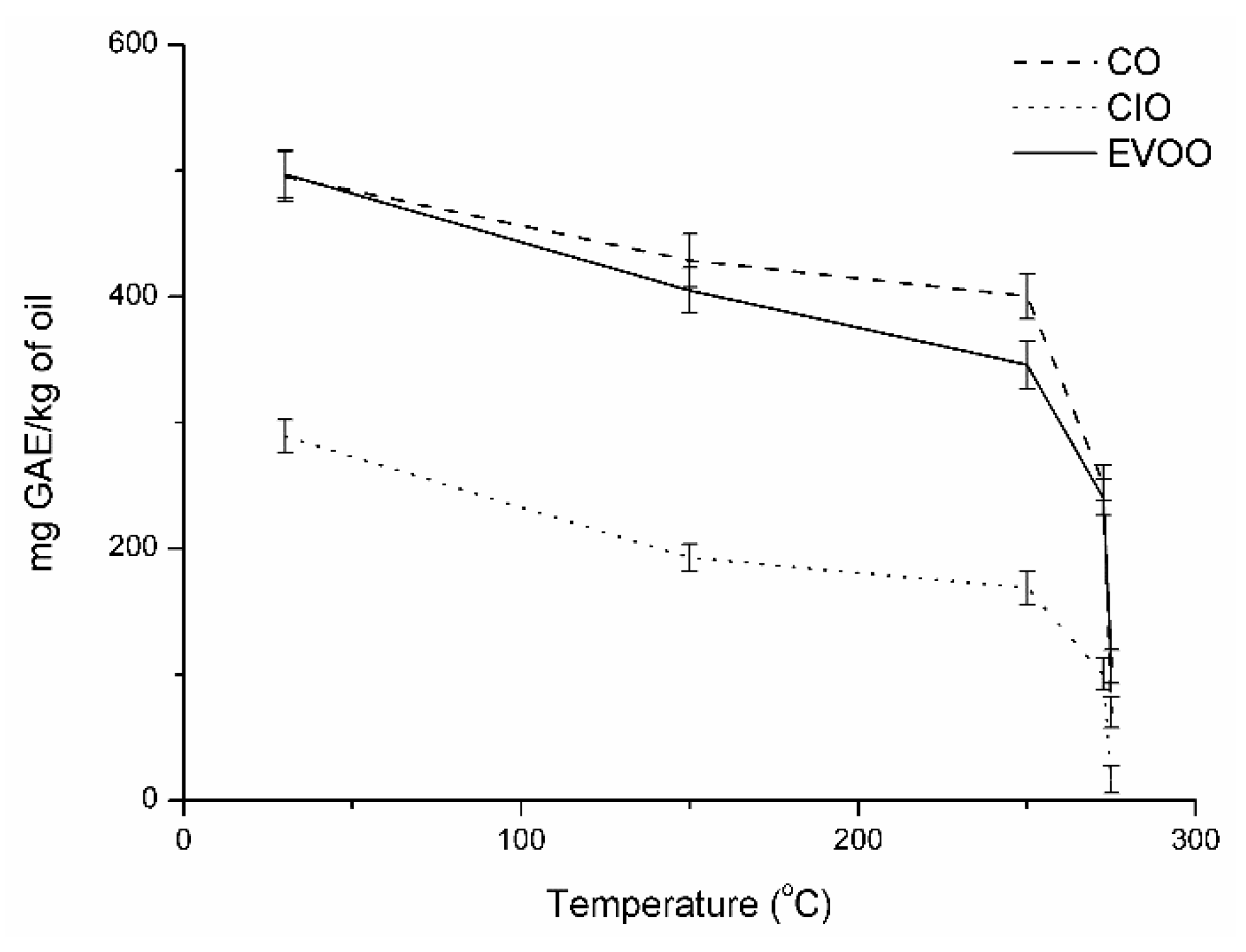
2.4.8. Total Phenolic Content
2.4.9. Using Free Radical Scavenging Assay to Determine Antioxidant Activity
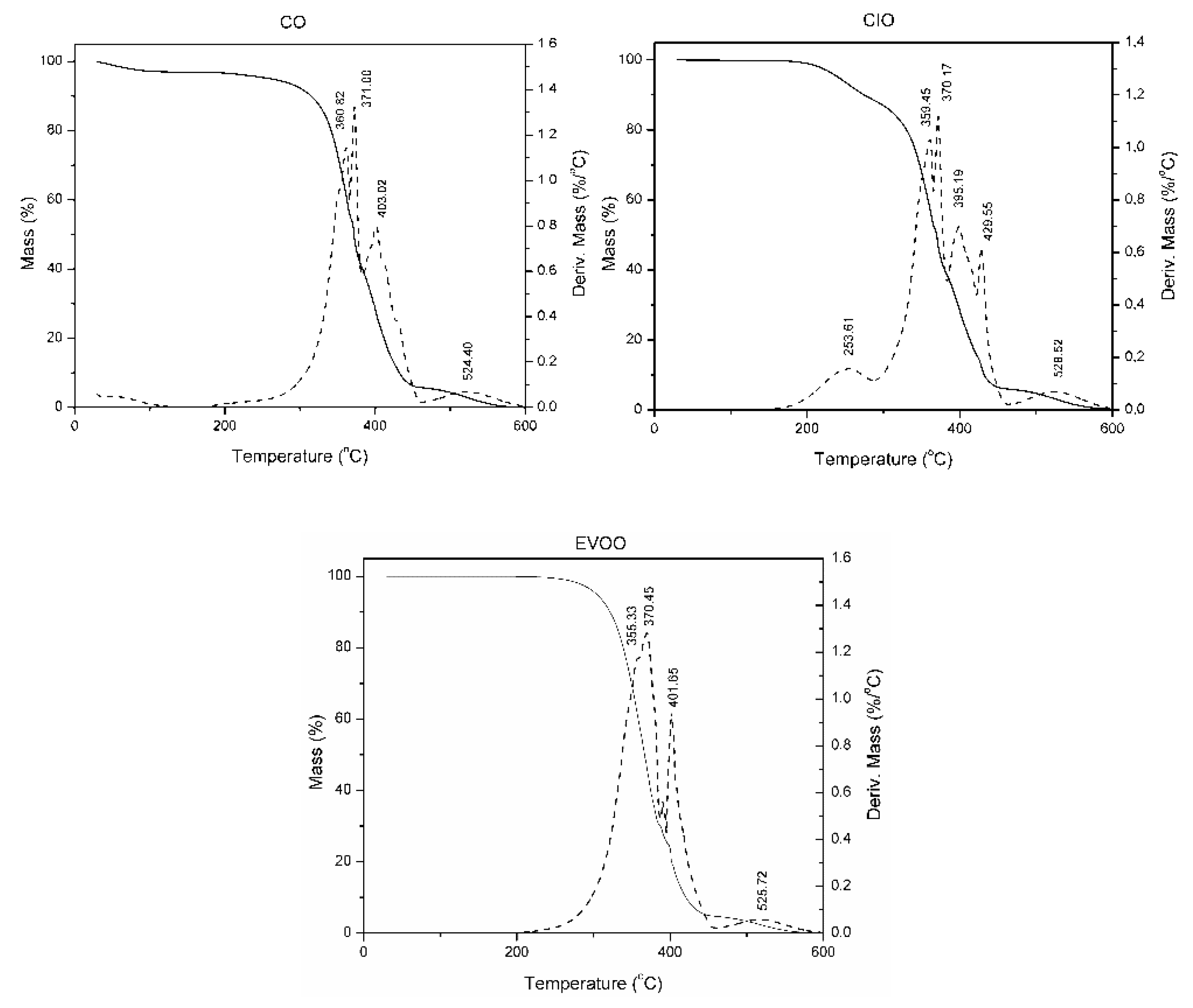
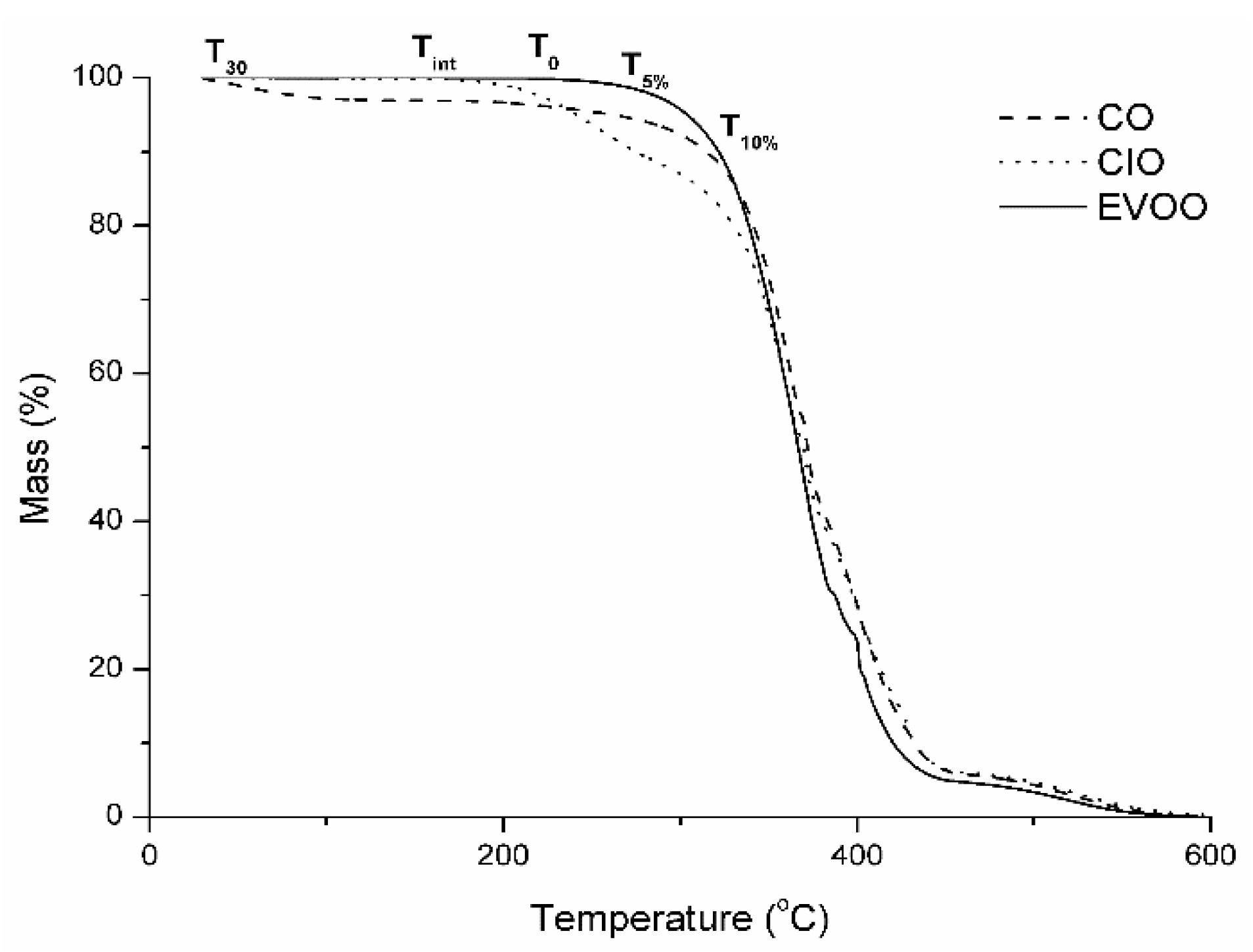
2.5. Thermal Analysis
2.6. Heating of Oils
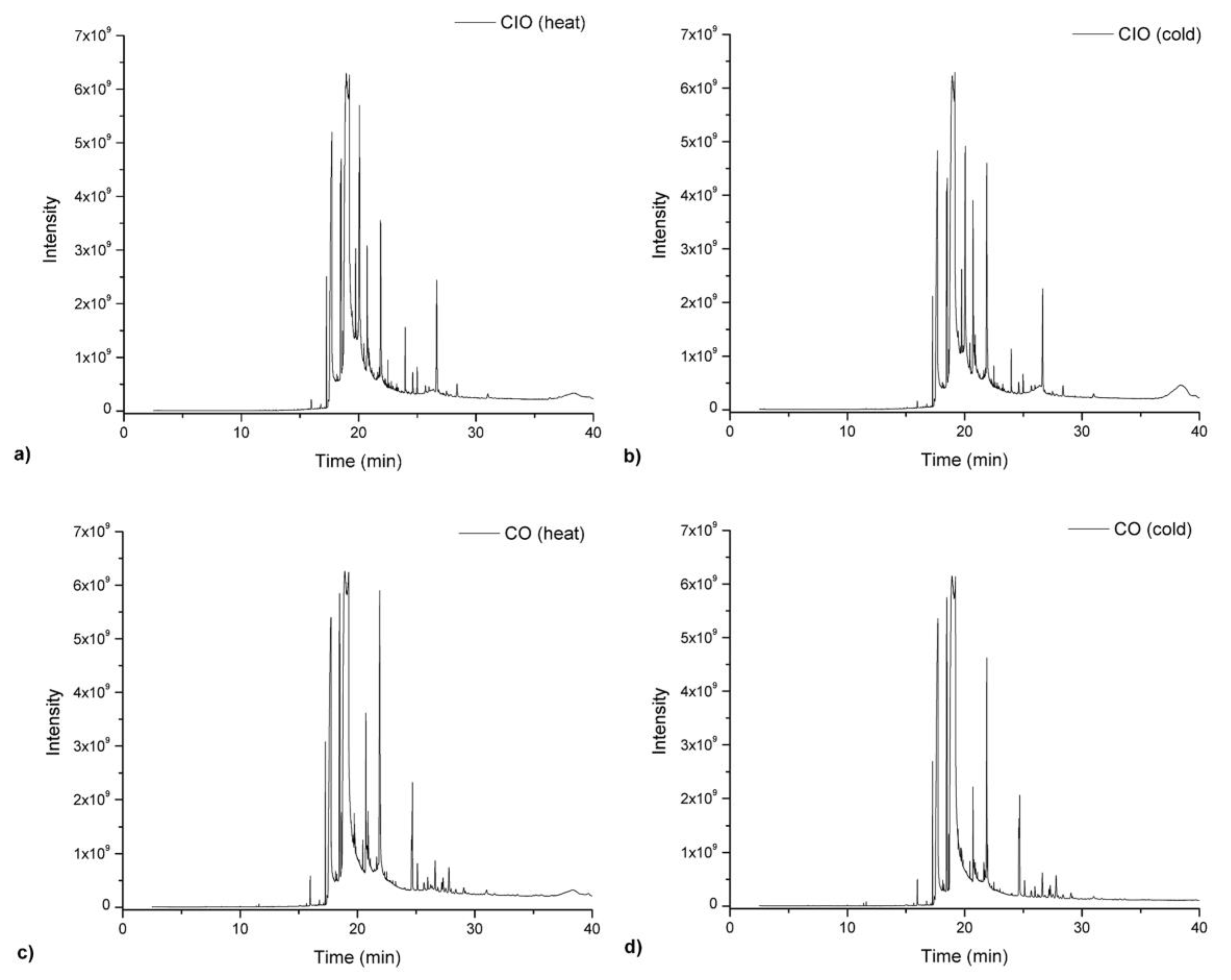
2.7. Determination of Other Minor Components Before and After Heating at 180 °C
2.8. Statistical Analysis
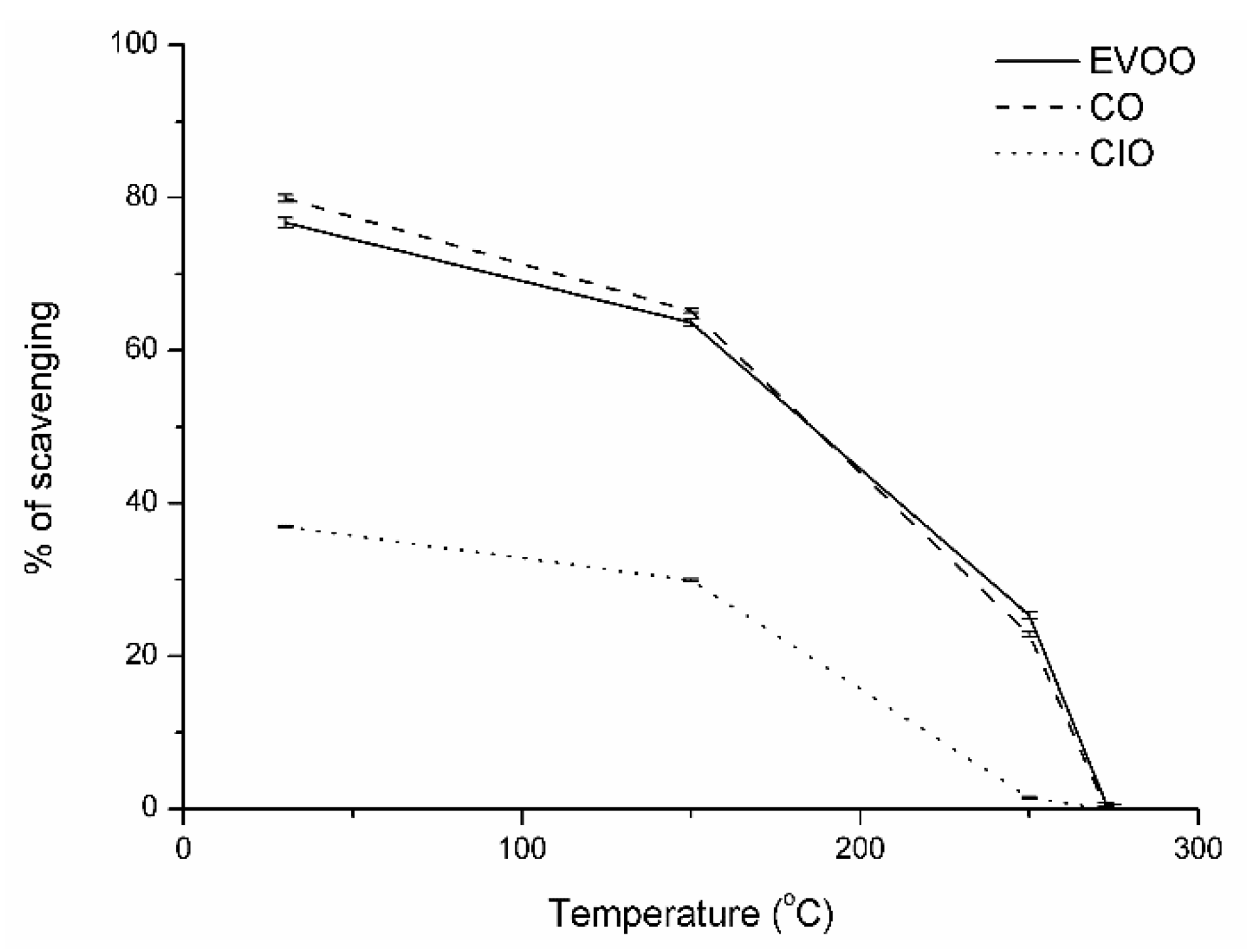
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mercy, B.A.; Elie, F.; Clergé, T.; Martin, F.; Felicité, M.T. Nutritive value of some Cucurbitaceae oil seeds from different regions in Cameroon. Afr. J. Biotechnol. 2005, 4, 1329–1334. [Google Scholar]
- Valentăo, P.; Fernandes, E.; Carvalho, F.; Aandrade, P.B.; Seabra, R.M.; Bastos, M.L. Antioxidative Properties of Cardoon (Cynara cardunculus L.) Infusion Against Superoxide Radical, Hydroxyl Radical, and Hypochlorous Acid. J. Agric. Food Chem. 2002, 50, 4989–4993. [Google Scholar] [CrossRef] [PubMed]
- Santos, K.A.; Filho, O.P.A.; Aguiar, C.M.; Milinsk, M.C.; Sampaio, S.C.; Palú, F.; da Silva, E.A. Chemical composition, antioxidant activity and thermal analysis of oil extracted from favela (Cnidoscolusquercifolius) seeds. Ind. Crop Prod. 2017, 97, 368–373. [Google Scholar] [CrossRef]
- Forero-Doria, O.; Gallego, J.; Valdés, O.; Pinzon-Topal, C.; Santos, L.S.; Guzmán, L. Relationship between oxidative stability and antioxidant activity of oil extracted from the peel of Mauritia flexuosa fruits. J. Therm. Anal. Calorim. 2016, 123, 2173–2178. [Google Scholar] [CrossRef]
- Farhoosh, R.; Hossein, M.; Khodaparast, H.; Sharif, A. Bene hull oil as a highly stable and antioxidative vegetable oil. Eur. J. Lipid Sci. Technol. 2009, 111, 1259–1265. [Google Scholar] [CrossRef]
- Cheikhousman, R.; Zude, M.; Bouveresse, D.J.R.; Léger, C.L.; Rutledge, D.N.; Birlouez-Aragon, I. Fluorescence spectroscopy for monitoring deterioration of extra virgin extra-virgin olive oil during heating. Anal. Bioanal. Chem. 2005, 382, 1438–1443. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.E.; Gil, R.; Acosta, M.G.; Saad, R.J.; Borkowski, E.; María, A.O.M. Diuretic activity of aqueous extract and betulin from Colliguajaintegerrima in rats. Pharm. Biol. 2009, 47, 274–278. [Google Scholar] [CrossRef]
- Pinto-Vitorino, G.; Toledo, I.B.; Cordoba, O.L.; Flores, M.L.; Cabrera, J.L. Análisisfitoquímico de Colliguayaintegerrima (Hook.) Gill. et Hook. (Euphorbiaceae), una planta de la Patagonia Argentina. Acta Farm. Bonaer. 2004, 23, 459–465. [Google Scholar]
- Portis, E.; Acquadro, A.; Comino, C.; Mauromicale, G.; Saba, E.; Lanteri, S. Genetic structure of island populations of wild cardoon [Cynara cardunculus L. var. sylvestris (Lamk) Fiori] detected by AFLPs and SSRs. Plant Sci. 2005, 169, 199–210. [Google Scholar]
- Wiklund, A. The genus Cynara L. (Asteraceae-Cardueae). Bot. J. Linn. Soc. 1992, 109, 75–123. [Google Scholar] [CrossRef]
- Fernández, J.; Curt, M.D.; Aguado, P.L. Industrial applications of Cynara cardunculus L. for energy and other uses. Ind. Crop Prod. 2006, 24, 222–229. [Google Scholar] [CrossRef]
- Acquadro, A.; Barchi, L.; Portis, E.; Mangino, G.; Valentino, D.; Mauromicale, G.; Lanteri, S. Genome reconstruction in Cynara cardunculus taxa gains access to chromosome-scale DNA variation. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.E.; Borkowski, E. Efecto de infusiones de Euphorbia schickendantzii y Colliguajaintegerrima, en la permeabilidad de piel de sapo. Bol. latinoam. Caribe plantas med. aromát. 2007, 6, 321–322. [Google Scholar]
- AOAC-Association of Official Analytical Chemists. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1995. [Google Scholar]
- Harhar, H.; Gharbya, S.; Kartaha, B.; Piochc, D.; Guillaumed, D.; Charrouf, Z. Effect of harvest date of Argania spinosa fruits on Argan oil quality. Ind. Crop Prod. 2014, 56, 156–159. [Google Scholar] [CrossRef]
- AOAC. Official Method Cd 3d-63. Official Methods and Recommended Practices of the American Oil Chemists’ Society; AOCS Press: Champaign, IL, USA, 1993. [Google Scholar]
- AOAC-Association of Official Analytical Chemists. Official Methods of Analysis, 18th ed.; Association of Official Analytical: Washington, DC, USA, 2005. [Google Scholar]
- Morrison, W.R.; Smith, L.M. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride-methanol. J. Lipid. Res. 1964, 5, 600–608. [Google Scholar]
- Fatemi, S.H.; Hammond, E.G. Analysis of oleate, linoleate and linolenate hydroperoxides in oxidized ester mixtures. Lipids 1980, 15, 379–385. [Google Scholar] [CrossRef]
- Singleton, V.L.; Joseph, A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Capannesi, C.; Palchetti, I.; Mascini, M.; Parenti, A. Electrochemical sensor and biosensor for polyphenols detection in olive oils. Food Chem. 2000, 71, 553–562. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Molyneux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Statgraphics®. Statgraphics Software, Version Centurion XV; StatPoint: Warrenton, VA, USA, 2013. [Google Scholar]
- Origin lab Corporation. OriginPro Software, Version 8.6; Origin lab Corporation: Northampton, MA, USA, 2011. [Google Scholar]
- Matthaus, B.; Özcan, M.M. Fatty acid and tocopherol contents of several soybean oils. Nat. Prod. Res. 2014, 28, 1–4. [Google Scholar] [CrossRef]
- Roman, O.; Heyd, B.; Broyart, B.; Castillo, R.; Maillard, M. Oxidative reactivity of unsaturated fatty acids from sunflower, high oleic sunflower and rapeseed oils subjected to heat treatment, under controlled conditions. LWT-Food Sci. Technol. 2013, 52, 49–59. [Google Scholar] [CrossRef]
- Navarro, S.L.B.; Capellini, M.C.; Aracava, K.K.; Rodrigues, C.E.C. Corngerm-bran oils extracted with alcoholic solvents Extraction yield, oil composition and evaluation of protein solubility of defatted meal. Food Bioprod. Process. 2016, 100, 185–194. [Google Scholar] [CrossRef]
- Matthaus, B.; Özcan, M.M.; Al-Juhaimi, F. Fatty acid and tocopherol contents of several seed oils. Asian J. Chem. 2014, 26, 6613–6615. [Google Scholar] [CrossRef]
- Bhutada, P.R.; Jadhav, A.J.; Pinjari, D.V.; Nemade, P.R.; Jain, R.D. Solvent assisted extraction of oil from Moringa oleifera Lam. Seeds. Ind. Crop. Prod. 2016, 82, 74–80. [Google Scholar] [CrossRef]
- Gil, A.; Serra-Majem, L.; Calder, P.C.; Uauy, R. Systematic reviews of the role of omega-3 fatty acids in the prevention and treatment of disease. Brit. J. Nutr. 2012, 107, S1–S2. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, R.; Morales, J.; Sanhueza, J.; Valenzuela, A. Docosahexaenoic acid (DHA), an essential fatty acid for the proper functioning of neuronal cells: Their role in mood disorders. Rev. Chil. Nutr. 2013, 40, 383–390. [Google Scholar] [CrossRef]
- Whitney, E.; Rolfes, S.R. Understanding Nutrition, 12th ed.; Wadsworth Cengage Learning: Wadsworth, OH, USA, 2011. [Google Scholar]
- Gunstone, F.D.; John, L.H.; Fred, B.P. The Lipid Handbook; Chapman & Hall Chemical Database: New York, NY, USA, 1994. [Google Scholar]
- Yahuaca-Juárez, B.; Martínez-Flores, H.E.; Huerta-Ruelas, J.A.; Vázquez-Landaverde, P.A.; Pless, R.C.; Tello-Santillán, R. Effect of thermal-alkaline processing conditions on the quality level of corn oil. CyTA-J. Food. 2013, 11, 1–7. [Google Scholar] [CrossRef][Green Version]
- EU Reg. 2015/1830. Official Journal of the European Union. Regulation No. 2015/1830, L66. 2015. Available online: https://www.fsai.ie/uploadedFiles/Reg2015_1830.pdf (accessed on 12 July 2018).
- Cui, Y.; Hao, P.; Liu, B.; Meng, X. Effect of traditional Chinese cooking methods on fatty acid profiles of vegetable oils. Food Chem. 2017, 233, 77–84. [Google Scholar] [CrossRef]
- Cerretani, L.; Bendini, A.; Barbieri, S.; Lercker, G. Preliminary observations on the change of some chemical characteristics of virgin olive oils subjected to a ‘‘soft deodorization’’ process. Riv. Ital. Sostanze Gr. 2008, 85, 75–82. [Google Scholar]
- Santos, J.C.O.; Souza, A.G. Thermal stability of edible oil by Thermal Analysis. J. Food Technol. 2007, 5, 79–81. [Google Scholar]
- Santos, J.C.O.; Santos, I.M.G.; Conceicăo, M.M.; Porto, S.L.; Trindade, M.F.S.; Souza, A.G.; Prasad, S.; Fernandez, V.J., Jr.; Araujo, A.S. Thermoanalytical, kinetic and rheological parameters of commercial edible oils. J. Therm. Anal. Calorim. 2004, 75, 419–428. [Google Scholar] [CrossRef]
- Dweck, J.; Sampaio, C.M.S. Analysis of the thermal decomposition of commercial vegetable oils in air by simultaneous TG/DTA. J. Therm. Anal. Calorim. 2004, 75, 385–391. [Google Scholar] [CrossRef]
- Servili, M.; Esposto, S.; Fabiani, R.; Urbani, S.; Taticchi, A.; Mariucci, F.; Selvaggini, R.; Montedoro, G.F. Phenolic compounds in olive oil: Antioxidant, health and organoleptic activities according to their chemical structure. Inflammopharmacology 2009, 17, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Bulotta, S.; Celano, M.; Lepore, S.M.; Montalcini, T.; Pujia, A.; Russo, D. Beneficial effects of the olive oil phenolic components oleuropein and hydroxytyrosol: Focus on protection against cardiovascular and metabolic diseases. J. Transl. Med. 2014, 12, 219. [Google Scholar] [CrossRef]
- Kanakis, P.; Termentzi, A.; Michel, T.; Gikas, E.; Halabalaki, M.; Skaltsounis, A.L. From Olive Drupes to Olive Oil. An HPLC-Orbitrap-based Qualitative and Quantitative Exploration of Olive Key Metabolites. Planta Med. 2013, 79, 1576–1587. [Google Scholar]
- Tuck, K.L.; Hayball, P.J. Major phenolic compounds in olive oil: Metabolism and health effects. J. Nutr. Biochem. 2002, 13, 636–644. [Google Scholar] [CrossRef]
- Mba, O.I.; Dumont, M.-J.; Ngadi, M. Thermostability and degradation kinetics of tocochromanols and carotenoids in palm oil, canola oil and their blends during deep-fat frying. LWT-Food Sci. Technol. 2017, 82, 131–138. [Google Scholar] [CrossRef]
- Tjaša, P.; Nataša, Š.; Nataša, P.U.; Blaž, C. DPPH assay of vegetable oils and model antioxidants in protic and aprotic solvents. Talanta 2013, 109, 13–19. [Google Scholar]
- Andrikopoulos, N.K.; Dedoussis, G.V.Z.; Falirea, A.; Kalogeropoulos, N.; Hatzinikola, H.S. Deteriorationof natural antioxidantspeciesof vegetable edibleoilsduringthedomesticdeep-frying and pan-fryingofpotatoes. Int. J. Food Sci. Nutr. 2002, 53, 351–363. [Google Scholar] [CrossRef]
- Naz, S.; Siddiqi, R.; Sheikh, H.; Sayeed, S.A. Deteriorationof olive, corn and soybeanoilsdueto air, light, heat and deep-frying. Food Res.Int. 2005, 38, 127–134. [Google Scholar] [CrossRef]
- Gabay, O.; Sanchez, C.; Salvat, C.; Chevy, F.; Breton, M.; Nourissat, G. Stigmasterol: A phytosterol with potential antiosteoarthritic properties. Am. J. Clin. Nutr. 2010, 18, 106–116. [Google Scholar]
- Seki, H.; Ohyama, K.; Sawai, S.; Mizutzni, M.; Ohnishi, T.; Sudo, H.; Akashi, T.; Aoki, T.; Saito, K.; Muranaka, T. Licorice β-amyrin 11-oxidase, a cytochrome P450 with a key role in the biosynthesis of the triterpene sweetener glycyrrhizin. Proc. Natl. Acad. Sci. USA 2008, 105, 14204–14209. [Google Scholar] [CrossRef] [PubMed]
- Soni, N.; Mehta, S.; Satpathy, G.; Gupta, R.K. Estimation of nutritional, phytochemical, antioxidant and antibacterial activity of dried fig (Ficuscarica). J. Pharmacogn. Phytochem. 2014, 3, 158–165. [Google Scholar]

| Parameters | Oils | ||
|---|---|---|---|
| CIO | CO | EVOO | |
| Cox value | 9.35 | 7.57 | 2.38 |
| AV [mg KOH/g oil] | 0.2 ± 0.01a | 0.2 ± 0.02a | 0.4 ± 0.09b |
| IV [g I2/100 g oil] | 124 ± 4.68b | 125 ± 3.89b | 79 ±1.68a |
| PV [meq O2/kg oil] | 19 ± 1.29c | 15 ± 0.21b | 3 ± 0.84a |
| SN [mg KOH/g oil] | 190 ± 5.21a | 196 ± 3.62a | 194 ± 2.01a |
| TPC [mg GAE/kg oil] | 289 ± 13a | 495 ± 19b | 497 ± 19b |
| Fatty acid (%) | |||
| Myristic, C14:0 | 0.06 ± 0.01a | 0.12 ± 0.09a | 0.01 ± 0.01a |
| Palmitic, C16:0 | 10.54 ± 0.35a | 10.42 ± 0.27a | 14.17 ± 0.24b |
| Palmitoleic, C16:1 ϖ-7 | 0.05 ± 0.01a | 0.06 ± 0.01a | 1.94 ± 0.11b |
| Margaric, C17:0 | 0.00 ± 0.009a | 0.05 ± 0.010a | 0.00 ± 0.042a |
| Stearic, C18:0 | 2.03 ± 0.58a | 2.81 ± 0.43a | 4.16 ± 0.18b |
| Oleic, C18:1 ϖ-9 | 23.50 ± 1.20b | 14.90 ± 1.58a | 62.34 ± 1.07c |
| Linoleic, C18:2 ϖ-6 | 31.11 ± 2.10b | 71.11 ± 3.08c | 15.63 ± 1.57a |
| Gamma-linolenic, C18:3 ϖ-6 | 0.48 ± 0.07c | 0.25 ± 0.04b | 0.00 ± 0.02a |
| Alpha-linolenic, C18:3 ϖ-3 | 26.39 ± 0.89b | 0.17 ± 0.09a | 0.58 ± 0.04a |
| Gondoic, C20:1 ϖ-9 | 5.35 ± 1.11b | 0.11 ± 0.08a | 0.00 ± 0.02a |
| Eicosadienoic C20:2 ϖ-6 | 0.48 ± 0.06b | 0.00 ± 0.09a | 0.00 ± 0.01a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abril, D.; Mirabal-Gallardo, Y.; González, A.; Marican, A.; Durán-Lara, E.F.; Silva Santos, L.; Valdés, O. Comparison of the Oxidative Stability and Antioxidant Activity of Extra-Virgin Olive Oil and Oils Extracted from Seeds of Colliguaya integerrima and Cynara cardunculus under Normal Conditions and After Thermal Treatment. Antioxidants 2019, 8, 470. https://doi.org/10.3390/antiox8100470
Abril D, Mirabal-Gallardo Y, González A, Marican A, Durán-Lara EF, Silva Santos L, Valdés O. Comparison of the Oxidative Stability and Antioxidant Activity of Extra-Virgin Olive Oil and Oils Extracted from Seeds of Colliguaya integerrima and Cynara cardunculus under Normal Conditions and After Thermal Treatment. Antioxidants. 2019; 8(10):470. https://doi.org/10.3390/antiox8100470
Chicago/Turabian StyleAbril, Diana, Yaneris Mirabal-Gallardo, Aymeé González, Adolfo Marican, Esteban F. Durán-Lara, Leonardo Silva Santos, and Oscar Valdés. 2019. "Comparison of the Oxidative Stability and Antioxidant Activity of Extra-Virgin Olive Oil and Oils Extracted from Seeds of Colliguaya integerrima and Cynara cardunculus under Normal Conditions and After Thermal Treatment" Antioxidants 8, no. 10: 470. https://doi.org/10.3390/antiox8100470
APA StyleAbril, D., Mirabal-Gallardo, Y., González, A., Marican, A., Durán-Lara, E. F., Silva Santos, L., & Valdés, O. (2019). Comparison of the Oxidative Stability and Antioxidant Activity of Extra-Virgin Olive Oil and Oils Extracted from Seeds of Colliguaya integerrima and Cynara cardunculus under Normal Conditions and After Thermal Treatment. Antioxidants, 8(10), 470. https://doi.org/10.3390/antiox8100470