S-Allylmercapro-N-Acetylcysteine Attenuates the Oxidation-Induced Lens Opacification and Retinal Pigment Epithelial Cell Death In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cells and Tissue Culture Conditions
2.3. Porcine Lenses Ex-Vivo Model
2.4. Glutathione Determination
2.5. Free Sulfhydryls Assay
2.6. Cell Survival Assay
2.7. Lens Opacity Measurement
2.8. Statistical Analysis
3. Results
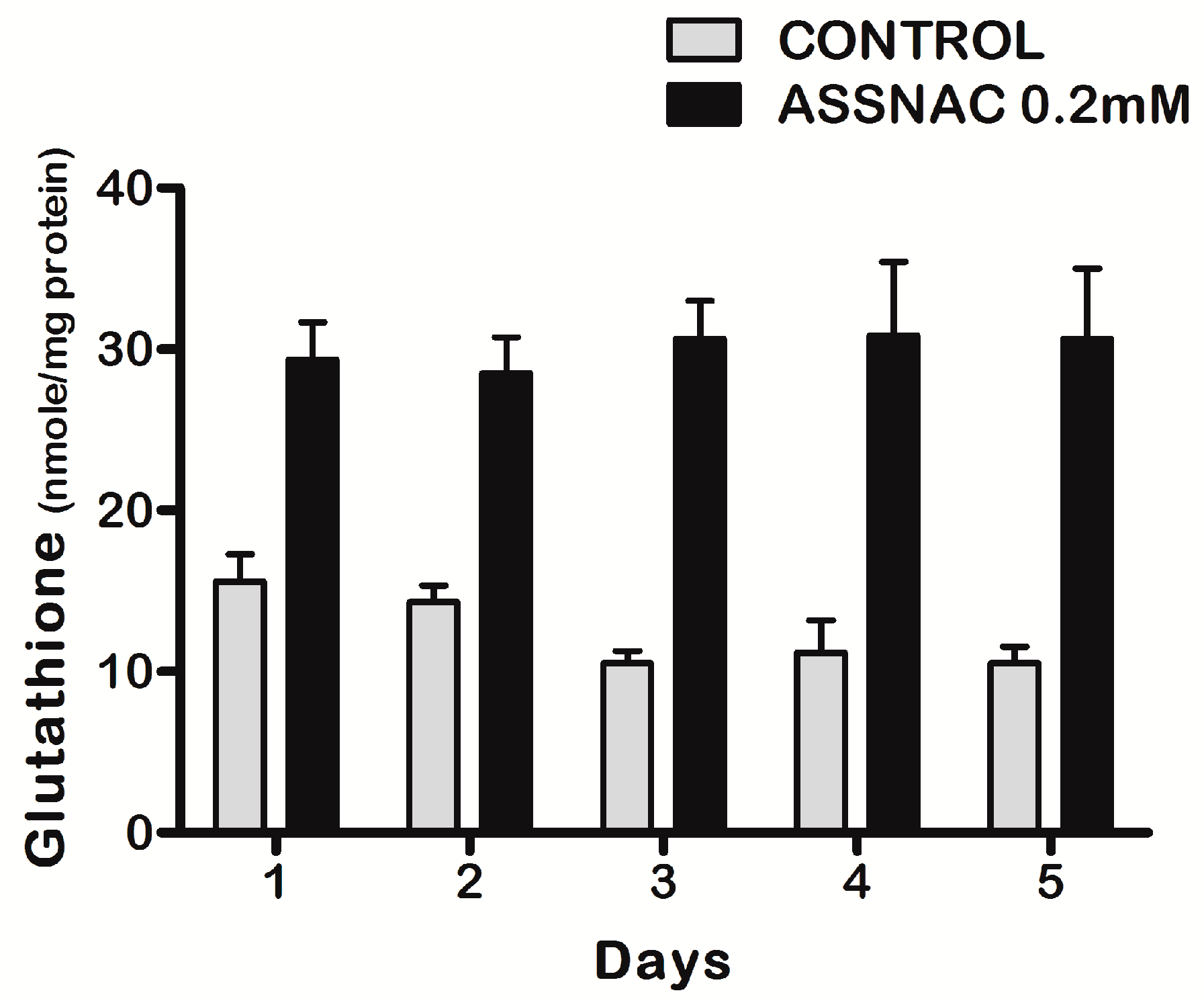
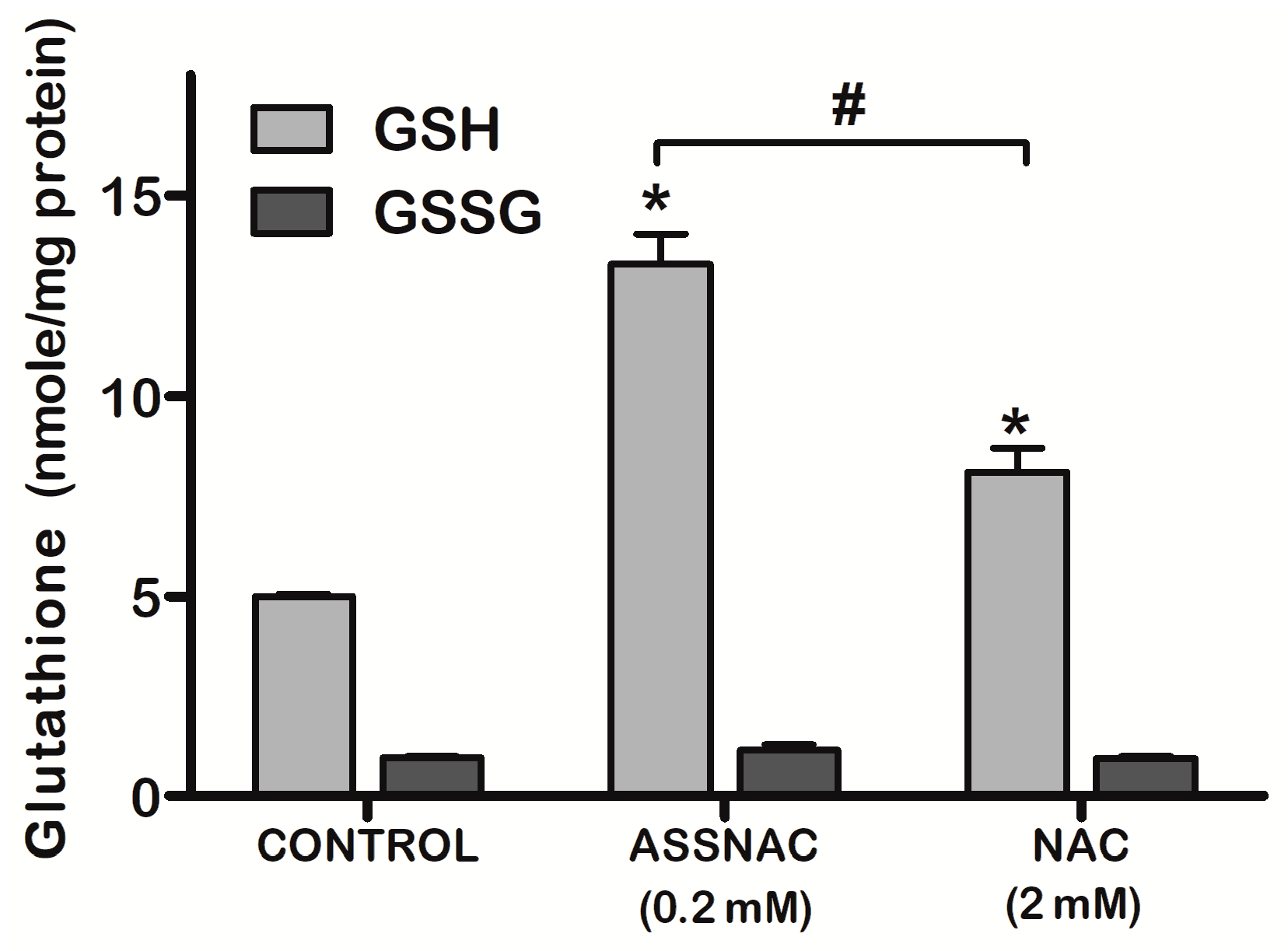
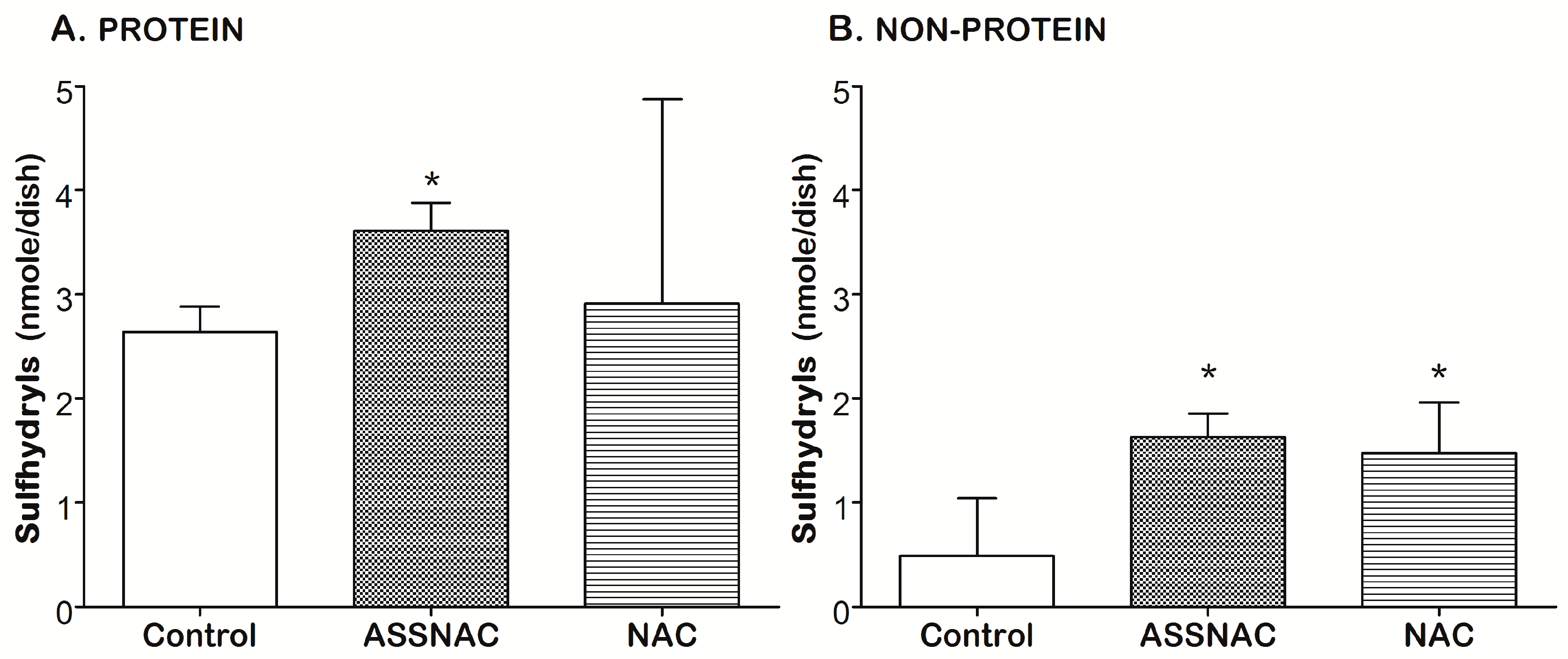
3.1. ASSNAC Up-Regulates Glutathione and Total Free Sulfhydryls Level in ARPE-19 Cells
3.2. ASSNAC Protects ARPE-19 Cells from Oxidation-Induced Cytotoxic Effect
3.3. ASSNAC Ex-Vivo Effect on Lens Opacity
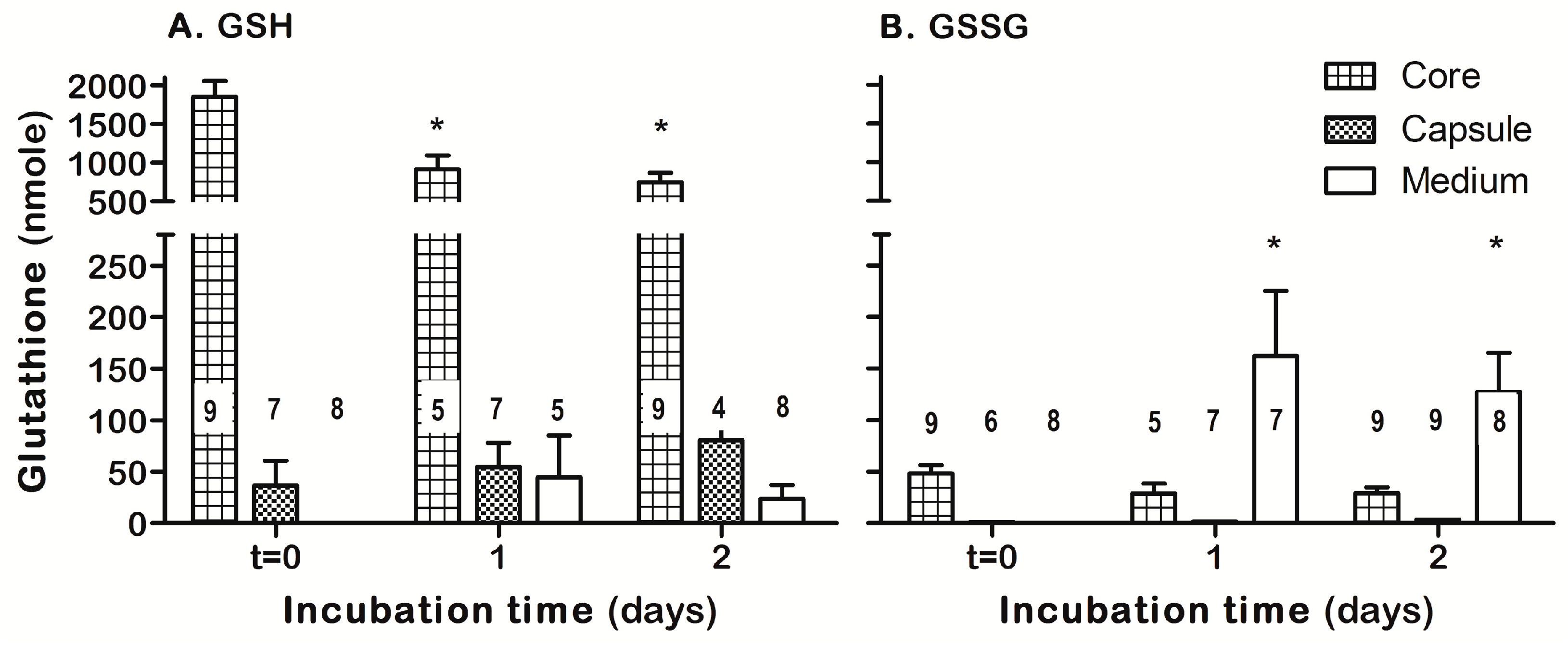
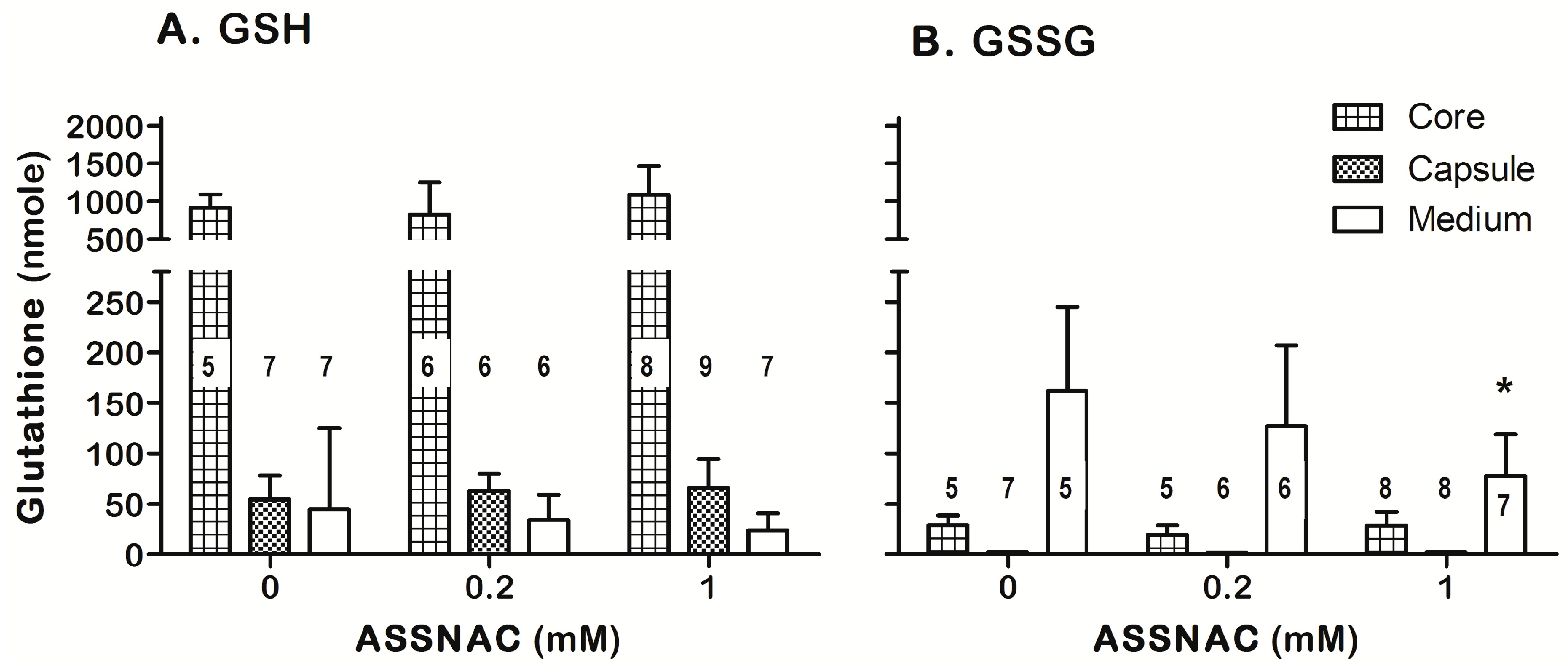
3.4. ASSNAC Ex-Vivo Effect on Lens Glutathione
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McCusker, M.M.; Durrani, K.; Payette, M.J.; Suchecki, J. An eye on nutrition: The role of vitamins, essential fatty acids, and antioxidants in age-related macular degeneration, dry eye syndrome, and cataract. Clin. Dermatol. 2016, 34, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Tu, G.; Zhang, Y.; Wei, W.; Li, L.; Zhang, Y.; Yang, J.; Xing, Y. Allicin attenuates H2O2-induced cytotoxicity in retinal pigmented epithelial cells by regulating the levels of reactive oxygen species. Mol. Med. Rep. 2016, 13, 2320–2326. [Google Scholar] [CrossRef] [PubMed]
- Kruk, J.; Kubasik-Kladna, K.; Aboul-Enein, H.Y. The role oxidative stress in the pathogenesis of eye diseases: Current status and a dual role of physical activity. Mini-Rev. Med. Chem. 2016, 16, 241–257. [Google Scholar] [CrossRef]
- Beatty, S.; Koh, H.; Henson, D.; Boulton, M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv. Ophthalmol. 2000, 45, 115–134. [Google Scholar] [CrossRef]
- Bhuyan, K.; Bhuyan, D. Molecular mechanism of cataractogenesis: 3. toxic metabolites of oxygen as initiators of lipid-peroxidation and cataract. Curr. Eye Res. 1984, 3, 67–81. [Google Scholar] [CrossRef]
- Shichi, H. Cataract formation and prevention. Expert Opin. Investig. Drugs 2004, 13, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Spector, A. Oxidative stress-induced cataract—Mechanism of action. FASEB J. 1995, 9, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Spector, A.; Garner, W. Hydrogen-peroxide and human cataract. Exp. Eye Res. 1981, 33, 673–681. [Google Scholar] [CrossRef]
- Singh, A.; Khan, S.A.; Choudhary, R.; Bodakhe, S.H. Cinnamaldehyde attenuates cataractogenesis via restoration of hypertension and oxidative stress in fructose-fed hypertensive rats. J. Pharmacopunct. 2016, 19, 137–144. [Google Scholar]
- Kagan, D.B.; Liu, H.; Hutnik, C.M. Efficacy of various antioxidants in the protection of the retinal pigment epithelium from oxidative stress. Clin. Ophthalmol. 2012, 6, 1471–1476. [Google Scholar]
- Schimel, A.M.; Abraham, L.; Cox, D.; Sene, A.; Kraus, C.; Dace, D.S.; Ercal, N.; Apte, R.S. N-acetylcysteine amide (NACA) prevents retinal degeneration by up-regulating reduced glutathione production and reversing lipid peroxidation. Am. J. Pathol. 2011, 178, 2032–2043. [Google Scholar] [CrossRef] [PubMed]
- Anbaraki, A.; Khoshaman, K.; Ghasemi, Y.; Yousefi, R. Preventive role of lens antioxidant defense mechanism against riboflavin-mediated sunlight damaging of lens crystallins. Int. J. Biol. Macromol. 2016, 91, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Raman, T.; Ramar, M.; Arumugam, M.; Nabavi, S.M.; Varsha, M.K.N.S. Cytoprotective mechanism of action of curcumin against cataract. Pharmacol. Rep. 2016, 68, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yang, X.; Cao, G. Acetyl-l-carnitine prevents homocysteine-induced suppression of Nrf2/Keap1 mediated antioxidation in human lens epithelial cells. Mol. Med. Rep. 2015, 12, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Shichi, H. Prevention of acetaminophen-induced cataract by a combination of diallyl disulfide and N-acetylcysteine. J. Ocul. Pharmacol. Ther. 1998, 14, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Suresha, B.S.; Srinivasan, K. Antioxidant potential of fungal metabolite nigerloxin during eye lens abnormalities in galactose-fed rats. Curr. Eye Res. 2013, 38, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- Orhan, H.; Marol, S.; Hepsen, I.; Sahin, G. Effects of some probable antioxidants on selenite-induced cataract formation and oxidative stress-related parameters in rats. Toxicology 1999, 139, 219–232. [Google Scholar] [CrossRef]
- Zhou, Y.; Guo, B.; Ye, M.; Liao, R.; Li, S. Protective effect of rutin against H2O2-induced oxidative stress and apoptosis in human lens epithelial cells. Curr. Eye Res. 2016, 41, 933–942. [Google Scholar] [CrossRef]
- Ma, Q. Role of Nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Izigov, N.; Farzam, N.; Savion, N. S-allylmercapto-N-acetylcysteine up-regulates cellular glutathione and protects vascular endothelial cells from oxidative stress. Free Radic. Biol. Med. 2011, 50, 1131–1139. [Google Scholar] [CrossRef]
- Savion, N.; Levine, A.; Kotev-Emeth, S.; Abu-Shach, U.B.; Broday, L. S-allylmercapto-N-acetylcysteine protects against oxidative stress and extends lifespan in caenorhabditis elegans. PLoS ONE 2018, 13, e0194780. [Google Scholar] [CrossRef] [PubMed]
- Savion, N.; Izigov, N.; Morein, M.; Pri-Chen, S.; Kotev-Emeth, S. S-allylmercapto-N-acetylcysteine (ASSNAC) protects cultured nerve cells from oxidative stress and attenuates experimental autoimmune encephalomyelitis. Neurosci. Lett. 2014, 583, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Pittala, V.; Fidilio, A.; Lazzara, F.; Platania, C.B.M.; Salerno, L.; Foresti, R.; Drago, F.; Bucolo, C. Effects of novel nitric oxide-releasing molecules against oxidative stress on retinal pigmented epithelial cells. Oxid. Med. Cell. Longev. 2017, 2017, 1420892. [Google Scholar] [CrossRef] [PubMed]
- Dinc, E.; Ayaz, L.; Kurt, A.H. Effects of bevacizumab, ranibizumab, and aflibercept on MicroRNA expression in a retinal pigment epithelium cell culture model of oxidative stress. J. Ocul. Pharmacol. Ther. 2018, 34, 346–353. [Google Scholar] [CrossRef]
- Li, Y.; Zou, X.; Gao, J.; Cao, K.; Feng, Z.; Liu, J. APR3 modulates oxidative stress and mitochondrial function in ARPE-19 cells. FASEB J. 2018, 32. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zheng, Y.; Wang, C.; Liu, Y. Glutathione depletion induces ferroptosis, autophagy, and premature cell senescence in retinal pigment epithelial cells. Cell Death Dis. 2018, 9, 753. [Google Scholar] [CrossRef] [PubMed]
- Sundararajan, M.; Thomas, P.A.; Babyshalini, K.; Geraldine, P. Identification of phytoconstituents and in-vitro evaluation of the putative anticataractogenic effect of an ethanolic root extract of leucas aspera. Biomed. Pharmacother. 2017, 85, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M. Determination of glutathione and glutathione disulfide in biological samples. Meth. Enzymol. 1985, 113, 548–555. [Google Scholar]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2006, 1, 3159–3165. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Kosower, E.M.; Kosower, N.S. Bromobimane probes for thiols. Meth. Enzymol. 1995, 251, 133–148. [Google Scholar]
- Babich, H.; Borenfreund, E. Cytotoxicity of T-2 toxin and its metabolites determined with the neutral red-cell viability assay. Appl. Environ. Microbiol. 1991, 57, 2101–2103. [Google Scholar] [PubMed]
- Bree, M.; Borchman, D. The optical properties of rat, porcine and human lenses in organ culture treated with dexamethasone. Exp. Eye Res. 2018, 170, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Cu, X.; Lou, M. The effect and recovery of long-term H2O2 exposure on lens morphology and biochemistry. Exp. Eye Res. 1993, 57, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Smith, A.J.O.; Lott, M.C.; Bao, Y.; Bowater, R.P.; Reddan, J.R.; Wormstone, I.M. Sulforaphane can protect lens cells against oxidative stress: Implications for cataract prevention. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5236–5248. [Google Scholar] [CrossRef] [PubMed]
- Babu, E.; Ananth, S.; Veeranan-Karmegam, R.; Coothankandaswamy, V.; Smith, S.B.; Boettger, T.; Ganapathy, V.; Martin, P.M. Transport via SLC5A8 (SMCT1) is obligatory for 2-oxothiazolidine-4-carboxylate to enhance glutathione production in retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5749–5757. [Google Scholar] [CrossRef] [PubMed]
- Gerona, G.; Lopez, D.; Palmero, M.; Maneu, V. Antioxidant N-acetyl-cysteine protects retinal pigmented epithelial cells from long-term hypoxia changes in gene expression. J. Ocul. Pharmacol. Ther. 2010, 26, 309–314. [Google Scholar] [CrossRef]
- Babizhayev, M.A. Generation of reactive oxygen species in the anterior eye segment. synergistic codrugs of N-acetylcarnosine lubricant eye drops and mitochondria-targeted antioxidant act as a powerful therapeutic platform for the treatment of cataracts and primary open-angle glaucoma. BBA Clin. 2016, 19, 49–68. [Google Scholar]
- Miranda, M.; Arnal, E.; Ahuja, S.; Alvarez-Nolting, R.; Lopez-Pedrajas, R.; Ekstrom, P.; Bosch-Morell, F.; van Veen, T.; Romero, F.J. Antioxidants rescue photoreceptors in rd1 mice: Relationship with thiol metabolism. Free Radic. Biol. Med. 2010, 48, 216–222. [Google Scholar] [CrossRef]
- Miyamoto, N.; Izumi, H.; Miyamoto, R.; Bin, H.; Kondo, H.; Tawara, A.; Sasaguri, Y.; Kohno, K. Transcriptional regulation of activating transcription factor 4 under oxidative stress in retinal pigment epithelial ARPE-19/HPV-16 cells. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1226–1234. [Google Scholar] [CrossRef]
- Zareba, M.; Raciti, M.; Henry, M.; Sarna, T.; Burke, J. Oxidative stress in ARPE-19 cultures: Do melanosomes confer cytoprotection? Free Radic. Biol. Med. 2006, 40, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Giddabasappa, A.; Bauler, M.; Yepuru, M.; Chaum, E.; Dalton, J.T.; Eswaraka, J. 17-beta estradiol protects ARPE-19 cells from oxidative stress through estrogen receptor-beta. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5278–5287. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, C.; Zeng, Y.; Yang, C.; Li, H.; Fan, H.; Hahn, C.E.W.; Farmery, A.D.; Chen, R. Cell culture pO2 monitoring with a fiber optic oxygen sensor. Biomed. Phys. Eng. Express 2016, 2, 057001. [Google Scholar] [CrossRef]
- Siegfried, C.J.; Shui, Y.; Holekamp, N.M.; Bai, F.; Beebe, D.C. Oxygen distribution in the human eye: Relevance to the etiology of open-angle glaucoma after vitrectomy. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5731–5738. [Google Scholar] [CrossRef] [PubMed]
- Oriowo, O.M.; Cullen, A.P.; Schirmer, K.; Chou, B.R.; Bols, N.C.; Sivak, J.G. Evaluation of a porcine lens and fluorescence assay approach for in vitro ocular toxicological investigations. Altern. Lab. Anim. 2002, 30, 505–513. [Google Scholar] [PubMed]
- Carey, J.W.; Pinarci, E.Y.; Penugonda, S.; Karacal, H.; Ercal, N. In vivo inhibition of 1-buthionine-(S,R)-sulfoximine-induced cataracts by a novel antioxidant, N-acetylcysteine amide. Free Radic. Biol. Med. 2011, 50, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.V.; Zigler, J.S.J. Zeta-crystallin from guinea pig lens is capable of functioning catalytically as an oxidoreductase. Arch. Biochem. Biophys. 1991, 284, 181–185. [Google Scholar] [CrossRef]
- Qin, C.; Tumminia, S.; Russell, P.; Rao, P.; Zigler, J. Investigations into the loss of glutathione from lenses in organ culture. Curr. Eye Res. 1996, 15, 719–725. [Google Scholar] [CrossRef]
- Dickinson, D.; Forman, H. Glutathione in defense and signaling - lessons from a small thiol. Ann. N. Y. Acad. Sci. 2002, 973, 488–504. [Google Scholar] [CrossRef]
- Yu, N.; Denagel, D.; Pruett, P.; Kuck, J. Disulfide bond formation in the eye lens. Proc. Natl. Acad. Sci. USA 1985, 82, 7965–7968. [Google Scholar] [CrossRef]
- Lou, M.; Dickerson, J.; Garadi, R. The role of protein-thiol mixed disulfides in cataractogenesis. Exp. Eye Res. 1990, 50, 819–826. [Google Scholar] [CrossRef]
- Pescosolido, N.; Barbato, A.; Giannotti, R.; Komaiha, C.; Lenarduzzi, F. Age-related changes in the kinetics of human lenses: Prevention of the cataract. Int. J. Ophthalmol. 2016, 9, 1506–1517. [Google Scholar] [PubMed]
- Goutham, G.; Manikandan, R.; Beulaja, M.; Thiagarajan, R.; Arulvasu, C.; Arumugam, M.; Setzer, W.N.; Daglia, M.; Nabavi, S.F.; Nabavi, S.M. A focus on resveratrol and ocular problems, especially cataract: From chemistry to medical uses and clinical relevance. Biomed. Pharmacother. 2017, 86, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Abu-Amero, K.K.; Kondkar, A.A.; Chalam, K.V. Resveratrol and ophthalmic diseases. Nutrients 2016, 8, 200. [Google Scholar] [CrossRef] [PubMed]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savion, N.; Dahamshi, S.; Morein, M.; Kotev-Emeth, S. S-Allylmercapro-N-Acetylcysteine Attenuates the Oxidation-Induced Lens Opacification and Retinal Pigment Epithelial Cell Death In Vitro. Antioxidants 2019, 8, 25. https://doi.org/10.3390/antiox8010025
Savion N, Dahamshi S, Morein M, Kotev-Emeth S. S-Allylmercapro-N-Acetylcysteine Attenuates the Oxidation-Induced Lens Opacification and Retinal Pigment Epithelial Cell Death In Vitro. Antioxidants. 2019; 8(1):25. https://doi.org/10.3390/antiox8010025
Chicago/Turabian StyleSavion, Naphtali, Samia Dahamshi, Milana Morein, and Shlomo Kotev-Emeth. 2019. "S-Allylmercapro-N-Acetylcysteine Attenuates the Oxidation-Induced Lens Opacification and Retinal Pigment Epithelial Cell Death In Vitro" Antioxidants 8, no. 1: 25. https://doi.org/10.3390/antiox8010025
APA StyleSavion, N., Dahamshi, S., Morein, M., & Kotev-Emeth, S. (2019). S-Allylmercapro-N-Acetylcysteine Attenuates the Oxidation-Induced Lens Opacification and Retinal Pigment Epithelial Cell Death In Vitro. Antioxidants, 8(1), 25. https://doi.org/10.3390/antiox8010025





