Effect of a Strawberry and Spinach Dietary Supplement on Spatial Learning in Early and Late Middle-Aged Female Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Diet
2.3. Apparatus & Procedure
Morris Water Maze
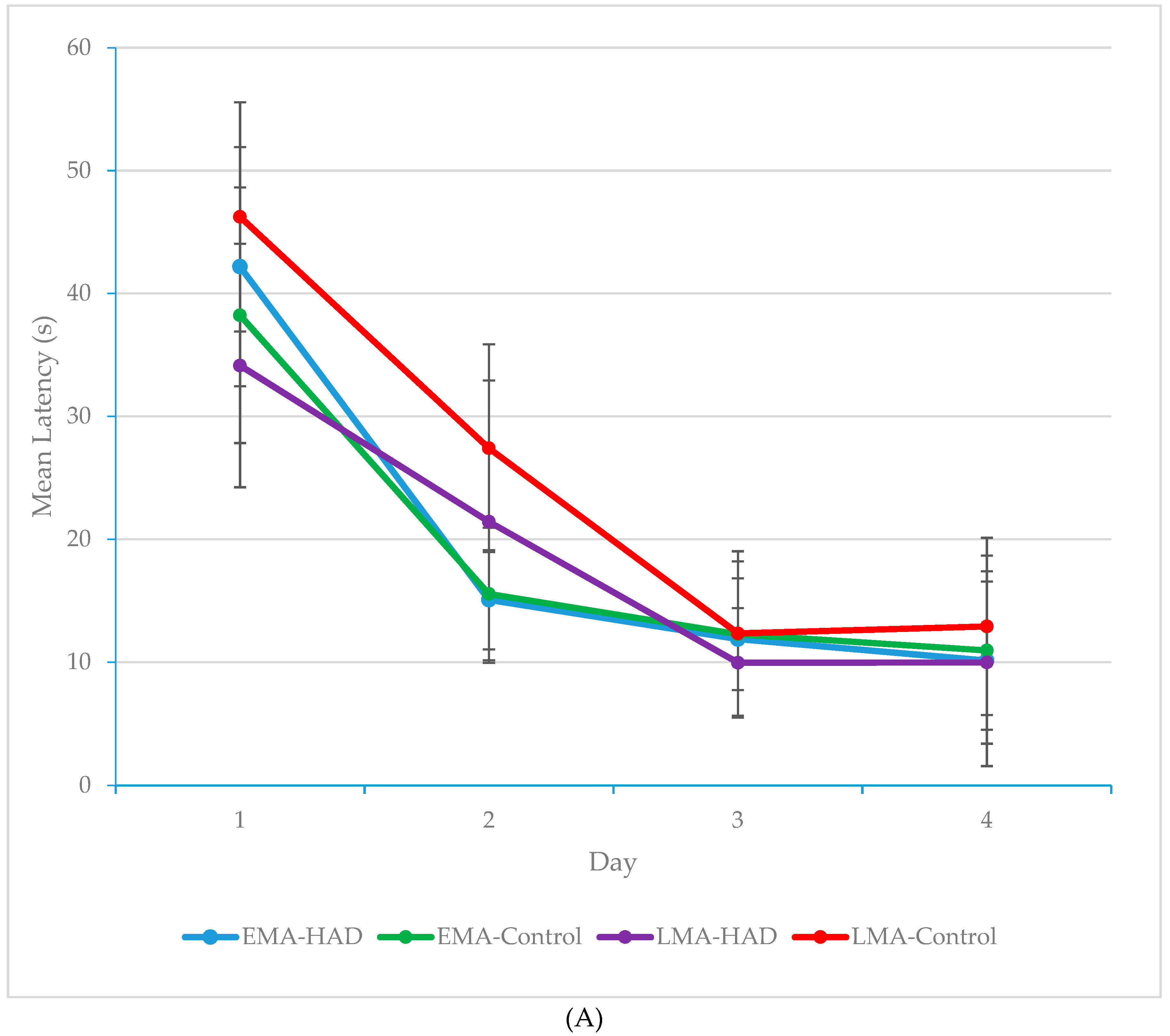
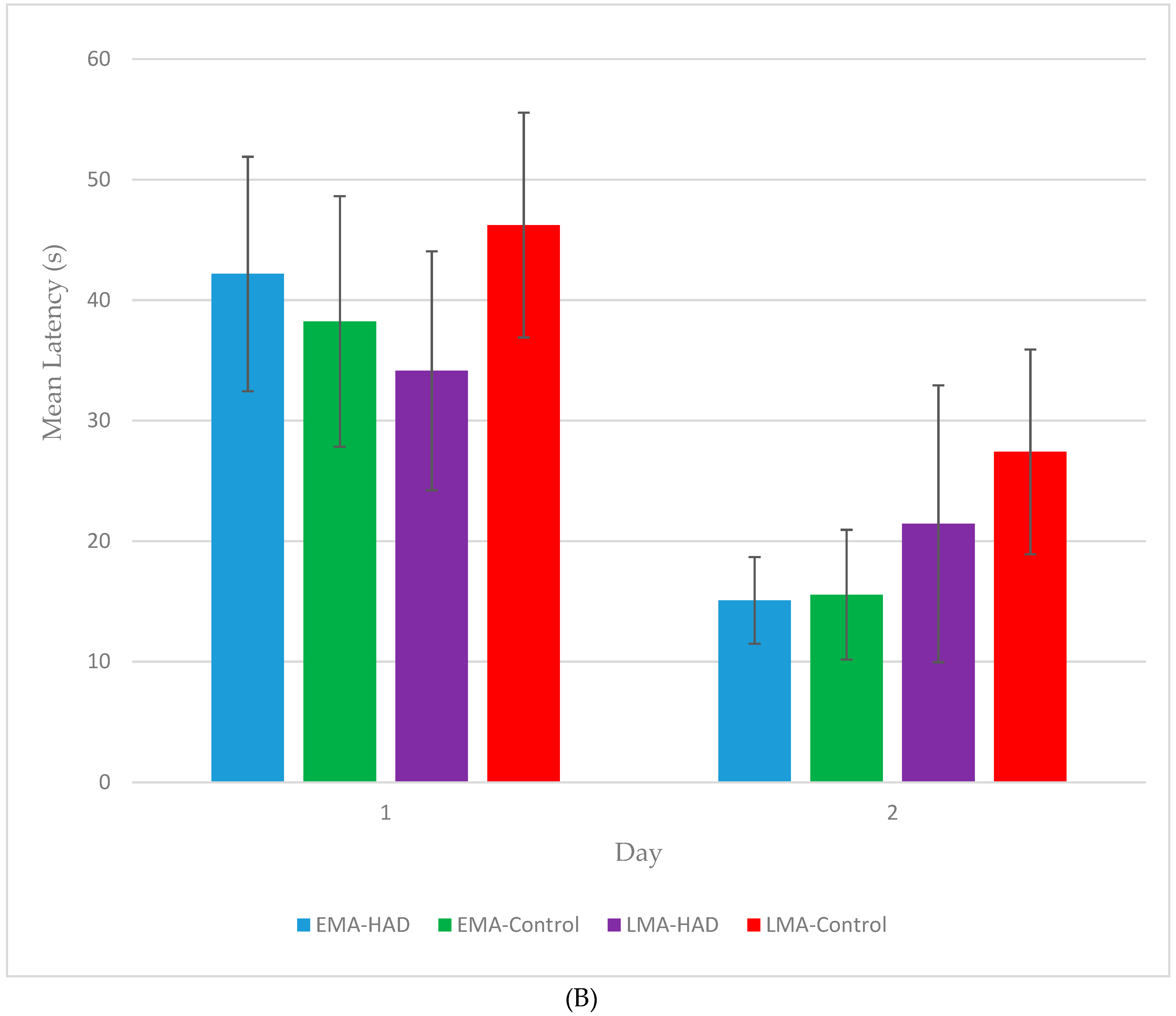
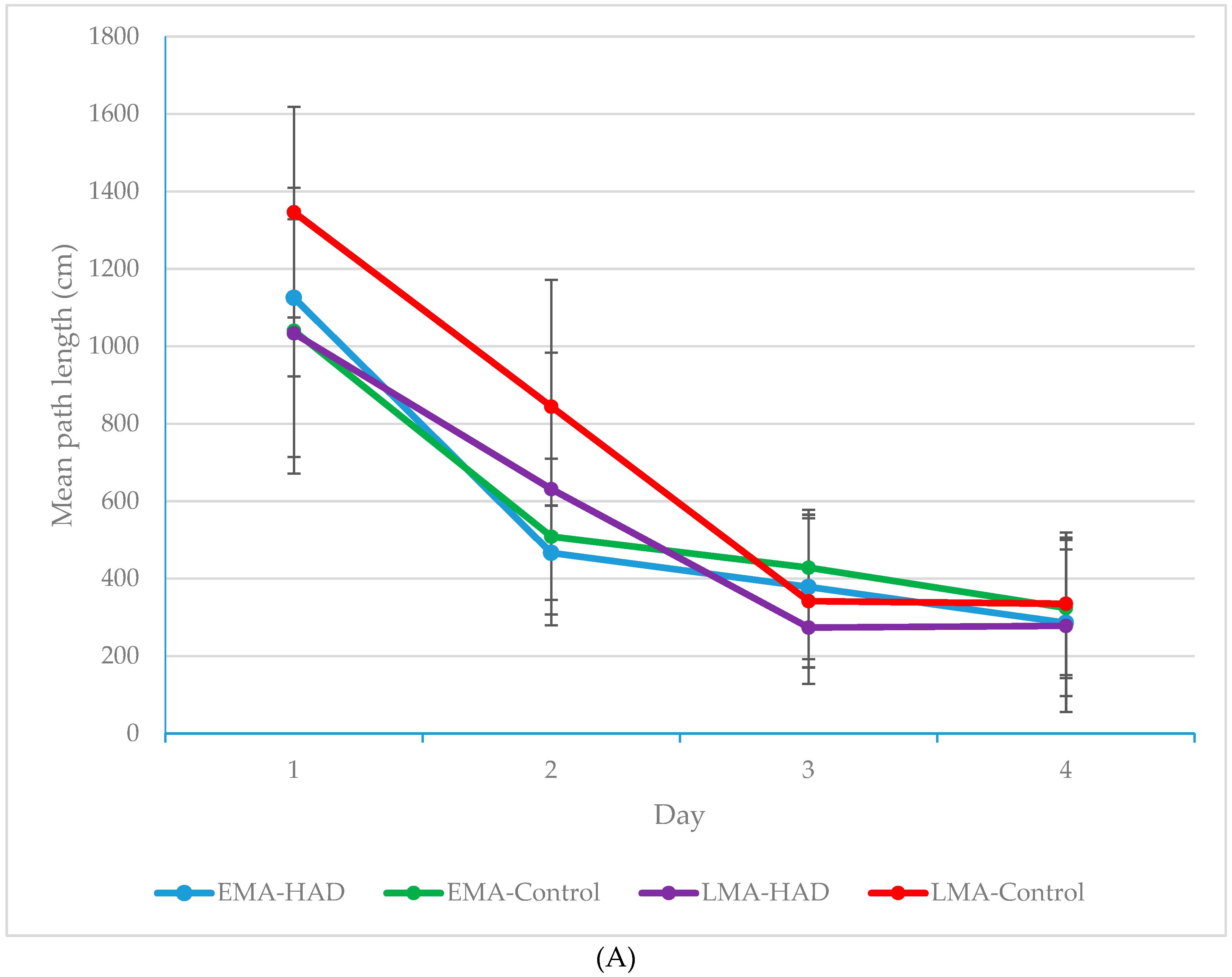
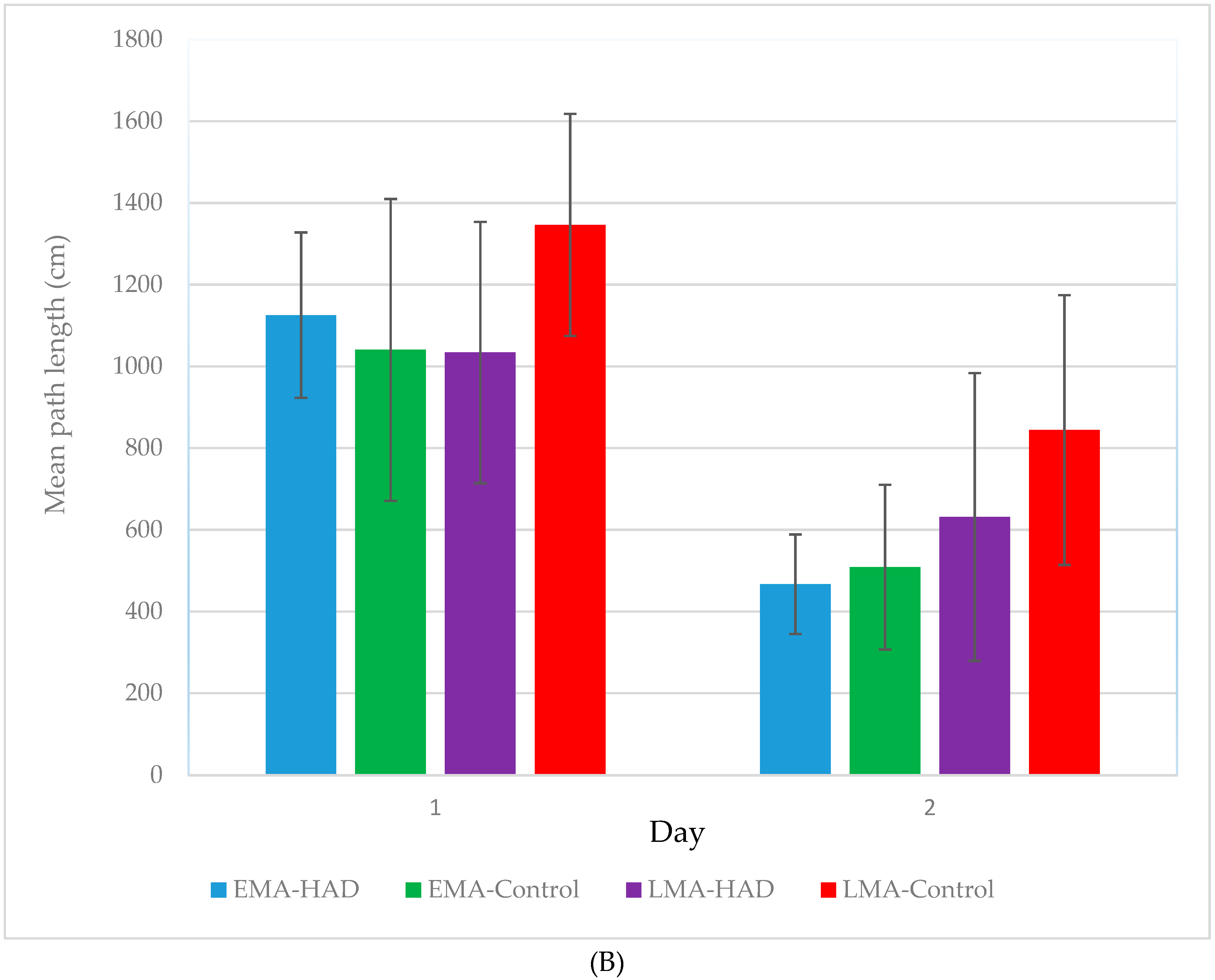
3. Results
3.1. Latency
Path Length
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Health and Aging; 11-7737; National Institute on Aging; National Institute of Health: Bethesda, MD, USA, 2011. [Google Scholar]
- Zyzak, D.R.; Otto, T.; Eichenbaum, H.; Gallagher, M. Cognitive Decline Associated with Normal Aging in Rats: A Neuropsychological Approach. Learn. Mem. 1995, 2, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, L.; Lövdén, M.; Riklund, K.; Lindenberger, U.; Bäckman, L. Memory Aging and Brain Maintenance. Trends Cogn. Sci. 2012, 16, 292–305. [Google Scholar] [CrossRef] [PubMed]
- Pilotti, M.; Meade, M.L.; Gallo, D.A. Implicit and Explicit Measures of Memory for Perceptual Information in Young Adults, Healthy Older Adults, and Patients with Alzheimer’s Disease. Exp. Aging Res. 2003, 29, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, E.S.; Barnes, C.A. Impact of Aging on Hippocampal Function: Plasticity, Network Dynamics, and Cognition. Prog. Neurobiol. 2003, 69, 143–179. [Google Scholar] [CrossRef]
- Hess, T.M. Memory and Aging in Context. Psychol. Bull. 2005, 131, 383–406. [Google Scholar] [CrossRef]
- Harman, D. Aging: A Theory Based on Free Radical and Radiation Chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harman, D. The Biologic Clock: The Mitochondria? J. Am. Geriatr. Soc. 1972, 20, 145–147. [Google Scholar] [CrossRef]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free Radicals, Antioxidants in Disease and Health. Free Radic. Antioxid. 2008, 4, 89. [Google Scholar]
- Sanz, A.; Stefanatos, R.K.A. The Mitochondrial Free Radical Theory of Aging: A Critical View. Curr. Aging Sci. 2008, 1, 10–21. [Google Scholar] [CrossRef]
- Bonda, D.J.; Wang, X.; Perry, G.; Nunomura, A.; Tabaton, M.; Zhu, X.; Smith, M.A. Oxidative Stress in Alzheimer Disease: A Possibility for Prevention. Neuropharmacology 2010, 59, 290–294. [Google Scholar] [CrossRef]
- Chen, Z.; Zhong, C. Oxidative Stress in Alzheimer’s Disease. Neurosci. Bull. 2014, 30, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Sánchez, F.F.; Gironès, X.; Ortega, A.; Alameda, F.; Lafuente, J.V. Oxidative Stress in Alzheimer’s Disease Hippocampus: A Topographical Study. J. Neurol. Sci. 2010, 299, 163–167. [Google Scholar] [CrossRef]
- Eichenbaum, H.; Otto, T.; Cohen, N.J. The Hippocampus—What Does It Do? Behav. Neural Biol. 1992, 57, 2–36. [Google Scholar] [CrossRef]
- Jarrard, L.E. What Does the Hippocampus Really Do? Behav. Brain Res. 1995, 71, 1–10. [Google Scholar] [CrossRef]
- Cantuti-Castelvetri, I.; Shukitt-Hale, B.; Joseph, J.A. Neurobehavioral Aspects of Antioxidants in Aging. Int. J. Dev. Neurosci. 2000, 18, 367–381. [Google Scholar] [CrossRef]
- Nicolle, M.; Gonzalez, J.; Sugaya, K.; Baskerville, K.; Bryan, D.; Lund, K.; Gallagher, M.; McKinney, M. Signatures of Hippocampal Oxidative Stress in Aged Spatial Learning-Impaired Rodents. Neuroscience 2001, 107, 415–431. [Google Scholar] [CrossRef]
- Dröge, W.; Schipper, H.M. Oxidative Stress and Aberrant Signaling in Aging and Cognitive Decline. Aging Cell 2007, 6, 361–370. [Google Scholar] [CrossRef]
- Serrano, F.; Klann, E. Reactive Oxygen Species and Synaptic Plasticity in the Aging Hippocampus. Ageing Res. Rev. 2004, 3, 431–443. [Google Scholar] [CrossRef]
- Shukitt-Hale, B.; Cheng, V.; Joseph, J.A. Effects of Blackberries on Motor and Cognitive Function in Aged Rats. Nutr. Neurosci. 2009, 12, 135–140. [Google Scholar] [CrossRef]
- Shukitt-Hale, B.; Carey, A.; Simon, L.; Mark, D.A.; Joseph, J.A. Effects of Concord Grape Juice on Cognitive and Motor Deficits in Aging. Nutrition 2006, 22, 295–302. [Google Scholar] [CrossRef]
- Willis, L.M.; Shukitt-Hale, B.; Joseph, J.A. Modulation of Cognition and Behavior in Aged Animals: Role for Antioxidant- and Essential Fatty Acid–Rich Plant Foods. Am. J. Clin. Nutr. 2009, 89, 1602S–1606S. [Google Scholar] [CrossRef] [PubMed]
- Patil, C.S.; Singh, V.P.; Satyanarayan, P.S.V.; Jain, N.K.; Singh, A.; Kulkarni, S.K. Protective Effect of Flavonoids against Aging- and Lipopolysaccharide-Induced Cognitive Impairment in Mice. Pharmacology 2003, 69, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P.E. Flavonoids: Modulators of Brain Function? Br. J. Nutr. 2008, 99. [Google Scholar] [CrossRef] [PubMed]
- Gildawie, K.R.; Galli, R.L.; Shukkit-Hale, B.; Carey, A.N. Protective Effects of Foods Containing Flavonoids on Age-Related Cognitive Decline. Curr. Nutr. Rep. 2018, 7, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Shukitt-Hale, B.; Carey, A.N.; Jenkins, D.; Rabin, B.M.; Joseph, J.A. Beneficial Effects of Fruit Extracts on Neuronal Function and Behavior in a Rodent Model of Accelerated Aging. Neurobiol. Aging 2007, 28, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.A.; Shukitt-Hale, B.; Denisova, N.A.; Prior, R.L.; Cao, G.; Martin, A.; Taglialatela, G.; Bickford, P.C. Long-Term Dietary Strawberry, Spinach, or Vitamin E Supplementation Retards the Onset of Age-Related Neuronal Signal-Transduction and Cognitive Behavioral Deficits. J. Neurosci. 1998, 18, 8114–8121. [Google Scholar] [CrossRef]
- Joseph, J.A.; Shukitt-Hale, B.; Denisova, N.A.; Bielinski, D.; Martin, A.; McEwen, J.J.; Bickford, P.C. Reversals of Age-Related Declines in Neuronal Signal Transduction, Cognitive, and Motor Behavioral Deficits with Blueberry, Spinach, or Strawberry Dietary Supplementation. J. Neurosci. 1999, 19, 8047–8055. [Google Scholar] [CrossRef]
- Bickford, P.C.; Gould, T.; Briederick, L.; Chadman, K.; Pollock, A.; Young, D.; Shukitt-Hale, B.; Joseph, J. Antioxidant-Rich Diets Improve Cerebellar Physiology and Motor Learning in Aged Rats. Brain Res. 2000, 866, 211–217. [Google Scholar] [CrossRef]
- Kiray, M.; Uysal, N.; Sönmez, A.; Açıkgöz, O.; Gönenç, S. Positive Effects of Deprenyl and Estradiol on Spatial Memory and Oxidant Stress in Aged Female Rat Brains. Neurosci. Lett. 2004, 354, 225–228. [Google Scholar] [CrossRef]
- Monteiro, S.C.; Matté, C.; Bavaresco, C.S.; Netto, C.A.; Wyse, A.T.S. Vitamins E and C Pretreatment Prevents Ovariectomy-Induced Memory Deficits in Water Maze. Neurobiol. Learn. Mem. 2005, 84, 192–199. [Google Scholar] [CrossRef]
- Xu, J.; Rong, S.; Xie, B.; Sun, Z.; Zhang, L.; Wu, H.; Yao, P.; Zhang, X.; Zhang, Y.; Liu, L. Rejuvenation of Antioxidant and Cholinergic Systems Contributes to the Effect of Procyanidins Extracted from the Lotus Seedpod Ameliorating Memory Impairment in Cognitively Impaired Aged Rats. Eur. Neuropsychopharmacol. 2009, 19, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Bouayed, J.; Bohn, T. Exogenous Antioxidants—Double-Edged Swords in Cellular Redox State: Health Beneficial Effects at Physiologic Doses versus Deleterious Effects at High Doses. Oxid. Med. Cell. Longev. 2010, 3, 228–237. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, M.A.; Maier, S.F.; Einstein, G.O. “Brain-Specific” Nutrients: A Memory Cure? Nutrients 2003, 19, 957–975. [Google Scholar] [CrossRef]
- Giampieri, F.; Alvarez-Suarez, J.M.; Battino, M. Strawberry and Human Health: Effects beyond Antioxidant Activity. J. Agric. Food Chem. 2014, 62, 3867–3876. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.; Marjan, Z.; Foong, C. Total Antioxidant Activity and Phenolic Content in Selected Vegetables. Food Chem. 2004, 87, 581–586. [Google Scholar] [CrossRef]
- Alzheimer’s Association. Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement. 2015, 11, 332. [Google Scholar]
- Vauzour, D. Effect of Flavonoids on Learning, Memory and Neurocognitive Performance: Relevance and Potential Implications for Alzheimer’s Disease Pathophysiology: Flavonoids and Memory. J. Sci. Food Agric. 2014, 94, 1042–1056. [Google Scholar] [CrossRef]
- Crichton, G.E.; Bryan, J.; Murphy, K.J. Dietary Antioxidants, Cognitive Function and Dementia—A Systematic Review. Plant Foods Hum. Nutr. 2013, 68, 279–292. [Google Scholar] [CrossRef]
- Rafnsson, S.B.; Dilis, V.; Trichopoulou, A. Antioxidant Nutrients and Age-Related Cognitive Decline: A Systematic Review of Population-Based Cohort Studies. Eur. J. Nutr. 2013, 52, 1553–1567. [Google Scholar] [CrossRef]
| Group | n | Day | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| EMA-Control | 10 | 28.02(3.68) | 30.26(5.28) | 29.52(4.63) | 27.53(4.59) |
| EMA-HAD | 10 | 27.16(3.48) | 28.40(4.61) | 28.08(3.54) | 28.36(4.35) |
| LMA-Control | 11 | 25.57(2.88) | 25.21(3.88) | 23.63(3.38) | 23.18(2.71) |
| LMA-HAD | 11 | 26.66(2.79) | 25.10(3.76) | 23.48(4.06) | 23.14(4.47) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Millin, P.M.; Rickert, G.T. Effect of a Strawberry and Spinach Dietary Supplement on Spatial Learning in Early and Late Middle-Aged Female Rats. Antioxidants 2019, 8, 1. https://doi.org/10.3390/antiox8010001
Millin PM, Rickert GT. Effect of a Strawberry and Spinach Dietary Supplement on Spatial Learning in Early and Late Middle-Aged Female Rats. Antioxidants. 2019; 8(1):1. https://doi.org/10.3390/antiox8010001
Chicago/Turabian StyleMillin, Paula M., and Gina T. Rickert. 2019. "Effect of a Strawberry and Spinach Dietary Supplement on Spatial Learning in Early and Late Middle-Aged Female Rats" Antioxidants 8, no. 1: 1. https://doi.org/10.3390/antiox8010001
APA StyleMillin, P. M., & Rickert, G. T. (2019). Effect of a Strawberry and Spinach Dietary Supplement on Spatial Learning in Early and Late Middle-Aged Female Rats. Antioxidants, 8(1), 1. https://doi.org/10.3390/antiox8010001





