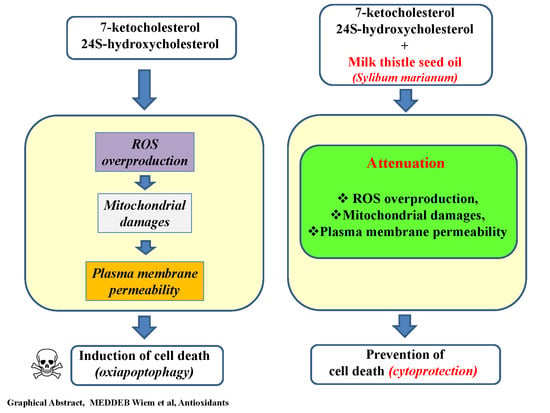
Cytoprotective Activities of Milk Thistle Seed Oil Used in Traditional Tunisian Medicine on 7-Ketocholesterol and 24S-Hydroxycholesterol-Induced Toxicity on 158N Murine Oligodendrocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cold Extraction of Oils
2.1.1. Seed Materials
2.1.2. Extraction of Oils
2.2. Cell Culture and Treatments
2.3. Determination of the Fatty Acid Profiles of Milk Thistle Oils and Nigella Oil by Gas Chromatography
2.4. Determination of the Tocopherol Profile of Milk Thistle Oils and Nigella Oil by High Pressure Liquid Chromatography
2.5. Analysis of Polyphenols of Milk Thistle Oils and Nigella Oil
2.6. Analysis of Phytosterols of Milk Thistle Oils and Nigella Oil
2.7. KRL (Kit Radicaux Libres) Test
2.8. Determination of Ferric Reducing Antioxidant Power (FRAP)
2.9. DPPH Assay
2.10. Crystal Violet Test
2.11. Measurement of Mitochondrial Activity with the MTT (Methylthiazolyldiphenyl-Tetrazolium Bromide) Test
2.12. Measurement of Superoxide Anions Production with Dihydroethidium
2.13. Measurement of Plasma Membrane Permeability with Propidium Iodide
2.14. Quantification of Apoptotic Cells after Nuclei Staining with Hoechst 33342
2.15. Analysis of Caspase-3 and Microtubule-Associated Protein 1 Light Chain 3 (LC3) by Polyacrylamide Gel Electrophoresis and Western Blotb
2.16. Statistical Analyses
3. Results and Discussion
3.1. Profiles of Fatty Acids, Phytosterols, Tocopherols, and Polyphenols Cold-Extracted from Milk Thistle Seed Oils from Different Area of Tunisia
3.2. Evaluation of the Antioxidant Properties of Milk Thistle Seed Oil from Different Area of Tunisia with the KRL, FRAP, and DPPH Tests
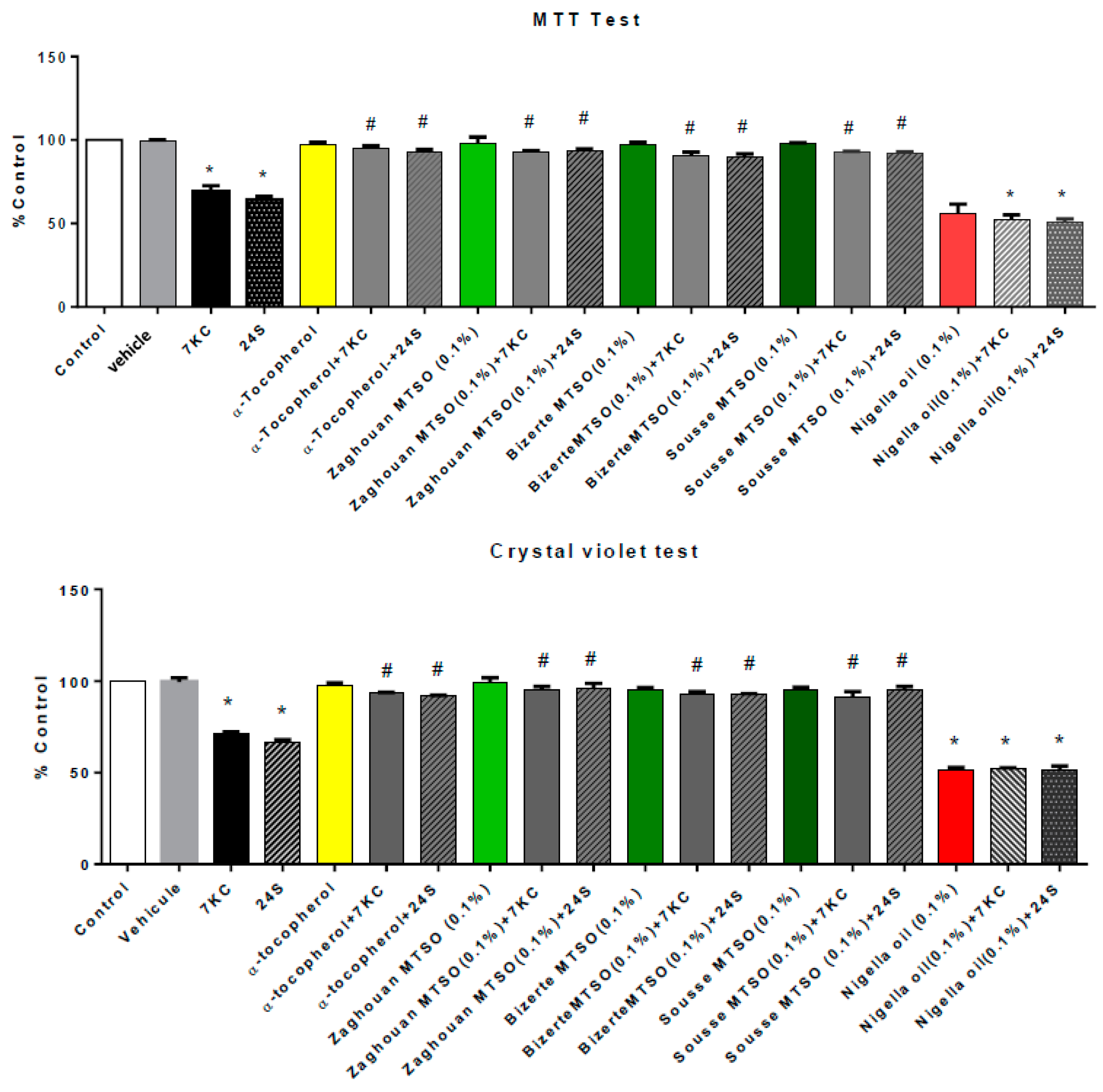
3.3. Effects of Milk Thistle Seed Oil on 7-Ketocholesterol- and 24S-Hydroxycholesterol-Induced Mitochondrial Dysfunction and Cell Growth Inhibition, Assessed with the MTT and Crystal Violet Tests
3.4. Effects of Milk Thistle Seed Oil on 7-Ketocholesterol and 24S-Hydroxycholesterol-Induced Overproduction of Reactive Oxygen Species
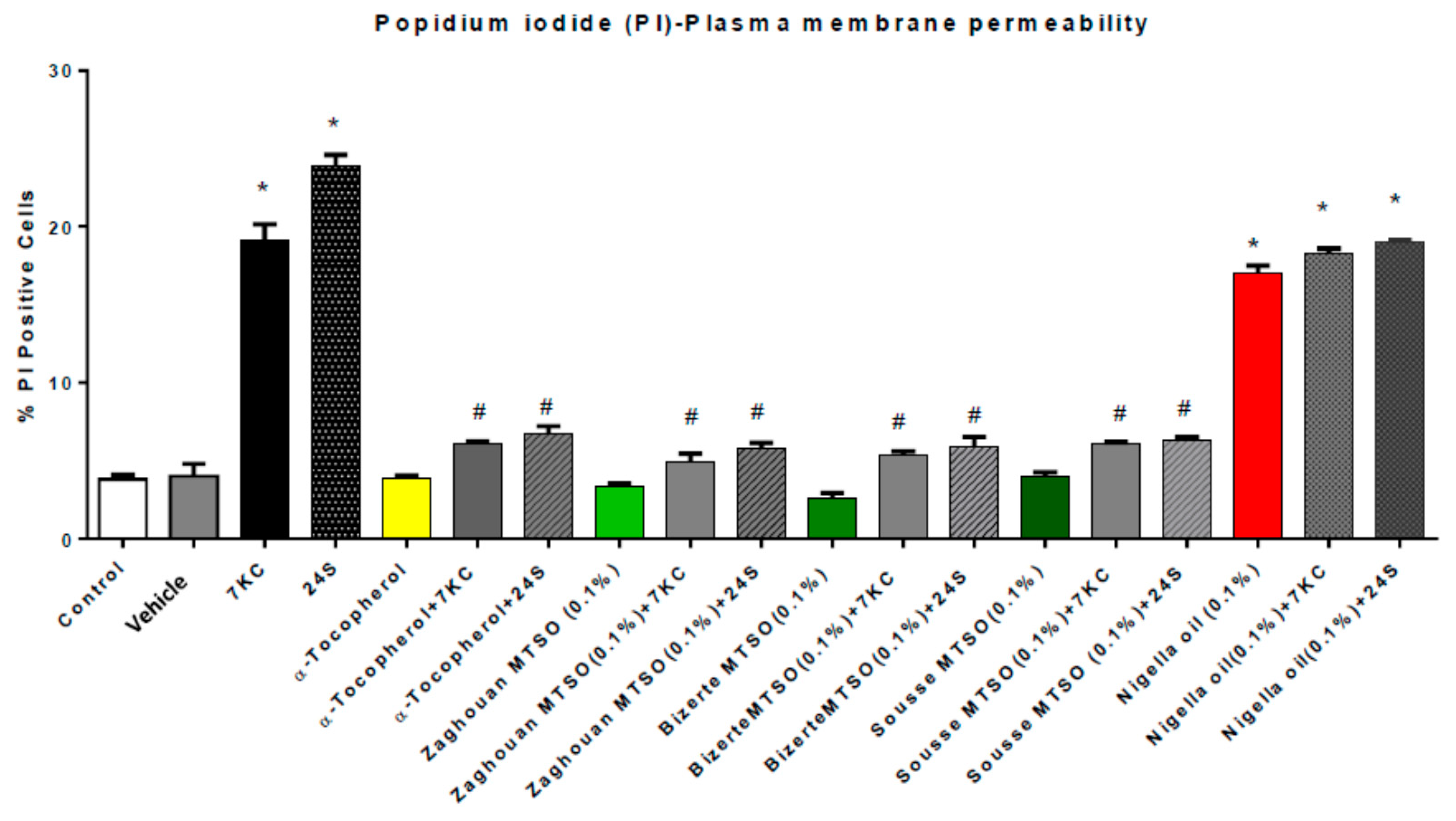
3.5. Effects of Milk Thistle Seed Oil from Different Areas of Tunisia on Plasma Membrane Permeability under Treatment with 7-Ketocholesterol and 24S-Hydroxycholesterol
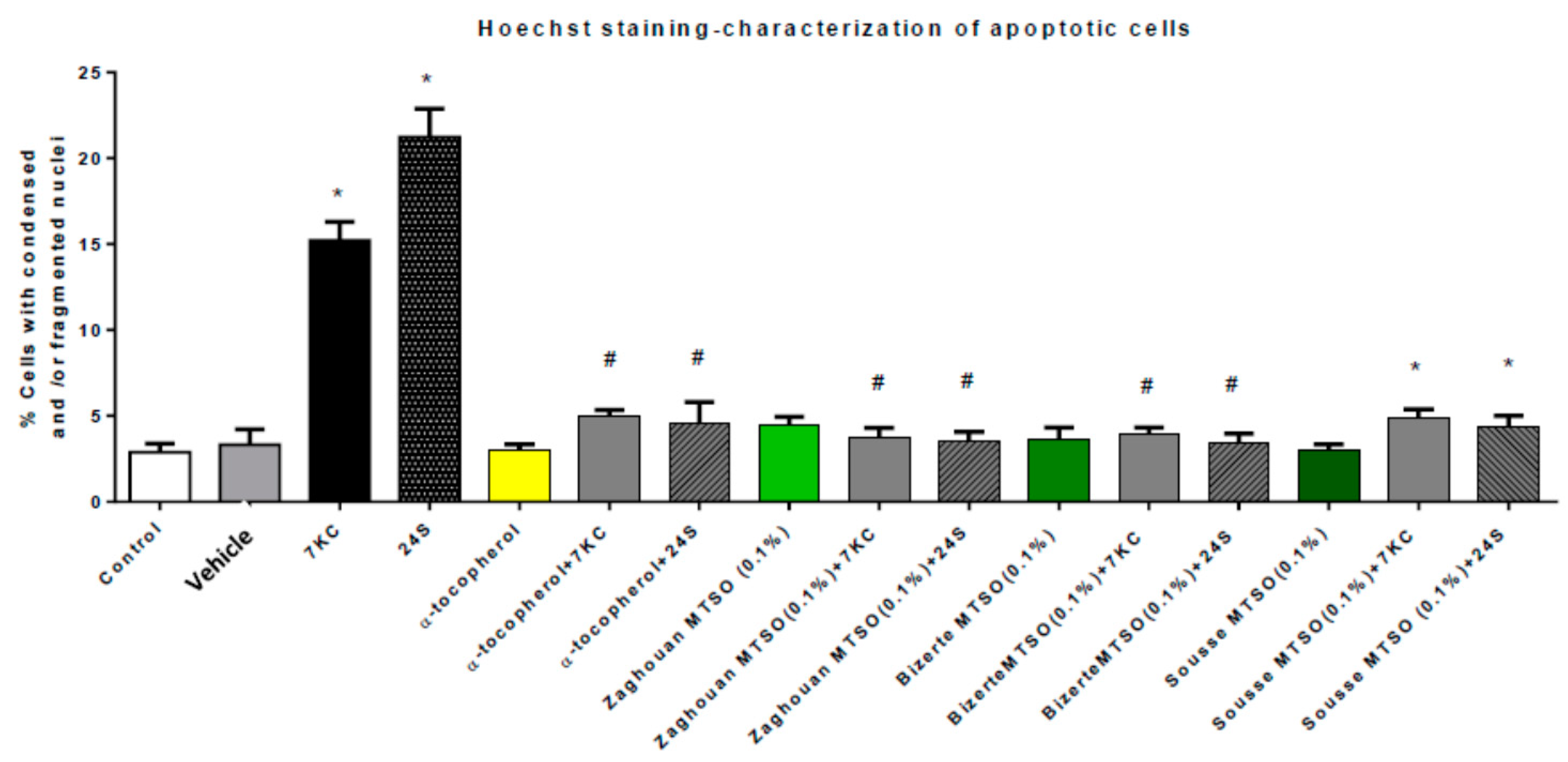
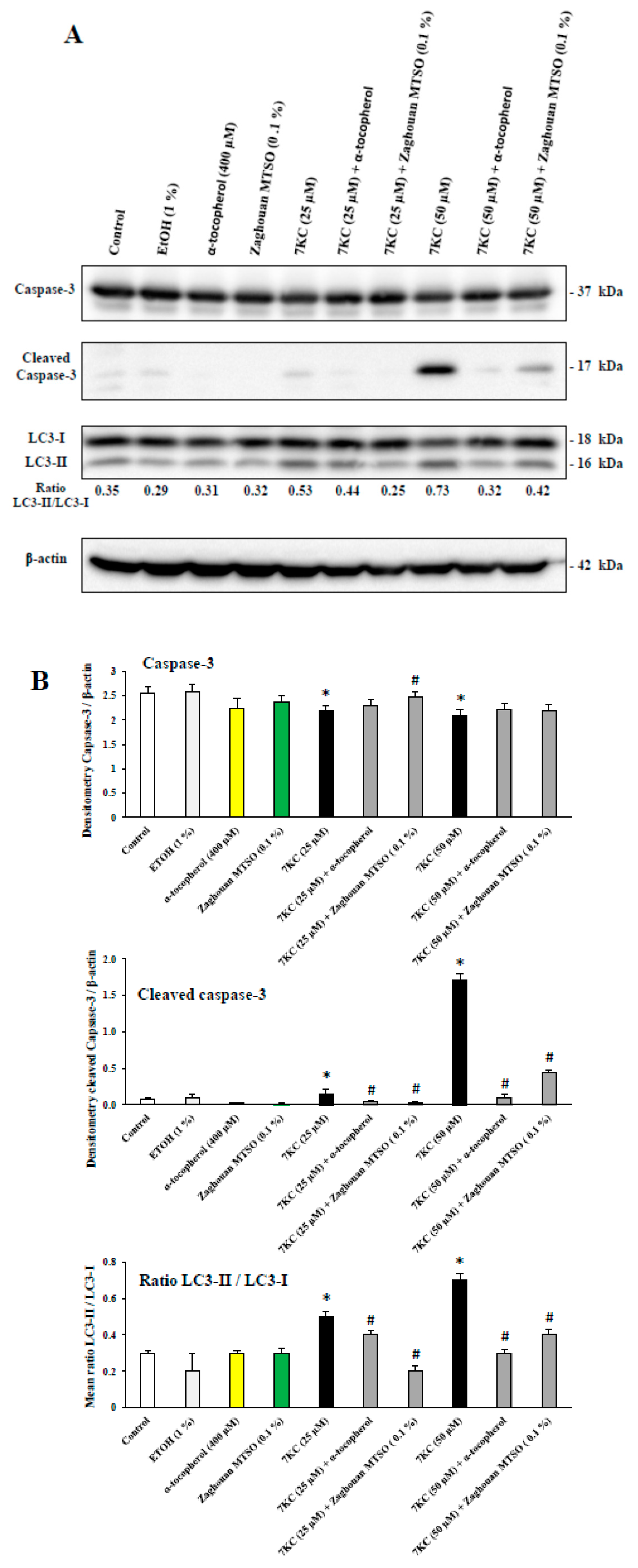
3.6. Effects of Milk Thistle Seed Oil on 7-Ketocholesterol- and 24S-Hydroxycholesterol-Induced Apoptosis and Autophagy
3.7. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Harman, D. The free radical theory of aging. Antioxid. Redox Signal. 2003, 5, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Tosato, M.; Zamboni, V.; Ferrini, A.; Cesari, M. The aging process and potential interventions to extend life expectancy. Clin. Interv. Aging 2007, 2, 401–412. [Google Scholar] [PubMed]
- Lordan, S.; Mackrill, J.J.; O’Brien, N.M. Oxysterols and mechanisms of apoptotic signaling: Implications in the pathology of degenerative diseases. J. Nutr. Biochem. 2009, 20, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Vejux, A.; Lizard, G. Cytotoxic effects of oxysterols associated with human diseases: Induction of cell death (apoptosis and/or oncosis), oxidative and inflammatory activities, and phospholipidosis. Mol. Asp. Med. 2009, 30, 153–170. [Google Scholar] [CrossRef] [PubMed]
- Leoni, V.; Caccia, C. Oxysterols as biomarkers in neurodegenerative diseases. Chem. Phys. Lipids 2011, 164, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Poli, G.; Biasi, F.; Leonarduzzi, G. Oxysterols in the pathogenesis of major chronic diseases. Redox Biol. 2013, 1, 125–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroepfer, G.J., Jr. Oxysterols: Modulators of cholesterol metabolism and other processes. Physiol. Rev. 2000, 80, 361–554. [Google Scholar] [CrossRef] [PubMed]
- Iuliano, L. Pathways of cholesterol oxidation via non-enzymatic mechanisms. Chem. Phys. Lipids 2011, 164, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Vejux, A.; Samadi, M.; Lizard, G. Contribution of cholesterol and oxysterols in the physiopathology of cataract: Implication for the development of pharmacological treatments. J. Ophthalmol. 2011, 2011, 471947. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, I.R.; Clark, M.E.; Lee, J.W.; Curcio, C.A. 7-ketocholesterol accumulates in ocular tissues as a consequence of aging and is present in high levels in drusen. Exp. Eye Res. 2014, 128, 151–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarrouk, A.; Vejux, A.; Mackrill, J.; O’Callaghan, Y.; Hammami, M.; O’Brien, N.; Lizard, G. Involvement of oxysterols in age-related diseases and ageing processes. Ageing Res. Rev. 2014, 18, 148–162. [Google Scholar] [CrossRef] [PubMed]
- El-Sayyad, H.; Bakr, E.; El-Ghawet, H.; El-Desoky, T. Overview of congenital, senile and metabolic cataract. J. Ocular Biol. 2015, 3, 12. [Google Scholar]
- Testa, G.; Staurenghi, E.; Zerbinati, C.; Gargiulo, S.; Iuliano, L.; Giaccone, G.; Fantò, F.; Poli, G.; Leonarduzzi, G.; Gamba, P. Changes in brain oxysterols at different stages of Alzheimer’s disease: Their involvement in neuroinflammation. Redox Biol. 2016, 10, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Balandrin, M.F.; Klocke, J.A.; Wurtele, E.S.; Bollinger, W.H. Natural plant chemicals: Sources of industrial and medicinal materials. Science 1985, 228, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Seifaddinipour, M.; Farghadani, R.; Namvar, F.; Mohamad, J.; Abdul Kadir, H. Cytotoxic Effects and Anti-Angiogenesis Potential of Pistachio (Pistacia vera L.) Hulls against MCF-7 Human Breast Cancer Cells. Molecules 2018, 23, 110. [Google Scholar] [CrossRef] [PubMed]
- Parry, J.H.Z.; Luther, M.; Su, L.; Zhou, K.; Yu, L. Characterization of cold-pressed onion, parsley, cardamom, mullein, roasted pumpkin, and milk thistle seed oils. J. Am. Oil Chem. Soc. 2006, 83, 847–854. [Google Scholar] [CrossRef]
- Rahal, N.B.; Barba, F.J.; Barth, D.; Chevalot, I. Supercritical CO2 extraction of oil, fatty acids and flavonolignans from milk thistle seeds: Evaluation of their antioxidant and cytotoxic activities in Caco-2 cells. Food Chem. Toxicol. 2015, 83, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Parry, J.; Yu, L. Fatty acid content and antioxidant properties of cold-pressed black raspberry seed oil and meal. J. Food Sci. 2004, 69. [Google Scholar] [CrossRef]
- Connor, W.E. Importance of n−3 fatty acids in health and disease. Am. J. Clin. Nutr. 2000, 71, 171S–175S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tapiero, H.; Ba, G.N.; Couvreur, P.; Tew, K. Polyunsaturated fatty acids (PUFA) and eicosanoids in human health and pathologies. Biomed. Pharmacother. 2002, 56, 215–222. [Google Scholar] [CrossRef]
- Singh, R.P.; Dhanalakshmi, S.; Tyagi, A.K.; Chan, D.C.; Agarwal, C.; Agarwal, R. Dietary feeding of silibinin inhibits advance human prostate carcinoma growth in athymic nude mice and increases plasma insulin-like growth factor-binding protein-3 levels. Cancer Res. 2002, 62, 3063–3069. [Google Scholar] [PubMed]
- Zi, X.; Feyes, D.K.; Agarwal, R. Anticarcinogenic effect of a flavonoid antioxidant, silymarin, in human breast cancer cells MDA-MB 468: Induction of G1 arrest through an increase in Cip1/p21 concomitant with a decrease in kinase activity of cyclin-dependent kinases and associated cyclins. Clin. Cancer Res. 1998, 4, 1055–1064. [Google Scholar] [PubMed]
- Deep, G.; Singh, R.; Agarwal, C.; Kroll, D.; Agarwal, R. Silymarin and silibinin cause G1 and G2–M cell cycle arrest via distinct circuitries in human prostate cancer PC3 cells: A comparison of flavanone silibinin with flavanolignan mixture silymarin. Oncogene 2006, 25, 1053–1069. [Google Scholar] [CrossRef] [PubMed]
- Vinh, P.Q.; Sugie, S.; Tanaka, T.; Hara, A.; Yamada, Y.; Katayama, M.; Deguchi, T.; Mori, H. Chemopreventive Effects of a Flavonoid Antioxidant Silymarin on N-Butyl-N-(4-hydroxybutyl) nitrosamine-induced Urinary Bladder Carcinogenesis in Male ICR Mice. Cancer Sci. 2002, 93, 42–49. [Google Scholar] [CrossRef]
- Tyagi, A.; Agarwal, C.; Harrison, G.; Glode, L.M.; Agarwal, R. Silibinin causes cell cycle arrest and apoptosis in human bladder transitional cell carcinoma cells by regulating CDKI–CDK–cyclin cascade, and caspase 3 and PARP cleavages. Carcinogenesis 2004, 25, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Kohno, H.; Tanaka, T.; Kawabata, K.; Hirose, Y.; Sugie, S.; Tsuda, H.; Mori, H. Silymarin, a naturally occurring polyphenolic antioxidant flavonoid, inhibits azoxymethane-induced colon carcinogenesis in male F344 rats. Int. J. Cancer 2002, 101, 461–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.-H.; Lin, J.-K.; Chen, W.-S.; Chiu, J.-H. Anti-angiogenic effect of silymarin on colon cancer lovo cell line. J. Surg. Res. 2003, 113, 133–138. [Google Scholar] [CrossRef]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Malewicz, B.; Wang, Z.; Jiang, C.; Guo, J.; Cleary, M.P.; Grande, J.P.; Lü, J. Enhancement of mammary carcinogenesis in two rodent models by silymarin dietary supplements. Carcinogenesis 2006, 27, 1739–1747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vejux, A.; Aires, V.; Delmas, D. Functional Foods and Cancer: Bioactive Compounds and Cancer Volume 4, 1st ed.; Martirosyan, D.M., Zhou, J.-K., Eds.; CreateSpace Independent Publishing Platform: Lavergne, TN, USA, 2017; p. 204. [Google Scholar]
- Kittur, S.; Wilasrusmee, S.; Pedersen, W.A.; Mattson, M.P.; Straube-West, K.; Wilasrusmee, C.; Jubelt, B.; Kittur, D.S. Neurotrophic and neuroprotective effects of milk thistle (Silybum marianum) on neurons in culture. J. Mol. Neurosci. 2002, 18, 265–269. [Google Scholar] [CrossRef]
- Teerapattarakan, N.; Benya-aphikul, H.; Tansawat, R.; Wanakhachornkrai, O.; Tantisira, M.H.; Rodsiri, R. Neuroprotective effect of a standardized extract of Centella asiatica ECa233 in rotenone-induced parkinsonism rats. Phytomedicine 2018, 44, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Baarine, M.; Ragot, K.; Genin, E.C.; El Hajj, H.; Trompier, D.; Andreoletti, P.; Ghandour, M.S.; Menetrier, F.; Cherkaoui-Malki, M.; Savary, S. Peroxisomal and mitochondrial status of two murine oligodendrocytic cell lines (158N, 158JP): Potential models for the study of peroxisomal disorders associated with dysmyelination processes. J. Neurochem. 2009, 111, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Ragot, K.; Mackrill, J.J.; Zarrouk, A.; Nury, T.; Aires, V.; Jacquin, A.; Athias, A.; de Barros, J.-P.P.; Véjux, A.; Riedinger, J.-M. Absence of correlation between oxysterol accumulation in lipid raft microdomains, calcium increase, and apoptosis induction on 158N murine oligodendrocytes. Biochem. Pharmacol. 2013, 86, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Nury, T.; Zarrouk, A.; Ragot, K.; Debbabi, M.; Riedinger, J.-M.; Vejux, A.; Aubourg, P.; Lizard, G. 7-Ketocholesterol is increased in the plasma of X-ALD patients and induces peroxisomal modifications in microglial cells: Potential roles of 7-ketocholesterol in the pathophysiology of X-ALD. J. Steroid Biochem. Mol. Biol. 2017, 169, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Ragot, K.; Delmas, D.; Athias, A.; Nury, T.; Baarine, M.; Lizard, G. α-Tocopherol impairs 7-ketocholesterol-induced caspase-3-dependent apoptosis involving GSK-3 activation and Mcl-1 degradation on 158N murine oligodendrocytes. Chem. Phys. Lipids 2011, 164, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Leoni, V.; Nury, T.; Vejux, A.; Zarrouk, A.; Caccia, C.; Debbabi, M.; Fromont, A.; Sghaier, R.; Moreau, T.; Lizard, G. Mitochondrial dysfunctions in 7-ketocholesterol-treated 158N oligodendrocytes without or with α-tocopherol: Impacts on the cellular profil of tricarboxylic cycle-associated organic acids, long chain saturated and unsaturated fatty acids, oxysterols, cholesterol and cholesterol precursors. J. Steroid Biochem. Mol. Biol. 2017, 169, 96–110. [Google Scholar] [PubMed]
- Zarrouk, A.; Nury, T.; Samadi, M.; O’Callaghan, Y.; Hammami, M.; O’Brien, N.M.; Lizard, G.; Mackrill, J.J. Effects of cholesterol oxides on cell death induction and calcium increase in human neuronal cells (SK-N-BE) and evaluation of the protective effects of docosahexaenoic acid (DHA; C22: 6 n-3). Steroids 2015, 99, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Nury, T.; Zarrouk, A.; Mackrill, J.J.; Samadi, M.; Durand, P.; Riedinger, J.-M.; Doria, M.; Vejux, A.; Limagne, E.; Delmas, D. Induction of oxiapoptophagy on 158N murine oligodendrocytes treated by 7-ketocholesterol-, 7β-hydroxycholesterol-, or 24 (S)-hydroxycholesterol: Protective effects of α-tocopherol and docosahexaenoic acid (DHA; C22: 6 n-3). Steroids 2015, 99, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Miguet, C.; Monier, S.; Bettaieb, A.; Athias, A.; Bessede, G.; Laubriet, A.; Lemaire, S.; Neel, D.; Gambert, P.; Lizard, G. Ceramide generation occurring during 7β-hydroxycholesterol-and 7-ketocholesterol-induced apoptosis is caspase independent and is not required to trigger cell death. Cell Death Differ. 2001, 8, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Kahn, E.; Ménétrier, F.; Vejux, A.; Montange, T.; Dumas, D.; Riedinger, J.-M.; Frouin, F.; Tourneur, Y.; Brau, F.; Stoltz, J.-F. Flow cytometry and spectral imaging multiphoton microscopy analysis of CD36 expression with quantum dots 605 of untreated and 7-ketocholesterol-treated human monocytic cells. Anal. Quant. Cytol. Histol. 2006, 28, 316–330. [Google Scholar] [PubMed]
- Vurusaner, B.; Gamba, P.; Testa, G.; Gargiulo, S.; Biasi, F.; Zerbinati, C.; Iuliano, L.; Leonarduzzi, G.; Basaga, H.; Poli, G. Survival signaling elicited by 27-hydroxycholesterol through the combined modulation of cellular redox state and ERK/Akt phosphorylation. Free Radic Biol. Med. 2014, 77, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Debbabi, M.; Zarrouk, A.; Bezine, M.; Meddeb, W.; Nury, T.; Badreddine, A.; Sghaier, R.; Bretillon, L.; Guyot, S.; Samadi, M. Comparison of the effects of major fatty acids present in the Mediterranean diet (oleic acid, docosahexaenoic acid) and in hydrogenated oils (elaidic acid) on 7-ketocholesterol-induced oxiapoptophagy in microglial BV-2 cells. Chem. Phys. Lipids 2017, 207, 151–170. [Google Scholar] [CrossRef] [PubMed]
- Moilanen, T.; Nikkari, T. The effect of storage on the fatty acid composition of human serum. Clin. Chim. Acta 1981, 114, 111–116. [Google Scholar] [CrossRef]
- Morrison, W.R.; Smith, L.M. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride–methanol. J. Lipid Res. 1964, 5, 600–608. [Google Scholar] [PubMed]
- Debbabi, M.; Nury, T.; Zarrouk, A.; Mekahli, N.; Bezine, M.; Sghaier, R.; Grégoire, S.; Martine, L.; Durand, P.; Camus, E. Protective effects of α-tocopherol, γ-tocopherol and oleic acid, three compounds of olive oils, and no effect of trolox, on 7-ketocholesterol-induced mitochondrial and peroxisomal dysfunction in microglial BV-2 cells. Int. J. Mol. Sci. 2016, 17, 1973. [Google Scholar] [CrossRef] [PubMed]
- Badreddine, A.; Zarrouk, A.; Karym, E.M.; Debbabi, M.; Nury, T.; Meddeb, W.; Sghaier, R.; Bezine, M.; Vejux, A.; Martine, L. Argan Oil-Mediated Attenuation of Organelle Dysfunction, Oxidative Stress and Cell Death Induced by 7-Ketocholesterol in Murine Oligodendrocytes 158N. Int. J. Mol. Sci. 2017, 18, 2220. [Google Scholar] [CrossRef] [PubMed]
- Mounts, T.; Abidi, S.; Rennick, K. Effect of genetic modification on the content and composition of bioactive constituents in soybean oil. J. Am. Oil Chem. Soc. 1996, 73, 581–586. [Google Scholar] [CrossRef]
- Park, S.W.; Addis, P. HPLC determination of C-7 oxidized cholesterol derivatives in foods. J. Food Sci. 1985, 50, 1437–1441. [Google Scholar] [CrossRef]
- Rossi, R.; Pastorelli, G.; Corino, C. Application of KRL test to assess total antioxidant activity in pigs: Sensitivity to dietary antioxidants. Res. Vet. Sci. 2013, 94, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Oyaizu, M. Studies on products of browning reaction. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Colin, D.; Limagne, E.; Jeanningros, S.; Jacquel, A.; Lizard, G.; Athias, A.; Gambert, P.; Hichami, A.; Latruffe, N.; Solary, E. Endocytosis of resveratrol via lipid rafts and activation of downstream signaling pathways in cancer cells. Cancer Prev. Res. 2011, 4, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- Lizard, G.; Gueldry, S.; Deckert, V.; Gambert, P.; Lagrost, L. Evaluation of the cytotoxic effects of some oxysterols and of cholesterol on endothelial cell growth: Methodological aspects. Pathol. Biol. 1997, 45, 281–290. [Google Scholar] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Zarrouk, A.; Vejux, A.; Nury, T.; El Hajj, H.I.; Haddad, M.; Cherkaoui-Malki, M.; Riedinger, J.-M.; Hammami, M.; Lizard, G. Induction of mitochondrial changes associated with oxidative stress on very long chain fatty acids (C22: 0, C24: 0, or C26: 0)-treated human neuronal cells (SK-NB-E). Oxid. Med. Cell. Longev. 2012, 2012, 623257. [Google Scholar] [CrossRef] [PubMed]
- Rothe, G.; Valet, G. Flow cytometric analysis of respiratory burst activity in phagocytes with hydroethidine and 2′, 7′-dichlorofluorescin. J. Leukoc. Biol. 1990, 47, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Lizard, G.; Fournel, S.; Genestier, L.; Dhedin, N.; Chaput, C.; Flacher, M.; Mutin, M.; Panaye, G.; Revillard, J.P. Kinetics of plasma membrane and mitochondrial alterations in cells undergoing apoptosis. Cytom. Part A 1995, 21, 275–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Mallah, H.M.; El-Shami, S.M.; Hassanein, M.M. Detailed studies on some lipids of Silybum marianum (L.) seed oil. Grasas y Aceites 2003, 54, 397–402. [Google Scholar]
- Fathi-Achachlouei, B.; Azadmard-Damirchi, S. Milk thistle seed oil constituents from different varieties grown in Iran. J. Am. Oil Chem. Soc. 2009, 86, 643–649. [Google Scholar] [CrossRef]
- Dabbour, I.; Al-Ismail, K.; Takruri, H.; Azzeh, F. Chemical characteristics and antioxidant content properties of cold pressed seed oil of wild milk thistle plant grown in Jordan. Pak. J. Nutr. 2014, 13, 67–78. [Google Scholar] [CrossRef]
- Hasanlou, T.; Bahmani, M.; Sepehrifar, R.; Kalantari, F. Determination of tocopherols and fatty acids in seeds of Silybum marianum (L.) gaerth. J. Med. Plants 2008, 7, 69–76. [Google Scholar]
- O’Brian, R.D. Fats and Oils: Formulating and Processing for Applications; Taylor & Francis Group, CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Benveniste, P. Biosynthesis and accumulation of sterols. Annu. Rev. Plant Biol. 2004, 55, 429–457. [Google Scholar] [CrossRef] [PubMed]
- Behrman, E.; Gopalan, V. Cholesterol and plants. J. Chem. Educ. 2005, 82, 1791. [Google Scholar] [CrossRef]
- Traber, M.G.; Stevens, J.F. Vitamins C and E: Beneficial effects from a mechanistic perspective. Free Radic. Biol. Med. 2011, 51, 1000–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cilla, A.; Alegría, A.; Attanzio, A.; Garcia-Llatas, G.; Tesoriere, L.; Livrea, M.A. Dietary phytochemicals in the protection against oxysterol-induced damage. Chem. Phys. Lipids 2017, 207, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Otaegui-Arrazola, A.; Menéndez-Carreño, M.; Ansorena, D.; Astiasarán, I. Oxysterols: A world to explore. Food Chem. Toxicol. 2010, 48, 3289–3303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lizard, G.; Deckert, V.; Dubrez, L.; Moisant, M.; Gambert, P.; Lagrost, L. Induction of apoptosis in endothelial cells treated with cholesterol oxides. Am. J. Pathol. 1996, 148, 1625–1638. [Google Scholar] [PubMed]
- Zahm, J.M.; Baconnais, S.; Monier, S.; Bonnet, N.; Bessede, G.; Gambert, P.; Puchelle, E.; Lizard, G. Chronology of cellular alterations during 7-ketocholesterol–induced cell death on A7R5 rat smooth muscle cells: Analysis by time lapse-video microscopy and conventional fluorescence microscopy. Cytom. Part A 2003, 52, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Zarrouk, A.; Nury, T.; Karym, E.-M.; Vejux, A.; Sghaier, R.; Gondcaille, C.; Andreoletti, P.; Trompier, D.; Savary, S.; Cherkaoui-Malki, M. Attenuation of 7-ketocholesterol-induced overproduction of reactive oxygen species, apoptosis, and autophagy by dimethyl fumarate on 158 N murine oligodendrocytes. J. Steroid Biochem. Mol. Biol. 2017, 169, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Monier, S.; Samadi, M.; Prunet, C.; Denance, M.; Laubriet, A.; Athias, A.; Berthier, A.; Steinmetz, E.; Jürgens, G.; Nègre-Salvayre, A. Impairment of the cytotoxic and oxidative activities of 7β-hydroxycholesterol and 7-ketocholesterol by esterification with oleate. Biochem. Biophys. Res. Commun. 2003, 303, 814–824. [Google Scholar] [CrossRef]
- Nury, T.; Zarrouk, A.; Vejux, A.; Doria, M.; Riedinger, J.M.; Delage-Mourroux, R.; Lizard, G. Induction of oxiapoptophagy, a mixed mode of cell death associated with oxidative stress, apoptosis and autophagy, on 7-ketocholesterol-treated 158N murine oligodendrocytes: Impairment by α-tocopherol. Biochem. Biophys. Res. Commun. 2014, 446, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Lütjohann, D.; Breuer, O.; Ahlborg, G.; Nennesmo, I.; Siden, A.; Diczfalusy, U.; Björkhem, I. Cholesterol homeostasis in human brain: Evidence for an age-dependent flux of 24S-hydroxycholesterol from the brain into the circulation. Proc. Natl. Acad. Sci. USA 1996, 93, 9799–9804. [Google Scholar] [CrossRef] [PubMed]
- Sottero, B.; Gamba, P.; Gargiulo, S.; Leonarduzzi, G.; Poli, G. Cholesterol oxidation products and disease: An emerging topic of interest in medicinal chemistry. Curr. Med. Chem. 2009, 16, 685–705. [Google Scholar] [CrossRef] [PubMed]
- Tritschler, H.-J.; Packer, L.; Medori, R. Oxidative stress and mitochondrial dysfunction in neurodegeneration. Biochem. Mol. Biol. Int. 1994, 34, 169–181. [Google Scholar] [PubMed]
- Rimbach, G.; Minihane, A.M.; Majewicz, J.; Fischer, A.; Pallauf, J.; Virgli, F.; Weinberg, P.D. Regulation of cell signalling by vitamin E. Proc. Nutr. Soc. 2002, 61, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Grimsrud, P.A.; Xie, H.; Griffin, T.J.; Bernlohr, D.A. Oxidative stress and covalent modification of protein with bioactive aldehydes. J. Biol. Chem. 2008, 283, 21837–21841. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Abdelmohsen, K.; Abe, A.; Abedin, M.J.; Abeliovich, H.; Acevedo Arozena, A.; Adachi, H.; Adams, C.M.; Adams, P.D.; Adeli, K. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 2016, 12, 1–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guina, T.; Biasi, F.; Calfapietra, S.; Nano, M.; Poli, G. Inflammatory and redox reactions in colorectal carcinogenesis. Ann. N. Y. Acad. Sci. 2015, 1340, 95–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dugas, B.; Charbonnier, S.; Baarine, M.; Ragot, K.; Delmas, D.; Ménétrier, F.; Lherminier, J.; Malvitte, L.; Khalfaoui, T.; Bron, A. Effects of oxysterols on cell viability, inflammatory cytokines, VEGF, and reactive oxygen species production on human retinal cells: Cytoprotective effects and prevention of VEGF secretion by resveratrol. Eur. J. Nutr. 2010, 49, 435–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, C.; Zhu, H.; Zhang, W.; Okon, I.; Wang, Q.; Li, H.; Le, Y.-Z.; Xie, Z. 7-Ketocholesterol induces autophagy in vascular smooth muscle cells through Nox4 and Atg4B. Am. J. Pathol. 2013, 183, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Kroemer, G. Autophagy in the pathogenesis of disease. Cell 2008, 132, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Vejux, A.; Malvitte, L.; Lizard, G. Side effects of oxysterols: Cytotoxicity, oxidation, inflammation, and phospholipidosis. Braz. J. Med. Biol. Res. 2008, 41, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Vejux, A.; Guyot, S.; Montange, T.; Riedinger, J.-M.; Kahn, E.; Lizard, G. Phospholipidosis and down-regulation of the PI3-K/PDK-1/Akt signalling pathway are vitamin E inhibitable events associated with 7-ketocholesterol-induced apoptosis. J. Nutr. Biochem. 2009, 20, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Zaoui, A.; Cherrah, Y.; Mahassini, N.; Alaoui, K.; Amarouch, H.; Hassar, M. Acute and chronic toxicity of Nigella sativa fixed oil. Phytomedicine 2002, 9, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Zaghlol, D.A.A.; Kamel, E.S.; Mohammed, D.S.; Abbas, N.H. The possible toxic effect of different doses of Nigella sativa oil on the histological structure of the liver and renal cortex of adult male albino rats. Egypt. J. Histol. 2012, 35, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Al-Ali, A.; Alkhawajah, A.A.; Randhawa, M.A.; Shaikh, N.A. Oral and intraperitoneal LD50 of thymoquinone, an active principle of Nigella sativa, in mice and rats. J. Ayub Med. Coll. Abbottabad 2008, 20, 25–27. [Google Scholar] [PubMed]
- Giacoppo, S.; Galuppo, M.; Lombardo, G.E.; Ulaszewska, M.M.; Mattivi, F.; Bramanti, P.; Mazzon, E.; Navarra, M. Neuroprotective effects of a polyphenolic white grape juice extract in a mouse model of experimental autoimmune encephalomyelitis. Fitoterapia 2015, 103, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Gundimeda, U.; McNeill, T.H.; Schiffman, J.E.; Hinton, D.R.; Gopalakrishna, R. Green tea polyphenols potentiate the action of nerve growth factor to induce neuritogenesis: Possible role of reactive oxygen species. J. Neurosci. Res. 2010, 88, 3644–3655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandenhaute, E.; Dehouck, L.; Boucau, M.-C.; Sevin, E.; Uzbekov, R.; Tardivel, M.; Gosselet, F.; Fenart, L.; Cecchelli, R.; Dehouck, M.-P. Modelling the neurovascular unit and the blood-brain barrier with the unique function of pericytes. Curr. Neurovasc. Res. 2011, 8, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Figueira, I.; Tavares, L.; Jardim, C.; Costa, I.; Terrasso, A.P.; Almeida, A.F.; Govers, C.; Mes, J.J.; Gardner, R.; Becker, J.D.; et al. Blood-brain barrier transport and neuroprotective potential of blackberry-digested polyphenols: An in vitro study. Eur. J. Nutr. 2017. [Google Scholar] [CrossRef] [PubMed]
- Gosselet, F. Modelling of the blood-brain barrier. Med. Sci. 2017, 33, 423–431. [Google Scholar]

| Fatty Acids | Origins of Milk Thistle Seed Oil (MTSO) | Nigella Seed Oil (NSO) | ||
|---|---|---|---|---|
| Sousse | Zaghouan | Bizerte | ||
| C12:0 | 0.00 | 0.00 | 0.11 ± 0.00 | 0.00 |
| C14:0 | 0.08 ± 0.00 | 0.07 ± 0.02 | 0.13 ± 0.00 | 0.16 ± 0.001 |
| C15:0 | 0.00 | 0.00 | 0.00 | 0.00 |
| C16:0 | 13.06 ± 0.07 | 6.25 ± 2.00 | 8.89 ± 0.14 | 12.29 ± 0.043 |
| C17:0 | 0.09 ± 0.00 | 0.08 ± 0.03 | 0.10 ± 0.00 | 0.07 ± 0.00 |
| C18:0 | 3.35 ± 0.11 | 4.03 ± 1.32 | 5.21 ± 0.02 | 3.37 ± 0.05 |
| C20:0 | 1.48 ± 0.06 | 2.33 ± 0.71 | 2.72 ± 0.09 | 0.22 ± 0.01 |
| C22:0 | 0.76 ± 0.00 | 1.86 ± 0.59 | 1.99 ± 0.11 | 0.05 ± 0.00 |
| C24:0 | 0.19 ± 0.00 | 0.40 ± 0.13 | 0.46 ± 0.03 | 0.00 |
| C16:1 n-7 | 19.24 ± 0.17 | 18.98 ± 0.80 | 19.63 ± 0.10 | 0.22 ± 0.00 |
| C16:1 n-9 | 0.00 | 0.00 | 0.00 | 0.00 |
| C18:1n-9 | 21.39 ± 0.02 | 15.78 ± 4.95 | 19.03 ± 0.01 | 22.76 ± 0.02 |
| C18:1 n-7 | 0.79 ± 0.01 | 0.40 ± 0.13 | 0.57 ± 0.01 | 1.09 ± 0.00 |
| C18:2 n-6 | 56.77 ± 0.57 | 48.70 ± 14.94 | 58.47 ± 0.20 | 54.56 ± 0.14 |
| C20:1 n-9 | 0.31 ± 0.00 | 0.72 ± 0.21 | 0.81 ± 0.01 | 0.32 ± 0.00 |
| C18:3 n-3 | 0.49 ± 0.01 | 0.11 ± 0.03 | 0.16 ± 0.00 | 1.54 ± 0.02 |
| C20:2 n-6 | 0.00 | 0.00 | 0.00 | 2.25 ± 0.02 |
| C22:1 n-9 | 0.00 | 0.06 ± 0.04 | 0.00 | 0.11 ± 0.02 |
| ∑SFA | 19.24 ± 0.17 | 18.98 ± 0.80 | 19.63 ± 0.10 | 16.18 ± 0.01 |
| ∑UFA | 80.83 ± 1.86 | 83.30 ± 4.39 | 79.12 ± 0.20 | 82.85 ± 0.14 |
| Area from Tunisia | Bizerte | Zaghouan | Sousse |
|---|---|---|---|
| Tocopherol | |||
| α | 47.65 ± 3.54 | 286.22 ± 25.49 | 278.47 ± 24.64 |
| β | 1.91 ± 0.21 | 3.58 ± 0.37 | 6.66 ± 0.74 |
| γ | 0.0 | 14.24 ± 1.25 | 23.94 ± 2.14 |
| δ | 0.0 | 14.24 ± 1.22 | 5.23 ± 0.61 |
| Total | 49.57 ± 5.11 | 318.29 ± 28.45 | 314.31 ± 30.77 |
| Sterols | Origins of Milk Thistle Seed Oil (MTSO) | Nigella Seed Oil (NSO) | ||
|---|---|---|---|---|
| Bizerte | Zaghouan | Sousse | ||
| Cholesterol | 11.47 ± 0.04 | 4.53 ± 0.01 | 9.53 ± 0.03 | 0.55 ± 0.01 |
| Campesterol | 4.75 ± 0.01 | 4.77 ± 0.02 | 10.89 ± 0.03 | 6.97 ± 0.06 |
| ∆7-Campesterol | 4.14 ± 0.01 | 4.81 ± 0.02 | 2.73 ± 0.02 | 0.18 ± 0.03 |
| Spinasterol | 0 | 0 | 0 | 0 |
| β-Sitosterol | 31.96 ± 0.13 | 32.78 ± 0.05 | 42.33 ± 0.01 | 34.91 ± 0.30 |
| Schotenol | 20.97 ± 0.09 | 24.54 ± 0.12 | 6.86 ± 0.03 | 0.51 ± 0.03 |
| Stigmasterol | 20.97 ± 0.09 | 5.91 ± 0.03 | 4.88 ± 0.01 | 6.92 ± 0.09 |
| β-amyrine | 4.72 ± 0.01 | 5.14 ± 0.04 | 3.08 ± 0.01 | 1.29 ± 0.08 |
| ∆5 avenasterol | 2.83 ± 0.03 | 3.03 ± 0.02 | 4.59 ± 0.02 | 8.50 ± 0.05 |
| cycloartenol | 2.14 ± 0.07 | 1.67 ± 0.03 | 1.51 ± 0.05 | 18.34 ± 0.21 |
| ∆7 avenasterol | 3.82 ± 0.05 | 4.68 ± 0.06 | 3.98 ± 0.07 | 1.33 ± 0.06 |
| 24-Methylene cycloartenol | 2.07 ± 0.03 | 2.40 ± 0.02 | 2.52 ± 0.01 | 12.55 ± 0.10 |
| 24-Methylene cholesterol | 0.25 ± 0.01 | 0.20 ± 0.01 | 0.77 ± 0.00 | 0.82 ± 0.03 |
| Campestanol | 0.50 ± 0.01 | 0.54 ± 0.02 | 0.91 ± 0.02 | 0.79 ± 0.01 |
| ∆7-Stigmasterol | 0 | 0 | 0 | 0 |
| Clerosterol | 0 | 0 | 0 | 0.85 ±0.05 |
| Graminasterol | 1.31 ± 0.04 | 1.54 ± 0.06 | 1.39 ± 0.02 | 1.80 ± 0.04 |
| Lupeol | 0 | 0 | 0 | 0 |
| Fucosterol | 1.77 ± 0.01 | 2.03 ± 0.05 | 2.79 ± 0.00 | 0.91 ± 0.12 |
| Citrostadienol | 1.57 ±0.01 | 1.43 ± 0.00 | 1.24 ± 0.00 | 2.78 ± 0.11 |
| Total content (mg/kg) | 5206.13 ± 24.23 | 5088.54 ± 71.96 | 5891.82 ± 118.12 | 2659.29 ± 189.56 |
| Polyphenols | Origins of Milk Thistle Seed Oil (MTSO) | Nigella Seed Oil (NSO) | ||
|---|---|---|---|---|
| Bizerte | Zaghouan | Sousse | ||
| Homovanillic acid | ND | 0.13 | ND | 0.19 |
| Vanillin | ND | 0.20 | 0.33 | 0.23 |
| p-Coumaric acid | ND | 0.07 | ND | ND |
| Quercetine-3β-glucoside | ND | 0.08 | ND | ND |
| Quercetin | ND | 0.12 | ND | 0.13 |
| Apigenin | ND | 0.09 | ND | ND |
| 2,6-Dihydroxybenzoïc acid | ND | ND | ND | 1.27 |
| Chlorogenic acid | ND | ND | ND | 0.09 |
| Ferrulic acid | ND | ND | ND | 0.09 |
| Thymoquinone | ND | ND | ND | 0.70 |
| Hydroxytyrosol | ND | ND | ND | ND |
| Tyrosol | ND | ND | ND | ND |
| Oleuropein | ND | ND | ND | ND |
| Luteoline | ND | ND | ND | ND |
| Protocatechic acid | ND | ND | ND | ND |
| Compounds | Antioxidant Activities (Trolox Equivalent) | ||
|---|---|---|---|
| KRL | FRAP | DPPH | |
| α-Tocopherol | 0.94 ± 0.01 | 0.80 ± 0.06 | 1.33 ± 0.03 |
| Resveratrol | 7.90 ± 0.05 | 5.27 ± 0.03 | 4.52 ± 0.04 |
| Silymarin | 3.67 ± 0.03 | 3.47± 0.07 | 2.96 ± 0.02 |
| Ferrulic acid | 4.73 ± 0.05 | 2.02 ± 0.04 | 3.36 ± 0.03 |
| Zaghouan MTSO | 144.25 ± 0.58 | 211.06 ± 0.42 | 216.16 ± 0.45 |
| Bizerte MTSO | 180.48 ± 0.49 | 125.122 ± 0.35 | 139.480 ± 0.53 |
| Sousse MTSO | 160.173 ± 0.33 | 122.14 ± 0.47 | 98.54 ± 0.29 |
| Nigella seed oil | ND | 312.29 ± 0.35 | 227.53 ± 0.28 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meddeb, W.; Rezig, L.; Zarrouk, A.; Nury, T.; Vejux, A.; Prost, M.; Bretillon, L.; Mejri, M.; Lizard, G. Cytoprotective Activities of Milk Thistle Seed Oil Used in Traditional Tunisian Medicine on 7-Ketocholesterol and 24S-Hydroxycholesterol-Induced Toxicity on 158N Murine Oligodendrocytes. Antioxidants 2018, 7, 95. https://doi.org/10.3390/antiox7070095
Meddeb W, Rezig L, Zarrouk A, Nury T, Vejux A, Prost M, Bretillon L, Mejri M, Lizard G. Cytoprotective Activities of Milk Thistle Seed Oil Used in Traditional Tunisian Medicine on 7-Ketocholesterol and 24S-Hydroxycholesterol-Induced Toxicity on 158N Murine Oligodendrocytes. Antioxidants. 2018; 7(7):95. https://doi.org/10.3390/antiox7070095
Chicago/Turabian StyleMeddeb, Wiem, Leila Rezig, Amira Zarrouk, Thomas Nury, Anne Vejux, Michel Prost, Lionel Bretillon, Mondher Mejri, and Gérard Lizard. 2018. "Cytoprotective Activities of Milk Thistle Seed Oil Used in Traditional Tunisian Medicine on 7-Ketocholesterol and 24S-Hydroxycholesterol-Induced Toxicity on 158N Murine Oligodendrocytes" Antioxidants 7, no. 7: 95. https://doi.org/10.3390/antiox7070095
APA StyleMeddeb, W., Rezig, L., Zarrouk, A., Nury, T., Vejux, A., Prost, M., Bretillon, L., Mejri, M., & Lizard, G. (2018). Cytoprotective Activities of Milk Thistle Seed Oil Used in Traditional Tunisian Medicine on 7-Ketocholesterol and 24S-Hydroxycholesterol-Induced Toxicity on 158N Murine Oligodendrocytes. Antioxidants, 7(7), 95. https://doi.org/10.3390/antiox7070095