Betalains in Some Species of the Amaranthaceae Family: A Review
Abstract
1. Introduction
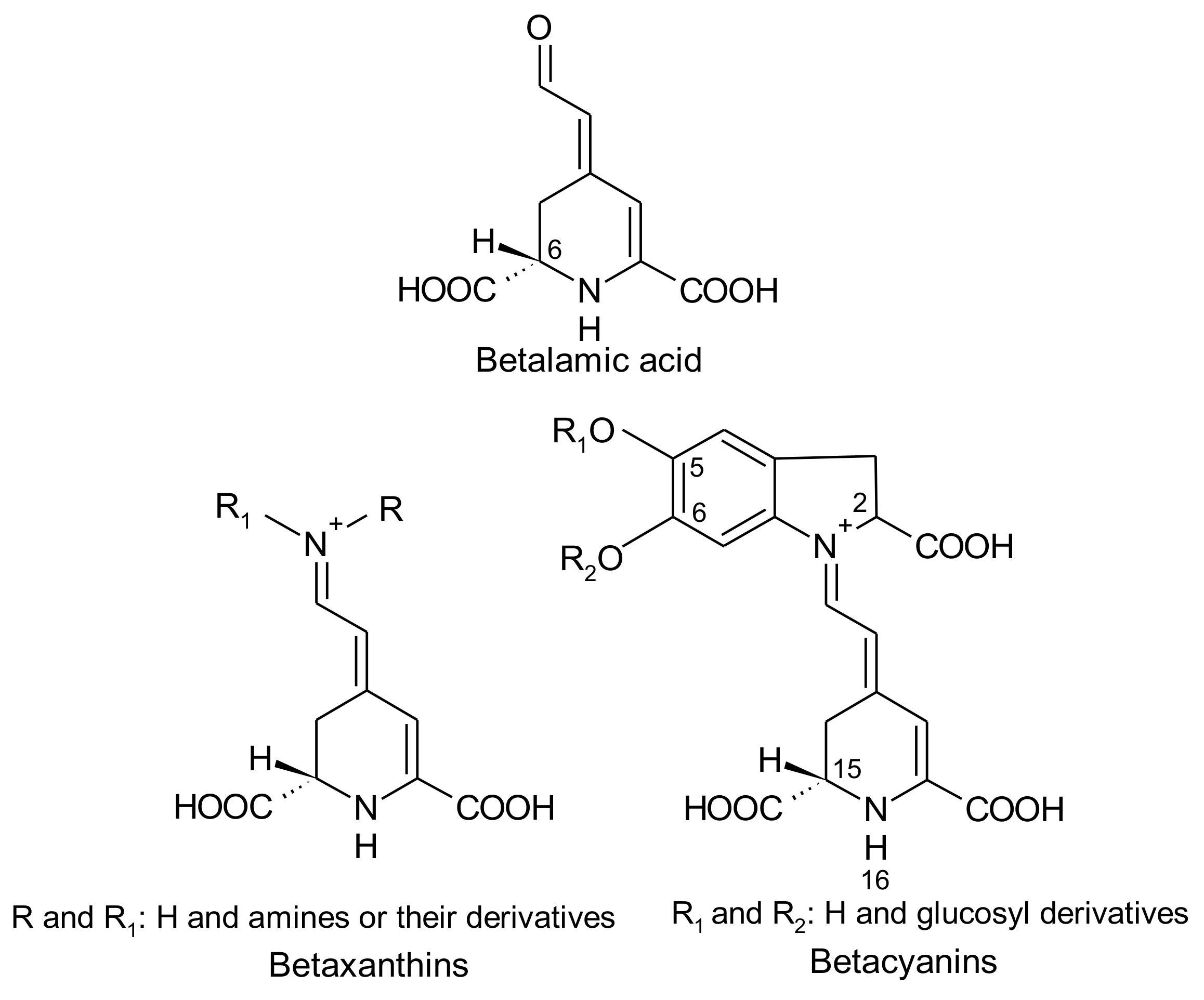
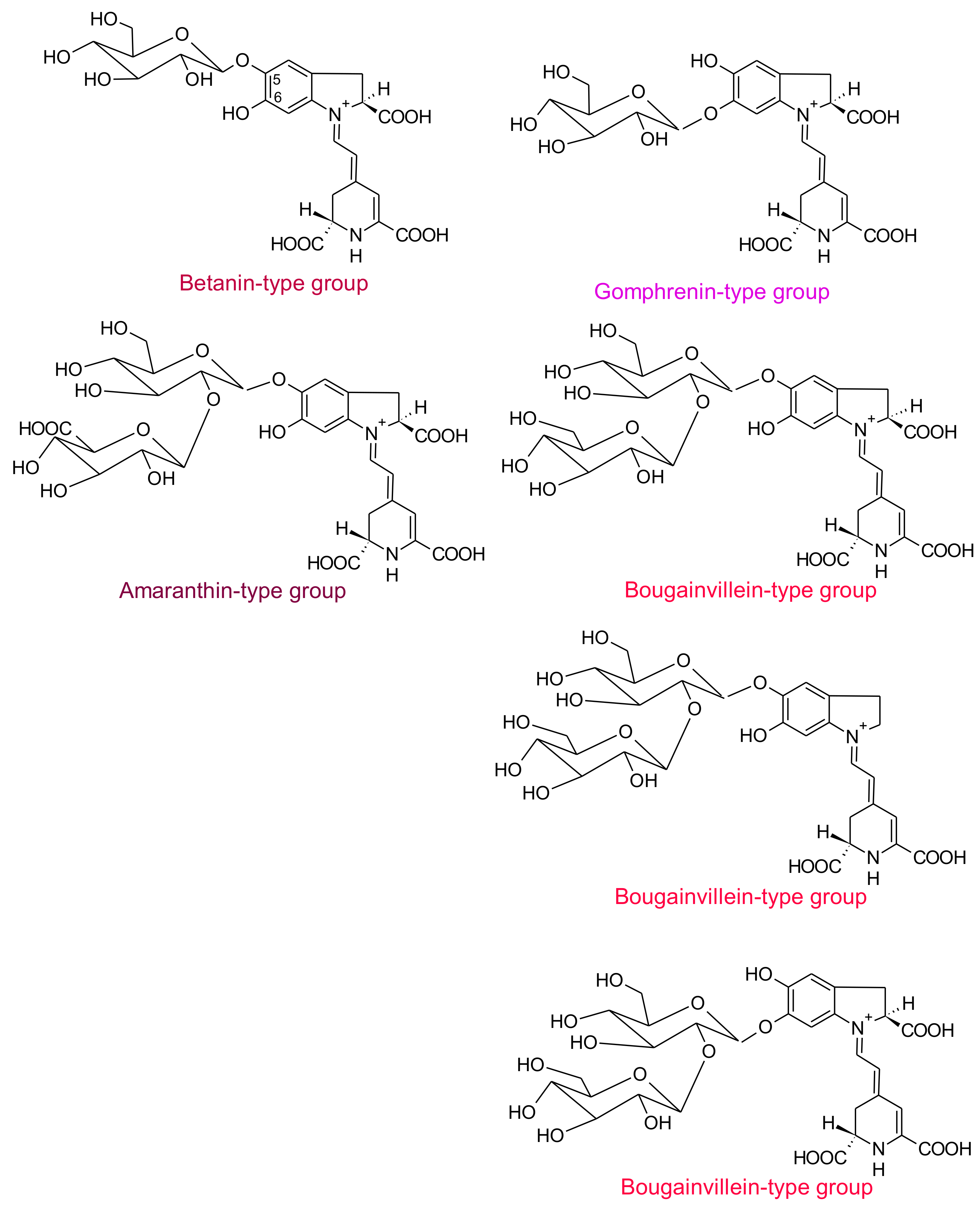
2. Betalains
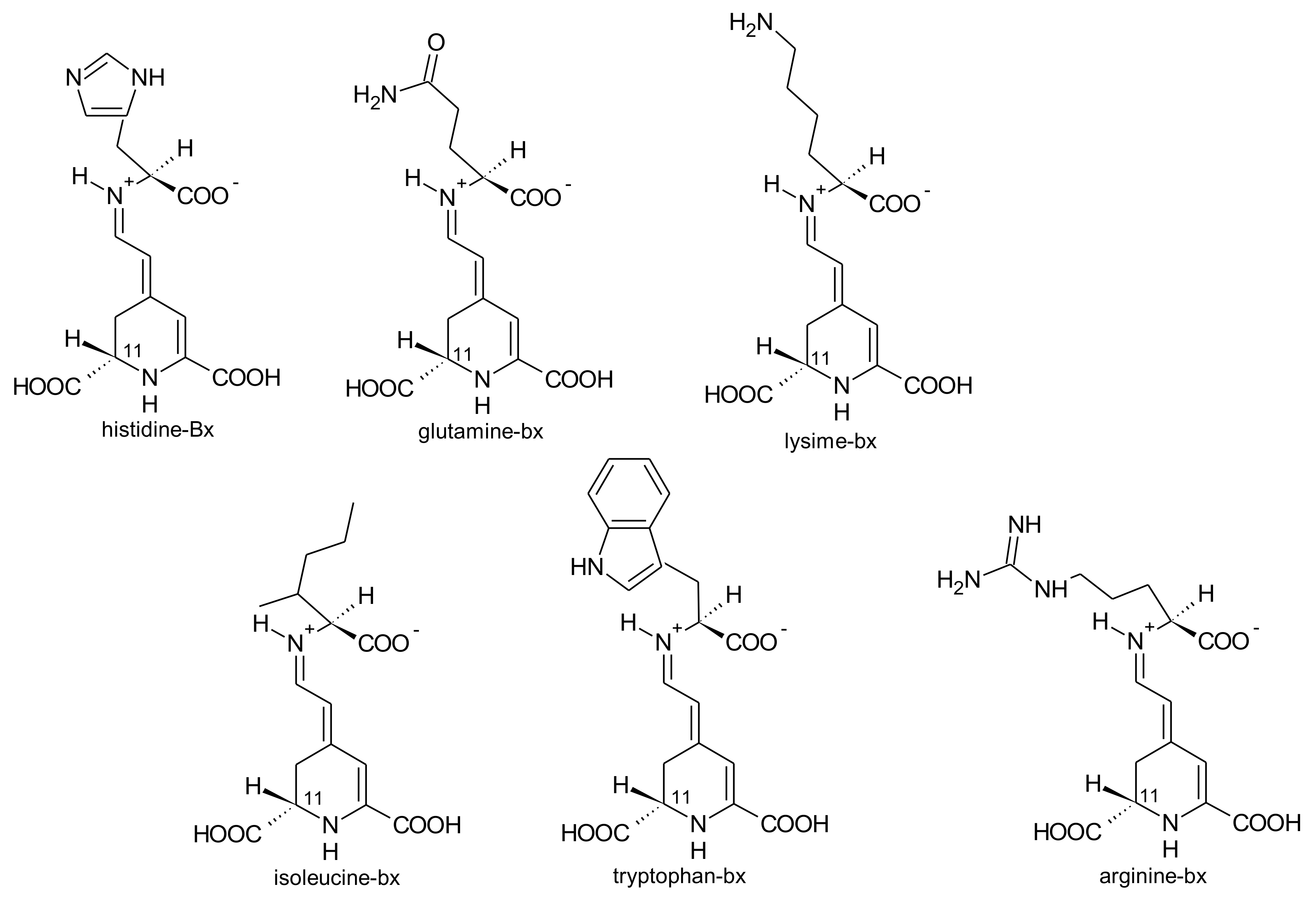
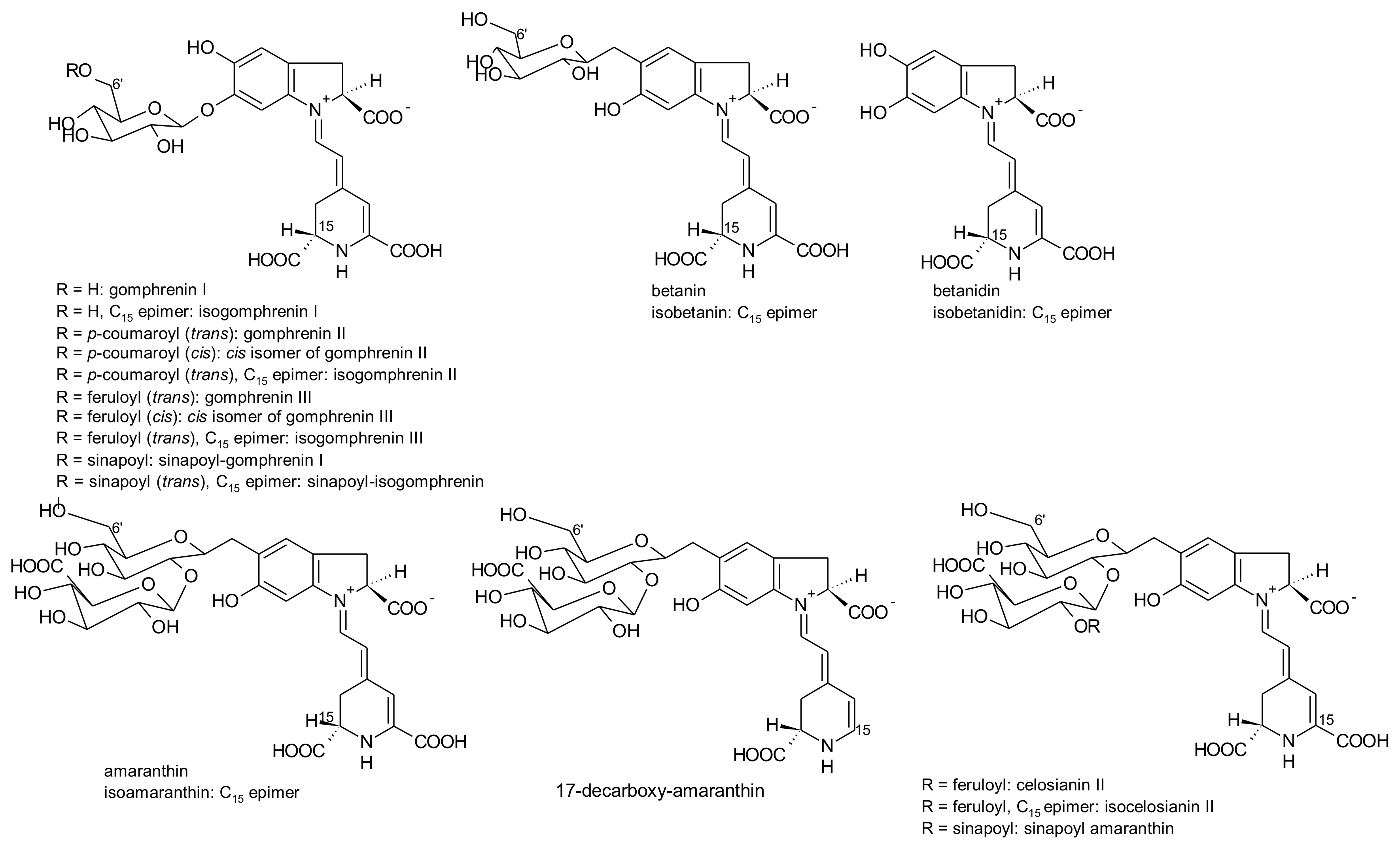
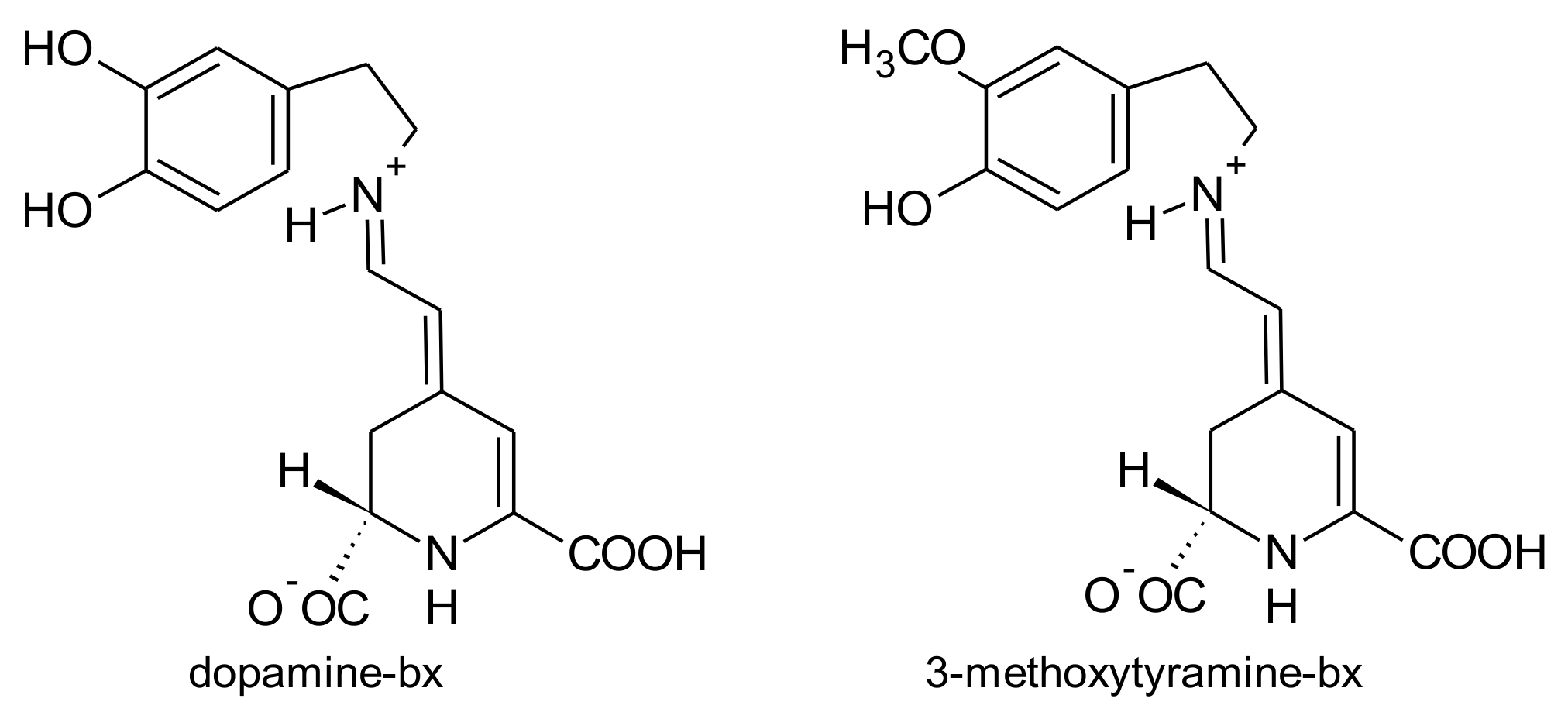
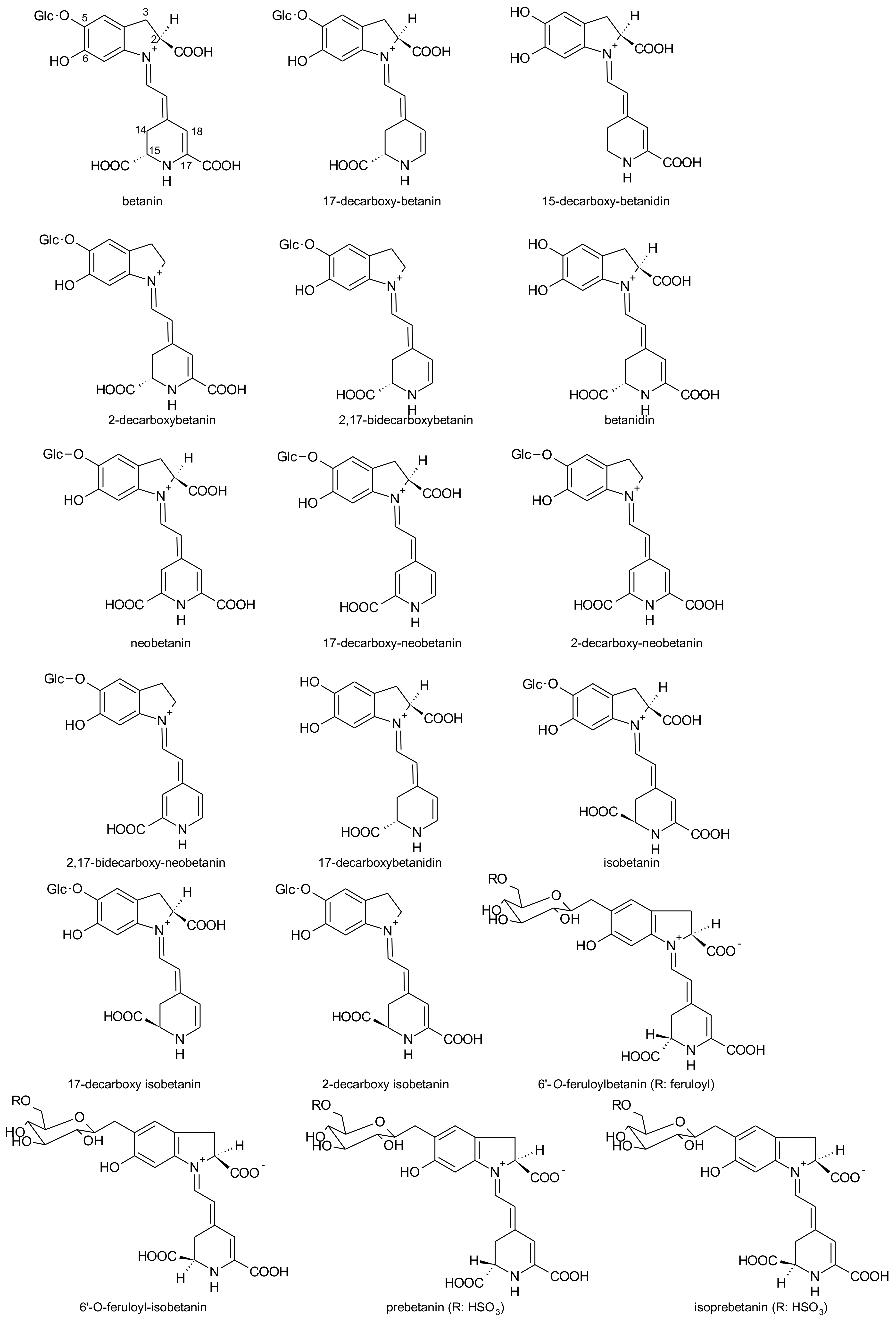
2.1. General Chemical Aspects of Betalains
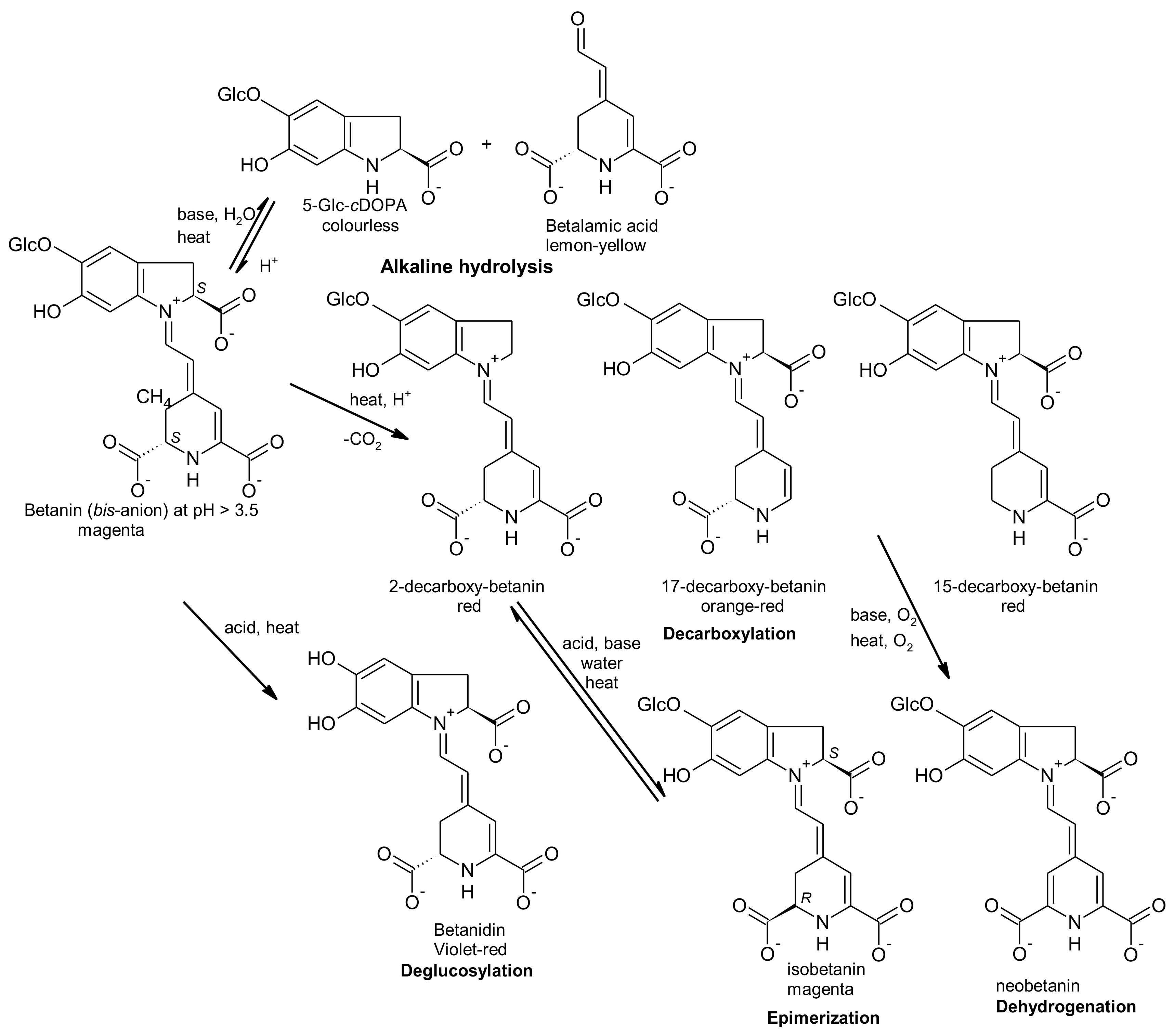
2.2. Stability of Betalains
2.3. Extraction, Separation, Identification and Quantification of Betalains
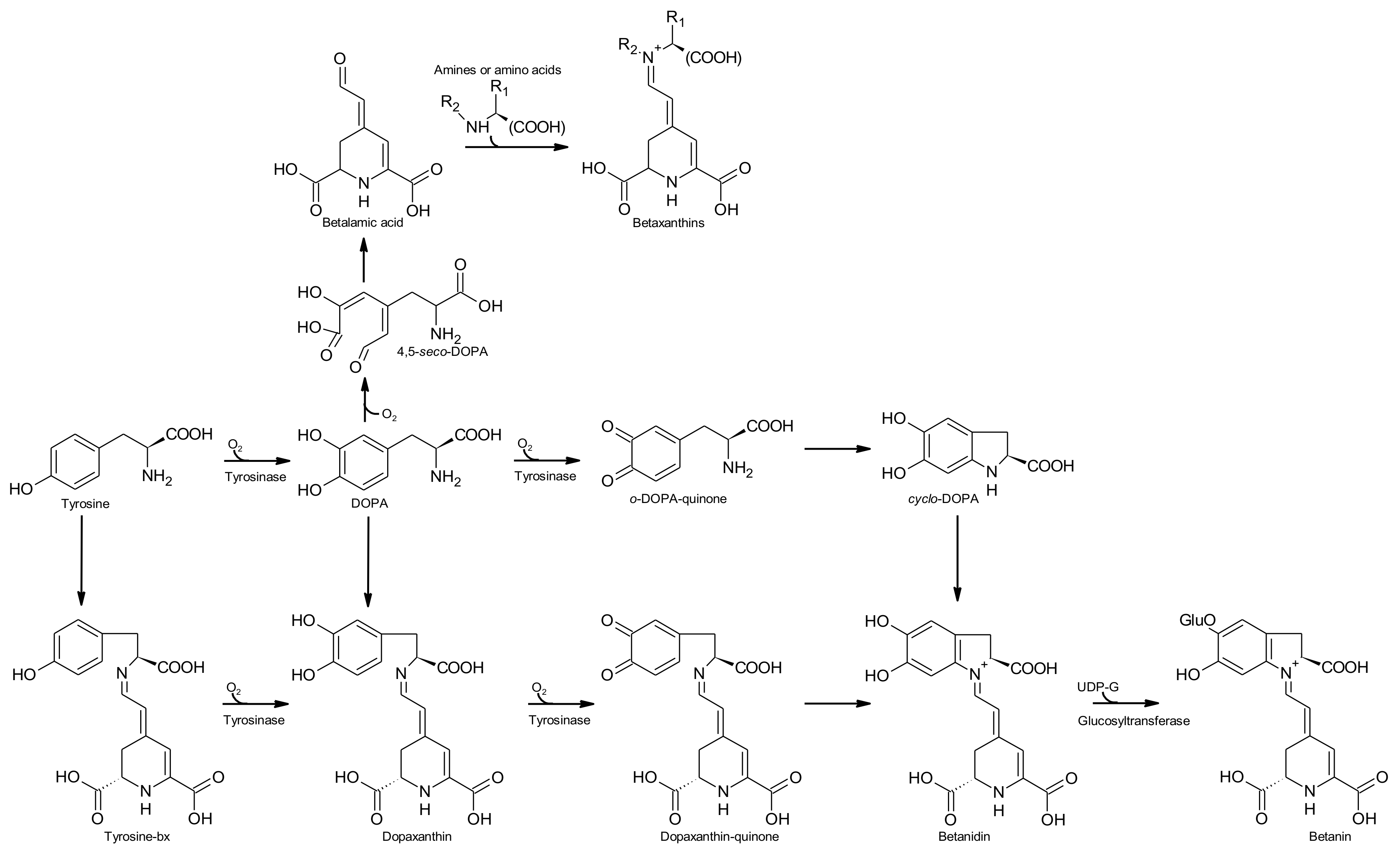
2.4. Biosynthesis of Betalains
2.5. Role of Betalains in Plants
2.6. Importance of Betalains for Consumers
2.7. Production of Betalains
2.8. Biological Properties of Betalains
3. Amaranthaceae
3.1. Gomphrena globosa L.
3.2. Alternanthera
3.3. Amaranthus
3.4. Beta vulgaris L.
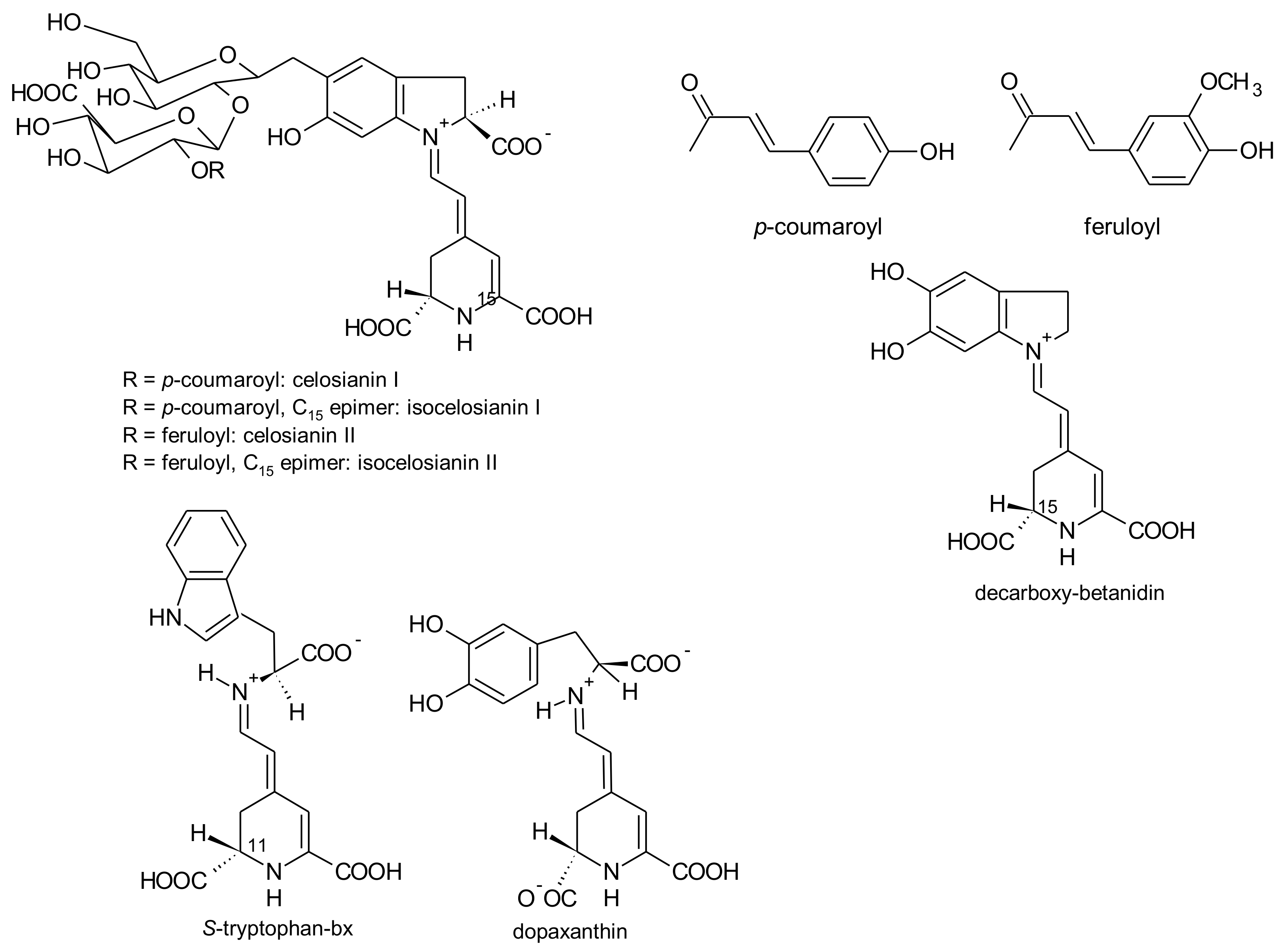
3.5. Celosia argentea L.
3.6. Chenopodium quinoa Willd
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Delgado-Vargas, F.; Jiménez, A.R.; Paredes-López, O. Natural pigments: Carotenoids, anthocyanins and betalains—Characterisitcs, biosynthesis, processing and stability. Crit. Rev. Food Sci. Nutr. 2000, 40, 173–189. [Google Scholar] [CrossRef] [PubMed]
- Stintzing, F.C.; Carle, R. Functional properties of anthocyanins and betalains in plants, food and in human nutrition. Trends Food Sci. Technol. 2004, 15, 19–38. [Google Scholar] [CrossRef]
- Clement, J.S.; Mabry, T.J. Pigment evolution in the Caryophyllales: A systematic overview. Bot. Acta 1996, 109, 360–367. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Trichterborn, J.; Carle, R. Characterisation of anthocyanin–betalain mixtures for food colouring by chromatic and HPLC-DAD-MS analyses. Food Chem. 2006, 94, 296–309. [Google Scholar] [CrossRef]
- Gandía-Herrero, F.; Escribano, J.; García-Carmona, F. Biological activities of plant pigments betalains. Crit. Rev. Food Sci. Nutr. 2016, 56, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Slimen, I.B.; Najar, T.; Abderrabba, M. Chemical and antioxidant properties of betalains. J. Agric. Food Chem. 2017, 65, 675–689. [Google Scholar] [CrossRef] [PubMed]
- Herbach, K.M.; SDtintzing, F.C.; Carle, R. Betalain stability and degradation-structural and chromatic aspects. J. Food Sci. 2006, 71, R41–R50. [Google Scholar] [CrossRef]
- Khan, M.I.; Giridhar, P. Plant betalains: Chemistry and biochemistry. Phytochemistry 2015, 117, 267–295. [Google Scholar] [CrossRef] [PubMed]
- Sigurdson, G.T.; Tang, P.; Giusti, M.M. Natural colorants: Food colorants from natural sources. Ann. Rev. Food Sci. Technol. 2017, 8, 261–280. [Google Scholar] [CrossRef] [PubMed]
- Strack, D.; Vogt, T.; Schliemann, W. Recent advances in betalain research. Phytochemistry 2003, 62, 247–269. [Google Scholar] [CrossRef]
- Azeredo, H.M.C. Betalains: Properties, sources, applications and stability—A review. Int. J. Food Sci. Technol. 2009, 44, 2365–2376. [Google Scholar] [CrossRef]
- Gandía-Herrero, F.; Escribano, J.; García-Carmona, F. Structural implications on color, fluorescence and antiradical activity in betalains. Planta 2010, 232, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I. Plant betalains: Safety, antioxidant activity, clinical efficacy and bioavailability. Compr. Rev. Food Sci. Food Saf. 2016, 15, 316–330. [Google Scholar] [CrossRef]
- Ngmwonglumlert, L.; Devahastin, S.; Chiewchan, N. Natural colorants: Pigment stability and extraction yield enhancement via utilization of appropriate pre-treatment and extraction methods. Crit. Rev. Food Sci. Nutr. 2017, 57, 3243–3259. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, L.C.P.; di Genova, B.M.; Dörr, F.A.; Pinto, E.; Bastos, E.L. Effect of dielectric microwave heating on the color and antiradical capacity of betanin. J. Food Eng. 2013, 118, 49–55. [Google Scholar] [CrossRef]
- Cardoso-Ugarte, G.A.; Sosa-Morales, M.E.; Ballard, T.; Liceaga, A.; San Martín-González, M.F. Microwave-assited extraction of betalains from red beet (Beta vulgaris). LWT Food Sci. Technol. 2014, 59, 276–282. [Google Scholar] [CrossRef]
- Celli, G.B.; Brooks, M.S.-L. Impact of extraction and processing conditions on betalains and comparison of proerties with anthocyanins—A current review. Food Res. Int. 2017, 100, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Mereddy, R.; Chan, A.; Fanning, K.; Nirmal, N.; Sultanbawa, Y. Betalain rich functional extract with reduced salts and nitrate content from red beetroot (Beta vulgaris L.) using membrane separation technology. Food Chem. 2017, 215, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Mulinacci, N.; Innocenti, M. Anthocyanins and betalains. Chapter 22. In Food Analysis by HPLC, 3rd ed.; Nollet, M.L.L., Toldrà, F., Eds.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Didcot, UK, 2013; pp. 757–776. [Google Scholar]
- Gandía-Herrero, F.; García-Carmona, F. Biosynthesis of betalains: Yellow and violet plant pigments. Trends Plant Sci. 2013, 18, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Schliemann, W.; Steiner, U.; Strack, D. Betanidin formation from dihydroxyphenylalanine in a model assay system. Phytochemistry 1998, 49, 1593–1598. [Google Scholar] [CrossRef]
- Niveyro, S.L.; Mortensen, A.; Fomsgaerd, I.S.; Salvo, A. Differences among five amaranth varieties (Amaranthus spp.) regarding secondary metabolites and foliar herbivory by chewing insects in the field. Arthropod Plant Interact. 2013, 7, 235–245. [Google Scholar] [CrossRef]
- Shao, L.; Chen, X.; Chen, Y.; Sun, B.; Chow, W.; Peng, C. Differential responses of photosystem II activity to photooxidation in red and green tissues of Amaranthus tricolour leaves. Pak. J. Bot. 2013, 45, 1905–1912. [Google Scholar]
- Shu, Z.; Shao, L.; Huang, H.-Y.; Zeng, X.-Q.; Lin, Z.-F.; Chen, G.-Y.; Peng, C.-L. Comparison of thermostability of PSII between the chromatic and green leaf cultivars of Amaranthus tricolour L. Phytosynthetica 2009, 47, 548–558. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Wagner, A.E.; Schini-Kert, V.B.; Rimbach, G. Betanin—A food colorant with biological activity. Mol. Nutr. Food Res. 2015, 59, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.-Z.; Sun, M.; Corke, H. Characterization and application of betalain pigments from plants of the Amaranthaceae. Trends Food Sci. Technol. 2005, 16, 370–376. [Google Scholar] [CrossRef]
- Gliszczyńska-Świgło, A.; Szymusiak, H.; Malinowska, P. Betanin, the main pigment of red beet: Molecular origin of its exceptionally high free radical-scavenging activity. Food Addit. Contam. 2006, 23, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Graf, B.L.; Rojas-Silva, P.; Rojo, L.E.; Delatorre-Herrera, J.; Baldeón, M.E.; Raskin, I. Innovations in health value and functional food development of quinoa (Chenopodium quinoa Willd.). Compr. Rev. Food Sci. Food Saf. 2015, 14, 431–445. [Google Scholar] [CrossRef] [PubMed]
- Hilou, A.; Nacoulma, O.G.; Guiguemde, T.R. In vivo antimalarial activities of extracts from Amaranthus spinosus L. and Boerhaavia erecta L. in mice. J. Ethnopharmacol. 2006, 103, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Koubaier, H.B.H.; Snoussi, A.; Essaidi, I.; Chaabouni, M.M.; Thonart, P.; Bouzouita, N. Betalain and phenolic compositions, antioxidant of Tunisian red beet (Beta vulgaris L. conditiva) roots and stems extracts. Int. J. Food Prop. 2014, 17, 1934–1945. [Google Scholar] [CrossRef]
- Lin, S.-M.; Lin, B.-H.; Hsieh, W.-M.; Ko, H.-J.; Liu, C.-D.; Chen, L.-G.; Chiou, R.Y.-Y. Structural identification and bioactivities of red-violet pigments present in Basella alba fruits. J. Agric. Food Chem. 2010, 58, 10364–10372. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, A.; Kovatcheva, P.; Tuneva, D.; Ilieva, M.; Bley, T. Radical scavenging activity and stability of betalains from Beta vulgaris hairy root culture in simulated conditions of human gastrointestinal tract. Plant Food Hum. Nutr. 2005, 60, 43–47. [Google Scholar] [CrossRef]
- Vidal, P.J.; López-Nicolás, J.M.; Gandía-Herrero, F.; García-Carmona, F. Inactivation of lipoxygenase and cyclooxigenase by natural betalains and semi-synthetic analogues. Food Chem. 2014, 154, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, V.; Ilieva, M.; Bley, T.; Pavlov, A. Betalain production in plant in vitro systems. Acta Physiol. Plant. 2008, 30, 581–593. [Google Scholar] [CrossRef]
- Gandía-Herrero, F.; García-Carmona, F.; Escribano, J. Development of a protocol for the semi-synthesis and purification of betaxanthins. Phytochem. Anal. 2006, 17, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Schliemann, W.; Kobayashi, N.; Strack, D. The decisive step in betaxanthin biosynthesis is a spontaneous reaction. Plant Physiol. 1999, 119, 1217–1232. [Google Scholar] [CrossRef] [PubMed]
- Cabanes, J.; Gandía-Herrero, F.; Escribano, J.; García-Carmona, F.; Jiménez-Atiénzar, M. One-step synthesis of betalains using a novel betalamic acid derivatized support. J. Agric. Food Chem. 2014, 62, 3776–3782. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, A.; Bley, T. Betalains biosynthesis by Beta vulgaris L. hairy root culture in a temporary immersion cultivation system. Proc. Biochem. 2006, 41, 848–852. [Google Scholar] [CrossRef]
- Weller, T.A.; Lasure, L.L. Betalains in beet root tissue culture. J. Food Sci. 1981, 47, 162–163. [Google Scholar] [CrossRef]
- Böhm, H.; Mäck, G. Betaxanthin formation and free amino acids in hairy roots of Beta vulgaris var. lutea depending on nutrient medium and glutamate or glutamine feeding. Phytochemistry 2004, 65, 1361–1368. [Google Scholar] [PubMed]
- Neelwarne, B.; Thimmaraju, R. Bioreactor for cultivation of red beet hairy roots and in situ recovery of primary and secondary metabolites. Eng. Life Sci. 2009, 9, 227–238. [Google Scholar] [CrossRef]
- Pavlov, A.; Kovatcheva, P.; Georgiev, V.; Koleva, I.; Ilieva, M. Biosynthesis and radical scavenging activity of betalains during the cultivation of red beet (Beta vulgaris) hairy root cultures. Z. Naturforsch. 2002, 57, 640–644. [Google Scholar] [CrossRef]
- Križnic, B.; Pavoković, D. Enhancement of betanin yield in transformed cells of sugar beet (Beta vulgaris L.). Acta Bot. Croat. 2010, 69, 173–182. [Google Scholar]
- Rudrappa, T.; Neelwarne, B.; Aswathanarayana, R.G. In situ and ex situ adsorption and recovery of betalains from hairy root cultures of Beta vulgaris. Biotechnol. Prog. 2004, 20, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.S.; Murthy, H.N.; Heo, J.W.; Paek, K.Y. Unduction of betalain pigmetantion in hairy roots of red beet under different radiation sources. Biol. Plant. 2003, 47, 149–152. [Google Scholar] [CrossRef]
- Wang, M.; Lopez-Nieves, S.; Goldman, I.L.; Maeda, H.A. Limited tyrosine utilization explains lower betalain contents in yellow than red table beet genotypes. J. Agric. Food Chem. 2017, 65, 4305–4313. [Google Scholar] [CrossRef]
- Polturak, G.; Aharoni, A. “La vie en rose”: Biosynthesis, sources and applications of betalain pigments. Mol. Plant 2018, 11, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.-Z.; Sun, M.; Corke, H. Antioxidant activity of betalains from plants of the Amaranthaceae. J. Agric. Food Chem. 2003, 51, 2288–2294. [Google Scholar] [CrossRef] [PubMed]
- Taira, J.; Tsuchida, E.; Katoh, M.C.; Uehara, M.; Ogi, T. Antioxidant capacity of betacyanins as radical scavengers for peroxyl radical and nitric oxide. Food Chem. 2015, 166, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Tesoriere, L.; Allegra, M.; Gentile, C.; Livrea, M.A. Betacyanins as phenol antioxidants. Chemistry and mechanistic aspects of the lipoperoxyl radical-scavenging activity in solution and liposomes. Free Radic. Res. 2009, 43, 706–717. [Google Scholar] [CrossRef] [PubMed]
- Gandía-Herrero, F.; Escribano, J.; García-Carmona, F. The role of phenolic hidroxi groups in the free radical scavenging activity of betalains. J. Nat. Prod. 2009, 72, 1142–1146. [Google Scholar] [CrossRef] [PubMed]
- Gandía-Herrero, F.; Escribano, J.; García-Carmona, F. Purification and antiradical properties of the structural unit of betalains. J. Nat. Prod. 2012, 75, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Wybraniec, S.; Starzak, K.; Skopińska, A.; Nemzer, B.; Pietrzkowski, Z.; Michałowsli, T. Studies on nonenzymatic oxidation mechanisms in neobetanin, betanin and decarboxylated betanins. J. Agric. Food Chem. 2013, 61, 6465–6476. [Google Scholar] [CrossRef] [PubMed]
- Kanner, J.; Harel, S.; Granit, R. Betalains—A new class of dietary cationized antioxidants. J. Agric. Food Chem. 2001, 49, 5178–5185. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.K.; Alexander-Lindo, R.L.; Nair, M.G. Relative inhibition of lipid peroxidation, cyclooxygenase enzymes and human tumor cell proliferation by natural food colors. J. Agric. Food Chem. 2005, 53, 9268–9273. [Google Scholar] [CrossRef] [PubMed]
- Esatbeyoglu, T.; Wagner, A.E.; Motafakkerazad, R.; Nakajima, Y.; Matsugo, S.; Rimbach, G. Free radical scavenging and antioxidant activity of betanin: Electron spin resonance spectroscopy studies and studies in cultured cells. Food Chem. Toxcicol. 2014, 73, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Pietrzkowski, Z.; Nemzer, B.; Spórna, A.; Stalica, P.; Tresher, W.; Keller, R.; Jiminez, R.; Michalowski, T.; Wybraniec, S. Influence of betalin-rich extracts on reduction of discomfort associated with osteoarthritis. New Med. 2010, 1, 12–17. [Google Scholar]
- Gentile, C.; Tesoriere, L.; Allegra, M.; Livrea, M.A.; D’Alessio, P. Antioxidant betalains from cactus pear (Opuntia ficus-indica) inhibit endothelial ICAM-1 expression. Ann. N. Y. Acad. Sci. 2004, 1028, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Wettasinghe, M.; Bolling, B.; Plhak, L.; Xiao, H.; Parkin, K. Phase II enzyme-inducing and antioxidant activities of beetroot (Beta vulgaris L.) extracts from phenotypes of different pigmentation. J. Agric. Food Chem. 2002, 50, 6704–6709. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Wettasinghe, M.; Bolling, B.W.; Ji, L.-L.; Parkin, K.L. Betalains, phase II enzyme-inducing components from red beetroot (Beta vulgaris L.) extracts. Nutr. Cancer 2005, 53, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Kujawska, M.; Ignatowicz, E.; Murias, M.; Ewertowsca, M.; Mikolajczyk, K.; Jodynis-Liebert, J. Protective effect of red beetroot against carbon tetrachloride- and N-nitrosodiethylamine-induced oxidative stress in rats. J. Agric. Food Chem. 2009, 57, 2570–2575. [Google Scholar] [CrossRef] [PubMed]
- Szaefer, H.; Krajka-Kuźniak, V.; Ignatowicz, E.; Adamska, T.; Baer-Dubowska, W. Evaluation of the effect of beetroot juice on DMBA-induced damage in liver and mammary gland of female Sprague-Dawley rats. Phytother. Res. 2014, 28, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Gengatharan, A.; Dykes, G.A.; Choo, E.S. Betalains: Natural plant pigments with potential application in functional foods. LWT Food Sci. Technol. 2015, 64, 645–649. [Google Scholar] [CrossRef]
- Clemente, A.C.; Desai, P.V. Evaluation of the haematological, hypoglycemic, hypolipidemic and antioxidant properties of Amaranthus tricolor leaf extract in rat. Trop. J. Pharm. Res. 2011, 10, 595–602. [Google Scholar]
- Zielinbska-Przyjemska, M.; Olejnik, A.; Dobrowolska-Zachwieja, A.; Grajek, W. In vitro effects of beetroot juice and chips on oxidative metabolism and apoptosis neutrophils from obese individuals. Phytother. Res. 2009, 23, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Mroczek, A. Phytochemistry and bioactivity of triterpene saponins from Amaranthaceae family. Phytochem. Rev. 2015, 14, 577–605. [Google Scholar] [CrossRef]
- Clifford, T.; Howatson, G.; West, D.J.; Stevenson, E.J. The potential benefits of red beetroot supplementation in health and disease. Nutrients 2015, 7, 2801–2822. [Google Scholar] [CrossRef] [PubMed]
- Deladino, L.; Alvarez, I.; de Ancos, B.; Sánchez-Moreno, C.; Molina-García, A.D.; Teixeira, A.S. Betalains and phenolic compounds of leaves and stems of Alternanthera brasiliana and Alternanthera tenella. Food Res. Int. 2017, 97, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.-J.; Sheu, C.-H.; Wu, P.-H.; Sun, Y.-F. Thermal and pH stability of betacyanin pigment of djulis (Chenopodium formosanum) in Taiwan and their relation to antixodant activity. J. Agric. Food Chem. 2010, 58, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.R.; Valentão, P.; Faria, J.; Ferreres, F.; Sousa, C.; Gil-Izquierdo, A.; Pinho, B.R.; Andrade, P.B. Phytochemical investigations and biological potential screening with cellular and non-cellular models of globe amaranth (Gomphrena globosa L.) inflorescences. Food Chem. 2012, 135, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.-Z.; Xing, J.; Sun, M.; Corke, H. Rapid identification of betacyanins from Amaranthus tricolor, Gomphrena globosa and Hylocereus polyrhizus by matrix-assisted laser desorption/ionization quadrupole ion trap time-of-flight mass spectrometry (MALDI-QIT-TOF-MS). J. Agric. Food Chem. 2006, 54, 6520–6526. [Google Scholar] [CrossRef] [PubMed]
- Dinda, B.; Ghosh, B.; Achari, B.; Arima, S.; Sato, N.; Harigaya, Y. Chemical constituents of Gomphrena globosa. II. Nat. Prod. Sci. 2006, 12, 89–93. [Google Scholar]
- Lans, C. Ethnomedicines used in Trinidad and Tobago for urinary problems and diabetes mellitus. J. Ethnobiol. Ethnomed. 2006, 2, 45. [Google Scholar] [CrossRef] [PubMed]
- Lans, C. Ethnomedicines used in Trinidad and Tobago for reproductive problems. J. Ethnobiol. Ethnomed. 2007, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Kugler, F.; Stintzing, F.C.; Carle, R. Characterisation of betalain patterns of differently coloured inflorescences from Gomphrena globosa L. and Bougainvillea sp. By HPLC-DAD-ESI-MSn. Anal. Bioanal. Chem. 2007, 387, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Heuer, S.; Wray, V.; Metzaer, J.W.; Strack, D. Betacyanins from flowers of Gomphrena globosa. Phytochemistry 1992, 31, 1801–1807. [Google Scholar] [CrossRef]
- Spórna-Kucab, A.; Hołda, E.; Wybraniec, S. High-speed counter-current chromatography in separation of betacyanins from flowers of red Gomphrena globosa L. cultivars. J. Chromatogr. B 2016, 1033–1034, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Roriz, C.L.; Barros, L.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Pterospartum tridentatum, Gomphrena globosa and Cymbopogon citratus. A phytochemical study focused on antioxidant compounds. Food Res. Int. 2014, 62, 684–693. [Google Scholar] [CrossRef]
- Ferreres, F.; Gil-Izquierdo, A.; Valentão, P.; Andrade, P.B. Structural characterization of phenolics and betacyanins in Gomphrena globosa by high-performance liquid chromatography-diode array detection/electrospray ionization multi-stage masss pectrometry. Rapid Commun. Mass Spectrom. 2011, 25, 3441–3446. [Google Scholar] [CrossRef] [PubMed]
- Roriz, C.L.; Barros, L.; Prieto, M.A.; Morales, P.; Ferreira, I.C.F.R. Floral parts of Gomphrena globosa L. as a novel alternative source of betacyanins: Optimization of the extraction using response surface methodology. Food Chem. 2017, 229, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Roriz, C.L.; Barros, L.; Prieto, M.A.; Barreiro, M.F.; Morales, P.; Ferreira, I.C.F.R. Modern extraction techniques optimized to extract betacyanins from Gomphrena globosa L. Ind. Crops Prod. 2017, 105, 29–40. [Google Scholar] [CrossRef]
- Cai, Y.-Z.; Sun, M.; Corke, H. Identification and distribution of simple and acylated betacyanins in the Amaranthaceae. J. Agric. Food Chem. 2001, 49, 1971–1978. [Google Scholar] [CrossRef] [PubMed]
- Aquino, D.F.; Piccinelli, C.; Soares, F.L.P.; Arena, A.C.; Salvador, M.J.; Kassuya, A.L. Anti-hyperalgesic and anti-inflammatory activity of Alternanthera maritima extract and 2″-O-α-l-rhamnopyranosylvitexin in mice. Inflammation 2015, 38, 2057–2066. [Google Scholar] [CrossRef] [PubMed]
- Guerra, R.N.M.; Pereira, H.-A.W.; Silveira, L.M.S.; Olea, R.S.G. Immunomodulatory properties of Alternathera tenella Colla aqueous extracts in mice. Braz. J. Med. Biol. Res. 2003, 36, 1215–1219. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Souza, J.G.; Tomei, R.R.; Kanashiro, A.; Kabeya, L.M.; Azzolini, A.E.C.S.; Dias, D.A.; Salvador, M.J.; Lucisano, Y. Ethanolic crude extract and flavonoids isolated from Alternanthera maritima: Neutrophil chemiluminescence inhibition and free radical scavenging activity. Z. Naturforsch. 2007, 62, 339–347. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, P.; Mishra, G.; Srivastav, S.; Jha, K.K.; Khosa, R.L. Phytopharmacological review of Alternanthera brasiliana (Amaranthaceae). Asian J. Plant Sci. Res. 2011, 1, 41–47. [Google Scholar]
- Samudrala, P.K.; Augustine, B.B.; Kasala, E.R.; Bodduluru, L.N.; Barua, C.; Lahkar, M. Evaluation of antitumor activity and antioxidant status of Alternanthera brasiliana against Ehrlich ascites carcinoma in Swiss albino mice. Pharmacogn. Res. 2015, 7, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Barua, C.C.; Talukdar, A.; Begum, S.A.; Sarma, D.K.; Pathak, D.C.; Barua, A.G.; Bora, R.S. Wound healing activity of methanolic extract of leaves of Alternanthera brasiliana Kuntze using in vivo and in vitro model. Indian J. Exp. Biol. 2009, 47, 1001–1005. [Google Scholar] [PubMed]
- Brochado, C.O.; Almeida, A.P.; Barreto, B.P.; Costa, L.P.; Ribeiro, L.S.; Pereira, R.L.C.; Koatz, V.L.G.; Costa, S.S. Flavonol robinobiosides and rutinosides from Alternanthera brasiliana (Amaranthaceae) and their effects on lymphocyte proliferation in vitro. J. Braz. Chem. Soc. 2003, 14, 449–451. [Google Scholar] [CrossRef]
- Lagrota, M.H.C.; Wigg, M.D.; Santos, M.M.G.; Miranda, M.M.F.S.; Camara, F.P.; Couceiro, J.N.S.S.; Costa, S.S. Inhibitory activity of extracts of Alternanthera brasiliana (Amaranthaceae) against the Herpes simplex virus. Phytother. Res. 1994, 8, 358–361. [Google Scholar] [CrossRef]
- Pereira, D.F.; Zanon, R.B.; Santos, M.; Boligon, A.A.; Athayde, M.L. Antioxidant activities and triterpenoids isolated from Alternanthera brasiliana (L.) Kuntze leaves. Nat. Prod. Res. 2013, 27, 1660–1663. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.M.; Kern, P.; Floriani, A.E.O.; Cechinel-Folho, V. Analgesic properties of a hydroalcoholic extract obtained from Alternanthera brasiliana. Phytother. Res. 1998, 12, 279–281. [Google Scholar] [CrossRef]
- Souza, G.C.; Haas, A.P.S.; von Poser, G.L.; Schapoval, E.E.S.; Elisabetsky, E. Ethnopharmacological studies of antimicrobial remedies in the South of Brazil. J. Ethnopharmacol. 2004, 90, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Bussmann, R.W.; Glenn, A.; Sharon, D. Antibacterial activity of medicinal plants of Northern Peru—Can traditional applications provide leads for modern science? Indian J. Tradit. Knowl. 2010, 9, 742–753. [Google Scholar]
- Biella, C.A.; Salvador, M.J.; Dias, D.A.; Dias-Baruffi, M.; Pereira-Crott, L.S. Evaluation of immunomodulatory and anti-inflammatory effects and phytochemical screening of Alternanthera tenella Colla (Amaranthaceae) aqueous extracts. Mem. Inst. Pswaldo Cruz 2008, 103, 569–577. [Google Scholar] [CrossRef]
- Harthman, V.C.; Souza, L.A. Ontogenia do fruto em desenvolvimento de Alternanthera tenella Colla e Amaranthus blitum Linnaeus (Amaranthaceae). Acta Bot. Bras. 2012, 26, 642–650. [Google Scholar] [CrossRef]
- Kleinowski, A.M.; Brandão, I.R.; Einhardt, A.M.; Ribeiro, M.V.; Peters, J.A.; Braga, E.J.B. Pigment production and growth of Alternanthera plants cultured in vitro in the presence of tyrosine. Braz. Arch. Biol. Technol. 2014, 57, 253–260. [Google Scholar] [CrossRef]
- Vendruscolo, G.S.; Mentz, L.A. Levantamento etnobotânico das plantas utilizadas como medicinais por moradores do bairro Ponta Grossa, Porto Alegre do Sul, Brasil. Iheringa Sér. Bot. 2006, 61, 83–103. [Google Scholar]
- Salvador, M.J.; Zucchi, O.L.A.D.; Candido, R.C.; Ito, I.Y.; Dias, D.A. In vitro antimicrobial activity of crude extracts and isolated constituents of Alternanthera maritima (Amaranthaceae). Pharm. Biol. 2004, 42, 138–148. [Google Scholar] [CrossRef]
- Salvador, M.J.; Ferreira, E.O.; Mertens-Talcott, S.U.; Castro, V.; Butterweck, V.; Derendorf, H.; Dias, D.A. Isolation and HPLC quantitative analysis of antioxidant flavnoids from Alternanthera tenella Colla. Z. Naturforsch. 2006, 61, 19–25. [Google Scholar]
- Reis, A.; Kleinowski, A.M.; Klein, F.R.S.; Telles, R.T.; Amarante, L.; Braga, E.J.B. Light quality on the in vitro growth and production of pigments in the genus Alternanthera. J. Crop Sci. Biotechnol. 2015, 18, 349–357. [Google Scholar] [CrossRef]
- Rodrigues-Brandão, I.; Kneinowski, A.M.; Einhardt, A.M.; Lima, M.C.; Amarante, M.C.L.L.; Peters, J.A.; Braga, E.J.B. Salicylic acid on antioxidant activity and betacyanins in production from leaves of Alternanthera tenella. Ciênc. Rural 2014, 44, 1893–1898. [Google Scholar] [CrossRef]
- Moraes, V.L.G.; Santos, L.F.M.; Castro, S.B.; Loureiro, L.H.; Lima, D.A.; Souza, M.L.M.; Yien, L.M.K.; Rossi-Bergmann, B.; Costa, S.S. Inhibition of lymphocyte activation by extracts and fractions of Kalanchoe, Alternanthera, Paullinia and Mikania species. Phytomedicine 1994, 1, 199–204. [Google Scholar] [CrossRef]
- Narayanan, M.R.; Anilkumar, N.; Balakrishnan, V.; Sivadasan, M.; Alfarhan, H.A.; Alatar, A. Wild edible plants used by the Kattunaikka, Paniya and Kuruma tribes of Wayanad District, Kerala, India. J. Med. Plant Res. 2011, 5, 3520–3529. [Google Scholar]
- Ramachandran, V. Wild edible plants of the Anamalais, Coimbatore district, Western Ghats, Tamil Nadu. Indian J. Tradit. Knowl. 2007, 6, 173–176. [Google Scholar]
- Peter, K.; Gandhi, P. Rediscovering the therapeutic potential of Amaranthus species a review. J. Basic Appl. Sci. 2017, 4, 196–205. [Google Scholar] [CrossRef]
- Rastogi, A.; Shukla, S. Amaranth: A new millennium crop of nutraceutical values. Crit. Rev. Food Sci. Nutr. 2013, 53, 109–125. [Google Scholar] [CrossRef] [PubMed]
- Achigan-Dako, E.G.; Sogbohossou, O.E.D.; Maundu, P. Current knowledge on Amaranthus spp.: Research avenues for improved nutritional value and yield in leafy amaranths in sub-Saharan Africa. Euphytica 2014, 197, 303–317. [Google Scholar] [CrossRef]
- Das, S. Systematics and taxonomic delimitation of vegetable, grain and weed amaranths: A morphological and biochemical approach. Genet. Resour. Crop Evol. 2012, 59, 289–303. [Google Scholar] [CrossRef]
- Caselato-Sousa, V.M.; Amaya-Farfán, J. State of knowledge on amaranth grain: A comprehensive review. J. Food Sci. 2012, 77, R93–R104. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Corke, H. Amaranthus betacyanin pigments applied in model food systems. J. Food Sci. 1999, 64, 869–873. [Google Scholar] [CrossRef]
- Cai, Y.; Sun, M.; Wu, H.; Huang, R.; Corke, H. Characterization and quantification of betacyanin pigments from diverse Amaranthus species. J. Agric. Food Chem. 1998, 46, 2063–2070. [Google Scholar] [CrossRef]
- Cai, Y.; Sun, M.; Corke, H. Colorant properties and stability of Amaranthus betacyanin pigments. J. Agric. Food Chem. 1998, 46, 4491–4495. [Google Scholar] [CrossRef]
- Khanam, U.K.S.; Oba, S. Bioactive substances in leaves of two amaranth species, Amaranthus tricolor and Amaranthus hypochondriacus. Can. J. Plant Sci. 2013, 93, 47–58. [Google Scholar] [CrossRef]
- Shukla, S.; Bhargava, A.; Chatterjee, A.; Srivastava, A.; Singh, S.P. Genotypic variability in vegetable amaranth (Amaranthus tricolor L.) for foliage and its contributing traits over successive cuttings and years. Euphytica 2006, 151, 103–110. [Google Scholar] [CrossRef]
- Sarker, U.; Islam, M.T.; Rabbani, M.G.; Oba, S. Variability, heritability and genetic association in vegetable amaranth (Amaranthus tricolor L.). Span. J. Agric. Res. 2015, 13. [Google Scholar] [CrossRef]
- Khandaker, L.; Akond Asmgm, A.S.M.G.; Ali Md, B.; Oba, S. Biomass yield and accumulations of bioactive compounds in red amaranth (Amaranthus tricolor L.) grown under colored shade polyethylene in spring season. Sci. Hortic. 2010, 123, 289–294. [Google Scholar] [CrossRef]
- Das, S.S.; Gauri, S.S.; Misra, B.B.; Biswas, M.; Dey, S. Purification and characterization of a betanidin glucosyltransferase from Amaranthus tricolor L. catalyzing non-specific biotransformation of flavonoids. Plant Sci. 2013, 211, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Q.; Zhang, Y.; Wang, C.; Su, P.; Liao, X.L.; Bai, L.Y. Preliminary study on component analysis of Amaranthus tricolor leaf extracts against plant pathogenic bacteria. Egypt. J. Biol. Pest Control 2016, 26, 643–650. [Google Scholar]
- Al-Dosari, M.S. The effectiveness of ethanolic extract of Amaranthus tricolor L.: A natural hepatoprotetive agent. Am. J. Chin. Med. 2010, 38, 1051–1064. [Google Scholar] [CrossRef] [PubMed]
- Amornrit, W.; Santiyanont, R. Effect of Amaranthus on advanced glycation end-products induced cytotoxicity and proinflammatory cytokine gene expression in SH-SY5Y cells. Molecules 2015, 20, 17288–17308. [Google Scholar] [CrossRef] [PubMed]
- Amornrit, W.; Santiyanont, R. Neuroprotective effect of Amaranthus lividus and Amaranthus tricolor and their effects on gene expression of RAGE during oxidative stress in SH-SY5Y cells. Gen. Mol. Res. 2016, 15, 15027562. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasam, B.; Zhang, Y.; Nair, M.G. Tumor cell proliferation and cyclooxygenase enzyme inhibitory compounds in Amaranthus tricolor. J. Agric. Food Chem. 2004, 52, 6939–6943. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Deng, Z.; Liu, R.; Zhu, H.; Draves, J.; Marcone, M.; Sun, Y.; Tsao, R. Characterization of phenolics, betacyanins and antioxidant activities of the seed, leaf, sprout, flower and stalk extracts of three Amaranthus species. J. Food Compos. Anal. 2015, 37, 75–81. [Google Scholar] [CrossRef]
- Khandaker, L.; Ali, M.B.; Oba, S. Total polyphenol and antioxidant activity of red amaranth (Amaranthus tricolor L.) as affected by different sunlight level. J. Jpn. Soc. Hortic. Sci. 2008, 77, 395–401. [Google Scholar] [CrossRef]
- Khanam, U.K.S.; Oba, S. Phenotypic plasticity of vegetable amaranth Amaranthus tricolor L. under a natural climate. Plant Prod. Sci. 2014, 17, 166–172. [Google Scholar] [CrossRef]
- Khandaker, L.; Babar, A.M.; Shynia, O. Influence of cultivar and growth stage on pigments and processing factors on betacyanins in red amaranth (Amaranthus tricolor L.). Food Sci. Technol. Int. 2009, 15, 259–265. [Google Scholar] [CrossRef]
- Ali, M.B.; Khandaker, L.; Oba, S. Comparative study on functional componenrs, antioxidant activity and color parameters of selected colored leafy vegetables as affected by photoperiods. J. Food Agric. Environ. 2009, 7, 392–398. [Google Scholar]
- Biswas, M.; Das, S.S.; Dey, S. Establishment of a stable Amaranthus tricolor callus line for production of food colorant. Food Sci. Biotechnol. 2013, 22, 1–8. [Google Scholar] [CrossRef]
- Bianco-Colomas, J.; Hugues, M. Establishment and characterization of a betacyanin producing cell line of Amaranthus tricolor: Inductive effects of light and cytokinins. J. Plant Physiol. 1990, 136, 734–739. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, S.; Cheng, C.; Guo, R.; Chen, Y.; Xie, L.; Mao, Y.; Lin, Y.; Zhang, Z.; Lai, Z. Cloning and expression analysis of betalain biosynthesis genes in Amaranthus tricolor. Biotechnol. Lett. 2016, 38, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Pin, P.A. Life Cycle and Flowering Time Control in Beet. Ph.D. Thesis, Swedish University of Agricultural Sciences, Umeå, Sweden, 2012. [Google Scholar]
- Lee, S.S.; Le, E.M.; Hong, S.H.; Bai, H.-W.; Lee, I.C.; Chung, B.Y. Gamma rays as an effective tool for removing undesirable color without adverse changes in biological activities of red beet extracts. Radiat. Phys. Chem. 2012, 81, 1147–1151. [Google Scholar] [CrossRef]
- Lee, E.J.; An, D.; Nguyen, C.T.T.; Patil, B.S.; Kim, J.; Yoo, K.S. Betalain and betaine composition of greenhouse- or field-produced beetroot (Beta vulgaris L.) and inhibition of HepG2 cell proliferation. J. Agric. Food Chem. 2014, 62, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, T.; Baczek, N.; Wiczkowski, W. Betalain profile, content and antuioxidant capacity of red beetroot dependent on the genotype and root part. J. Funct. Food 2016, 27, 249–261. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Schieber, A.; Carle, R. Identification of betalains from yellow beet (Beta vulgaris L.) and cactus pear [Opuntia ficus-indica (L.) Mill.] by high-performance liquid chromatography-electrospray ionization mass spectrometry. J. Agric. Food Chem. 2002, 50, 2302–2307. [Google Scholar] [CrossRef] [PubMed]
- Slatnar, A.; Stampar, F.; Vebaric, R.; Jakopic, J. HPLC-MSn identification of betalains profile of different beetroot (Beta vulgaris L. ssp. vulgaris) parts and cultivars. J. Food Sci. 2015, 80, 1952–1958. [Google Scholar] [CrossRef] [PubMed]
- Mukundan, U.; Bhide, V.; Singh, G.; Curtis, W.R. pH-mediated release of betalains from transformed root cultures of Beta vulgaris L. Appl. Microbiol. Biotechnol. 1998, 50, 241–245. [Google Scholar] [CrossRef]
- Pavlov, A.; Georgiev, V.; Ilieva, M. Betalain biosynthesis by red beet (Beta vulgaris L.) hairy root culture. Proc. Biochem. 2005, 40, 1531–1533. [Google Scholar] [CrossRef]
- Pavlov, A.; Georgiev, M.; Bley, T. Batch and fed-batch production of betalains by red beet (Beta vulgaris) hairy roots in a bubble column reactor. Z. Naturforsch. 2007, 62, 439–446. [Google Scholar] [CrossRef]
- Pavoković, D.; Rusak, G.; Besendorfer, V.; Krsnik-Rasol, M. Light-dependent betanin production by transformed cells of sugar beet. Food Technol. Biotechnol. 2009, 47, 153–158. [Google Scholar]
- Liu, X.; Gao, Y.; Xu, H.; Wang, Q.; Yang, B. Imapct of high-pressure carbon dioxide combined with thermal treatment on degradation of red beet (Beta vulgaris L.) pigments. J. Agric. Food Chem. 2008, 56, 6480–6487. [Google Scholar] [CrossRef] [PubMed]
- Guldiken, B.; Toydemir, G.; Memis, K.N.; Okur, S.; Boyacioglu, D.; Capanoglu, E. Home-processed red beetroot (Beta vulgaris L.) products: Changes in antioxidant properties and bioaccessibility. Int. J. Mol. Sci. 2016, 17, 858. [Google Scholar] [CrossRef] [PubMed]
- Stagnari, F.; Galieni, A.; Speca, S.; Pisante, M. Water stress effects on growth, yield and quality traits of red beet. Sci. Hortic. 2014, 165, 13–22. [Google Scholar] [CrossRef]
- Stagnari, F.; Galieni, A.; Cafiero, G.; Pisante, M. Application of photo-selective films to manipulate wavelenght of transmitted radiation and photosynthate composition in red beet (Beta vulgaris var. conditiva Alef.). J. Sci. Food Agric. 2014, 94, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Popa, A.; Moldovan, B.; David, L. Betanin from red beet (Beta vulgaris L.). Rev. Chim. 2015, 66, 413–416. [Google Scholar]
- Escribano, J.; Pedreño, M.A.; Garcia-Carmona, F.; Muñoz, R. Characterization of the antiradical activity of betalains from Beta vulgaris L. roots. Phytochem. Anal. 1998, 9, 124–127. [Google Scholar] [CrossRef]
- Gandía-Herrero, F.; Cabanes, J.; Escribano, J.; García-Carmona, F.; Jiménez-Atiénzar, M. Encapsulation of the most potent antioxidant betalains in edible matrixes as powders of different colors. J. Agric. Food Chem. 2013, 61, 4294–4302. [Google Scholar] [CrossRef] [PubMed]
- Slavov, A.; Karagyozov, V.; Denev, P.; Kratchanova, M.; Kratchanov, C. Antioxidant activity of red beet juices obtained after microwave and thermal pretreatments. Czech J. Food Sci. 2013, 31, 139–147. [Google Scholar] [CrossRef]
- Čanadanović-Brunet, J.M.; Savatović, S.S.; Ćetković, G.S.; Vulić, J.J.; Djilas, S.M.; Markov, S.L.; Cvetković, G.S. Antioxidant and antimicrobial activities of beet root pomace extracts. Czech J. Food Sci. 2011, 29, 575–585. [Google Scholar] [CrossRef]
- Vulić, J.J.; Čanadanović-Brunet, J.; Cvetković, G.; Tumbas, V.; Djilas, S.; Četojević-Simin, D.; Čanadanović, V. Antioxidant and cell growth activities of beet root pomace extracts. J. Funct. Food. 2012, 4, 570–578. [Google Scholar] [CrossRef]
- Bucur, L.; Tarălungă, G.; Schroder, V. The betalains content and antioxidant capacity of red beet (Beta vulgaris L. subsp. vulgaris) root. Farmacia 2016, 64, 198–201. [Google Scholar]
- Pitalua, E.; Jimenez, M.; Vernon-Carter, E.J.; Beristain, C.I. Antioxidantive activity of microcapsules with beetroot juice using gum Arabic as wall material. Food Bioprod. Process. 2010, 88, 253–258. [Google Scholar] [CrossRef]
- Schliemann, W.; Cai, Y.; Degenkolb, T.; Schmidt, J.; Corke, H. Betalains of Celosia argentea. Phytochemistry 2001, 58, 159–165. [Google Scholar] [CrossRef]
- Iamonico, D. About the circumscription of Celosia argentea (Amaranthaceae) and the related Linnaean taxa. Phytotaxa 2013, 90, 61–64. [Google Scholar] [CrossRef]
- Chaturvedi, M.; Datta, K.; Nath, P.; Pal, M. Cytopalynology of Celosia L. Grana 1993, 32, 169–175. [Google Scholar] [CrossRef]
- Feng, N.; Xue, Q.; Guo, Q.; Zhao, R.; Guo, M. Genetic diversity and population structure of Celosia argentea and related species revealed by SRAP. Biochem. Genet. 2009, 47, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.F. A cytological study of Celosia argentea, C. argentea var. cristata and their hybrids. Bot. Gaz. 1954, 115, 323–336. [Google Scholar] [CrossRef]
- Grant, W.F. Speciation and basic chromosome number in the genus Celosia. Can. J. Bot. 1961, 39, 45–50. [Google Scholar] [CrossRef]
- Grant, W.F. Speciation and nomenclature in the genus Celosia. Can. J. Bot. 1962, 40, 1355–1363. [Google Scholar] [CrossRef]
- Nath, P.; Phri, D.; Pal, M. Nuclear DNA content in Celosia (Amaranthaceae). Plant Syst. Evol. 1992, 182, 253–257. [Google Scholar] [CrossRef]
- Nath, P.; Phri, D.; Jha, S.S.; Pal, M. Seed protein electrophoresis of wild and cultivated species of Celosia (Amaranthaceae). Genet. Resour. Crop Evol. 1997, 44, 241–245. [Google Scholar] [CrossRef]
- Mastuti, R.; Arumingtyas, E.L.; Fatinah, A.A. Genetic diversity of Celosia variants in East Java based on polyphenol oxidase—PPO genes. Procedia Chem. 2015, 14, 361–366. [Google Scholar] [CrossRef][Green Version]
- Surse, S.N.; Shrivastava, B.; Sharma, P.; Gide, P.S.; Attar, S. Celosia cristata: Potent pharmacotherapeutic herb—A review. Int. J. Pharm. Phytopharm. Res. 2014, 3, 444–446. [Google Scholar]
- Nidavani, R.B.; Mahalakshmi, A.M.; Shalawadi, M. Towards a better understanding of an updated of ethnopharmacology of Celosia argentea L. Int. J. Pharm. Pharm. Sci. 2013, 5 (Suppl. 3), 54–59. [Google Scholar]
- Cai, Y.; Sun, M.; Schliemann, W.; Corke, H. Chemical stability and colorant properties of betaxanthin pigments from Celosia argentea. J. Agric. Food Chem. 2001, 49, 4429–4435. [Google Scholar] [CrossRef] [PubMed]
- Molehin, O.R.; Adefegha, S.A.; Oboh, G.; Saliu, J.A.; Athayde, M.L.; Boligon, A.A. Comparative study on the phenolic content, antioxidant properties and HPLC fingerprinting of three varieties of Celosia species. J. Food Biochem. 2014, 38, 575–583. [Google Scholar] [CrossRef]
- Odukoya, O.A.; Inya-Agha, S.I.; Segun, F.I.; Sodifiya, M.O.; Ilori, O.O. Antioxidant activities of selected Nigerian green leafy vegetables. Am. J. Food Technol. 2007, 2, 169–175. [Google Scholar] [CrossRef]
- Rehman, A.; Imran, M.; Mehboob, S.; Khan, N.A.; Alam, W.; Riaz, K. Etiology, pathogenicity and management of collar rot in cockscomb (Celosia argentea). Int. J. Agric. Biol. 2015, 17, 9–14. [Google Scholar]
- Bakar, D.A.; Ahmed, B.A.; Taha, R.M. In vitro callus induction and plant regeneration of Celosia argentea—An important medicinal plant. Braz. Arch. Biol. Technol. 2014, 57, 860–866. [Google Scholar] [CrossRef]
- Rub, R.A.; Pati, M.J.; Siddiqui, A.A.; Moghe, A.S.; Shaikh, N.N. Characterization of anticancer principles of Celosia argentea (Amaranthaceae). Pharmacogn. Res. 2016, 8, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Xin, H.-L.; Guo, M.L. Review on research of the phytochemistry and pharmacological activities of Celosia argentea. Braz. J. Pharmacogn. 2016, 26, 787–796. [Google Scholar] [CrossRef]
- Wu, Q.B.; Wang, Y.; Liang, L.; Jiang, Q.; Guo, M.L.; Zhang, J.J. Novel triterpenoid saponins from the seeds of Celosia argentea L. Nat. Prod. Res. 2013, 27, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Saritha, P.; Sreeramulu, A. Allelopathic effects of Celosia argentea L. root extracts on crop plant seed germination. Int. J. Life Sci. Biotechnol. Pharma Res. 2013, 2, 142–154. [Google Scholar]
- Perveen, S.; Yousaf, M.; Zahoor, A.F.; Rasool, N.; Jabber, A. Extraction, isolation and identification of various environment friendly components from cock’s comb (Celosia argentea) leaves for allelopathic potential. Toxicol. Environ. Chem. 2014, 96, 1523–1534. [Google Scholar] [CrossRef]
- Gnanamani, A.; Priya, K.S.; Radhakrishan, N.; Babu, M. Antibacterial activity of two plant extracts on eight burn pathogens. J. Ethnopharmacol. 2003, 86, 59–61. [Google Scholar] [CrossRef]
- Wiart, C.; Mogana, S.; Khalifah, S.; Mahan, M.; Ismail, S.; Buckle, M.; Narayana, A.K.; Sulaiman, M. Antimicrobial screening of plants used for traditional medicine in the sate of Perak, Peninsular Malaysia. Fitoterapia 2004, 75, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Bhujbal, S.S.; Chitlange, S.S.; Suralkar, A.A.; Shinde, D.B.; Patil, M.J. Anti-inflammatory activity of an isolated flavonoid fraction from Celosia argentea Linn. J. Med. Plant Res. 2008, 2, 52–54. [Google Scholar]
- Hase, K.; Kadota, S.; Basnet, P.; Takahashi, T.; Namba, T. Protective effect of celosian, an acidic polysaccharide, on chemically and immunologically induced liver injuries. Biol. Pharm. Bull. 1996, 19, 567–572. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hase, K.; Kadota, S.; Basnet, P.; Takahashi, T.; Namba, T. Hepatoprotective effects of traditional medicines. Isolation of the active constituent from seeds of Celosia argentea. Phytother. Res. 1996, 10, 387–392. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Fujii, H.; Hase, K.; Ohnishi, Y.; Sakukawa, R.; Kadota, S.; Namba, T.; Saiki, I. Anti-metastatic and immunomodulating properties of the water extract from Celosia argentea seeds. Biol. Pharm. Bull. 1998, 21, 1154–1159. [Google Scholar] [CrossRef] [PubMed]
- Rub, R.A.; Patil, M.J.; Shaikh, N.N.; Haikh, T.; Ahmed, J.; Siddiqui, A.A. Immunomodulatory profile of Celosia argentea–Activity of isolated compounds I and II. Int. J. Adv. Biotechnol. Res. 2015, 6, 270–277. [Google Scholar]
- Vetrichelvan, T.; Jegadeesan, M.; Devi, B.A.U. Anti-diabetic activity of alcoholic extract of Celosia argentea Linn. seeds in rats. Biol. Pharm. Bull. 2002, 25, 526–528. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Ding, X.; Ouyang, M.-A.; Wu, Z.-J.; Xie, L.-H. A new phenolic glycoside and cytotoxic constituents from Celosia argentea. J. Asian Nat. Prod. Res. 2010, 12, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.-Z.; Di, H.; Bao, R.; Chu, G.-H.; Tang, X.-H.; Feng, M.; Diao, L.-J.; Lu, L. The healing effect of Celosia argentea leaf extract on burn wounds: An in vivo and in vitro evaluation. Int. J. Clin. Exp. Med. 2016, 9, 21018–21027. [Google Scholar]
- Priya, K.S.; Arumugam, G.; Rathinam, B.; Wells, A.; Babu, M. Celosia argentea Linn. leaf extract improves wound healing in a rat burn wound model. Wound Repair Regen. 2004, 12, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Telagari, M.; Hullatti, K. In vitro α-amylase and α-glucosidase inhibitory activity of Adiantum caudatum Linn. and Celosia argentea Linn. Extracts and fractions. Indian J. Pharmacol. 2015, 47, 425–429. [Google Scholar] [PubMed]
- Saqib, F.; Janbaz, K.H.J.; Sherwani, M.K. In vitro inhibitory potential of methanolic extract of Celosia argentea var. cristata on tyrosinase, acetylcholinesterase and butyrylcholinesterase enzymes. Bangladesh J. Pharmacol. 2015, 10, 449–454. [Google Scholar]
- Xue, Q.; Sun, Z.L.; Guo, M.L.; Wang, Y.; Zhang, G. Two new compounds from Semen Celosiae and their protective effects against CCl4-induced hepatotoxity. Nat. Prod. Res. 2011, 25, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Malomo, S.O.; Ore, A.; Yakubu, M.T. In vitro and in vivo antioxidant activities ofthe aqueous extract of Celosia argentea leaves. Indian J. Pharmacol. 2011, 43, 278–285. [Google Scholar] [PubMed]
- Iwalewa, E.O.; Adewunni, C.O.; Omisone, N.O.A.; Adebanji, O.A.; Azike, C.K.; Adigun, A.O.; Adesina, O.A.; Olowoyo, O.G. Pro- and antioxidant effects and cytoprotective potentials of nine edible vegetables in Southwest Nigeria. J. Med. Food 2005, 8, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Prakash, D.; Nath, P.; Pal, M. Composition and variation in vitamin C, carotenoids, protein, nitrate and oxalate contents in Celosia leaves. Plant Food Hum. Nutr. 1995, 47, 221–226. [Google Scholar] [CrossRef]
- Suzuki, H.; Morita, H.; Iwasaki, S.; Kobayashi, J. New antimitotic bicyclic peptides, celogentins D-H and J, from the seeds of Celosia argentea. Tetrahedron 2003, 59, 5307–5315. [Google Scholar] [CrossRef]
- Morita, H.; Takeya, K. Bioactive cyclic peptides from higher plants. Heterocycles 2010, 80, 739–764. [Google Scholar] [CrossRef]
- Zhang, S.-M.; Wang, X.-F.; Feng, J.; Sun, Z.-L. Chemical constituents of the seeds of Celosia cristata. Chem. Nat. Prod. 2016, 52, 827–829. [Google Scholar] [CrossRef]
- Morita, H.; Suzuki, H.; Kobayashi, J. Celogenamide A, a new cyclic peptide from the seeds of Celosia argentea. J. Nat. Prod. 2004, 67, 1628–1630. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Shimbo, K.; Shigemori, H.; Kobayashi, J. Antimitotic activity of moroidin, a bicyclic peptide from the seeds of Celosia argentea. Bioorg. Med. Chem. Lett. 2000, 10, 469–471. [Google Scholar] [CrossRef]
- Suzuki, H.; Morita, H.; Shiro, M.; Kobayashi, J. Celogentin K, a new cyclic peptide from the seeds of Celosia argentea and X-ary strucutre of moroidin. Tetrahedron 2004, 60, 2489–2495. [Google Scholar] [CrossRef]
- Bobayashi, J.; Suzuki, H.; Shimbo, K.; Takeya, K.; Morita, H. Celogentins A–C, new antimitotic bicyclic peptides from the seeds of Celosia argentea. J. Org. Chem. 2001, 66, 6626–6633. [Google Scholar] [CrossRef]
- Piattelli, M.; Minale, L. Pigments of Centrospermae II. Distribution of betacyanins. Phytochemistry 1964, 3, 547–557. [Google Scholar] [CrossRef]
- Cai, Y.; Sun, M.; Corke, H. HPLC characterization of betalains from plants in the Amaranthaceae. J. Chromatogr. Sci. 2005, 43, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Guadarrama-Flores, B.; Rodríguez-Monroy, M.; Cruz-Sosa, F.; García-Carmona, F.; Gandía-Herrero, F. Production of dihydroxylated betalains and dopamine in cell suspension cultures of Celosia argentea var. plumosa. J. Agric. Food Chem. 2015, 63, 2741–2749. [Google Scholar] [CrossRef] [PubMed]
- Nicola, M.G.; Amico, V.; Piatteli, M. Effect of white amd far-red light on betalain formation. Phytochemistry 1974, 13, 439–442. [Google Scholar] [CrossRef]
- Rink, E.; Böhm, H. Effect of DOPA feeding on betaxanthins in various species of Centrospermae. Phytochemistry 1991, 30, 1109–1112. [Google Scholar] [CrossRef]
- Singh, O.S.; Chhabra, N. Effect of plant growth regulators on betacyanin synthesis in Celosia argentea var. cristata in the dark. Z. Pflanzenphysiol. 1976, 78, 189–196. [Google Scholar] [CrossRef]
- Bodhipadma, K.; Noichinda, S.; Yadbuntung, I.; Buaeiam, W.; Leung, D.W.M. Comparison of in vitro and in vivo inflorescence of common cockscomb (Celosia argentea var. cristata). Sci. Asia 2010, 36, 68–71. [Google Scholar] [CrossRef]
- Taha, R.M.; Wafa, S.N. Plant regeneration and cellular behaviour studies in Celosia cristata grown in vivo and in vitro. Sci. World J. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Yaacob, J.S.; Saleh, A.; Elias, H.; Abdullah, S.; Mahmad, N.; Mohamed, N. In vitro regeneration and acclimatization protocols of selected ornamental plants (Agapanthus praecox, Justicia betonica and Celosia cristata). Sains Malays. 2014, 43, 715–722. [Google Scholar]
- Corio-Costet, M.F.; Chapuis, L.; Delbecque, J.P. Chenopodium album L. (Fat Hen): In vitro cell culture and production of secondary metabolites (phytosterols and ecdysteroids). In Biotechnology in Agriculture and Forestry, vol 41. Medicinal and Aromatic Plants X; Bajaj, Y.P.S., Ed.; Springer: Berlin, Germany, 1998; pp. 97–112. [Google Scholar]
- Filho, A.M.M.; Pirozi, M.R.; Borges, J.T.S.; Sant’Ana, H.M.P.; Chaves, J.B.P.; Coimbra, J.S.R. Quinoa: Nutritional, functional and antinutritional aspects. Crit. Rev. Food Sci. Nutr. 2017, 57, 1618–1630. [Google Scholar] [CrossRef] [PubMed]
- Nowak, V.; Du, J.; Charrondière, U.R. Assessment of the nutritional composition of quinoa (Chenopodium quinoa Willd.). Food Chem. 2016, 193, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, K.B.; Biondi, S.; Oses, R.; Acuña-Rodríguez, I.S.; Antognoni, F.; Martinez-Mosqueira, E.A.; Coulibaly, A.; Canahua-Murillo, A.; Pinto, M.; Zurita-Silva, A.; et al. Quinoa biodiversity and sustainability for food security under climate change. A review. Agron. Sustain. Dev. 2014, 34, 349–359. [Google Scholar] [CrossRef]
- Tang, Y.; Li, X.; Zhang, B.; Chen, P.X.; Liu, R.; Tsao, R. Characterisation of phenolics, betanins and antioxidant activities in seeds of three Chenopodium quinoa Willd. genotypes. Food Chem. 2015, 166, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Escribano, J.; Cabanes, J.; Jiménez-Atiénzar, M.; Ibañez-Tremolada, M.; Gómez-Pando, L.R.; García-Carmona, F.; Gandía-Herrero, F. Characterization of betalains, saponins and antioxidant power in differently colored quinoa (Chenopodium quinoa) varieties. Food Chem. 2017, 234, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Adolf, V.I.; Jacobsen, S.-E.; Shabala, S. Salt tolerance mechanisms in quinoa (Chenopodium quinoa Willd.). Environ. Exper. Bot. 2013, 92, 43–54. [Google Scholar] [CrossRef]
- Jacobsen, S.J.; Sørensen, M.; Pedersen, S.M.; Weiner, J. Feeding the world: Genetically modified crops versus agricultural biodiversity. Agron. Sustain. Dev. 2013, 33, 651–662. [Google Scholar] [CrossRef]
- Zurita-Silva, A.; Fuentes, F.; Zamora, P.; Jacobsen, S.-E.; Schwember, A.R. Breeding quinoa (Chenopodium quinoa Willd.): Potential and perspectives. Mol. Breed. 2014, 34, 13–30. [Google Scholar] [CrossRef]
- Koyro, H.-W.; Eisa, S.S. Effects of salinity on composition, viability and germination of seeds of Chenopodium quinoa Willd. Plant Soil 2008, 302, 79–90. [Google Scholar] [CrossRef]
- Bazile, D.; Martínez, E.A.; Fuentes, F. Diversity of quinoa in a biogeographical Island: A review of constraints and potential from arid to temperate regions of Chile. Not. Bot. Horti Agrobot. 2014, 42, 289–298. [Google Scholar] [CrossRef]
- Jacobsen, S.-E. The worldwide potential for quinoa (Chenopodium quinoa Willd.). Food Rev. Int. 2003, 19, 167–177. [Google Scholar] [CrossRef]
- Ruiz, K.B.; Biondi, S.; Martínez, E.A.; Orsini, F.; Antognoni, F.; Jacobsen, S.-E. Quinoa—A model crop for understanding salt-tolerance mechanisms in halophytes. Plant Biosyst. 2016, 150, 357–371. [Google Scholar] [CrossRef]
- Ng, S.-C.; Anderson, A.; Coker, J.; Ondrus, M. Characteerization of lipid oxidation products in quinoa (Chenopodium quinoa). Food Chem. 2007, 101, 185–192. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, F. Formulation and quality attributes of quinoa food products. Food Bioproc. Technol. 2016, 9, 49–68. [Google Scholar] [CrossRef]
- Comino, I.; Moreno, M.L.; Real, A.; Rodríguez-Herrera, A.; Barro, F.; Sousa, C. The gluten-free diet: Testing alternative cereals tolerated by celiac patients. Nutrients 2013, 5, 4250–4268. [Google Scholar] [CrossRef] [PubMed]
- Jancurová, M.; Minarovičová, L.; Dandar, A. Quinoa—A review. Czeck J. Food Sci. 2009, 27, 71–79. [Google Scholar] [CrossRef]
- Zevallos, V.F.; Ellis, H.J.; Suligoj, T.; Herencia, L.I.; Ciclitira, P.J. Variable activation of immune response by quinoa (Chenopodium quinoa Wild.) prolamins in celiac disease. Am. J. Clin. Nutr. 2012, 96, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Mujica, A. Andean grains and legumes. In Neglected Crops: 1492 from a Different Perspective; Hernando Bermujo, J.E., Leon, J., Eds.; FAO: Rome, Italy, 1994; Volume 26, pp. 131–148. Available online: http://www.hort.purdue.edu/newcrop/1492/grains.html (accessed on 25 August 2017).
- Szakiel, A.; Paczkawski, C.; Henry, M. Influence of environmental abiotic factors on the content of saponins in plants. Phytochem. Rev. 2011, 10, 471–491. [Google Scholar] [CrossRef]
- Vega-Gálvez, A.; Miranda, M.; Vergara, J.; Uribe, E.; Puente, L.; Martínez, E.A. Nutritions facts and functional potential of quinoa (Chenopodium quinoa Willd.), an ancient Andean grain: A review. J. Sci. Food Agric. 2010, 90, 2541–2547. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Caravaca, A.M.; Iafelice, G.; Lavini, A.; Pulvento, C.; Caboni, M.F.; Marconi, E. Phenolic compounds and saponins in quinoa samples (Chenopodium quinoa Willd.) grown under different saline and ninsaline irrigation regimens. J. Agric. Food Chem. 2012, 60, 4620–4627. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Tsao, R. Phytochemicals in quinoa and amaranth grains and their antioxidant, anti-inflammatory and potential health beneficial effects: A review. Mol. Nutr. Food Res. 2017, 61, 1600767. [Google Scholar] [CrossRef] [PubMed]
- Repo-Carrasco-Valencia, R.; Hellström, J.K.; Pihlava, J.-M.; Mattila, P.H. Flavonoids and other phenolic compounds in Andean indigenous grains: Quinoa (Chenopodium quinoa), kañiva (Chenopodium pallidicaule) and kiwicha (Amaranthus caudatus). Food Chem. 2010, 120, 128–133. [Google Scholar] [CrossRef]
- Pasko, P.; Baiton, H.; Zagrodzki, P.; Gorinstein, S.; Fołta, M.; Zachwieja, Z. Anthocyanins, total polyphenols and antioxidant activity in amaranth and quinoa seeds and sprouts during their grow. Food Chem. 2009, 115, 994–998. [Google Scholar] [CrossRef]
- Abderrahim, F.; Huanatico, E.; Segura, R.; Arribas, S.; Gonzalez, M.C.; Condezo-Hoyos, L. Physical features, phenolic compounds, betalains and total antioxidant capacity for coloured quinoa seeds (Chenopodium quinoa Willd.) from Peruvian Altiplano. Food Chem. 2015, 183, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Jubete, L.; Wijngaard, H.; Arendt, E.K.; Gallagher, E. Polyphenol composition and in vitro antioxidant activity of amaranth, quinoa buckwheat and wheat as affected by sprouting and baking. Food Chem. 2010, 119, 770–778. [Google Scholar] [CrossRef]
- Miranda, M.; Vega-Gálvez, A.; Uribe, E.; López, J.; Martínez, E.; Rodriguez, M.J.; Quispe, I.; di Scala, K. Physico-chemical analysis, anrioxidant capacity and vitamins of six ecotypes of Chilean quinoa (Chenopodium quinoa Willd.). Procedia Food Sci. 2011, 1, 1439–1446. [Google Scholar] [CrossRef]
- Yao, Y.; Yang, X.; Shi, Z.; Ren, G. Anti-inflammatory activity of saponins from quinoa (Chenopodium quinoa Willd.) seeds in lipopolysaccharide-stimulated RAW 264.7 macrophage cells. J. Food Sci. 2014, 79, H1018–H1023. [Google Scholar] [CrossRef] [PubMed]
- Farinazzi-Machado, F.M.V.; Barbalho, S.M.; Oshiiwa, M.; Goulart, R.; Pessan Junior, O. Use of cereal bars with quinoa (Chenopodium quinoa W.) to reduce risk factors related to cardiovascular diseases. Food Sci. Technol. 2012, 32, 239–244. [Google Scholar] [CrossRef]
- De Carvalho, F.G.; Ovidio, P.P.; Padovan, G.J.; Jordao Junior, A.A.; Marchini, J.S.; Navarro, A.M. Metabolic parameters of postmenopausal women after quinoa or corn flakes intake-a prospective and double-blind study. Int. J. Food Sci. Nutr. 2014, 65, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Navruz-Varli, N.; Sanlier, N. Nutritional and health benefits of quinoa (Chenopodium quinoa Willd.). J. Cereal Sci. 2016, 69, 371–376. [Google Scholar] [CrossRef]
- Dinan, L.; Lanfort, L.R. Effects and applications of arthropod steroid hormones (ecdysteroids) in mammals. J. Endocrinol. 2006, 191, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Foucault, A.S.; Even, P.; Lafont, R.; Dioh, W.; Veillet, S.; Tome, D.; Huneau, J.F.; Herman, W.H. Quinoa extract enriched in 20-hydroxyecdysone affects energy homeostasis and intestinal fat absorption in mice fed a high-fat diet. Physiol. Behav. 2014, 128, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Yu, Y.; Zhang, X.H.; Ribnicky, D.; Cefalu, W.T. Ecdysterone enhances muscle insulin signaling by modulating acylcarnitine profile and mitochondrial oxidative phosphorylation complexes in mice fed a high-fat diet. Diabetes 2011, 1–10. [Google Scholar] [CrossRef]
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miguel, M.G. Betalains in Some Species of the Amaranthaceae Family: A Review. Antioxidants 2018, 7, 53. https://doi.org/10.3390/antiox7040053
Miguel MG. Betalains in Some Species of the Amaranthaceae Family: A Review. Antioxidants. 2018; 7(4):53. https://doi.org/10.3390/antiox7040053
Chicago/Turabian StyleMiguel, Maria Graça. 2018. "Betalains in Some Species of the Amaranthaceae Family: A Review" Antioxidants 7, no. 4: 53. https://doi.org/10.3390/antiox7040053
APA StyleMiguel, M. G. (2018). Betalains in Some Species of the Amaranthaceae Family: A Review. Antioxidants, 7(4), 53. https://doi.org/10.3390/antiox7040053




