Characterization of Phenolic Compounds in Wine Lees
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Wine Lees and Sample Preparation
2.3. Measurement of Total Phenolics Content
2.4. Measurement of Total Tannin Content
2.5. Phloroglucinol Analysis
2.6. Antioxidant Activities
2.7. Statistical Analysis
3. Results and Discussion
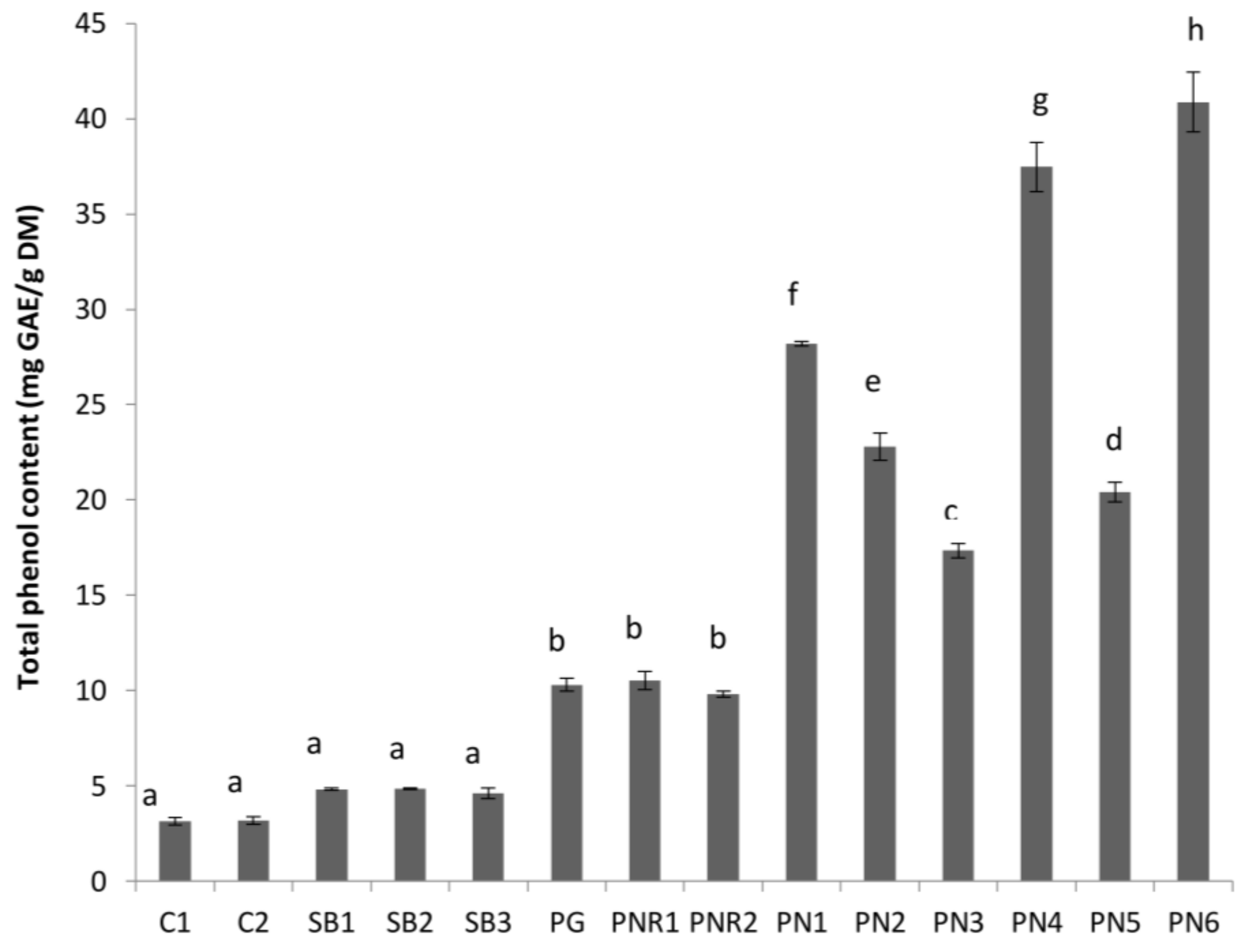
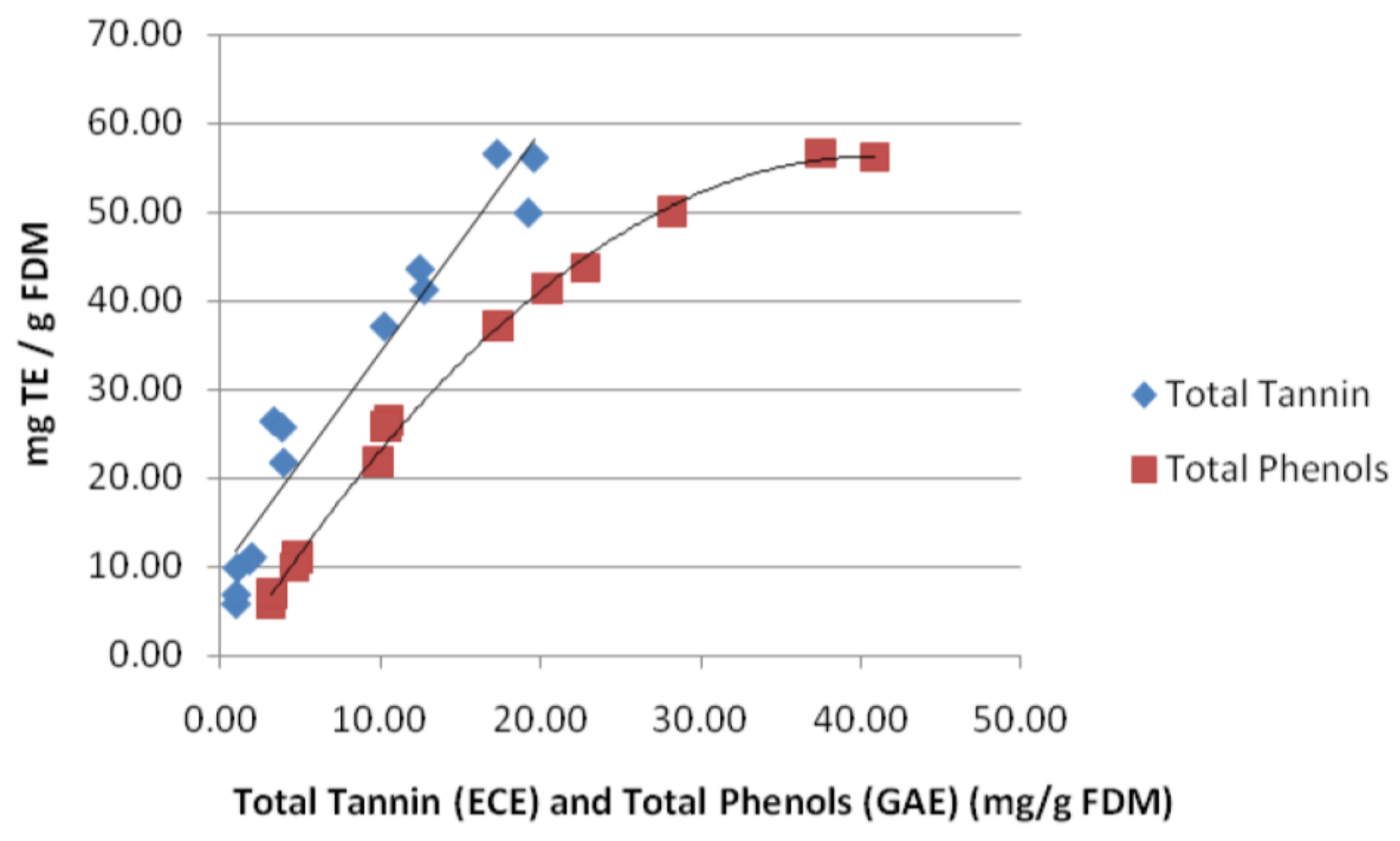
3.1. Total Phenol Content (TPC) and Total Tannin Content (TTC)
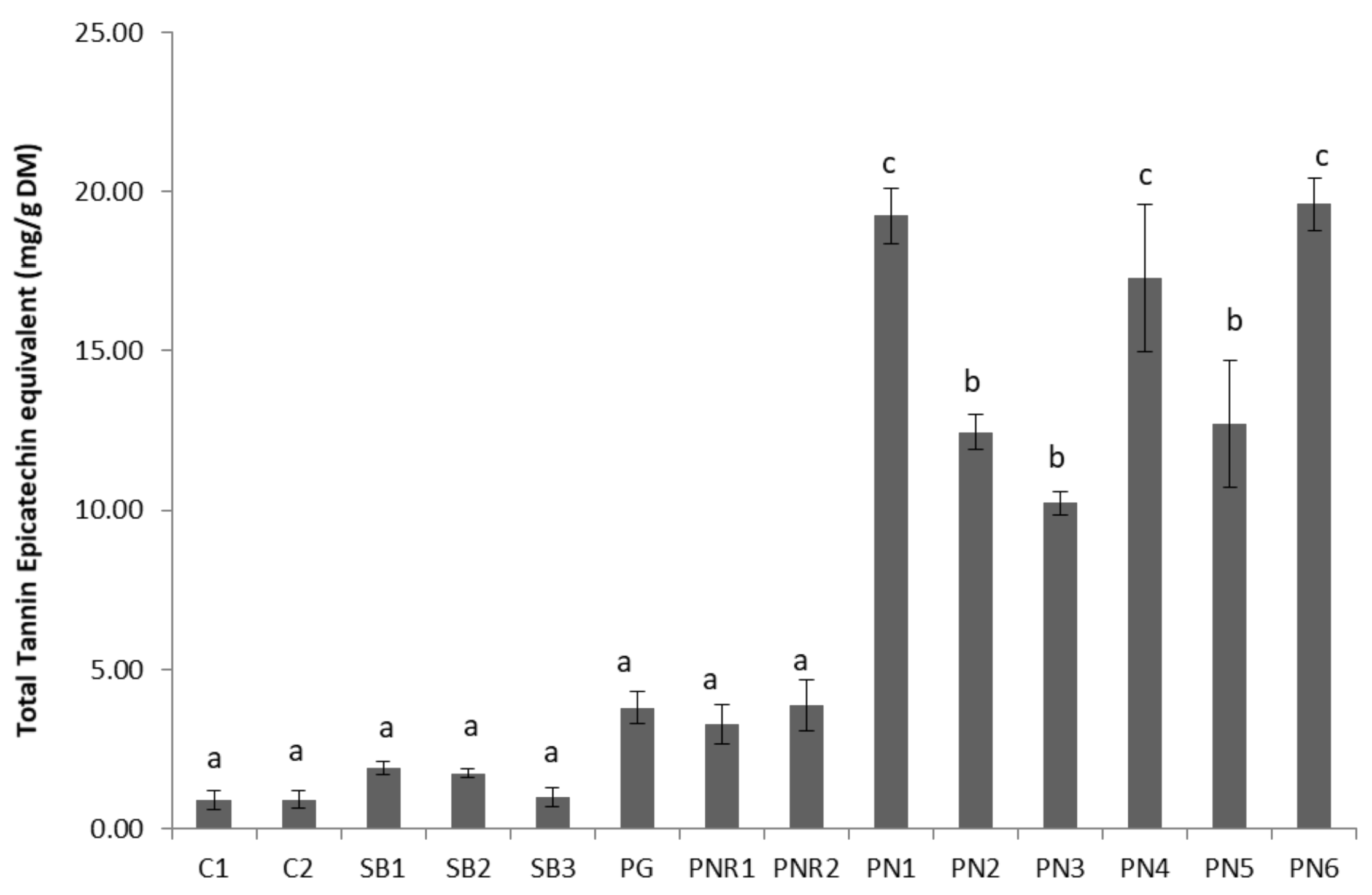
3.2. Total Tannin Content (TTC)
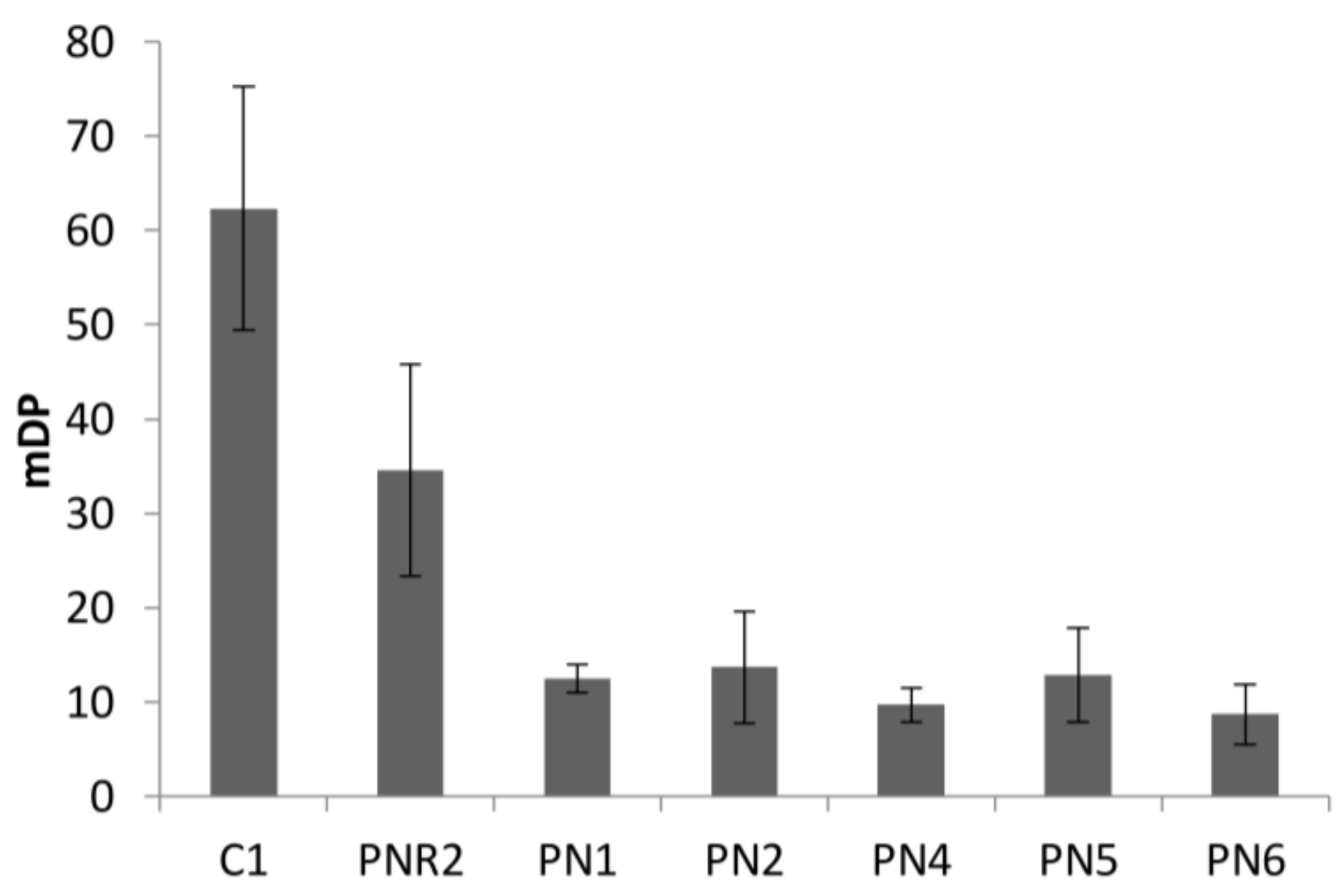
3.3. Polymeric Tannin Profile in Wine Lees
3.4. Antioxidant Activity
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Galanakis, C.M. Handbook of Grape Processing by-Products: Sustainable Solutions; Elsevier Science: New York, NY, USA, 2017. [Google Scholar]
- Lafka, T.I.; Sinanoglou, V.; Lazos, E.S. On the extraction and antioxidant activity of phenolic compounds from winery wastes. Food Chem. 2007, 104, 1206–1214. [Google Scholar] [CrossRef]
- Sacchi, K.L.; Bisson, L.F.; Adams, D.O. A review of the effect of winemaking techniques on phenolic extraction in red wines. Am. J. Enol. Vitic. 2005, 56, 197–206. [Google Scholar]
- Stintzing, F.C.; Carle, R. Functional properties of anthocyanins and betalains in plants, food, and in human nutrition. Trends Food Sci. Technol. 2004, 15, 19–38. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Shyu, Y.S.; Hsu, C.K. Grape wine lees improves the rheological and adds antioxidant properties to ice cream. LWT Food Sci. Technol. 2009, 42, 312–318. [Google Scholar] [CrossRef]
- Moldes, A.B.; Vazquez, M.; Dominguez, J.M.; Diaz-Fierros, F.; Barral, M.T. Negative effect of discharging vinification lees on soils. Bioresour. Technol. 2008, 99, 5991–5996. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, L.; Cadena, E.; Martinez-Blanco, J.; Gasol, C.M.; Rieradevall, J.; Gabarrell, X.; Gea, T.; Sort, X.; Sanchez, A. Recovery of organic wastes in the Spanish wine industry. Technical, economic and environmental analyses of the composting process. J. Clean. Prod. 2009, 17, 830–838. [Google Scholar] [CrossRef]
- Mercurio, M.D.; Dambergs, R.G.; Herderich, M.J.; Smith, P.A. High throughput analysis of red wine and grape phenolics-adaptation and validation of methyl cellulose precipitable tannin assay and modified Somers color assay to a rapid 96 well plate format. J. Agric. Food Chem. 2007, 55, 4651–4657. [Google Scholar] [CrossRef] [PubMed]
- Makkar, H.P.S.; Blummel, M.; Borowy, N.K.; Becker, K. Gravimetric-determination of tannins and their correlations with chemical and protein precipitation methods. J. Sci. Food Agric. 1993, 61, 161–165. [Google Scholar] [CrossRef]
- Sarneckis, C.J.; Dambergs, R.G.; Jones, P.; Mercurio, M.; Herderich, M.J.; Smith, P.A. Quantification of condensed tannins by precipitation with methylcellulose: Development and validation of an optimised tool for grape and wine analysis. Aust. J. Grape Wine Res. 2006, 12, 39–49. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Jones, G.P. Analysis of proanthocyanidin cleavage products following acid-catalysis in the presence of excess phloroglucinol. J. Agric. Food Chem. 2001, 49, 1740–1746. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A procedure to measure the antiradical efficiency of polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Krishnaiah, D.; Sarbatly, R.; Nithyanandam, R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. 2011, 89, 217–233. [Google Scholar] [CrossRef]
- Salvado, Z.; Arroyo-Lopez, F.N.; Barrio, E.; Querol, A.; Guillamon, J.M. Quantifying the individual effects of ethanol and temperature on the fitness advantage of Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Pinelo, M.; Arnous, A.; Meyer, A.S. Upgrading of grape skins: Significance of plant cell-wall structural components and extraction techniques for phenol release. Trends Food Sci. Technol. 2006, 17, 579–590. [Google Scholar] [CrossRef]
- Jackson, D.I.; Lombard, P.B. Environmental and management practices affecting grape composition and wine quality—A review. Am. J. Enol. Vitic. 1993, 44, 409–430. [Google Scholar]
- Kahkonen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Girard, B.; Mazza, G.; Reynolds, A.G. Changes in anthocyanins and color characteristics of pinot noir wines during different vinification processes. J. Agric. Food Chem. 1997, 45, 2003–2008. [Google Scholar] [CrossRef]
- Cosme, F.; Ricardo-Da-Silva, J.M.; Laureano, O. Tannin profiles of Vitis vinifera l. Cv. Red grapes growing in Lisbon and from their monovarietal wines. Food Chem. 2009, 112, 197–204. [Google Scholar] [CrossRef]
- Singleton, V.L.; Trousdale, E.K. Anthocyanin-tannin interactions explaining differences in polymeric phenols between white and red wines. Am. J. Enol. Vitic. 1992, 43, 63–70. [Google Scholar]
- Gris, E.F.; Mattivi, F.; Ferreira, E.A.; Vrhovsek, U.; Pedrosa, R.C.; Bordignon-Luiz, M.T. Proanthocyanidin profile and antioxidant capacity of Brazilian Vitis vinifera red wines. Food Chem. 2011, 126, 213–220. [Google Scholar] [CrossRef]
- Bordiga, M.; Travaglia, F.; Locatelli, M.; Coisson, J.D.; Arlorio, M. Characterisation of polymeric skin and seed proanthocyanidins during ripening in six Vitis vinifera L. Cv. Food Chem. 2011, 127, 180–187. [Google Scholar] [CrossRef]
- Lee, J.M. Degradation kinetics of grape skin and seed proanthocyanidins in a model wine system. Food Chem. 2010, 123, 51–56. [Google Scholar] [CrossRef]
- Villano, D.; Fernandez-Pachon, M.S.; Moya, M.L.; Troncoso, A.M.; Garcia-Parrilla, M.C. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta 2007, 71, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Larrauri, J.A.; Sanchez-Moreno, C.; Ruperez, P.; Saura-Calixto, F. Free radical scavenging capacity in the aging of selected red Spanish wines. J. Agric. Food Chem. 1999, 47, 1603–1606. [Google Scholar] [CrossRef] [PubMed]

| Sample Code | Sample Description |
|---|---|
| C1 | Lincoln University Chardonnay 1 |
| C2 | Lincoln University Chardonnay 2 |
| SB1 | Lincoln University Sauvignon Blanc, natural yeast ferment 1 |
| SB2 | Lincoln University Sauvignon Blanc, natural yeast ferment 2 |
| SB3 | Lincoln University Sauvignon Blanc, inoculated with commercial yeast |
| PG | Lincoln University Pinot Gris HB clone, macerated on skins |
| PNR1 | Lincoln University Pinot Noir Rosé, 1-week pre-ferment maceration |
| PNR2 | Lincoln University Pinot Noir Rosé, 48 h pre-ferment maceration |
| PN1 | Waipara Pinot Noir, no pre-ferment maceration |
| PN2 | Central Otago Pinot Noir, pumped over |
| PN3 | Waipara Pinot Noir, 1-week pre-ferment maceration |
| PN4 | Waipara Pinot Noir, 5 days pre-fermentation maceration with oak chips |
| PN5 | Central Otago Pinot Noir, hand plunged |
| PN6 | Central Otago Pinot Noir, No cap management |
| Sample | Terminal Units (No PG) (%) | Extension Units (PG) (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| C | EC | ECG | EGC | C | EC | ECG | EGC | |
| White wine lees | ||||||||
| C1 | 50.7 | 38.0 | 11.3 | 0.0 | 0.0 | 15.8 | 0.0 | 84.2 |
| PG | 52.9 | 37.1 | 8.1 | 0.0 | 0.9 | 51.3 | 2.1 | 45.6 |
| Rosé wine lees | ||||||||
| PNR2 | 56.8 | 40.6 | 8.6 | 0.0 | 1.1 | 59.2 | 2.8 | 36.9 |
| Red wine lees | ||||||||
| PN1 | 59.8 | 35.9 | 4.4 | 0.0 | 1.5 | 74.8 | 1.8 | 22.0 |
| PN2 | 55.1 | 36.2 | 8.7 | 0.0 | 1.4 | 63.4 | 4.6 | 30.7 |
| PN4 | 55.9 | 36.4 | 7.1 | 0.0 | 1.2 | 63.2 | 2.8 | 32.8 |
| PN5 | 61.6 | 48.4 | 10.5 | 0.0 | 1.3 | 63.0 | 3.4 | 32.4 |
| PN6 | 58.1 | 41.8 | 8.7 | 0.0 | 1.2 | 61.8 | 3.0 | 34.0 |
| Sample | EC50 (mg Lees Extracts/g DPPH) | ORAC (mg Trolox/g FD Material) |
|---|---|---|
| C1 | 12,166.3 ± 728.9 | 5.8 ± 0.10 |
| C2 | 12,608.3 ± 870.7 | 6.9 ± 0.74 |
| SB1 | 13,780.7 ± 895.4 | 11.1 ± 0.41 |
| SB2 | 15,030.0 ± 847.6 | 10.8 ± 0.17 |
| SB3 | 7803.3 ± 237.5 | 9.9 ± 0.39 |
| PG | 6724.1 ± 80.8 | 25.8 ± 1.70 |
| PNR1 | 6548.6 ± 1180.7 | 26.5 ± 3.65 |
| PNR2 | 6996.9 ± 323.4 | 21.8 ± 4.10 |
| PN1 | 5090.4 ± 56.6 | 50.0 ± 3.12 |
| PN2 | 5490.4 ± 279.0 | 43.7 ± 1.53 |
| PN3 | 7150.8 ± 230.5 | 37.2 ± 0.19 |
| PN4 | 5009.6 ± 48.5 | 56.6 ± 2.77 |
| PN5 | 6262.0 ± 177.9 | 41.3 ± 0.81 |
| PN6 | 5332.8 ± 20.2 | 56.2 ± 3.39 |
| Trolox | 104.5 ± 6.4 | - |
| Gallic acid | 58.2 ± 3.2 | - |
| Correlation | TPC | TTC | DPPH | ORAC |
|---|---|---|---|---|
| TTC | 0.955 | |||
| (0.000) | ||||
| DPPH | −0.808 | −0.819 | ||
| (0.000) | (0.000) | |||
| ORAC | 0.959 | 0.954 | −0.894 | |
| (0.000) | (0.000) | (0.000) | ||
| mDP | −0.404 | −0.464 | 0.354 | −0.371 |
| (0.050) | (0.022) | (0.089) | (0.075) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhijing, Y.; Shavandi, A.; Harrison, R.; Bekhit, A.E.-D.A. Characterization of Phenolic Compounds in Wine Lees. Antioxidants 2018, 7, 48. https://doi.org/10.3390/antiox7040048
Zhijing Y, Shavandi A, Harrison R, Bekhit AE-DA. Characterization of Phenolic Compounds in Wine Lees. Antioxidants. 2018; 7(4):48. https://doi.org/10.3390/antiox7040048
Chicago/Turabian StyleZhijing, Ye, Amin Shavandi, Roland Harrison, and Alaa El-Din A. Bekhit. 2018. "Characterization of Phenolic Compounds in Wine Lees" Antioxidants 7, no. 4: 48. https://doi.org/10.3390/antiox7040048
APA StyleZhijing, Y., Shavandi, A., Harrison, R., & Bekhit, A. E.-D. A. (2018). Characterization of Phenolic Compounds in Wine Lees. Antioxidants, 7(4), 48. https://doi.org/10.3390/antiox7040048






