Monocyte Subsets in Atherosclerosis and Modification with Exercise in Humans
Abstract
1. Introduction
2. Monocytes Contribute to the Pathogenesis of Atherosclerosis
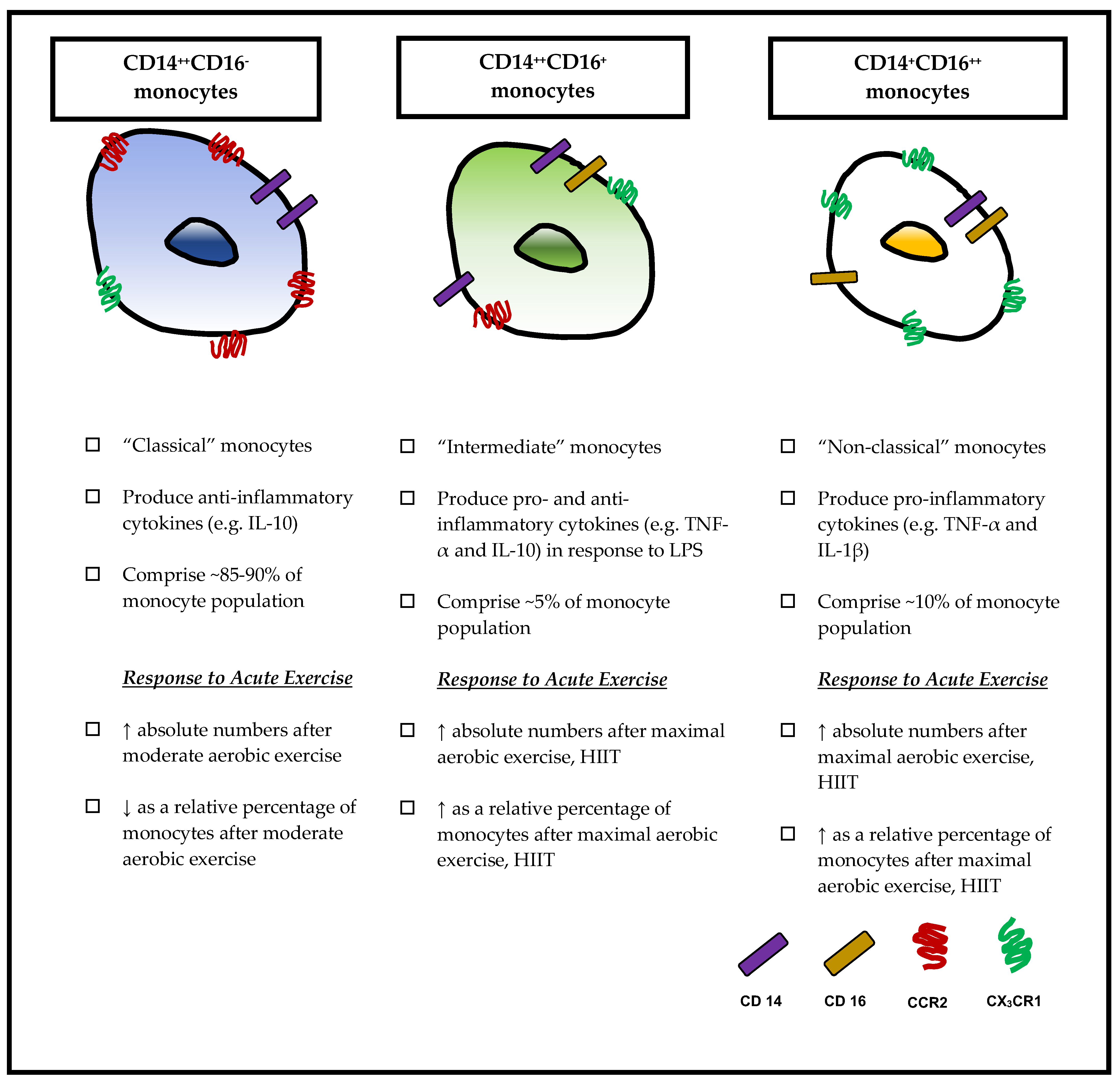
3. Monocyte Subsets
3.1. Classical Monocytes (CD14++CD16−)
3.2. Non-Classical Monocytes (CD14+CD16++)
3.3. Intermediate Monocytes (CD14++CD16+)
4. Role of Monocyte Subsets in Atherosclerosis
5. Exercise Modulates Monocyte Subsets
5.1. Effects of Acute Exercise
5.2. Effects of Chronic Exercise Training
6. Conclusions
Funding
Conflicts of Interest
References
- Ziegler-Heitbrock, L.; Ancuta, P.; Crowe, S.; Dalod, M.; Grau, V.; Hart, D.N.; Leenen, P.J.; Liu, Y.J.; MacPherson, G.; Randolph, G.J.; et al. Nomenclature of monocytes and dendritic cells in blood. Blood 2010, 116, e74–e80. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.L.; Yeap, W.H.; Tai, J.J.; Ong, S.M.; Dang, T.M.; Wong, S.C. The three human monocyte subsets: Implications for health and disease. Immunol. Res. 2012, 53, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Heitbrock, L.; Hofer, T.P. Toward a refined definition of monocyte subsets. Front. Immunol. 2013, 4, 23. [Google Scholar] [CrossRef]
- Libby, P. Inflammation in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2045–2051. [Google Scholar] [CrossRef]
- Nguyen, P.K.; Terashima, M.; Fair, J.M.; Varady, A.; Taylor-Piliae, R.E.; Iribarren, C.; Go, A.S.; Haskell, W.L.; Hlatky, M.A.; Fortmann, S.P.; et al. Physical activity in older subjects is associated with increased coronary vasodilation: The ADVANCE study. JACC Cardiovasc. Imaging 2011, 4, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Shibata, S.; Fujimoto, N.; Hastings, J.L.; Carrick-Ranson, G.; Bhella, P.S.; Hearon, C.M., Jr.; Levine, B.D. The effect of lifelong exercise frequency on arterial stiffness. J. Physiol. 2018, 596, 2783–2795. [Google Scholar] [CrossRef]
- Ashor, A.W.; Lara, J.; Siervo, M.; Celis-Morales, C.; Oggioni, C.; Jakovljevic, D.G.; Mathers, J.C. Exercise modalities and endothelial function: A systematic review and dose-response meta-analysis of randomized controlled trials. Sports Med. 2015, 45, 279–296. [Google Scholar] [CrossRef]
- Roth, G.A.; Forouzanfar, M.H.; Moran, A.E.; Barber, R.; Nguyen, G.; Feigin, V.L.; Naghavi, M.; Mensah, G.A.; Murray, C.J. Demographic and epidemiologic drivers of global cardiovascular mortality. N. Engl. J. Med. 2015, 372, 1333–1341. [Google Scholar] [CrossRef]
- Libby, P. Atherosclerosis: Disease biology affecting the coronary vasculature. Am. J. Cardiol. 2006, 98, 3Q–9Q. [Google Scholar] [CrossRef]
- Szmitko, P.E.; Wang, C.H.; Weisel, R.D.; de Almeida, J.R.; Anderson, T.J.; Verma, S. New markers of inflammation and endothelial cell activation: Part I. Circulation 2003, 108, 1917–1923. [Google Scholar] [CrossRef]
- Bosco, M.C.; Puppo, M.; Blengio, F.; Fraone, T.; Cappello, P.; Giovarelli, M.; Varesio, L. Monocytes and dendritic cells in a hypoxic environment: Spotlights on chemotaxis and migration. Immunobiology 2008, 213, 733–749. [Google Scholar] [CrossRef]
- Hoefer, I.E.; van Royen, N.; Rectenwald, J.E.; Deindl, E.; Hua, J.; Jost, M.; Grundmann, S.; Voskuil, M.; Ozaki, C.K.; Piek, J.J.; et al. Arteriogenesis proceeds via ICAM-1/Mac-1-mediated mechanisms. Circ. Res. 2004, 94, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Schuler, P.; Assefa, D.; Ylanne, J.; Basler, N.; Olschewski, M.; Ahrens, I.; Nordt, T.; Bode, C.; Peter, K. Adhesion of monocytes to medical steel as used for vascular stents is mediated by the integrin receptor Mac-1 (CD11b/CD18; αM β2) and can be inhibited by semiconductor coating. Cell Commun. Adhes. 2003, 10, 17–26. [Google Scholar] [CrossRef] [PubMed]
- De Groot, E.; van Leuven, S.I.; Duivenvoorden, R.; Meuwese, M.C.; Akdim, F.; Bots, M.L.; Kastelein, J.J. Measurement of carotid intima-media thickness to assess progression and regression of atherosclerosis. Nat. Clin. Pract. Cardiovasc. Med. 2008, 5, 280–288. [Google Scholar] [CrossRef]
- Jonasson, L.; Holm, J.; Skalli, O.; Bondjers, G.; Hansson, G.K. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis 1986, 6, 131–138. [Google Scholar] [CrossRef]
- Polverini, P.J.; Cotran, P.S.; Gimbrone, M.A., Jr.; Unanue, E.R. Activated macrophages induce vascular proliferation. Nature 1977, 269, 804–806. [Google Scholar] [CrossRef] [PubMed]
- Kruth, H.S. Macrophage foam cells and atherosclerosis. Front. Biosci. 2001, 6, D429–D455. [Google Scholar] [CrossRef]
- Ancuta, P.; Rao, R.; Moses, A.; Mehle, A.; Shaw, S.K.; Luscinskas, F.W.; Gabuzda, D. Fractalkine preferentially mediates arrest and migration of CD16+ monocytes. J. Exp. Med. 2003, 197, 1701–1707. [Google Scholar] [CrossRef]
- Timmerman, K.L.; Flynn, M.G.; Coen, P.M.; Markofski, M.M.; Pence, B.D. Exercise training-induced lowering of inflammatory (CD14+CD16+) monocytes: A role in the anti-inflammatory influence of exercise? J. Leukoc. Biol. 2008, 84, 1271–1278. [Google Scholar] [CrossRef]
- Kitchens, R.L. Role of CD14 in cellular recognition of bacterial lipopolysaccharides. Chem. Immunol. 2000, 74, 61–82. [Google Scholar]
- Tapping, R.I.; Tobias, P.S. Soluble CD14-mediated cellular responses to lipopolysaccharide. Chem. Immunol. 2000, 74, 108–121. [Google Scholar] [PubMed]
- Ma, S.; Suzuki, K. Toll-like receptor 4: Target of lipotoxicity and exercise-induced anti-inflammatory effect? Ann. Nutr. Food Sci. 2018, 2, 1027–1028. [Google Scholar]
- Ranoa, D.R.; Kelley, S.L.; Tapping, R.I. Human lipopolysaccharide-binding protein (LBP) and CD14 independently deliver triacylated lipoproteins to Toll-like receptor 1 (TLR1) and TLR2 and enhance formation of the ternary signaling complex. J. Biol. Chem. 2013, 288, 9729–9741. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Heitbrock, H.W.; Strobel, M.; Kieper, D.; Fingerle, G.; Schlunck, T.; Petersmann, I.; Ellwart, J.; Blumenstein, M.; Haas, J.G. Differential expression of cytokines in human blood monocyte subpopulations. Blood 1992, 79, 503–511. [Google Scholar]
- Wong, K.L.; Tai, J.J.; Wong, W.C.; Han, H.; Sem, X.; Yeap, W.H.; Kourilsky, P.; Wong, S.C. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood 2011, 118, e16–e31. [Google Scholar] [CrossRef]
- Skrzeczynska-Moncznik, J.; Bzowska, M.; Loseke, S.; Grage-Griebenow, E.; Zembala, M.; Pryjma, J. Peripheral blood CD14high CD16+ monocytes are main producers of IL-10. Scand. J. Immunol. 2008, 67, 152–159. [Google Scholar] [CrossRef]
- Zawada, A.M.; Rogacev, K.S.; Schirmer, S.H.; Sester, M.; Bohm, M.; Fliser, D.; Heine, G.H. Monocyte heterogeneity in human cardiovascular disease. Immunobiology 2012, 217, 1273–1284. [Google Scholar] [CrossRef]
- Bzowska, M.; Nogiec, A.; Skrzeczynska-Moncznik, J.; Mickowska, B.; Guzik, K.; Pryjma, J. Oxidized LDLs inhibit TLR-induced IL-10 production by monocytes: A new aspect of pathogen-accelerated atherosclerosis. Inflammation 2012, 35, 1567–1584. [Google Scholar] [CrossRef]
- Berg, K.E.; Ljungcrantz, I.; Andersson, L.; Bryngelsson, C.; Hedblad, B.; Fredrikson, G.N.; Nilsson, J.; Bjorkbacka, H. Elevated CD14++CD16− monocytes predict cardiovascular events. Circ. Cardiovasc. Genet. 2012, 5, 122–131. [Google Scholar] [CrossRef]
- Weber, C.; Belge, K.U.; von Hundelshausen, P.; Draude, G.; Steppich, B.; Mack, M.; Frankenberger, M.; Weber, K.S.; Ziegler-Heitbrock, H.W. Differential chemokine receptor expression and function in human monocyte subpopulations. J. Leukoc. Biol. 2000, 67, 699–704. [Google Scholar] [CrossRef]
- Belge, K.U.; Dayyani, F.; Horelt, A.; Siedlar, M.; Frankenberger, M.; Frankenberger, B.; Espevik, T.; Ziegler-Heitbrock, L. The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. J. Immunol. 2002, 168, 3536–3542. [Google Scholar] [CrossRef] [PubMed]
- Kaushansky, K. Lineage-specific hematopoietic growth factors. N. Engl. J. Med. 2006, 354, 2034–2045. [Google Scholar] [CrossRef] [PubMed]
- Steppich, B.; Dayyani, F.; Gruber, R.; Lorenz, R.; Mack, M.; Ziegler-Heitbrock, H.W. Selective mobilization of CD14+CD16+ monocytes by exercise. Am. J. Physiol. Cell Physiol. 2000, 279, C578–C586. [Google Scholar] [CrossRef] [PubMed]
- Grage-Griebenow, E.; Flad, H.D.; Ernst, M. Heterogeneity of human peripheral blood monocyte subsets. J. Leukoc. Biol. 2001, 69, 11–20. [Google Scholar]
- Grage-Griebenow, E.; Zawatzky, R.; Kahlert, H.; Brade, L.; Flad, H.; Ernst, M. Identification of a novel dendritic cell-like subset of CD64+/CD16+ blood monocytes. Eur. J. Immunol. 2001, 31, 48–56. [Google Scholar] [CrossRef]
- Patel, A.A.; Zhang, Y.; Fullerton, J.N.; Boelen, L.; Rongvaux, A.; Maini, A.A.; Bigley, V.; Flavell, R.A.; Gilroy, D.W.; Asquith, B.; et al. The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. J. Exp. Med. 2017, 214, 1913–1923. [Google Scholar] [CrossRef]
- Cros, J.; Cagnard, N.; Woollard, K.; Patey, N.; Zhang, S.Y.; Senechal, B.; Puel, A.; Biswas, S.K.; Moshous, D.; Picard, C.; et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity 2010, 33, 375–386. [Google Scholar] [CrossRef]
- Zawada, A.M.; Rogacev, K.S.; Rotter, B.; Winter, P.; Marell, R.R.; Fliser, D.; Heine, G.H. SuperSAGE evidence for CD14++CD16+ monocytes as a third monocyte subset. Blood 2011, 118, e50–e61. [Google Scholar] [CrossRef]
- Rogacev, K.S.; Cremers, B.; Zawada, A.M.; Seiler, S.; Binder, N.; Ege, P.; Grosse-Dunker, G.; Heisel, I.; Hornof, F.; Jeken, J.; et al. CD14++CD16+ monocytes independently predict cardiovascular events: A cohort study of 951 patients referred for elective coronary angiography. J. Am. Coll. Cardiol. 2012, 60, 1512–1520. [Google Scholar] [CrossRef]
- Shantsila, E.; Tapp, L.D.; Wrigley, B.J.; Montoro-Garcia, S.; Ghattas, A.; Jaipersad, A.; Lip, G.Y. The effects of exercise and diurnal variation on monocyte subsets and monocyte-platelet aggregates. Eur. J. Clin. Investig. 2012, 42, 832–839. [Google Scholar] [CrossRef]
- Cappellari, R.; D’Anna, M.; Bonora, B.M.; Rigato, M.; Cignarella, A.; Avogaro, A.; Fadini, G.P. Shift of monocyte subsets along their continuum predicts cardiovascular outcomes. Atherosclerosis 2017, 266, 95–102. [Google Scholar] [CrossRef]
- Nocon, M.; Hiemann, T.; Muller-Riemenschneider, F.; Thalau, F.; Roll, S.; Willich, S.N. Association of physical activity with all-cause and cardiovascular mortality: A systematic review and meta-analysis. Eur. J. Cardiovasc. Prev. Rehabil. 2008, 15, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.K.; Dykes, R.; Douglas, J.E.; Krishnaswamy, G.; Berk, S. Long-term exercise and atherogenic activity of blood mononuclear cells in persons at risk of developing ischemic heart disease. JAMA 1999, 281, 1722–1727. [Google Scholar] [CrossRef]
- Gabriel, H.; Urhausen, A.; Brechtel, L.; Muller, H.J.; Kindermann, W. Alterations of regular and mature monocytes are distinct, and dependent of intensity and duration of exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1994, 69, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Durrer, C.; Francois, M.; Neudorf, H.; Little, J.P. Acute high-intensity interval exercise reduces human monocyte Toll-like receptor 2 expression in type 2 diabetes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 312, R529–R538. [Google Scholar] [CrossRef]
- Lancaster, G.I.; Khan, Q.; Drysdale, P.; Wallace, F.; Jeukendrup, A.E.; Drayson, M.T.; Gleeson, M. The physiological regulation of toll-like receptor expression and function in humans. J. Physiol. 2005, 563, 945–955. [Google Scholar] [CrossRef]
- Gleeson, M.; McFarlin, B.; Flynn, M. Exercise and Toll-like receptors. Exerc. Immunol. Rev. 2006, 12, 34–53. [Google Scholar]
- Oliveira, M.; Gleeson, M. The influence of prolonged cycling on monocyte Toll-like receptor 2 and 4 expression in healthy men. Eur. J. Appl. Physiol. 2010, 109, 251–257. [Google Scholar] [CrossRef]
- Simpson, R.J.; McFarlin, B.K.; McSporran, C.; Spielmann, G.; Hartaigh, B.; Guy, K. Toll-like receptor expression on classic and pro-inflammatory blood monocytes after acute exercise in humans. Brain Behav. Immun. 2009, 23, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Radom-Aizik, S.; Zaldivar, F.P., Jr.; Haddad, F.; Cooper, D.M. Impact of brief exercise on circulating monocyte gene and microRNA expression: Implications for atherosclerotic vascular disease. Brain Behav. Immun. 2014, 39, 121–129. [Google Scholar] [CrossRef]
- LaVoy, E.C.; Bollard, C.M.; Hanley, P.J.; O’Connor, D.P.; Lowder, T.W.; Bosch, J.A.; Simpson, R.J. A single bout of dynamic exercise by healthy adults enhances the generation of monocyte-derived-dendritic cells. Cell Immunol. 2015, 295, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Slusher, A.L.; Zuniga, T.M.; Acevedo, E.O. Maximal Exercise Alters the Inflammatory Phenotype and Response of Mononuclear Cells. Med. Sci. Sports Exerc. 2018, 50, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Booth, S.; Florida-James, G.D.; McFarlin, B.K.; Spielmann, G.; O’Connor, D.P.; Simpson, R.J. The impact of acute strenuous exercise on TLR2, TLR4 and HLA.DR expression on human blood monocytes induced by autologous serum. Eur. J. Appl. Physiol. 2010, 110, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Mills, P.J. Effects of an exercise challenge on mobilization and surface marker expression of monocyte subsets in individuals with normal vs. elevated blood pressure. Brain Behav. Immun. 2008, 22, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, S.; Hulteng, E.; Hong, S. Inflammation and exercise: Inhibition of monocytic intracellular TNF production by acute exercise via β2-adrenergic activation. Brain Behav. Immun. 2017, 61, 60–68. [Google Scholar] [CrossRef]
- Graff, R.M.; Kunz, H.E.; Agha, N.H.; Baker, F.L.; Laughlin, M.; Bigley, A.B.; Markofski, M.M.; LaVoy, E.C.; Katsanis, E.; Bond, R.A.; et al. β2-Adrenergic receptor signaling mediates the preferential mobilization of differentiated subsets of CD8+ T-cells, NK-cells and non-classical monocytes in response to acute exercise in humans. Brain Behav. Immun. 2018, 74, 143–153. [Google Scholar] [CrossRef]
- Rooney, B.V.; Bigley, A.B.; LaVoy, E.C.; Laughlin, M.; Pedlar, C.; Simpson, R.J. Lymphocytes and monocytes egress peripheral blood within minutes after cessation of steady state exercise: A detailed temporal analysis of leukocyte extravasation. Physiol. Behav. 2018, 194, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, M.P.; DiCostanzo, A.C.; Wheatley, C.M.; Kim, C.H.; Bornschlegl, S.; Gastineau, D.A.; Johnson, B.D.; Dietz, A.B. A systems biology approach to investigating the influence of exercise and fitness on the composition of leukocytes in peripheral blood. J. Immunother. Cancer 2017, 5, 30. [Google Scholar] [CrossRef] [PubMed]
- De Matos, M.A.; Duarte, T.C.; Ottone Vde, O.; Sampaio, P.F.; Costa, K.B.; de Oliveira, M.F.; Moseley, P.L.; Schneider, S.M.; Coimbra, C.C.; Brito-Melo, G.E.; et al. The effect of insulin resistance and exercise on the percentage of CD16+ monocyte subset in obese individuals. Cell Biochem. Funct. 2016, 34, 209–216. [Google Scholar] [CrossRef]
- Boesten, L.S.; Zadelaar, A.S.; van Nieuwkoop, A.; Gijbels, M.J.; de Winther, M.P.; Havekes, L.M.; van Vlijmen, B.J. Tumor necrosis factor-alpha promotes atherosclerotic lesion progression in APOE*3-Leiden transgenic mice. Cardiovasc. Res. 2005, 66, 179–185. [Google Scholar] [CrossRef]
- Canault, M.; Peiretti, F.; Mueller, C.; Kopp, F.; Morange, P.; Rihs, S.; Portugal, H.; Juhan-Vague, I.; Nalbone, G. Exclusive expression of transmembrane TNF-α in mice reduces the inflammatory response in early lipid lesions of aortic sinus. Atherosclerosis 2004, 172, 211–218. [Google Scholar] [CrossRef]
- Xiao, N.; Yin, M.; Zhang, L.; Qu, X.; Du, H.; Sun, X.; Mao, L.; Ren, G.; Zhang, C.; Geng, Y.; et al. Tumor necrosis factor-alpha deficiency retards early fatty-streak lesion by influencing the expression of inflammatory factors in apoE-null mice. Mol. Genet. Metab. 2009, 96, 239–244. [Google Scholar] [CrossRef]
- Kleinbongard, P.; Heusch, G.; Schulz, R. TNFalpha in atherosclerosis, myocardial ischemia/reperfusion and heart failure. Pharmacol. Ther. 2010, 127, 295–314. [Google Scholar] [CrossRef]
- Zhao, S.P.; Dong, S.Z. Effect of tumor necrosis factor alpha on cholesterol efflux in adipocytes. Clin. Chim. Acta Int. J. Clin. Chem. 2008, 389, 67–71. [Google Scholar] [CrossRef] [PubMed]
- McKellar, G.E.; McCarey, D.W.; Sattar, N.; McInnes, I.B. Role for TNF in atherosclerosis? Lessons from autoimmune disease. Nat. Rev. Cardiol. 2009, 6, 410–417. [Google Scholar] [CrossRef]
- Ramji, D.P.; Davies, T.S. Cytokines in atherosclerosis: Key players in all stages of disease and promising therapeutic targets. Cytokine Growth Factor Rev. 2015, 26, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Hürlimann, D.; Forster, A.; Noll, G.; Enseleit, F.; Chenevard, R.; Distler, O.; Bechir, M.; Spieker, L.E.; Neidhart, M.; Michel, B.A.; et al. Anti-tumor necrosis factor-alpha treatment improves endothelial function in patients with rheumatoid arthritis. Circulation 2002, 106, 2184–2187. [Google Scholar] [CrossRef] [PubMed]
- Irace, C.; Mancuso, G.; Fiaschi, E.; Madia, A.; Sesti, G.; Gnasso, A. Effect of anti TNFalpha therapy on arterial diameter and wall shear stress and HDL cholesterol. Atherosclerosis 2004, 177, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Child, M.; Leggate, M.; Gleeson, M. Effects of Two Weeks of High-intensity Interval Training (HIIT) on Monocyte TLR2 and TLR4 Expression in High BMI Sedentary Men. Int. J. Exerc. Sci. 2013, 6, 81–90. [Google Scholar]
- Gano, L.B.; Donato, A.J.; Pierce, G.L.; Pasha, H.M.; Magerko, K.A.; Roeca, C.; Seals, D.R. Increased proinflammatory and oxidant gene expression in circulating mononuclear cells in older adults: Amelioration by habitual exercise. Physiol. Genom. 2011, 43, 895–902. [Google Scholar] [CrossRef]
- Nahrendorf, M.; Swirski, F.K. Lifestyle effects on hematopoiesis and atherosclerosis. Circ. Res. 2015, 116, 884–894. [Google Scholar] [CrossRef] [PubMed]
- Stewart, L.K.; Flynn, M.G.; Campbell, W.W.; Craig, B.A.; Robinson, J.P.; McFarlin, B.K.; Timmerman, K.L.; Coen, P.M.; Felker, J.; Talbert, E. Influence of exercise training and age on CD14+ cell-surface expression of toll-like receptor 2 and 4. Brain Behav. Immun. 2005, 19, 389–397. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aw, N.H.; Canetti, E.; Suzuki, K.; Goh, J. Monocyte Subsets in Atherosclerosis and Modification with Exercise in Humans. Antioxidants 2018, 7, 196. https://doi.org/10.3390/antiox7120196
Aw NH, Canetti E, Suzuki K, Goh J. Monocyte Subsets in Atherosclerosis and Modification with Exercise in Humans. Antioxidants. 2018; 7(12):196. https://doi.org/10.3390/antiox7120196
Chicago/Turabian StyleAw, Ning Hong, Elisa Canetti, Katsuhiko Suzuki, and Jorming Goh. 2018. "Monocyte Subsets in Atherosclerosis and Modification with Exercise in Humans" Antioxidants 7, no. 12: 196. https://doi.org/10.3390/antiox7120196
APA StyleAw, N. H., Canetti, E., Suzuki, K., & Goh, J. (2018). Monocyte Subsets in Atherosclerosis and Modification with Exercise in Humans. Antioxidants, 7(12), 196. https://doi.org/10.3390/antiox7120196






