Exercise and Redox Status Responses Following Alpha-Lipoic Acid Supplementation in G6PD Deficient Individuals
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Experimental Design
2.3. Physical Characteristics and Physical Activity Level
2.4. Exercise Performance
2.5. Blood Collection and Handling
2.6. Blood Sample Analysis
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO Working Group. Glucose-6-phosphate dehydrogenase deficiency. Bull. World Health Organ. 1989, 67, 601–611. [Google Scholar]
- Deneke, S.M.; Fanburg, B.L. Regulation of cellular glutathione. Am. J. Physiol. 1989, 257 Pt 1, L163–L173. [Google Scholar] [CrossRef]
- Ji, L.L. Antioxidants and Oxidative Stress in Exercise. Proc. Soc. Exp. Biol. Med. 1999, 222, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Ninfali, P.; Bresolin, N. Muscle Glucose 6-Phosphate Dehydrogenase (G6PD) Deficiency and Oxidant Stress During Physical Exercise. Cell Biochem. Funct. 1995, 13, 297–298. [Google Scholar] [CrossRef] [PubMed]
- Bresolin, N.; Bet, L.; Moggio, M.; Meola, G.; Comi, G.; Scarlato, G. Muscle G6PD deficiency. Lancet 1987, 330, 212–213. [Google Scholar] [CrossRef]
- Bresolin, N.; Bet, L.; Moggio, M.; Meola, G.; Fortunato, F.; Comi, G.; Adobbati, L.; Geremia, L.; Pittalis, S.; Scarloto, G. Muscle glucose 6-phosphate dehydrogenase deficiency. J. Neurol. 1989, 236, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Ninfali, P.; Bresolin, N.; Baronciani, L.; Fortunato, F.; Comi, G.; Magnani, M.; Scarlato, G. Glucose-6-phosphate dehydrogenase Lodi 844 C: A study on its expression in blood cells and muscle. Enzyme 1991, 45, 180–187. [Google Scholar] [PubMed]
- Bresolin, N.; Comi, P.G.; Ninfali, P.; Meola, G.; Gallanti, A.; Ravasio, A.; Piscaglia, M.G.; Felisari, G.; Bardoni, A.; Scarlato, G. Muscular G6PD deficiency: A definite clinical syndrome? Clin. Neuropathol. 1992, 11, 89. [Google Scholar]
- Nikolaidis, M.G.; Jamurtas, A.Z.; Paschalis, V.; Kostaropoulos, I.A.; Kladi-Skandali, A.; Balamitsi, V.; Koutedakis, Y.; Kouretas, D. Exercise-induced oxidative stress in G6PD-deficient individuals. Med. Sci. Sports Exerc. 2006, 38, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Jamurtas, A.Z.; Fatouros, I.G.; Koukosias, N.; Manthou, E.; Tofas, T.; Yfanti, C.; Nikolaidis, M.G.; Koutedakis, Y. Effect of exercise on oxidative stress in individuals with glucose-6-phosphate dehydrogenase deficiency. In Vivo 2006, 20, 875–880. [Google Scholar] [PubMed]
- Theodorou, A.A.; Nikolaidis, M.G.; Paschalis, V.; Sakellariou, G.K.; Fatouros, I.G.; Koutedakis, Y.; Jamurtas, A.Z. Comparison between glucose-6-phosphate dehydrogenase-deficient and normal individuals after eccentric exercise. Med. Sci. Sports Exerc. 2010, 42, 1113–1121. [Google Scholar] [PubMed]
- Packer, L.; Witt, E.H.; Tritschler, H.J. Alpha-lipoic acid as a biological antioxidant. Free Radic. Biol. Med. 1995, 19, 227–250. [Google Scholar] [CrossRef]
- Roy, S.; Sen, C.K.; Tritschler, H.J.; Packer, L. Modulation of cellular reducing equivalent homeostasis by alpha-lipoic acid. Mechanisms and implications for diabetes and ischemic injury. Biochem. Pharmacol. 1997, 53, 393–399. [Google Scholar] [CrossRef]
- Sen, C.K.; Roy, S.; Packer, L. Therapeutic potential of the antioxidant and redox properties of alpha-lipoic acid. In Oxidative Stress Cancer, AIDS and Neurodegenerative Diseases, 1st ed.; Montagnier, L., Olivier, R., Pasquier, C., Eds.; Dekker: New York, NY, USA, 1997; pp. 251–267. ISBN 9780824798628. [Google Scholar]
- Han, D.; Handelman, G.; Marcocci, L.; Sen, C.K.; Roy, S.; Kobuchi, H.; Flohe, L.; Packer, L. Lipoic acid increases de novo synthesis of cellular glutathione by improving cysteine utilization. Biofactors 1997, 6, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K. Nutritional biochemistry of cellular glutathione. J. Nutr. Biochem. 1997, 8, 660–672. [Google Scholar] [CrossRef]
- Biewenga, G.P.; Haenen, G.R.; Bast, A. The pharmacology of the antioxidant lipoic acid. Gen. Pharmacol. 1997, 29, 315–331. [Google Scholar] [CrossRef]
- Petersen Shay, K.; Moreau, R.F.; Smith, E.J.; Smith, A.R.; Hagen, T.M. Alpha-lipoic acid as a dietary supplement. Molecular mechanisms and therapeutic potential. Biochim. Biophys. Acta 2009, 1790, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Georgakouli, K.; Deli, C.K.; Zalavras, A.; Fatouros, I.G.; Kouretas, D.; Koutedakis, Y.; Jamurtas, A.Z. A-lipoic acid supplementation up-regulates antioxidant capacity in adults with G6PD deficiency. Food. Chem. Toxicol. 2013, 61, 69–73. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. US National Institutes of Health. Available online: http://www.clinicaltrials.gov (accessed on 9 September 2018).
- Papathanasiou, G.; Georgoudis, G.; Papandreou, M.; Spyropoulos, P.; Georgakopoulos, D.; Kalfakakou, V.; Evangelou, A. Reliability measures of the short International Physical Activity Questionnaire (IPAQ) in Greek young adults. Hell. J. Cardiol. 2009, 50, 283–294. [Google Scholar]
- Sedlak, T.W.; Saleh, M.; Higginson, D.S.; Paul, B.D.; Juluri, K.R.; Snyder, S.H. Bilirubin and glutathione have complementary antioxidant and cytoprotective roles. Proc. Natl. Acad. Sci. USA 2009, 106, 5171–5176. [Google Scholar] [CrossRef] [PubMed]
- Sautin, Y.Y.; Johnson, R.J. Uric acid: The oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids 2008, 27, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Ghiselli, A.; Serafini, M.; Natella, F.; Scaccini, C. Total antioxidant capacity as a tool to assess redox status: Critical view and experimental data. Free Radic. Biol. Med. 2000, 29, 1106–1114. [Google Scholar] [CrossRef]
- Balakrishnan, S.D.; Anuradha, C.V. Exercise, depletion of antioxidants and antioxidant manipulation. Cell Biochem. Funct. 1998, 16, 269–275. [Google Scholar] [CrossRef]
- Wayner, D.D.; Burton, G.W.; Ingold, K.U.; Barclay, L.R.; Locke, S.J. The relative contributions of vitamin E, urate, ascorbate and proteins to the total peroxyl radical-trapping antioxidant activity of human blood plasma. Biochim. Biophys. Acta 1987, 924, 408–419. [Google Scholar] [CrossRef]
- Peeling, P.; Dawson, B.; Goodman, C.; Landers, G.; Wiegerinck, E.T.; Swinkels, D.W.; Trinder, D. Training surface and intensity: Inflammation, hemolysis, and hepcidin expression. Med. Sci. Sports Exerc. 2009, 41, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Bilmen, S.; Aksu, T.A.; Gümüşlü, S.; Korgun, D.K.; Canatan, D. Antioxidant capacity of G-6-PD-deficient erythrocytes. Clin. Chim. Acta 2001, 303, 83–86. [Google Scholar] [CrossRef]
- Gurbuz, N.; Yalcin, O.; Aksu, T.A.; Baskurt, O.K. The relationship between the enzyme activity, lipid peroxidation and red blood cells deformability in hemizygous and heterozygous glucose-6-phosphate dehydrogenase deficient individuals. Clin. Hemorrheol. Microcirc. 2004, 31, 235–242. [Google Scholar]
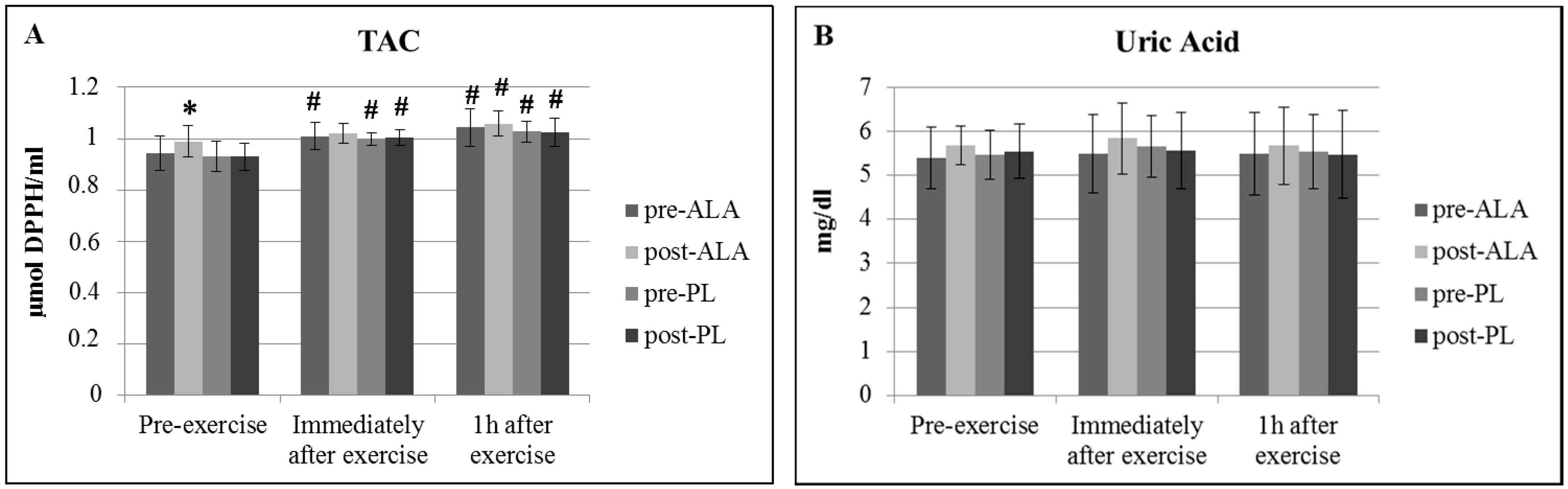
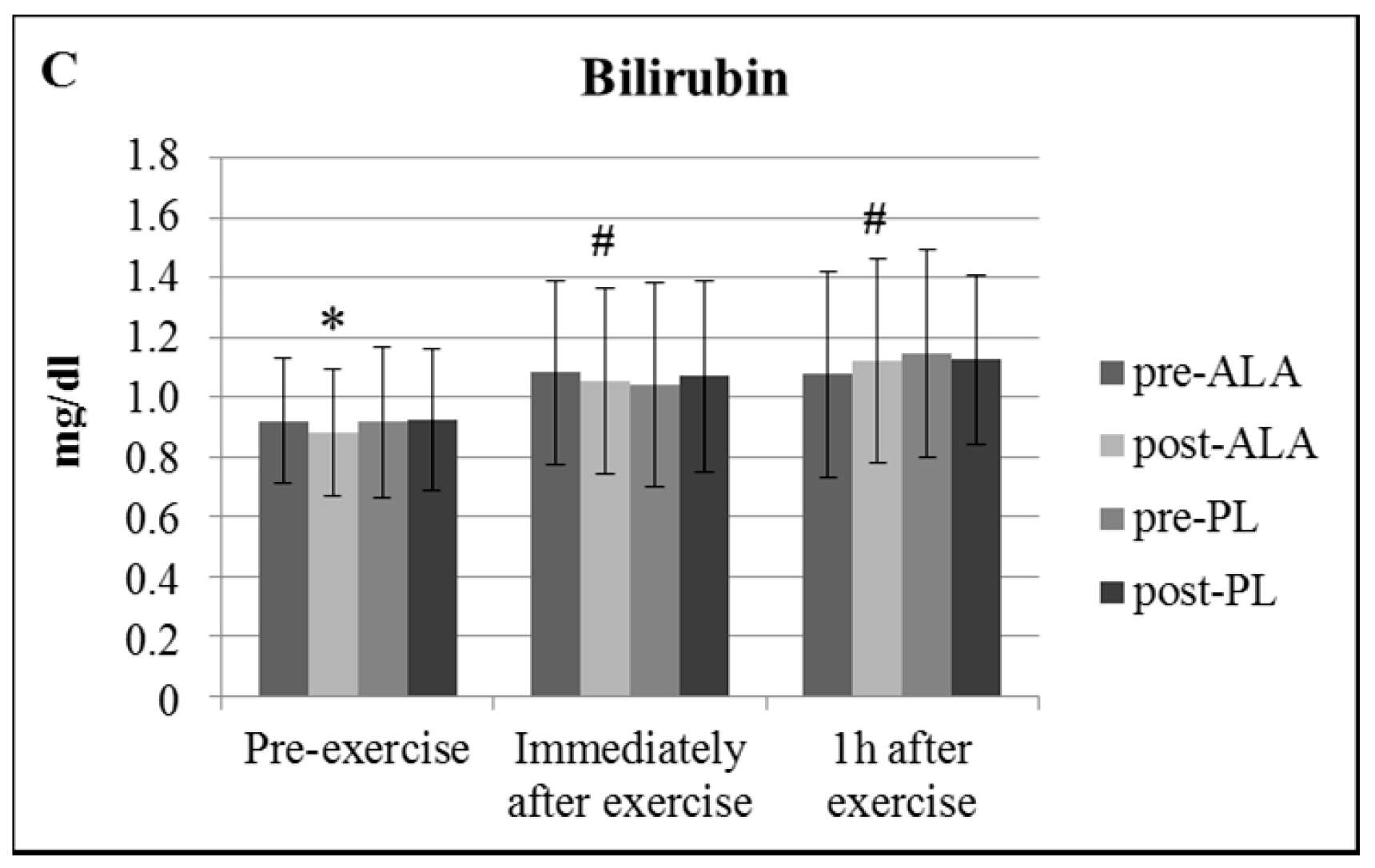

| Parameter | ALA | PL | ||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Body Weight (kg) | 81.64 ± 5.65 | 81.67 ± 5.32 | 81.53 ± 5.96 | 82.00 ± 5.86 |
| BMI (kg/m2) | 25.99 ± 1.50 | 26.02 ± 1.40 | 25.94 ± 1.58 | 26.09 ± 1.53 |
| % Body Fat | 25.84 ± 2.16 | 25.36 ± 2.32 | 25.43 ± 2.18 | 26.37 ± 1.92 |
| Waist Circumference (cm) | 89.36 ± 8.37 | 89.14 ± 9.00 | 88.57 ± 8.78 | 88.64 ± 8.00 |
| Hip Circumference (cm) | 104.7 ± 6.7 | 104.1 ± 6.7 | 104.6 ± 6.4 | 104.3 ± 6.4 |
| WHR | 0.853 ± 0.017 | 0.855 ± 0.018 | 0.846 ± 0.016 | 0.849 ± 0.014 |
| Systolic BP (mm Hg) | 130.9 ± 11.7 | 130.0 ± 12.7 | 130.6 ± 11.1 | 129.1 ± 12.0 |
| Diastolic BP (mm Hg) | 82.0 ± 7.4 | 80.0 ± 5.9 | 81.7 ± 6.5 | 78.6 ± 4.4 |
| Resting HR | 63.43 ± 2.57 | 59.29 ± 1.91 * | 62.14 ± 2.53 | 62.00 ± 2.73 |
| IPAQ (METs/min) | 1226 ± 336 | 1199 ± 281 | 922 ± 323 | 981 ± 336 |
| Parameter | ALA | PL | ||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Time to exhaustion (s) | 198.9 ± 37.0 | 246.9 ± 37.6 | 207.9 ± 37.4 | 267.4 ± 36.4 |
| MVC (Extensors) | ||||
| Left | 206.8 ± 18.1 | 205.3 ± 23.6 | 196.3 ± 18.8 | 202.8 ± 22.0 |
| Right | 213.8 ± 15.7 | 231.5 ± 22.5 | 209.8 ± 10.7 | 220.5 ± 11.1 |
| MVC (Flexors) | ||||
| Left | 170.7 ± 14.4 | 169.3 ± 10.4 | 164.8 ± 10.9 | 172.7 ± 12.4 |
| Right | 169.2 ± 18.8 | 169.0 ± 13.8 | 167.7 ± 14.3 | 172.2 ± 14.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgakouli, K.; Fatouros, I.G.; Fragkos, A.; Tzatzakis, T.; Deli, C.K.; Papanikolaou, K.; Koutedakis, Y.; Jamurtas, A.Z. Exercise and Redox Status Responses Following Alpha-Lipoic Acid Supplementation in G6PD Deficient Individuals. Antioxidants 2018, 7, 162. https://doi.org/10.3390/antiox7110162
Georgakouli K, Fatouros IG, Fragkos A, Tzatzakis T, Deli CK, Papanikolaou K, Koutedakis Y, Jamurtas AZ. Exercise and Redox Status Responses Following Alpha-Lipoic Acid Supplementation in G6PD Deficient Individuals. Antioxidants. 2018; 7(11):162. https://doi.org/10.3390/antiox7110162
Chicago/Turabian StyleGeorgakouli, Kalliopi, Ioannis G. Fatouros, Apostolos Fragkos, Theofanis Tzatzakis, Chariklia K. Deli, Konstantinos Papanikolaou, Yiannis Koutedakis, and Athanasios Z. Jamurtas. 2018. "Exercise and Redox Status Responses Following Alpha-Lipoic Acid Supplementation in G6PD Deficient Individuals" Antioxidants 7, no. 11: 162. https://doi.org/10.3390/antiox7110162
APA StyleGeorgakouli, K., Fatouros, I. G., Fragkos, A., Tzatzakis, T., Deli, C. K., Papanikolaou, K., Koutedakis, Y., & Jamurtas, A. Z. (2018). Exercise and Redox Status Responses Following Alpha-Lipoic Acid Supplementation in G6PD Deficient Individuals. Antioxidants, 7(11), 162. https://doi.org/10.3390/antiox7110162







