New Insights into the Potential of Endogenous Redox Systems in Wheat Bread Dough
Abstract
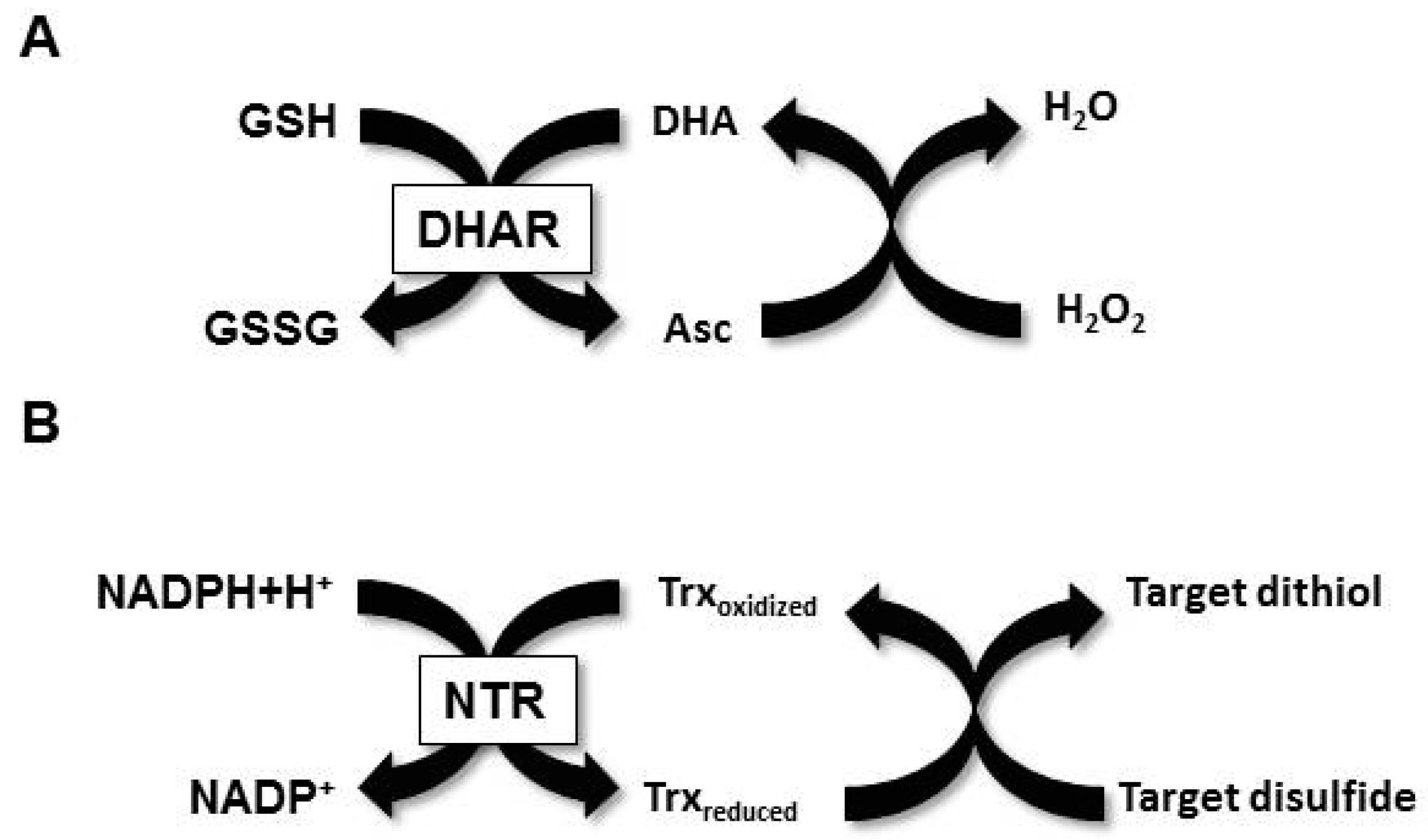
:1. Introduction
2. Materials and Methods
3. Results and Discussion
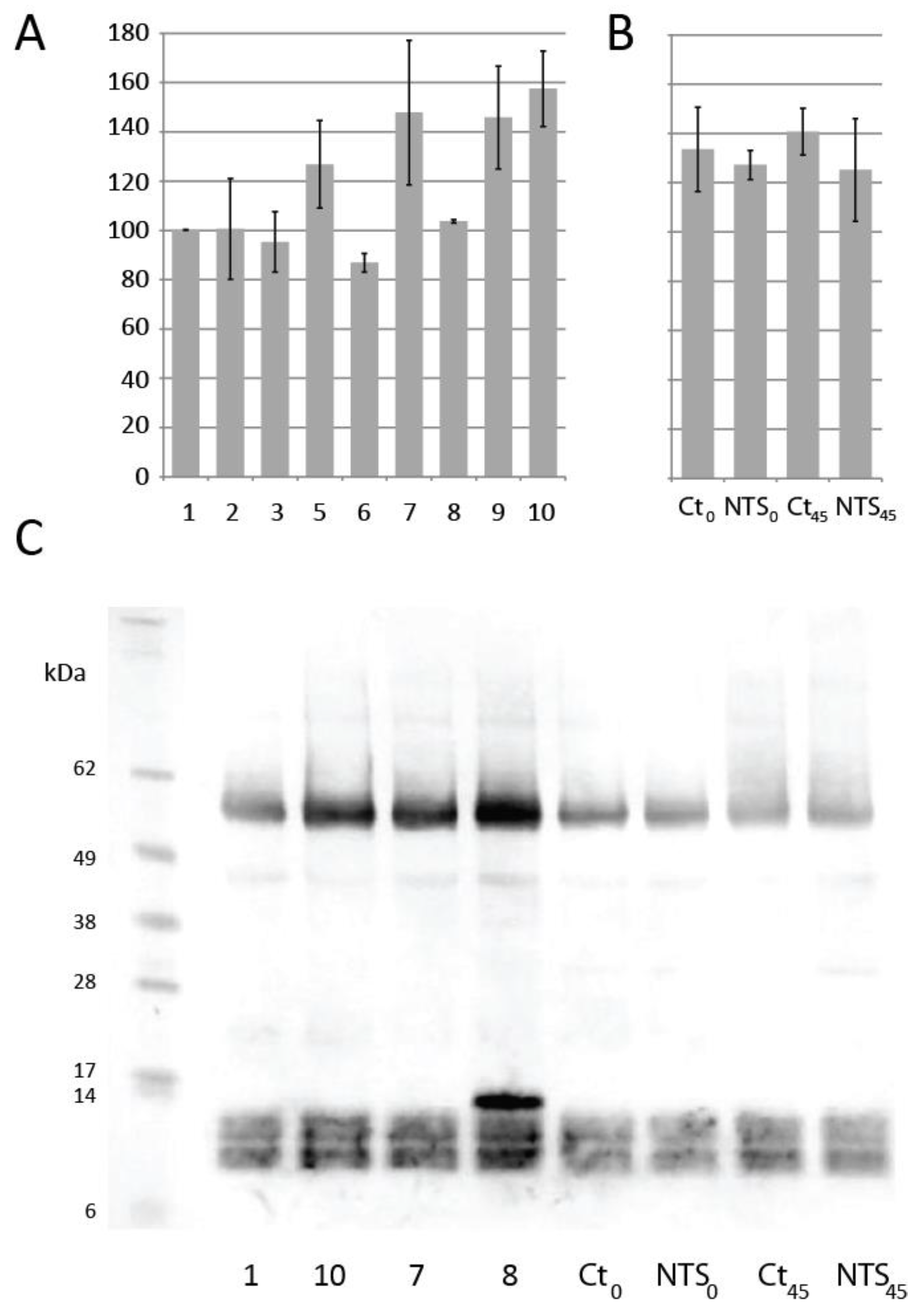
3.1. Effect of NTS on Dough Rheological Properties
3.2. Mini Breads and Buns
3.3. Protein Extraction and Dough Thiol Content
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Trx | thioredoxin |
| NTR | NADPH-dependent thioredoxin reductase |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| GSH | glutathione |
| NTS | NADPH-dependent NTR and Thioredoxin system |
References
- Shewry, P.R.; Halford, N.G.; Belton, P.S.; Tatham, A.S. The structure and properties of gluten: An elastic protein from wheat grain. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2002, 357, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Aamodt, A.; Magnus, E.M.; Faergestad, E.M. Effect of flour quality, ascorbic acid, and DATEM on dough rheological parameters and hearth loaves characteristics. J. Food Sci. 2003, 68, 2201–2210. [Google Scholar] [CrossRef]
- Reinbold, J.; Rychlik, M.; Asam, S.; Wieser, H.; Koehler, P. Concentrations of total glutathione and cysteine in wheat flour as affected by sulfur deficiency and correlation to quality parameters. J. Agric. Food Chem. 2008, 56, 6844–6850. [Google Scholar] [CrossRef] [PubMed]
- Berland, S.; Launay, B. Rheological properties of wheat flour doughs in steady and dynamic shear: Effect of water content and some additives. Cereal Chem. 1995, 72, 48–52. [Google Scholar]
- Grosch, W.; Wieser, H. Redox reactions in wheat dough as affected by ascorbic acid. J. Cereal Sci. 1999, 29, 1–16. [Google Scholar] [CrossRef]
- Dong, W.; Hoseney, R.C. Effects of certain breadmaking oxidants and reducing agents on dough rheological properties. Cereal Chem. 1995, 72, 58–64. [Google Scholar]
- Yano, H. Improvements in the Bread-Making Quality of Gluten-Free Rice Batter by Glutathione. J. Agric. Food Chem. 2010, 58, 7949–7954. [Google Scholar] [CrossRef]
- L-Cysteine. Code of Federal Regulations, 21CFR184.1271. Available online: https://www.ecfr.gov/cgi-bin/text-idx?SID=dc75a9bfd74137ed4b5dafeedd8db200&mc=true&node=se21.3.184_11271&rgn=div8 (accessed on 1 October 2018).
- Every, D.; Simmons, L.D.; Ross, M.P. Distribution of redox enzymes in millstreams and relationships to chemical and baking properties of flour. Cereal Chem. 2006, 83, 62–68. [Google Scholar] [CrossRef]
- Gütle, D.D.; Roret, T.; Hecker, A.; Reski, R.; Jacquot, J.P. Dithiol disulphide exchange in redox regulation of chloroplast enzymes in response to evolutionary and structural constraints. Plant Sci. 2017, 255, 1–11. [Google Scholar] [CrossRef]
- Gelhaye, E.; Rouhier, N.; Navrot, N.; Jacquot, J.P. The plant thioredoxin system. Cell Mol. Life Sci. 2005, 62, 24–35. [Google Scholar] [CrossRef]
- Geigenberger, P.; Thormählen, I.; Daloso, D.M.; Fernie, A.R. The Unprecedented Versatility of the Plant Thioredoxin System. Trends Plant Sci. 2017, 22, 249–262. [Google Scholar] [CrossRef]
- Montrichard, F.; Alkhalfioui, F.; Yano, H.; Vensel, W.H.; Hurkman, W.J.; Buchanan, B.B. Thioredoxin targets in plants: The first 30 years. J. Proteom. 2009, 72, 452–474. [Google Scholar] [CrossRef]
- Hägglund, P.; Bunkenborg, J.; Maeda, K.; Svensson, B. Identification of thioredoxin targets using a quantitative proteomics approach based on isotope-coded affinity tags—The ICAT switch. J. Proteom. Res. 2008, 7, 5270–5276. [Google Scholar] [CrossRef]
- Del Val, G.; Yee, B.C.; Lozano, R.M.; Buchanan, B.B. Thioredoxin treatment increases digestibility and lowers allergenicity of milk. J. Allergy Clin. Immunol. 1999, 103, 690–697. [Google Scholar] [CrossRef]
- Li, Y.C.; Ren, J.P.; Cho, M.J.; Zhou, S.M.; Kim, Y.B.; Guo, H.X.; Wong, J.H.; Niu, H.B.; Kim, H.K.; Morigasaki, S.; et al. The level of expression of thioredoxin is linked to fundamental properties and applications of wheat seeds. Mol. Plant 2009, 2, 430–441. [Google Scholar] [CrossRef]
- Maeda, K.; Hägglund, P.; Finnie, C.; Svensson, B.; Henriksen, A. Structural basis for target protein recognition by the protein disulfide reductase thioredoxin. Structure 2006, 14, 1701–1710. [Google Scholar] [CrossRef] [PubMed]
- Kirkensgaard, K.; Hägglund, P.; Finnie, C.; Svensson, B.; Henriksen, A. Crystal structure of Hordeum vulgare NADPH-dependent thioredoxin reductase 2. Unwinding the reaction mechanism. Acta Crystallogr. D 2009, 65, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Shahpiri, A.; Svensson, B.; Finnie, C. The NADPH-dependent thioredoxin reductase/thioredoxin system in germinating barley seeds: Gene expression, protein profiles, and interactions between isoforms of thioredoxin h and thioredoxin reductase. Plant Physiol. 2008, 146, 789–799. [Google Scholar] [CrossRef]
- Cecere, F.; Iuliano, A.; Albano, F.; Zappelli, C.; Castellano, I.; Grimaldi, P.; Masullo, M.; De Vendittis, E.; Ruocco, M.R. Diclofenac-induced apoptosis in the neuroblastoma cell line SH-SY5Y: Possible involvement of the mitochondrial superoxide dismutase. J. Biomed. Biotechnol. 2010, 2010, 801726–801737. [Google Scholar] [CrossRef] [PubMed]
- Andoh, T.; Chock, P.B.; Chiueh, C.C. The roles of thioredoxin in protection against oxidative stress-induced apoptosis in SH-SY5Y cells. J. Biol. Chem. 2002, 277, 9655–9660. [Google Scholar] [CrossRef] [PubMed]
- Das, K.C.; Lewis-Molock, Y.; White, C.W. Elevation of manganese superoxide dismutase gene expression by thioredoxin. Am. J. Respir. Cell Mol. Biol. 1997, 17, 713–726. [Google Scholar] [CrossRef] [PubMed]
- Orumets, K.; Kevvai, K.; Nisamedtinov, I.; Tamm, T.; Paalme, T. YAP1 over-expression in Saccharomyces cerevisiae enhances glutathione accumulation at its biosynthesis and substrate availability levels. Biotechnol. J. 2012, 7, 566–568. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.M.; Hägglund, P.; Christensen, H.E.M.; Svensson, B. Inactivation of barley limit dextrinase inhibitor by thioredoxin-catalysed disulfide reduction. FEBS Lett. 2012, 586, 2479–2482. [Google Scholar] [CrossRef] [PubMed] [Green Version]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navrot, N.; Buhl Holstborg, R.; Hägglund, P.; Povlsen, I.L.; Svensson, B. New Insights into the Potential of Endogenous Redox Systems in Wheat Bread Dough. Antioxidants 2018, 7, 190. https://doi.org/10.3390/antiox7120190
Navrot N, Buhl Holstborg R, Hägglund P, Povlsen IL, Svensson B. New Insights into the Potential of Endogenous Redox Systems in Wheat Bread Dough. Antioxidants. 2018; 7(12):190. https://doi.org/10.3390/antiox7120190
Chicago/Turabian StyleNavrot, Nicolas, Rikke Buhl Holstborg, Per Hägglund, Inge Lise Povlsen, and Birte Svensson. 2018. "New Insights into the Potential of Endogenous Redox Systems in Wheat Bread Dough" Antioxidants 7, no. 12: 190. https://doi.org/10.3390/antiox7120190
APA StyleNavrot, N., Buhl Holstborg, R., Hägglund, P., Povlsen, I. L., & Svensson, B. (2018). New Insights into the Potential of Endogenous Redox Systems in Wheat Bread Dough. Antioxidants, 7(12), 190. https://doi.org/10.3390/antiox7120190




