Effect of Extract and Ellagic Acid from Geranium schiedeanum on the Antioxidant Defense System in An Induced-Necrosis Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of G. schiedeanum Extract
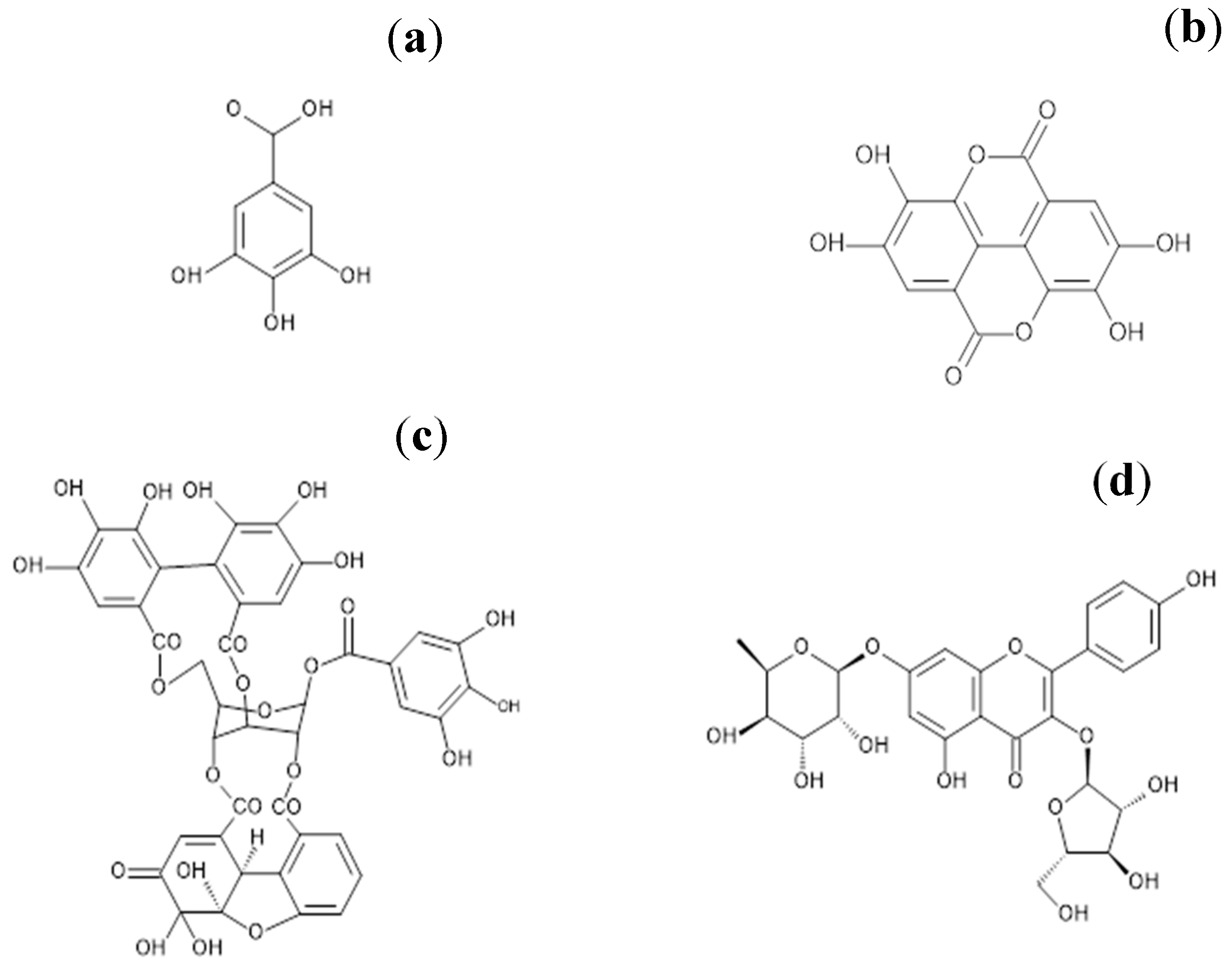
2.2. Purification of the Main Active Ingredients of G. schiedeanum
2.3. Animals and Treatment with the Extract of G. schiedeanum and Active Metabolites
2.4. Determination of Antioxidant Enzymes by Western Blot
2.5. Determination of Reduced Glutathione/Oxidized Glutathione (GSH/GSSG)
2.6. Sample Processing
2.7. Determination in Serum of Aspartate Aminotransferase (AST), Alanine Aminotransferase (ALT), and Total Bilirubin (BILT)
2.8. Statistical Analysis
3. Results and Discussion
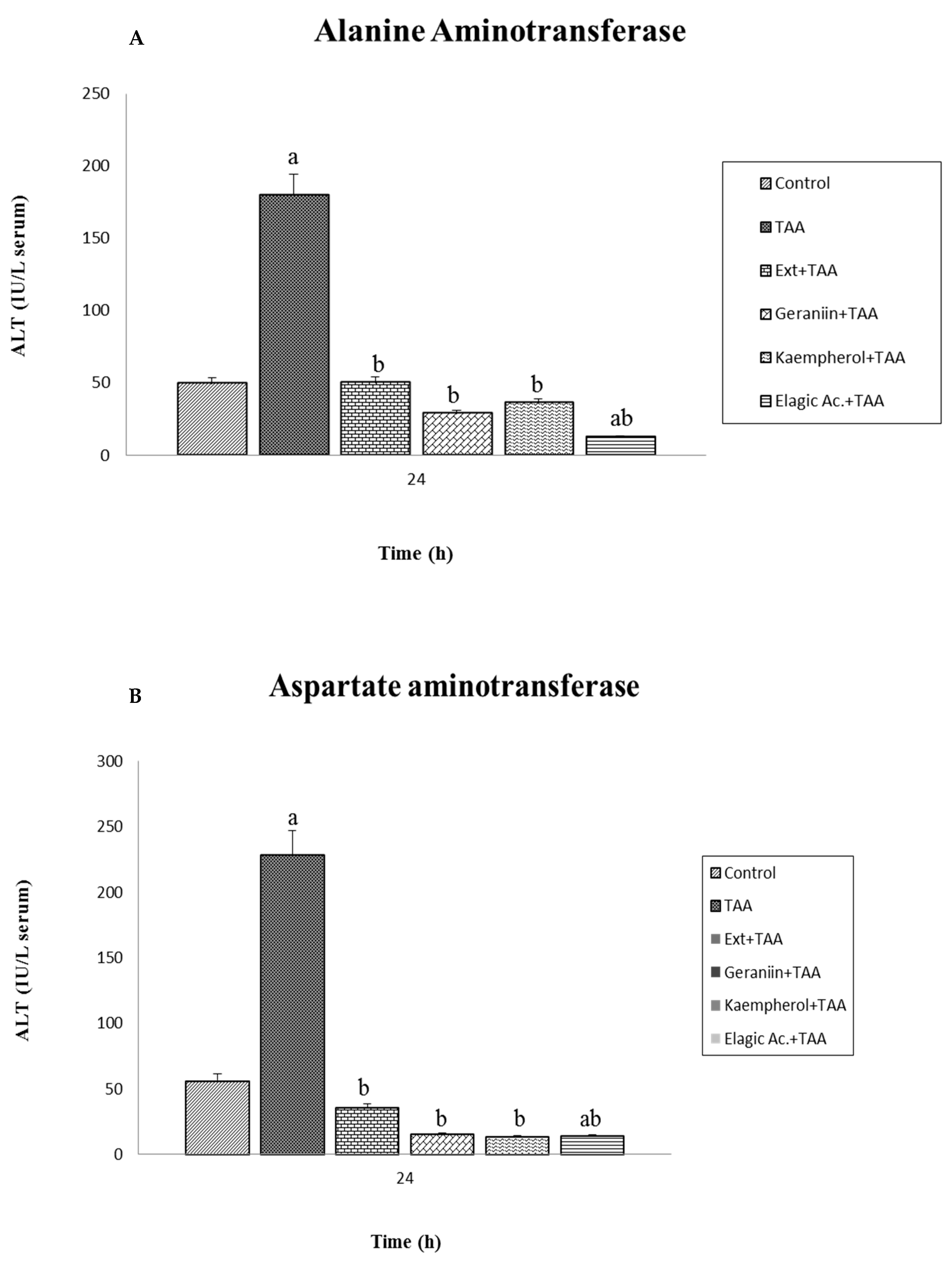
3.1. Effect of Pretreatment with G. schiedeanum Extract on EADS Enzymes in Rat Liver after Intoxication with TAA
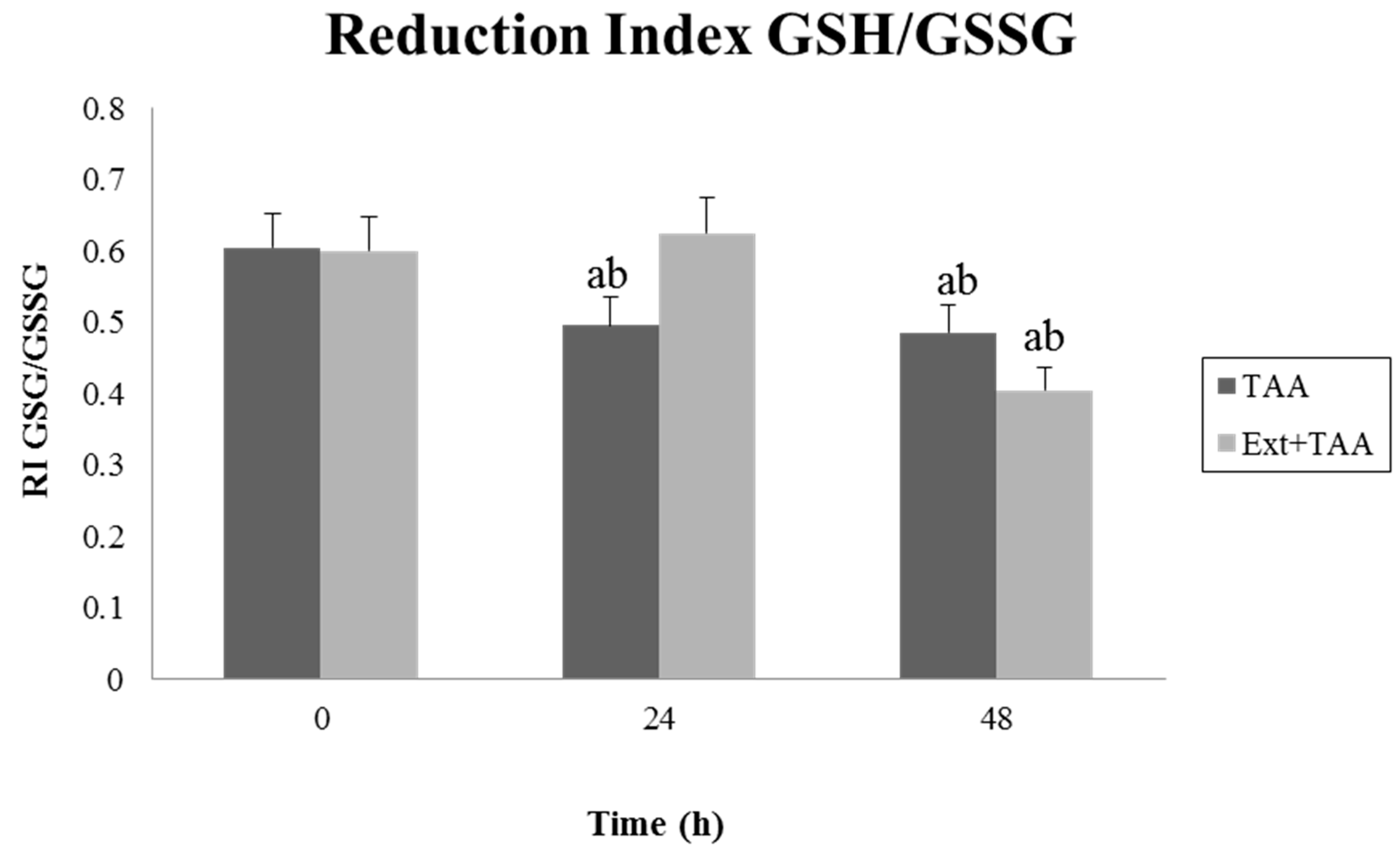
3.2. Effect of Pretreatment with the Extract of G. schiedeanum on the GSH/GSSG Reduction Index (RI) in TAA-Intoxicated Rats
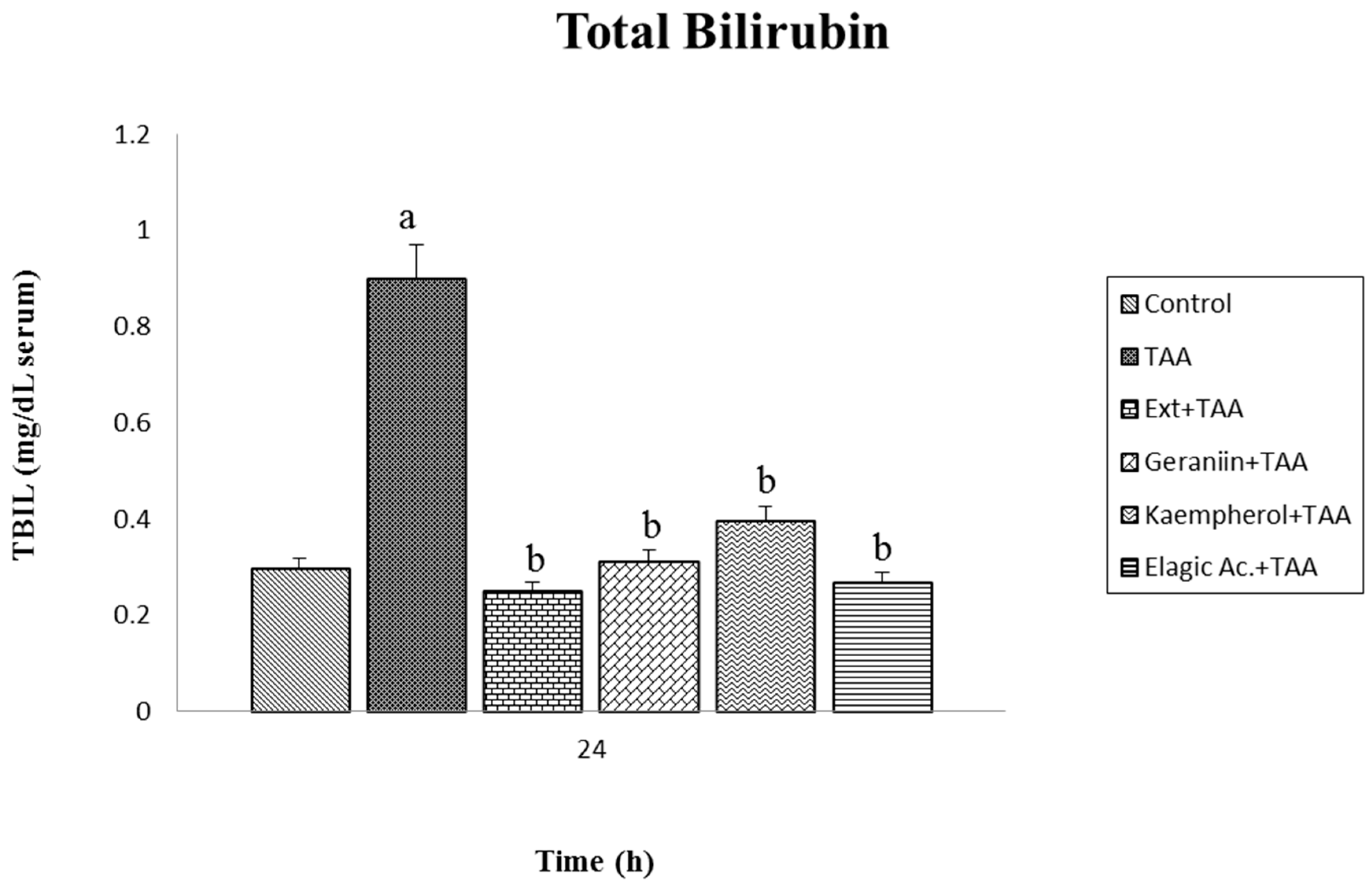
3.3. Effect of Pretreatment G. schiedeanum Extract and Active Metabolites in liver Damage
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chilakapati, J.; Korrapati, M.C.; Hill, R.A.; Warbritton, A.; Latendresse, J.R.; Mehendale, H.M. Toxicokinetics and toxicity of thioacetamide sulfoxide: A metabolite of thioacetamide. Toxicology 2007, 230, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.L.; Abdulla, M.A.; Chua, K.H.; Kuppusamy, U.R.; Tan, Y.S.; Sabaratnam, V. Hepatoprotective Effects of Panus giganteus (Berk.) Corner against Thioacetamide-(TAA-) Induced Liver Injury in Rats. Evid. Based Complement. Altern. Med. 2012, 2012, 170303. [Google Scholar] [CrossRef] [PubMed]
- Miliauskas, G.; Mulder, E.; Linssen, J.P.; Houben, J.H.; van Beek, T.A.; Venskutonis, P.R. Evaluation of antioxidative properties of Geranium macrorrhizum and Potentilla fruticosa extracts in Dutch style fermented sausages. Meat Sci. 2007, 77, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Miguel, O.G.; Calixto, J.B.; Santos, A.R.; Messana, I.; Ferrari, F.; Cechinel Filho, V.; Pizzolatti, M.G.; Yunes, R.A. Chemical and preliminary analgesic evaluation of geraniin and furosin isolated from Phyllanthus sellowianus. Planta Med. 1996, 62, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Toshkova, R.; Nikolova, N.; Ivanova, E.; Ivancheva, S.; Serkedjieva, J. In vitro investigation on the effect of a plant preparation with antiviral activity on the functions of mice phagocyte cells. Die Pharm. 2004, 59, 150–154. [Google Scholar]
- Shim, J.U.; Oh, P.S.; Lim, K.T. Anti-inflammatory activity of ethanol extract from Geranium sibiricum Linne. J. Ethnopharmacol. 2009, 126, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Gayosso-De-Lucio, J.; Torres-Valencia, M.; Rojo-Domínguez, A.; Nájera-Peña, H.; Aguirre-López, B.; Salas-Pacheco, J.; Avitia-Domínguez, C.; Téllez-Valencia, A. Selective inactivation of triosephosphate isomerase from Trypanosoma cruzi by brevifolin carboxylate derivatives isolated from Geranium bellum Rose. Bioorg. Med. Chem. Lett. 2009, 19, 5936–5939. [Google Scholar] [CrossRef] [PubMed]
- Gayosso-De-Lucio, J.; Bautista, M.; Velazquez-Gonzalez, C.; De la O Arciniega, M.; Morales-Gonzalez, J.A.; Benedi, J. Chemical composition and hepatotoxic effect of Geranium schiedeanum in a thioacetamide-induced liver injury model. Pharmacogn. Mag. 2014, 10, S574–S580. [Google Scholar] [CrossRef] [PubMed]
- Morales-González, A.; Bautista, M.; Mandrigal-Santillán, E.; Posadas-Mondragón, A.; Anguiano-Robledo, L.; Mandrigal-Bajaidar, E.; Álvarez-González, I.; Fregoso-Aguilar, T.; Gayosso-Islas, E.; Sánchez-Moreno, C.; et al. Nrf2 modulates cell proliferation and antioxidants defenses during liver regeneration induced by partial hepatectomy. Int. J. Clin. Exp. Pathol. 2017, 10, 7801–7811. [Google Scholar]
- Vázquez-Velasco, M.; González-Torres, L.; López-Gasco, P.; Bastida, S.; Benedí, J.; Sánchez-Reus, M.I.; González-Muñoz, M.J.; Sánchez-Muniz, F.J. Liver oxidation and inflammation in Fa/Fa rats fed glucomannan/spirulina-surimi. Food Chem. 2014, 159, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Rej, R.; Horder, M. Aspartate aminotransferase. l-aspartate: 2-oxoglutarate aminotranferase, EC 2.6.2.1. Routine U.V. method. In Methods of Enzymatic Analysis; Verlag-Chemie: Weinheim, Germany, 1984; pp. 416–424. [Google Scholar]
- Murray, R. Alanine aminotransferase. In Clinical Chemistry: Theory, Analysis, and Correlation, 2nd ed.; Kaplan, A., Pesce, A.J., Eds.; Princeton: St Louis, MO, USA; Toronto, ON, Canada, 1984; pp. 1088–1090. [Google Scholar]
- Martinek, R.G. Improved micro-method for determination of serum bilirubin. Clin. Chim. Acta 1966, 13, 161–170. [Google Scholar] [CrossRef]
- Li, S.; Tan, H.Y.; Wang, N.; Zhang, Z.J.; Lao, L.; Wong, C.W.; Feng, Y. The role of oxidative Stress and antioxidants in liver diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. Regulation of glutathione synthesis. Mol. Aspects Med. 2009, 30, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Mari, M.; Morales, A.; Colell, A.; Garcia-Ruiz, C.; Fernandez-Checa, J.C. Mitochondrial glutathione, a key survival antioxidant. Antioxid. Redox. Signal 2009, 11, 2685–2700. [Google Scholar] [CrossRef] [PubMed]
- Mato, J.M.; Corrales, F.J.; Lu, S.C.; Avila, M.A. S-Adenosylmethionine: A control switch that regulates liver function. FASEB J. 2002, 16, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Mikstacka, R.; Gnojkowski, J.; Baer-Dubowska, W. Effect of natural phenols on the catalytic activity of cytochrome P450 2E1. Acta Biochim. Pol. 2002, 49, 917–925. [Google Scholar] [PubMed]
- Qureshi, N.; Kuchekar, B.; Logade, N.; Haleem, M. Antioxidant and hepatoprotective activity of Cordia macleodii Leaves. Saudi Pharm. J. 2009, 17, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, G.R.; Naso, D.; Cangeri, F.; Porawski, M.; Marcolin, É.; Kretzmann, N.A.; Ferraz, A.D.; Richter, M.F.; Marroni, C.A.; Marroni, N.P. Treatment with aqueous extract from Croton cajucara Benth reduces hepatic oxidative stress in streptozotocin-diabetic rats. J. Biomed. Biotechnol. 2012, 2012, 902351. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Henning, S.M.; Zhang, Y.; Suchard, M.; Li, Z.; Heber, D. Pomegranate juice ellagitannin metabolites are present in human plasma and some persist in urine for up to 48 hours. J. Nutr. 2006, 136, 2481–2485. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.D.; Rodrigues, A.S.; Gaspar, J.; Maia, R.; Laires, A.; Rueff, J. Involvement of rat cytochrome 1A1 in the biotransformation of kaempferol to quercetin: Relevance to the genotoxicity of kaempferol. Mutagenesis 1997, 12, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Syamasundar, K.V.; Singh, B.; Thakur, R.S.; Husain, A.; Kiso, Y.; Hikino, H. Antihepatotoxic principles of Phyllanthus niruri herbs. J. Ethnopharmacol. 1985, 14, 41–44. [Google Scholar] [CrossRef]
- Tatsimo, S.J.; de Dieu Tamokou, J.; Havyarimana, L.; Csupor, D.; Forgo, P.; Hohmann, J.; Kuiate, J.R.; Tane, P. Antimicrobial and antioxidant activity of kaempferol rhamnoside derivatives from Bryophyllum pinnatum. BMC Res. Notes 2012, 5, 158. [Google Scholar] [CrossRef] [PubMed]
- Pérez Escandón, B.E.; Villavicencio Nieto, M.Á.; Ramírez Aguirre, A. Lista de las Plantas útiles del Estado de Hidalgo; Universidad Autónoma del Estado de Hidalgo: Pachuca, Mexico, 2003; ISBN 970-769-017-8. [Google Scholar]
- Walle, U.K.; Walle, T. Bioavailable flavonoids: Cytochrome P450-mediated metabolism of methoxyflavones. Drug Metab. Dispos. 2007, 35, 1985–1989. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Zhao, B.; Waterman, M.R. Moonlighting cytochrome P450 monooxygenases. IUBMB Life 2011, 63, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Aniya, Y.; Koyama, T.; Miyagi, C.; Miyahira, M.; Inomata, C.; Kinoshita, S.; Ichiba, T. Free radical scavenging and hepatoprotective actions of the medicinal herb, Crassocephalum crepidioides from the Okinawa Islands. Biol. Pharm. Bull. 2005, 28, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Harish, R.; Shivanandappa, T. Antioidant activity and hepatoprotective potential of Phyllantus niruri. Food Chem. 2006, 95, 180–185. [Google Scholar] [CrossRef]
- Amin, Z.A.; Bilgen, M.; Alshawsh, M.A.; Ali, H.M.; Hadi, A.H.; Abdulla, M.A. Protective Role of Phyllanthus niruri Extract against Thioacetamide-Induced Liver Cirrhosis in Rat Model. Evid. Based Complement. Altern. Med. 2012, 2012, 241583. [Google Scholar] [CrossRef]
- Girish, C.; Pradhan, S.C. Hepatoprotective activities of picroliv, curcumin, and ellagic acid compared to silymarin on carbon-tetrachloride-induced liver toxicity in mice. J. Pharmacol. Pharmacother. 2012, 3, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Morales-González, J.A.; Mandrigal-Santillán, E.; Morales-González, A.; Bautista, M.; Gayosso-Islas, E.; Sánchez-Moreno, C. What is Known Regarding the Participation of Factor Nrf-2 in Liver Regeneration? Cells 2015, 4, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Keum, Y.S.; Choi, B.Y. Molecular and chemical regulation of the Keap1-Nrf2 signaling pathway. Molecules 2014, 19, 100074–100089. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Shuen, T.; Lou, H. Dietary poliphenols and their biological significance. Int. J. Mol. Sci. 2007, 8, 950–988. [Google Scholar] [CrossRef]
- Xiong, Z.E.; Dong, W.G.; Wang, B.Y.; Tong, Q.Y.; Li, Z.Y. Curcumin attenuates chronic ethanol-induced liver injury by inhibition of oxidative stress via mitogen-activated protein kinase/nuclear factor E2-related factor 2 pathway in mice. Pharmacogn. Mag. 2015, 11, 707–715. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas-Mendoza, N.; Vázquez-Velasco, M.; González-Torres, L.; Benedí, J.; Sánchez-Muniz, F.J.; Morales-González, J.A.; Jaramillo-Morales, O.A.; Valadez-Vega, C.; Bautista, M. Effect of Extract and Ellagic Acid from Geranium schiedeanum on the Antioxidant Defense System in An Induced-Necrosis Model. Antioxidants 2018, 7, 178. https://doi.org/10.3390/antiox7120178
Vargas-Mendoza N, Vázquez-Velasco M, González-Torres L, Benedí J, Sánchez-Muniz FJ, Morales-González JA, Jaramillo-Morales OA, Valadez-Vega C, Bautista M. Effect of Extract and Ellagic Acid from Geranium schiedeanum on the Antioxidant Defense System in An Induced-Necrosis Model. Antioxidants. 2018; 7(12):178. https://doi.org/10.3390/antiox7120178
Chicago/Turabian StyleVargas-Mendoza, Nancy, Miguel Vázquez-Velasco, Laura González-Torres, Juana Benedí, Francisco José Sánchez-Muniz, Jose Antonio Morales-González, Osmar Antonio Jaramillo-Morales, Carmen Valadez-Vega, and Mirandeli Bautista. 2018. "Effect of Extract and Ellagic Acid from Geranium schiedeanum on the Antioxidant Defense System in An Induced-Necrosis Model" Antioxidants 7, no. 12: 178. https://doi.org/10.3390/antiox7120178
APA StyleVargas-Mendoza, N., Vázquez-Velasco, M., González-Torres, L., Benedí, J., Sánchez-Muniz, F. J., Morales-González, J. A., Jaramillo-Morales, O. A., Valadez-Vega, C., & Bautista, M. (2018). Effect of Extract and Ellagic Acid from Geranium schiedeanum on the Antioxidant Defense System in An Induced-Necrosis Model. Antioxidants, 7(12), 178. https://doi.org/10.3390/antiox7120178





