Possible Reactions of Dietary Phenolic Compounds with Salivary Nitrite and Thiocyanate in the Stomach
Abstract
:1. Introduction
2. Reactions of Phenolic Compounds with Nitrite
2.1. Nitric Oxide (•NO) Formation
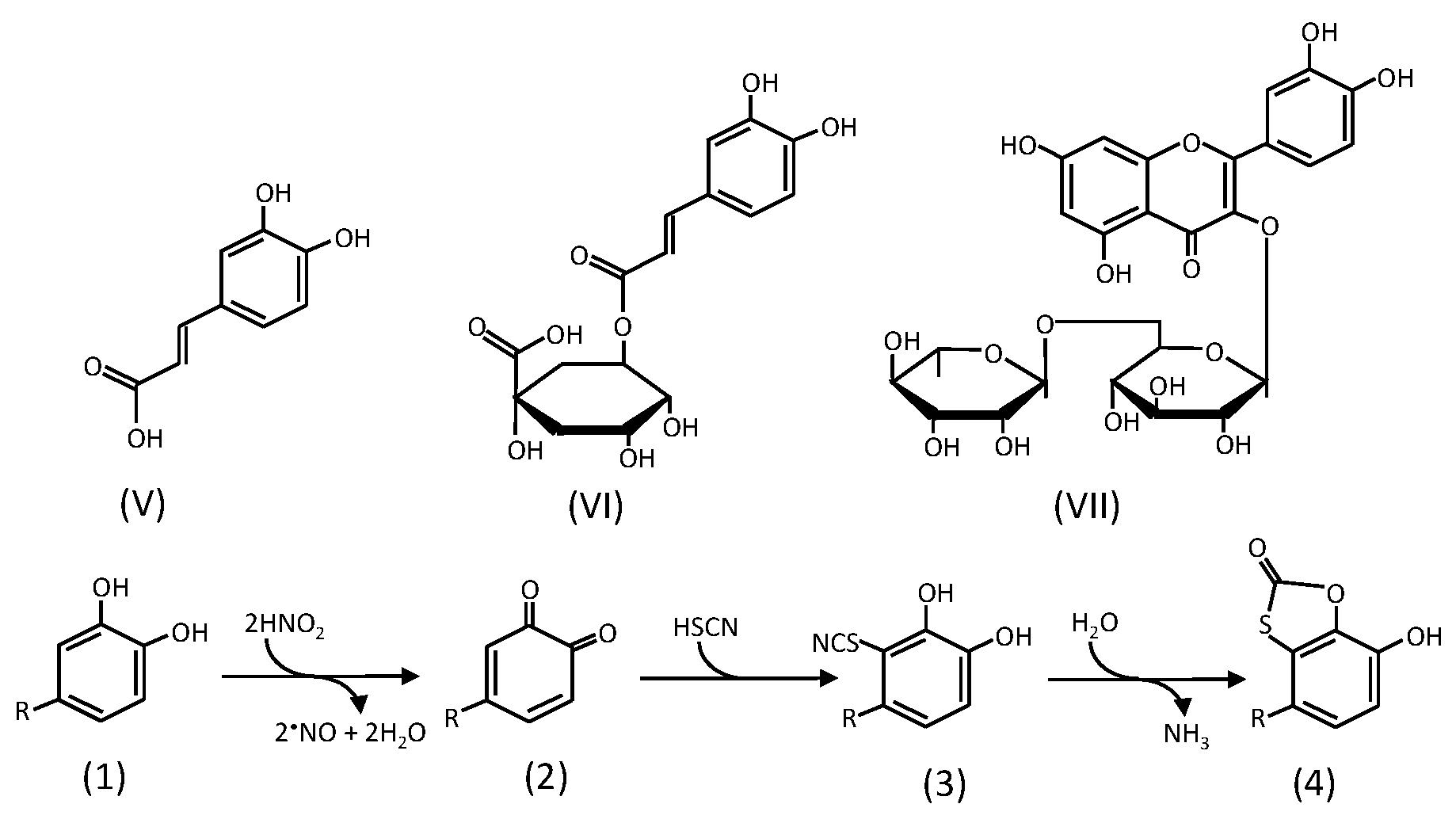
2.2. Formation of Oxathiolone Derivatives
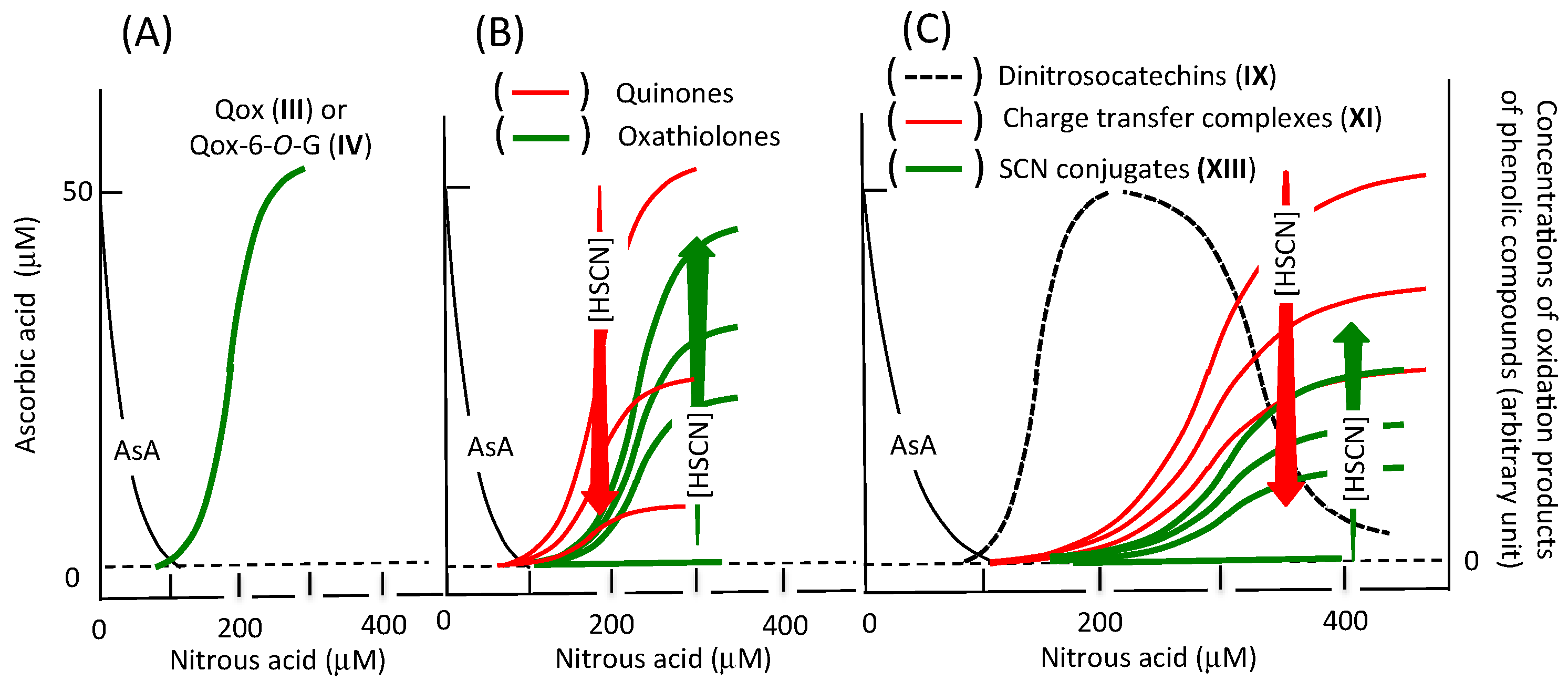
2.3. Reactions of Catechins with Nitrous Acid
2.4. Interactions of Floavonoids with Starch
3. Summarization of the Reactions in Nitrous Acid-Flavonoid Systems
Acknowledgments
Conflicts of Interest
References
- Ferreyra, M.L.F.; Sebastián, P.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Samanta, A.; Das, G.; Das, S.K. Role of flavonoids in plants. Int. J. Pharm. Sci. Tech. 2011, 6, 12–35. [Google Scholar]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.H.; Jiang, Y.M.; Shi, J.; Tomás-Barbera N, F.A.; Datta, N.; Singanusong, R.; Chen, S.S. Flavonoids in food and their health benefits. Plant Foods Hum. Nutr. 2004, 59, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Pirouzpanah, S.; Hanasee, J.; Razavieh, S.-V.; Rashidi, M.-R. Inhibitory effects of flavonoids on aldehyde oxidase activity. J. Enzym. Inhib. Med. Chem. 2009, 24, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Takahama, U.; Ansai, T.; Hirota, S. Nitrogen oxides toxicology of the aerodigestive tract. In Advances in Molecular Toxicology; Fishbein, J.C., Heilman, J.M., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2013; Volume 7, pp. 129–177. [Google Scholar]
- Duncan, C.; Dougall, H.; Johnston, P.; Green, S.; Brogan, R.; Leifert, C.; Smith, L.; Golden, M.; Benjamin, N. Chemical generation of nitric oxide in the mouth from the enterosalivary circulation of dietary nitrate. Nat. Med. 1995, 1, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Doel, J.J.; Benjamin, N.; Hector, M.P.; Rogers, M.; Allaker, R.P. Evaluation of bacterial nitrate reduction in the human oral cavity. Eur. J. Oral Sci. 2005, 113, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Wainright, T. The chemistry of nitrosoamine formation: Relevance to malting and brewing. J. Inst. Brewing 1986, 92, 49–64. [Google Scholar] [CrossRef]
- Takahama, U.; Oniki, T.; Hirota, S. Oxidation of quercetin by salivary components. Quercetin-dependent reduction of salivary nitrite under acidic conditions producing nitric oxide. J. Agric. Food Chem. 2002, 50, 4317–4322. [Google Scholar] [CrossRef] [PubMed]
- Peri, L.; Pietraforte, D.; Scorza, G.; Napolitano, A.; Fogliano, V.; Minetti, M. Apples increase nitric oxide production by human saliva at acidic pH of the stomach. Free Radic. Biol. Med. 2005, 39, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Whittle, B.J.; Lopez-Belmonte, J.; Moncada, S. Regulation of gastric mucosal integrity by endogenous nitric oxide: Interactions with prostanoids and sensory neuropeptides in the rat. Br. J. Pharmacol. 1990, 99, 607–611. [Google Scholar] [CrossRef]
- Björne, H.; Petersson, J.; Phillipson, M.; Weitzberg, E.; Holm, L.; Lundberg, J.O. Nitrite in saliva increases gastric mucosal blood flow and mucus thickness. J. Clin. Investig. 2004, 113, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Rocha, B.S.; Gago, B.; Barbosa, R.M.; Laranjinha, J. Dietary polyphenols generate nitric oxide from nitrite in the stomach and induce smooth muscle relaxation. Toxicology 2009, 265, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.; Ferreira, N.R.; Rocha, B.S.; Barbosa, R.M.; Laranjinha, J. The redox interplay between nitrite and nitric oxide: From the gut to the brain. Redox Biol. 2013, 1, 276–284. [Google Scholar] [CrossRef]
- Machha, A.; Schechter, A.N. Dietary nitrite and nitrate: A review of potential mechanisms of cardiovascular benefits. Eur. J. Nutr. 2011, 50, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Weitzberg, E.; Galdwin, M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Cosby, K.; Partovi, K.S.; Crawford, J.H.; Patel, R.P.; Reiter, C.D.; Martyr, S.; Yang, B.K.; Waclawiw, M.A.; Zalos, G.; Xu, X.; et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat. Med. 2003, 9, 1498–1505. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.J.; Patel, N.; Loukogeorgakis, S.; Okorie, M.; Aboud, Z.; Misra, S.; Rashid, R.; Miall, P.; Deanfield, J.; Benjamin, N.; et al. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension 2008, 51, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Kapil, V.; Milsom, A.B.; Okorie, M.; Maleki-Toyserkani, S.; Akram, F.; Rehman, F.; Arghandawi, S.; Pearl, V.; Benjamin, N.; Loukogeorgakis, S.; et al. Inorganic nitrate supplementation lowers blood pressure in humans. Role for nitrite-derived NO. Hypertension 2010, 56, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Apostoli, G.L.; Solomon, A.; Smallwood, M.J.; Winyard, P.G.; Emerson, M. Role of inorganic nitrate and nitrite in driving nitric oxide-cGMP-mediated inhibition of platelet aggregation in vitro and in vivo. J. Thromb. Haemost. 2014, 12, 1880–1889. [Google Scholar] [CrossRef] [PubMed]
- Kadan, M.; Doğanci, S.; Yildirim, V.; Özgür, G.; Erol, G.; Karabacak, K.; Avcu, F. In vitro effect of sodium nitrite on platelet aggregation in human platelet rich plasma—Preliminary report. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 3935–3939. [Google Scholar] [PubMed]
- Napoli, C.; Paolisso, G.; Casamassimi, A.; Al-Omran, M.; Barbieri, M.; Sommese, L.; Infante, T.; Ignarro, L.J. Effects of nitric oxide on cell proliferation. J. Am. Coll. Cardiol. 2013, 62, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Jeremy, J.Y.; Rowe, D.; Emsley, A.M.; Newby, A.C. Nitric oxide and the proliferation of vascular smooth muscle cells. Cardiovasc. Res. 1999, 43, 580–594. [Google Scholar] [CrossRef]
- Ling, W.C.; Murugan, D.D.; Yeh Siang Lau, Y.S.; Vanhoutte, P.M.; Mustafa, M.R. Sodium nitrite exerts an antihypertensive effect and improves endothelial function through activation of eNOS in the SHR. Sci. Rep. 2016, 6, 33048. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, N.; O’Driscoll, F.; Dougall, H.; Duncan, C.; Smith, L.; Golden, M.; McKenzie, H. Stomach NO synthesis. Nature 1994, 368, 502. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.N.; Weitzberg, E.; Lundberg, J.K.; Alving, K. Intragastric nitric oxide production in humans: Measurements in expelled air. Gut 1994, 35, 1543–1546. [Google Scholar] [CrossRef] [PubMed]
- McKnight, G.M.; Smith, L.M.; Drummond, R.S.; Golden, M.; Benjamin, N. Chemical synthesis of nitric oxide in the stomach from dietary nitrite in humans. Gut 1997, 40, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Lidder, S.; Webb, A.J. Vascular effects of dietary nitrate (as found in green leafy vegetables and beetroot) via the nitrate-nitrite-nitric oxide pathway. Br. J. Clin. Pharmacol. 2013, 75, 677–696. [Google Scholar] [CrossRef] [PubMed]
- Zweier, J.L.; Samouilov, A.; Kuppusamy, P. Non-enzymatic nitric oxide synthesis in biological systems. Biochim. Biophys. Acta 1999, 1411, 250–262. [Google Scholar] [CrossRef]
- Iijima, K.; Fyfe, V.; McColl, K.E. Studies of nitric oxide generation from salivary nitrite in human gastric juice. Scand. J. Gastroenterol. 2003, 38, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Takahama, U.; Tanaka, M.; Oniki, T.; Hirota, S.; Yamauchi, R. Formation of the thiocyanate conjugate of chlorogenic acid in coffee under acidic conditions in the presence of thiocyanate and nitrite: Possible occurrence in the stomach. J. Agric. Food Chem. 2007, 55, 4169–4176. [Google Scholar] [CrossRef] [PubMed]
- Gago, B.; Lundberg, J.O.; Barbosa, R.M.; Laranjinha, J. Red wine-dependent reduction of nitrite to nitric oxide in the stomach. Free Radic. Biol. Med. 2007, 43, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Takahama, U.; Tanaka, M.; Hirota, S. Formation of nitric oxide, ethyl nitrite and an oxathiolone derivative of caffeic acid in a mixture of saliva and white wine. Free Radic. Res. 2010, 44, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Takahama, U.; Tanaka, M.; Hirota, S. Proanthocyanidins in buckwheat flour can reduce salivary nitrite to nitric oxide in the stomach. Plant Foods Hum. Nutr. 2010, 65, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Takahama, U.; Yamamoto, A.; Hirota, S.; Oniki, T. Quercetin-dependent reduction of salivary nitrite to nitric oxide under acidic conditions and interaction between quercetin and ascorbic acid during the reduction. J. Agric. Food Chem. 2003, 51, 6014–6020. [Google Scholar] [CrossRef] [PubMed]
- Takahama, U.; Tanaka, M.; Hirota, S. Interaction between ascorbic acid and chlorogenic acid during the formation of nitric oxide in acidified saliva. J. Agric. Food Chem. 2008, 56, 10406–10413. [Google Scholar] [CrossRef] [PubMed]
- Waring, A.J.; Drake, I.M.; Schorah, C.J.; White, K.L.; Lynch, D.A.; Axon, A.T.; Dixon, M.F. Ascorbic acid and total vitamin C concentrations in plasma, gastric juice, and gastrointestinal mucosa: Effects of gastritis and oral supplementation. Gut 1996, 38, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Rathbone, B.J.; Johnson, A.W.; Wyatt, J.I.; Kelleher, J.; Heatley, R.V.; Losowsky, M.S. Ascorbic acid: A factor concentrated in human gastric juice. Clin. Sci. 1989, 76, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.W.; Patchett, S.E.; Perrett, D.; Katelaris, P.H.; Domizio, P.; Farthing, M.J.G. The relation between gastric vitamin C concentrations, mucosal histology, and CagA seropositivity in the human stomach. Gut 1998, 43, 322–326. [Google Scholar] [CrossRef]
- Ly, T.N.; Hazama, C.; Shimoyamada, M.; Ando, H.; Kato, K.; Yamauchi, R. Antioxidative compounds from the outer scale of onion. J. Agric. Food Chem. 2005, 53, 8183–8189. [Google Scholar] [CrossRef] [PubMed]
- Veljovic-Jovanovic, S.; Morina, F.; Yamauchi, R.; Hirota, S.; Takahama, U. Interactions between (+)-catechin and quercetin during their oxidation by nitrite under the conditions simulating the stomach. J. Agric. Food Chem. 2014, 62, 4951–4959. [Google Scholar] [CrossRef] [PubMed]
- Takahama, U.; Yamauchi, R.; Hirota, S. Antioxidative flavonoids in adzuki-meshi (rice boiled with adzuki bean) react with nitrite under simulated stomach conditions. J. Funct. Food 2016, 26, 657–666. [Google Scholar] [CrossRef]
- Schreier, P.; Miller, E. Studies on flavonol degradation by peroxidase (donor: H2O2-oxidoreductase, EC 1.11.1.7): Part 2—Quercetin. Food Chem. 1985, 18, 301–317. [Google Scholar] [CrossRef]
- Hirota, S.; Takahama, U.; Ly, T.N.; Yamauchi, R. Quercetin-dependent inhibition of nitration induced by peroxidase/H2O2/nitrite systems in human saliva and characterization of the oxidation product formed during the inhibition. J. Agric. Food Chem. 2005, 53, 3265–3272. [Google Scholar] [CrossRef]
- Dufour, C.; Loonis, M. Flavonoids and their oxidation products protect efficiently albumin-bound linoleic acid in a model of plasma oxidation. Biochim. Biophys. Acta 2007, 1770, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Timbola, A.K.; de Souza, C.D.; Giacomelli, C.; Spinelli, A. Electrochemical oxidation of quercetin in hydro-alcoholic solution. J. Braz. Chem. Soc. 2006, 17, 139–148. [Google Scholar] [CrossRef]
- Ramos, F.A.; Takaishi, Y.; Shirotori, M.; Kawaguchi, Y.; Tsuchiya, K.; Shibata, H.; Higuti, T.; Tadokoro, T.; Takeuchi, M. Antibacterial and antioxidant activities of quercetin oxidation products from yellow onion (Allium cepa) skin. J. Agric. Food Chem. 2006, 54, 3551–3557. [Google Scholar] [CrossRef]
- Takahama, U.; Hirota, S. Deglucosidation of quercetin glucosides to the aglycone and formation of antifungal agents by peroxidase-dependent oxidation of quercetin on browning of onion scales. Plant Cell Physiol. 2000, 41, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Morina, F.; Takahama, U.; Yamauchi, R.; Hirota, S.; Veljovic-Jovanovic, S. Quercetin 7-O-glucoside suppresses nitrite-induced formation of dinitrosocatechins and their quinones in catechin/nitrite systems under stomach simulating conditions. Food Funct. 2015, 6, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Takahama, U. Spectrophotometric study on the oxidation of rutin by horseradish peroxidase and characteristics of the oxidized products. Biochim. Biophys. Acta 1986, 882, 445–451. [Google Scholar] [CrossRef]
- Takahama, U.; Tanaka, M.; Hirota, S.; Yamauchi, R. Formation of an oxathiolone compound from rutin in acidic mixture of saliva and buckwheat dough: Possibility of its occurrence in the stomach. Food Chem. 2009, 116, 214–219. [Google Scholar] [CrossRef]
- Moridani, M.Y.; Scobie, H.; Jamshidzadeh, A.; Salehi, P.; O’Brien, P.J. Caffeic acid, chlorogenic acid, and dihydrocaffeic acid metabolism: Glutathione conjugate formation. Drug Metab. Dispos. 2001, 29, 1432–1439. [Google Scholar] [PubMed]
- Brittner, S. When quinones meet amino acids: Chemical, physical and biological consequences. Amino Acids 2006, 30, 205–224. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 3rd ed.; Oxford University Press: Oxford, UK, 1999; pp. 564–572. [Google Scholar]
- Tsuge, K.; Kataoka, M.; Seto, Y. Cyanide and Thiocyanate levels in blood and saliva of healthy adult volunteers. J. Health Sci. 2000, 46, 343–350. [Google Scholar] [CrossRef]
- Konieczny, M.T.; Konieczny, W.; Sabisz, M.; Skladanowski, A.; Wakieć, R.; Augustynowicz-Kopeć, E.; Zwolska, Z. Synthesis of isomeric, oxathiolone fused chalcones, and comparison of their activity toward various microorganisms and human cancer cells line. Chem. Pharm. Bull. 2007, 55, 817–820. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Roh, E.; Lee, H.Y.; Lee, I.-J.; Ahn, B.; Jung, S.-H.; Lee, H.; Han, S.-B.; Kim, Y. Benzoxathiole derivative blocks lipopolysaccharide-induced nuclear factor-κB activation and nuclear factor-κB- regulated gene transcription through inactivating inhibitory κB kinase β. Mol. Pharmacol. 2008, 73, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Takahama, U.; Koga, Y.; Hirota, S.; Yamauchi, R. Inhibition of xanthine oxidase activity by an oxathiolanone derivative of quercetin. Food Chem. 2011, 126, 1808–1811. [Google Scholar] [CrossRef] [PubMed]
- Takahama, U.; Yamauchi, R.; Hirota, S. Reactions of (+)-catechin with salivary nitrite and thiocyanate under conditions simulating the gastric lumen: Production of dinitrosocatechin and its thiocyanate conjugate. Free Radic. Res. 2014, 48, 956–966. [Google Scholar] [CrossRef]
- Hirota, S.; Takahama, U. Reactions of apple fruit polyphenols with nitrite under conditions of the gastric lumen: Generation of nitric oxide and formation of nitroso catechins. Food Sci. Technol. Res. 2014, 20, 439–447. [Google Scholar] [CrossRef]
- Hirota, S.; Takahama, U. Reactions of polyphenols in masticated apple fruit with nitrite under stomach simulating conditions: Formation of nitroso compounds and thiocyanate conjugate. Food Res. Int. 2015, 75, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Panzella, L.; Manini, P.; Napolitano, A.; d’Ischia, M. The acid-promoted reaction of the green tea polyphenol epigallocatechin gallate with nitrite ions. Chem. Res. Toxicol. 2005, 18, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.H.; Munerol, B.; Pollard, S.; Youdim, K.A.; Pannala, A.S.; Kuhnle, G.G.C.; Debnam, E.S.; Rice-Evans, C.; Spencer, J.P.E. The reaction of flavanols with nitrous acid protects against N-nitrosamine formation and leads to the formation of nitroso derivatives which inhibit cancer cell growth. Free Radic. Biol. Med. 2006, 40, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, S.V.; Steeken, S.; Simic, M.G.; Hara, Y. Antioxidant properties of flavonoids: Reduction potentials and electron transfer reactions of flavonoid radicals. In Flavonoids in Health and Disease; Rice-Evans, C.A., Packer, L., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1988; pp. 137–161. [Google Scholar]
- Morina, F.; Takahama, U.; Mojović, M.; Popvić- Bijelić, A.; Veljović-Jovanivić, S. Formation of stable radicals in catechin/nitrous acid systems: Participation of dinitrosocatechin. Food Chem. 2016, 194, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Takahama, U.; Hirota, S. Effects of starch on nitrous acid-induced oxidation of kaempferol and inhibition of α-amylase-catalyzed digestion of starch by kaempferol under conditions simulating the stomach and the intestine. Food Chem. 2013, 141, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Takahama, U.; Yamauchi, R.; Hirota, S. Interactions of starch with a cyanidin-catechin pigment (vignacyanidin) isolated from Vigna angularis bean. Food Chem. 2013, 141, 2600–2605. [Google Scholar] [CrossRef] [PubMed]
- Takahama, U.; Yamauchi, R.; Hirota, S. Isolation and characterization of a cyanidin-catechin pigment from adzuki bean (Vigna angularis). Food Chem. 2013, 141, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Xu, Y.; Lu, Y.-H. Inhibitory effects of Citrus flavonoids on starch digestion and antihyperglycemic effects in HepG2 cells. J. Agric. Food Chem. 2012, 60, 9609–9619. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-P.; He, H.; Lu, Y.-H. Flour flavonoid compounds from Phyllostachys edulis leaf extract retard the digestion of starch and its working mechanisms. J. Agric. Food Chem. 2014, 62, 7760–7770. [Google Scholar] [CrossRef] [PubMed]
- Takahama, U.; Hirota, S. Fatty acids, epicatechin-dimethylgallate, and rutin interact with buckwheat starch inhibiting its digestion by amylase: Implication for the decrease in glycemic index by buckwheat flour. J. Agric. Food Chem. 2010, 58, 12431–12439. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Hayatsu, T.; Negishi, T.; Hayatsu, H. Inhibition of N-nitrosation of secondary amines in vitro by tea extracts and catechins. Mutat. Res. 1998, 412, 91–98. [Google Scholar] [CrossRef]
- Masuda, S.; Uchida, S.; Terashima, Y.; Kuramoto, H.; Serizawa, M.; Deguchi, Y.; Yanai, K.; Sugiyama, C.; Oguni, I.; Kinae, N. Effect of green tea on the formation of nitrosamines, and cancer mortality. J. Health Sci. 2006, 52, 211–220. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahama, U.; Hirota, S. Possible Reactions of Dietary Phenolic Compounds with Salivary Nitrite and Thiocyanate in the Stomach. Antioxidants 2017, 6, 53. https://doi.org/10.3390/antiox6030053
Takahama U, Hirota S. Possible Reactions of Dietary Phenolic Compounds with Salivary Nitrite and Thiocyanate in the Stomach. Antioxidants. 2017; 6(3):53. https://doi.org/10.3390/antiox6030053
Chicago/Turabian StyleTakahama, Umeo, and Sachiko Hirota. 2017. "Possible Reactions of Dietary Phenolic Compounds with Salivary Nitrite and Thiocyanate in the Stomach" Antioxidants 6, no. 3: 53. https://doi.org/10.3390/antiox6030053
APA StyleTakahama, U., & Hirota, S. (2017). Possible Reactions of Dietary Phenolic Compounds with Salivary Nitrite and Thiocyanate in the Stomach. Antioxidants, 6(3), 53. https://doi.org/10.3390/antiox6030053



