Abstract
Oligodendrocyte damage and loss are key features of multiple sclerosis (MS) pathology. Oligodendrocytes appear to be particularly vulnerable to reactive oxygen species (ROS) and cytokines, such as tumor necrosis factor-α (TNF), which induce cell death and prevent the differentiation of oligodendrocyte progenitor cells (OPCs). Here, we investigated the efficacy of sulforaphane (SFN), monomethyl fumarate (MMF) and Protandim to induce Nrf2-regulated antioxidant enzyme expression, and protect oligodendrocytes against ROS-induced cell death and ROS-and TNF-mediated inhibition of OPC differentiation. OLN-93 cells and primary rat oligodendrocytes were treated with SFN, MMF or Protandim resulting in significant induction of Nrf2-driven (antioxidant) proteins heme oygenase-1, nicotinamide adenine dinucleotide phosphate (NADPH): quinone oxidoreductase-1 and p62/SQSTM1, as analysed by Western blotting. After incubation with the compounds, oligodendrocytes were exposed to hydrogen peroxide. Protandim most potently promoted oligodendrocyte cell survival as measured by live/death viability assay. Moreover, OPCs were treated with Protandim or vehicle control prior to exposing them to TNF or hydrogen peroxide for five days, which inhibited OPC differentiation. Protandim significantly promoted OPC differentiation under influence of ROS, but not TNF. Protandim, a combination of five herbal ingredients, potently induces antioxidants in oligodendrocytes and is able to protect oligodendrocytes against oxidative stress by preventing ROS-induced cell death and promoting OPC differentiation.
1. Introduction
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system (CNS). Histopathological hallmarks include focal demyelinated lesions characterized by oligodendrocyte (OL) cell death, axonal damage, gliosis, microglial activation, and infiltration of lymphocytes and macrophages [1,2]. In chronic demyelinating lesions in advanced stages of the disease, OL loss is extensive and widespread [3,4,5]. As oligodendrocyte progenitor cells (OPCs) were detected in chronic lesions [5,6], it appears that premyelinating OLs fail to differentiate and ensheath the axon with myelin in chronic MS. Evidence is emerging that both pro-inflammatory cytokines and oxidative stress play a key role in OL cell pathology in MS.
Over the past decade, studies have shown that ROS-induced oxidative damage to cells in the CNS significantly contribute to demyelination and neurodegeneration in MS [7,8,9]. Markers of lipid and DNA oxidative damage have been detected in several CNS cells, including neurons, axons, myelin, and OLs in early active lesions with the highest levels of expression in cells that morphologically resembled apoptotic OLs [10,11]. Furthermore, expression of genes involved in ROS production, in particular the subunits of ROS-synthesizing enzyme nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX), were profoundly upregulated in cases of acute MS, specifically in activated microglia and macrophages in close vicinity of OLs [12].
In vitro studies demonstrated enhanced susceptibility of OLs to ROS-induced oxidative damage and cell death compared to astrocytes [13,14,15] and microglia [16,17]. OPCs are even more vulnerable than mature OLs to oxidative stress in vitro [18,19,20,21,22]. Factors contributing to the increased susceptibility of OLs to oxidative stress include the high intracellular iron content of the cells and relatively low levels of endogenous antioxidant proteins, which are lower in OPCs compared with mature OLs [14,15,23,24]. Furthermore, it was demonstrated that ROS are able to block OL maturation in vitro by arresting them in the progenitor phase by decreasing the expression of genes involved in OL differentiation and increasing the expression of genes known to inhibit differentiation [25]. Not only ROS, but also cytokines, such as tumor necrosis factor-α (TNF), may contribute to OL pathology in MS. In several studies, TNF was shown to selectively damage OLs and myelin in vitro [26,27] and was toxic to OL cultures [28,29]. Furthermore, TNF inhibited the differentiation of OPCs into mature OLs [30,31]. Although the mechanisms and time frame of ROS- and cytokine-induced cell death may differ, it is conceivable that both factors contribute to OL pathology in MS. Thus, a therapeutic compound that is able to protect OLs from both ROS- and cytokine-induced damage would have clinical value in MS [32]. Activation of the nuclear factor erythroid-2 related factor-2 (Nrf2) pathway involves the transcription of multiple (antioxidant) proteins, including heme oxygenase 1 (HO-1) and NAD(P)H: quinone oxidoreductase 1 (NQO-1), which protect cells from ROS- and cytokine-induced damage and cell death. Nrf2-activating compounds, like sulforaphane (SFN) [33,34,35,36,37,38], fumaric acid esters [39,40,41,42], and Protandim® [43,44,45] are known to induce antioxidant enzymes in various cell types, have cytoprotective properties and reduce clinical signs in the experimental autoimmune encephalomyelitis animal model for MS. The fumaric acid ester dimethylfumarate (DMF) (and its active metabolite monomethylfumarate (MMF), in particular, have shown efficacy in MS, as the DMF-containing oral formulation Tecfidera™ was successful in two phase 3 clinical trials and is now used in the clinic to treat relapsing-remitting MS patients.
Here, we explored the efficacy of these cytoprotective compounds in protecting OLs against an oxidative and inflammatory insult. Our data showed that Protandim, a phytochemical compound consisting of five herbal ingredients, robustly increased antioxidant protein production in primary rat OLs. Treatment of Protandim protected OLs against oxidative insults and counteracted ROS-induced inhibition of OPC differentiation. Altogether, the results indicate that Protandim may have therapeutic potential for protecting OLs against oxidative insult in MS.
2. Materials and Methods
2.1. Cell Cultures
OLN-93 cells, a cell line established from spontaneously transformed rat brain glial cultures [46], were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS, Hyclone, Logan, UT, USA), penicillin, and streptomycin at 37 °C. OLN93 cells were plated in 96-wells plates (25,000 cells/well; Greiner Bio-One, Frickenhausen, Germany) or 24-well plates (50,000 cells/well; Greiner Bio-One). Primary Oligodendrocytes. Primary OLs were isolated from 0-day-old to 2-day-old Sprague Dawley rats, as described previously [47]. Animal experiments were approved by the Animal Experiments Review Board of the VU University Medical Center. Isolated OPCs were plated in 96-well plates (25,000 cells/well; Greiner Bio-One), 24-well plates (50,000 cells/well; Greiner Bio-One) or ibidi μ-Slide 8 well plates (30,000 cells/well; Ibidi, Martinsried, Germany) pre-coated with poly-l-lysine (PLL, 5 µg/mL; Sigma-Aldrich, St. Louis, MO, USA) for functional, protein or immunocytochemical analysis, respectively. For the differentiation studies, OLs were synchronized to OPCs by culturing in SATO medium [47] supplemented with the growth factors bFGF-2 (10 ng/mL; Peprotech, Rocky Hill, NJ, USA) and PDGF-AA (10 ng/mL; Peprotech) for 2 days before the cells were treated. For the studies with mature OLs, cells were directly incubated in SATO medium supplemented with 0.5% fetal calf serum (FCS) and cultured for 7 days (Bodinco, Alkmaar, the Netherlands). The plated OL cultures were estimated to be ~90% pure; ~5% of cells were astrocytes and ~5% microglia.
2.2. Functional Analysis of Oligodendrocytes
Primary OLs were allowed to mature for 7 days in SATO medium supplemented with 0.5% FCS. The cells were then treated for 24 h with sulforaphane (Sigma-Aldrich) (5 µM), monomethyl fumarate (Sigma-Aldrich) (90 µM), Protandim (LifeVantage, Sandy, UT, USA) (60 µg/mL) or their respective vehicle control, dimethylsulfoxide (DMSO) or ethanol (EtOH), followed by 4 h treatment with tert-butyl hydrogen peroxide (Sigma-Aldrich) or glucose oxidase (1:750,000; Sigma-Aldrich), two different methods of ROS exposure. Cell viability after tert-butyl hydrogen peroxide and glucose oxidase treatment was assessed using the LIVE/DEAD viability/cytotoxicity kit (Invitrogen, Carlsbad, CA, USA) according to manufacturer′s protocol. Fluorescent signals of alive and dead cells were measured with a fluorometer (FLUOstar Galaxy, BMG Lab technologies, Offenburg, Germany) and the ratio between live and dead cells was calculated. To assess the protective function of Protandim on OPC maturation under the inhibitory influence of inflammation or oxidative stress, OPCs were synchronized by growth factors for 2 days and subsequently treated with 30 µg/mL of Protandim or EtOH, as vehicle control. After 24 h, the medium was removed and replaced by SATO medium without vehicle control or Protandim, supplemented with 0.5% FCS in the absence or presence of either 10 ng/mL TNF or 10 µM tert-butyl hydrogen peroxide for 5 days.
2.3. Immunocytochemistry
To ensure purity of OPC culture and to determine differentiation of OPCs under experimental conditions, cells grown on PLL-coated ibidi slides were processed for detection of OL-specific antigens. Briefly, cells fixed with 4% paraformaldehyde were blocked with 10% goat serum and 0.1% Triton-X in phosphate-buffered saline (PBS) at room temperature for 45 min, and thereafter incubated with primary antibody overnight at 4 °C. Cells were then washed with PBS and incubated with secondary antibody for 30 min. After washing, cells were incubated with 4,6-diamidino-2-phenylidole (DAPI, 10 mg/mL; Sigma-Aldrich, St. Louis, MO, USA) for 5 min, washed again and kept in PBS. Primary antibodies used for the recognition of OL antigens were MBP (1:200) and Olig2 antibody (1:200), followed by goat anti-rat IgG Alexa Fluor or goat anti-rabbit Alexa Fluor (see ”antibody characterization” below). All secondary antibodies were diluted 1:200 in PBS with 1% goat serum and 0.1% Triton-X (Sigma-Aldrich). Five random fields per ibidi well/coverslip were scanned using a 10x lens of a Leica fluorescence microscope (DM6000), and the total number of cells, the number of cells labeled with each antibody, and the total number of MBP+/Olig2+ cells or MBP−/Olig2+ cells per field were counted with ImageJ and compared. These determinations were performed on cultures from three separate preparations.
2.4. Western Blotting
Protein isolation from primary rat OLs was performed using Laemmli buffer. Western blotting was performed, as described earlier [48,49]. Proteins were separated on 10% SDS-PAGE gels and transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad Laboratories, Berkeley, CA, USA). After blocking in Odyssey blocking buffer (LI-COR Biosciences, Lincoln, AK, USA), membranes were incubated with primary antibodies overnight in Odyssey blocking buffer at 4 °C. Primary antibodies were detected by incubation with appropriate IRDye secondary antibodies (LI-COR Biosciences) for 1 h at room temperature in Odyssey blocking buffer and quantified using the Odyssey infrared imaging system (LI-COR Biosciences). Actin quantification was used to correct for total protein loading variation. Primary antibodies used were rabbit anti-HO-1 (1:1000), mouse anti-NQO-1 (1:1000), mouse anti-p62/SQSTM1 (1:1000) and mouse anti-actin (1:5000) (see “antibody characterization” below and in Supplementary Table S1).
2.5. Antibody Characterization
Primary antibodies used were rat anti-MBP (catalog no. MCA409S; RRID: AB_325004; Abd Serotec, Oxfordshire, UK), rabbit anti-Olig2 (catalog no. AB9610; RRID: AB_570666; Millipore, Billerica, MA, USA), rabbit anti-HO-1 (catalog no. ADI-OSA-150F; RRID: AB_1505620; Enzo Life Sciences, Farmingdale, NY, USA), mouse anti-NQO-1 (catalog no. ab28947; RRID: AB_881738; Abcam, Cambridge, UK), and mouse anti-p62/SQSTM1 (catalog no. ab56416; RRID: AB_945626), mouse anti-β-actin (catalog no. A5441; RRID: AB_476744; Sigma-Aldrich, St. Louis, MO, USA). The anti-MBP antibody was previously shown to stain myelin membrane formation in rat oligodendrocytes isolated from the brains of newborn pups using immunocytochemistry [50]. The anti-HO-1 antibody was demonstrated to stain a band at ~32 kDa in primary rat cerebellar neurons, whilst the anti-NQO-1 antibody was shown to stain NQO-1 at ~30 kDa human oral squamous cell carcinomas [51]. Rat pancreatic islet cells were stained by the anti-p62 antibody at approximately 62 kDa [52] and β-actin in mouse brain cortical cells was identified by the anti-β-actin antibody at a height of 42 kDa in a paper by Cheishvili et al. [53]. The secondary Alexa antibodies (goat anti-rat 488 (catalog no. A-11006; RRID: AB_10561520; Thermo Fischer Scientific, Waltham, MA, USA), goat anti-mouse 555 (catalog No.A-21428; RRID: 2535849; Thermo Fischer Scientific) were used at 1:200 dilution.
2.6. Glutathione Assay
Cells were plated at 25,000 per well in a 96-well plate (Greiner Bio-One) and allowed to mature for 7 days in SATO medium supplemented with 0.5% FCS. Following 24 h treatment with sulforaphane (5 μM), monomethyl fumarate (Sigma-Aldrich) (90 µM), Protandim (LifeVantage) (60 µg/mL), OLs were washed with PBS, and total glutathione was measured in a white 96-wells plate (Greiner Bio-One), using GSH-Glo, according to manufacturer’s instructions (Promega, Madison, WI, USA). Luminescence was measured with a FLUOstar Galaxy fluorometer.
2.7. Statistical Analysis
Results were analyzed using one-way analysis of variance (ANOVA) or student’s t-test for an average of 3 independent experiments. p < 0.05.
3. Results
3.1. SFN, MMF and Protandim Increase Antioxidant Enzymes and p62 Expression in OLN-93 Cells
OLN-93 cells most closely resemble 5-day to 10-day old (postnatal) cultured rat brain oligodendrocytes in terms of their morphological features and antigenic properties. To investigate and compare the efficacy of the Nrf2-activating compounds, and optimize compound concentrations for further experiments with primary rat oligodendrocytes, we first incubated OLN-93 cells with two different concentrations of SFN, MMF or Protandim or their respective vehicle control (DMSO or EtOH) for 24 h. The concentrations used were based on prior publications with the compounds [37,54,55] and dose-ranging experiments we performed in OLN-93 cells (data not shown). The concentrations were well-tolerated by the OLN-93 cells. MMF and Protandim dose-dependently increased the protein expression of HO-1 (Figure 1A) and NQO-1 (Figure 1B) compared to the highest concentration of their respective vehicle control. SFN, MMF and Protandim significantly increased HO-1 expression, whereas only SFN and Protandim significantly enhanced the expression of NQO-1. Furthermore, only SFN and MMF increased the expression of p62 in OLN-93 cells significantly (Figure 1C). Although 5 µM SFN appears to be optimal for both NQO-1 and p62 expression, a general dose-dependent trend can be observed with MMF and Protandim. Protein expression of HO-1, NQO-1 and p62 under EtOH- or DMSO-treated conditions were comparable to each other and untreated control.
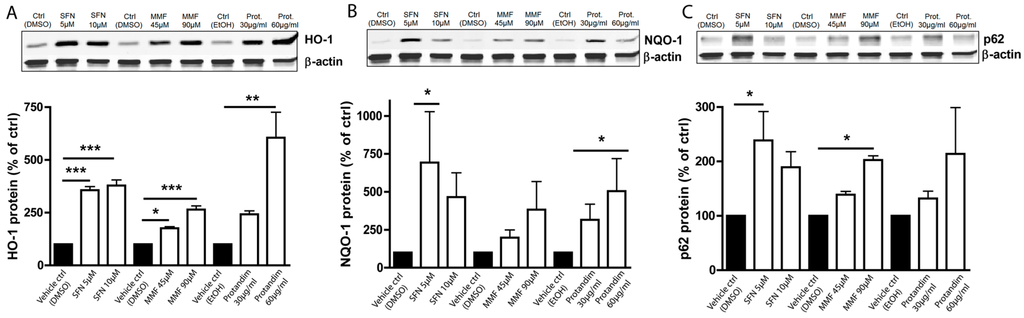
Figure 1.
Nrf2-activators dose-dependently increase antioxidant protein expression in OLN-93 cells. HO-1 (A), NQO-1 (B) and p62 (C) protein expression levels after 24 h treatment in the OLN-93 oligodendrocyte cell line with 5 µM or 10 µM SFN, 45 µM or 90 µM MMF, 30 µg/mL or 60 µg/mL Protandim or their respective DMSO or EtOH vehicle control. Protein levels were assayed by Western blotting. Data are presented as percentage of control and expressed as the mean ± SEM of 3 independent experiments. All statistics reflect one-way ANOVA tests with post hoc Bonferroni correction; * p < 0.05, ** p < 0.01; *** p < 0.001.
3.2. SFN and Protandim Prevent ROS-Induced Cell Death of OLN-93 Cells
To elucidate the cytoprotective potential of SFN, MMF and Protandim to protect OLN-93 cells from oxidative stress, the cells were incubated for 24 h with either SFN (5 µM), MMF (90 µM), Protandim (60 µg/mL) or their respective vehicle control prior to exposing them to 200 µM tert-butyl hydrogen peroxide for 4 h. OLN-93 cell viability under EtOH- or DMSO-treated conditions were comparable to each other and untreated control. Furthermore, SFN and Protandim, but not MMF, significantly increased survival of OLN-93 cells (SFN: 65% ± 4.25% vs. 16% ± 8% of vehicle control; Protandim: 56% ± 1.7% vs. 19% ± 5% of vehicle control) (Figure 2).
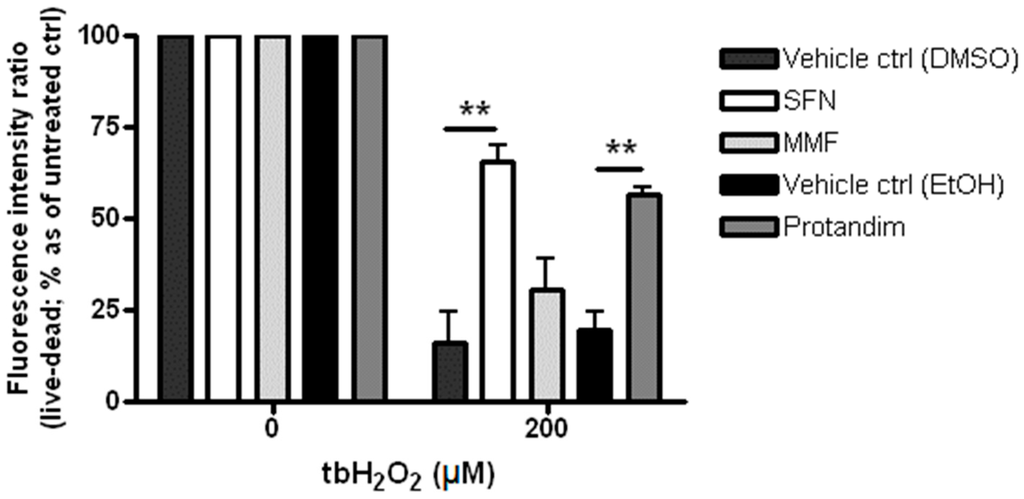
Figure 2.
Sulforaphane and Protandim promote viability of OLN-93 cells under tert-butyl hydrogen peroxide-induced oxidative insult. OLN-93 cells were treated with 5 µM SFN, 90 µM MMF, 60 µg/mL Protandim or their respective DMSO or EtOH vehicle control for 24 h. After removal of medium, cells were subsequently exposed to either control medium or medium with 200 µM tert-butyl hydrogen peroxide. Cell viability was measured using an Invitrogen live/dead cytotoxicity kit. Data are presented as percentage of control and expressed as the mean ± SEM of 3 independent experiments. Statistics reflect one-way ANOVA test with post hoc Bonferroni correction; ** p < 0.01.
3.3. SFN and Protandim Increase Antioxidant Enzymes and p62 Expression in Mature Primary Rat OLs
To investigate the potency of Nrf2-activating compounds in enhancing antioxidant proteins and p62 expression in mature primary rat OLs, we incubated mature rat OLs for 24 h with the most potent and tolerable concentrations of SFN, MMF or Protandim or their respective vehicle control, as determined by prior experiments with OLN-93 cells and viability assays with mature rat OLs. The concentrations used were 5 μM of SFN, 90 μM of MMF and 60 μg/mL of Protandim. Both SFN and Protandim significantly increased HO-1 protein expression in OLs, while NQO-1 and p62 protein levels were only significantly enhanced upon Protandim treatment. In contrast to OLN-93 cells, MMF treatment did not result in increased HO-1, NQO-1 and p62 protein expression (Figure 3). Furthermore, protein expression of HO-1, NQO-1 and p62 under EtOH- or DMSO-treated conditions were comparable to each other and untreated control.
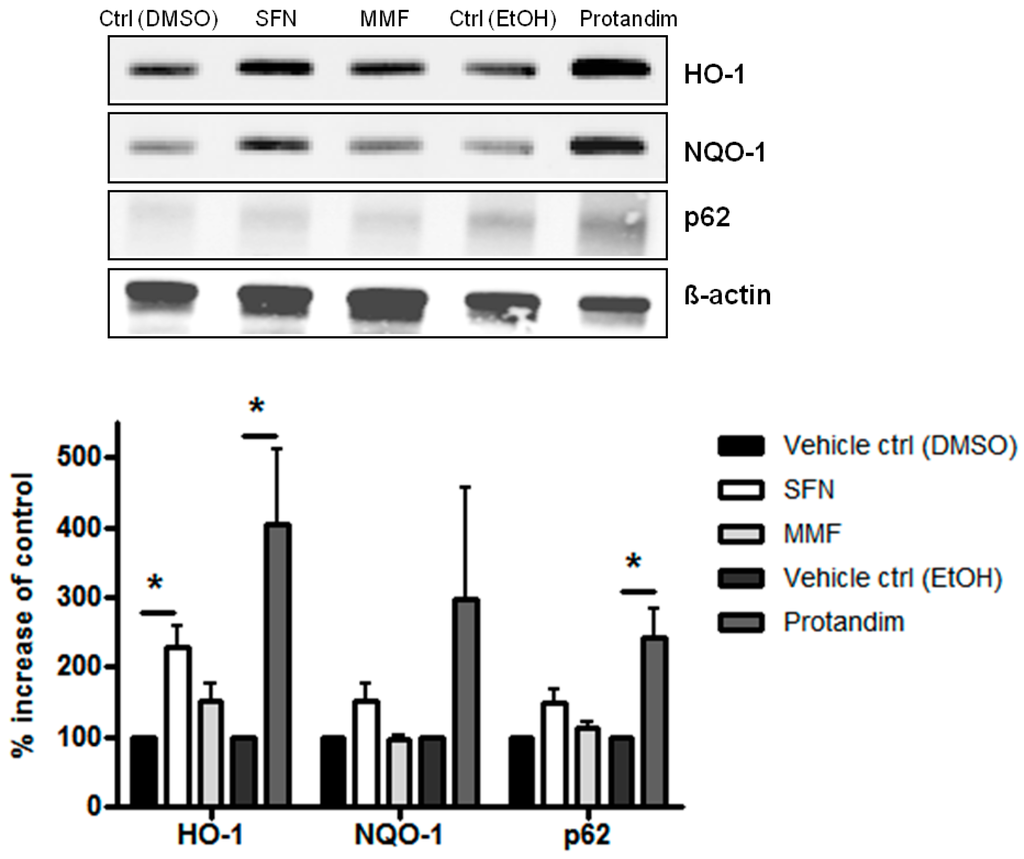
Figure 3.
Nrf2-activators dose-dependently increase antioxidant protein expression in mature primary rat OLs. HO-1, NQO-1 and p62 protein expression levels after 24 h treatment in mature primary rat OLs, differentiated for 7 days, with 5 µM SFN, 90 µM MMF,30 µg/mL Protandim or their respective DMSO or EtOH vehicle control. Protein levels were assayed by Western blotting. Data are presented as percentage of control and expressed as the mean ± SEM of 3 independent experiments. Statistics reflect one-way ANOVA test with post hoc Bonferroni correction; * p < 0.05.
3.4. Protandim Increases Glutathione Levels in Mature Primary Rat OLs and Protects OLs from ROS-Induced Cell Death
Glutathione is one of the most abundant mammalian intracellular thiol-containing antioxidants and represents a key buffer to maintain the cellular redox balance [56,57,58]. Hence, the capacity of the Nrf2-activators to enhance glutathione levels in mature OLs was investigated by incubating the cells for 24 h with SFN, MMF or Protandim or vehicle control. Protandim treatment significantly increased glutathione levels in primary OLs (Figure 4). Glutathione levels under EtOH- or DMSO-treated conditions were comparable to each other. To elucidate the cytoprotective potential of the Nrf2-activating compounds, primary rat OLs were incubated for 24 h with SFN, MMF, Protandim or vehicle control. Thereafter, the medium with compounds was removed and the cells exposed to an oxidative attack for 4 h. Only Protandim increased the percentage viability of primary rat OLs compared with vehicle control under both tert-butyl hydrogen peroxide exposure (Protandim: 71% ± 2% vs. 30% ± 7.5% of vehicle control) and glucose oxidase exposure (Protandim: 62% ± 2.5% vs. 20% ± 4.5% of vehicle control; Figure 5). OL viability under EtOH- or DMSO-treated conditions were comparable to each other and untreated control.
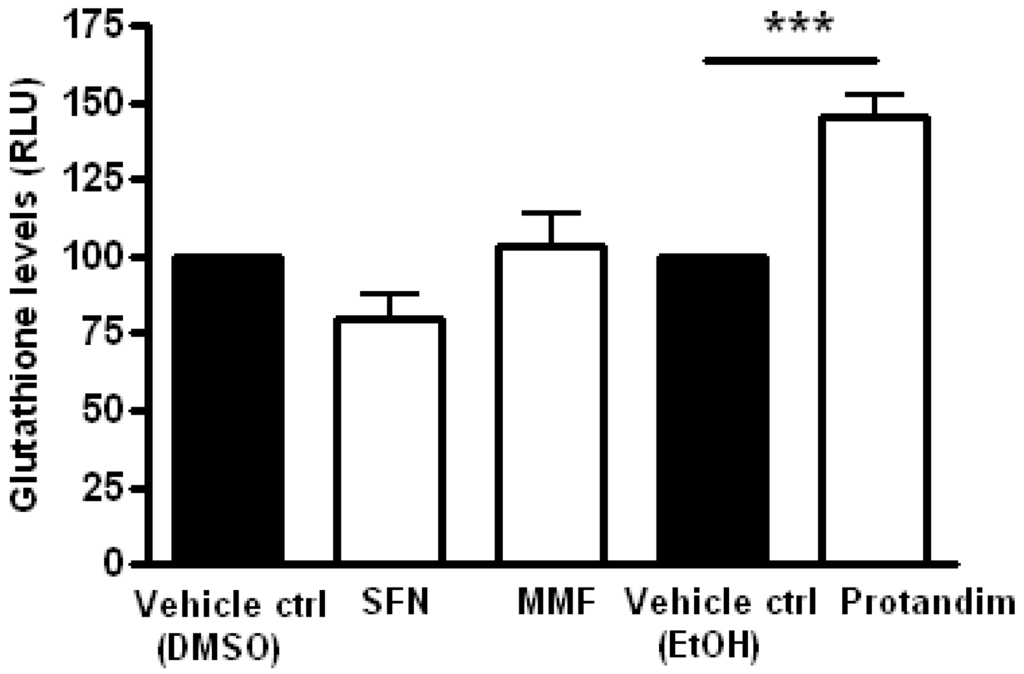
Figure 4.
Protandim increases total glutathione levels in mature primary rat OLs. Mature primary rat OLs differentiated for 7 days were treated for 24 h with 5 µM SFN, 90 µM MMF,30 µg/mL Protandim or their respective DMSO or EtOH control. Total glutathione levels were assayed using the GSH-Glo kit from Promega. Data are presented as percentage of control and expressed as the mean ± SEM of 3 independent experiments. Statistics reflect one-way ANOVA test with post hoc Bonferroni correction; *** p < 0.001.
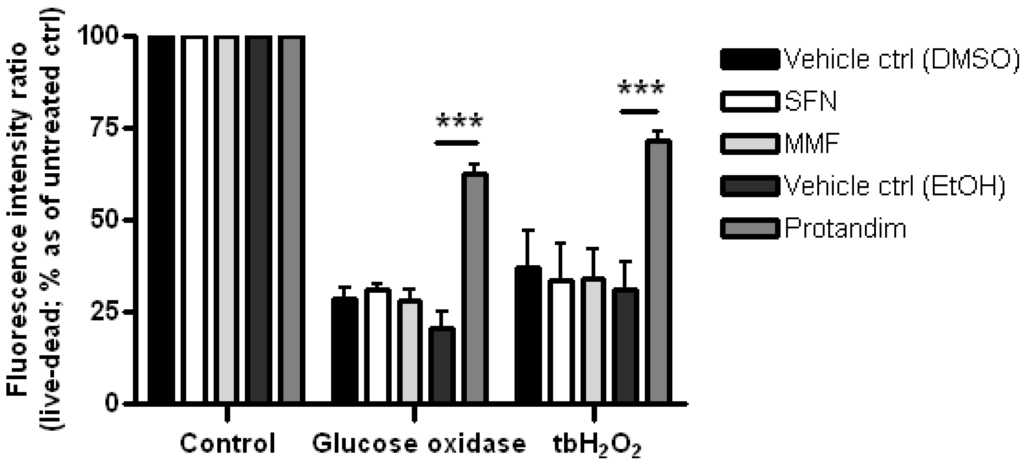
Figure 5.
Protandim promotes viability of mature primary rat OLs under oxidative insult. Mature primary rat OLs differentiated for 7 days were treated for 24 h with 5 µM SFN, 90 µM MMF,30 µg/mL Protandim or their respective DMSO or EtOH vehicle control. After removal of medium, cells were subsequently exposed to either control medium or medium with 100 µM tert-butyl hydrogen peroxide (tbH2O2) or glucose oxidase (1:750,000). Cell viability was measured using an Invitrogen live/dead cytotoxicity kit. Data are presented as percentage of control and expressed as the mean ± SEM of 3 independent experiments. Statistics reflect one-way ANOVA test with post hoc Bonferroni correction; *** p < 0.05.
3.5. Protandim Promotes Differentiation of OPCs under Oxidative Conditions
In order to investigate the protective potential of SFN, MMF and Protandim on OPC differentiation under oxidative stress and inflammation, we first determined the efficacy of the compounds to promote antioxidant enzyme expression in OPCs. Hereto, primary OPCs were incubated for 24 h with SFN (5 µM), MMF (90 µM) and Protandim (30 µg/mL) or vehicle control. Protandim and SFN significantly enhanced HO-1 protein expression in OPCs (Figure 6). NQO-1 levels were undetectable by Western blotting in OPCs (data not shown). ROS are known to inhibit OPC differentiation [25]. Based on the beneficial effects of Protandim, we next explored the potential of Protandim to promote differentiation of OPCs under oxidative conditions. OPCs were incubated for 24 h with Protandim (30 µg/mL) or vehicle control prior to 5 day exposure with medium containing 0.5% FCS or medium with 10 µM tertbutyl-hydrogen peroxide and 0.5% FCS, but no vehicle control or Protandim. Exposure to 10 µM tertbutyl-hydrogen peroxide resulted in significant and consistent inhibition of OPC maturation, and did not result in significant OPC cell death (data not shown). The ratio of MBP-positive and -negative Olig2-positive cells indicates the percentage of differentiated, MBP-expressing OLs per condition. Protandim treatment slightly increased the percentage of MBP-positive OLs compared to vehicle control under normal conditions (38% ± 3.35% and 31% ± 0.9%, respectively), but this was not significant. Tert-butyl hydrogen peroxide treatment significantly reduced the percentage of MBP-positive OLs compared to untreated vehicle control (25% ± 1.25% and 31% ± 0.9%, respectively). Protandim treatment significantly enhanced the percentage of MBP-positive OLs compared to vehicle control under oxidative stress (34% ± 1.25% and 25% ± 1.75%, respectively) (Figure 7). The percentage of MBP-positive OLs in untreated medium was comparable to that in vehicle control-treated medium.

Figure 6.
Nrf2-activators dose-dependently increase HO-1 protein expression in primary rat OPCs. After 2 day de-differentiation of OLs with growth factors bFGF-2 and PDGF-AA, primary rat OPCs were treated with 5 µM SFN, 90 µM MMF,30 µg/mL Protandim or their respective DMSO or EtOH vehicle control for 24 h. Protein levels were assayed by Western blotting. Data are presented as percentage of control and expressed as the mean ± SEM of 3 independent experiments. Statistics reflect one-way ANOVA test with post hoc Bonferroni correction; * p < 0.05.

Figure 7.
Protandim promotes differentiation of primary rat OPCs under oxidative stress. After 2 day de-differentiation of OLs with growth factors bFGF-2 and PDGF-AA, primary rat OPCs were incubated with 30 µg/mL Protandim or vehicle control (EtOH) for 24 h. After removal of medium, cells were subsequently exposed to either control medium or medium with 10 µM tert-butyl hydrogen peroxide (tbH2O2) for 5 days. MBP and Olig2 expression were assayed by immunocytochemistry. Data are presented as percentage of control and expressed as the mean ± SEM of 3 independent experiments. Statistics reflect student’s t-test, one-tailed; * p < 0.05.
Inflammatory mediators, such as TNF, are known to inhibit OPC differentiation [30,31]. We incubated OPCs with Protandim (30 µg/mL) or vehicle control and subsequently exposed OPCs to either control medium or medium containing 10 ng/mL TNF in the absence of vehicle control or Protandim for 5 days. This low dosage did not lead to significant OPC cell death (data not shown). TNF significantly decreased the percentage of MBP-positive OLs compared to untreated vehicle control (20% ± 2% and 28% ± 2.8%, respectively). Protandim marginally increased the percentage of MBP-positive oligodendrocytes compared to vehicle control upon TNF treatment (25% ± 2% and 20% ± 2%, respectively), albeit not significantly (Figure 8). The percentage of MBP-positive OLs in untreated medium was comparable to that in vehicle control-treated medium.

Figure 8.
Protandim marginally promotes differentiation of primary rat OPCs in the presence of TNF. After 2 days of de-differentiation, primary rat OPCs were incubated with 30 µg/mL Protandim or vehicle control (EtOH) for 24 h. After removal of medium, cells were subsequently exposed to either control medium or medium with 10 ng/mL TNF for 5 days. MBP and Olig2 expression were assayed by immunocytochemistry. Data are presented as percentage of control and expressed as the mean ± SEM of 3 independent experiments. Statistics reflect student’s t-test, one-tailed; * p < 0.05.
4. Discussion
In this study, we explored the efficacy of several cytoprotective compounds in protecting OLs against an oxidative and inflammatory insult. We show that the cytoprotective compounds sulforaphane (SFN), monomethyl fumarate (MMF) and Protandim increase the expression of proteins involved in antioxidant protection in OLN-93 cells, whereas Protandim and SFN promote antioxidant enzyme production in mature primary rat OLs and primary rat OPCs. Importantly, SFN and Protandim pre-treatment rescued OLN-93 cells from an oxidative attack. Protandim, but not SFN, significantly enhanced glutathione levels in primary rat OLs and rescued primary rat OLs from an oxidative insult. It is known that pro-inflammatory mediators, such as TNF and ROS inhibit OPC differentiation [25,31]. Here, we show that Protandim, a well-defined combination of five widely studied herbal ingredients and a potent inducer of the Nrf-2 pathway [44], attenuates ROS-induced OPC inhibition.
Early active demyelinating MS lesions are characterized by massive influx of monocyte-derived macrophages and activated microglia. These inflammatory cells produce cytotoxic mediators, such as ROS and inflammatory molecules, including TNF, resulting in OL damage and loss, demyelination and axonal damage [1,2]. To date, evidence is accumulating that compounds boosting endogenous antioxidant production show beneficial effects in various in vitro and in vivo models of neuroinflammation and oxidative stress [33,34,35,36,37,42,43,44,45,59], including the experimental autoimmune encephalomyelitis (EAE) animal model for MS [38,39,60]. In addition, as clinical efficacy of Tecfidera™ in MS was shown to be partly attributable to Nrf2-activation, it suggests that boosting the endogenous antioxidant system in MS has therapeutic potential. Indeed, reports have shown that ROS-induced oxidative damage may play a key role in demyelination in MS [7,8,9,10,11,12] and that Nrf2-driven genes are upregulated in white matter lesions [61].
Notably, all three compounds investigated in our study increased the expression of the cytoprotective proteins HO-1, NQO-1 and p62 in OLN-93 cells. Yet, only SFN and Protandim pre-treatment were able to rescue OLN-93 cells from hydrogen peroxide-induced cell death. This suggests that protein levels of HO-1, NQO-1 and p62 induced by MMF may have been insufficient to prevent ROS-induced cell death. Using primary mature OLs we observed that Protandim treatment resulted in a more pronounced increase in HO-1, NQO-1 and p62 protein expression compared with MMF and SFN. In addition, Protandim treatment significantly increased intracellular glutathione levels, whereas both MMF and SFN did not affect glutathione levels. Glutathione is the most abundant mammalian intracellular thiol-containing antioxidant and represents a key buffer to maintain the cellular redox balance [56,57,58]. Protandim pre-treatment, not MMF or SFN, protected mature primary rat OLs from hydrogen peroxide- and glucose oxidase-induced cell death. These findings indicate that high endogenous levels of glutathione might be essential in protecting OLs against an oxidative attack.
Furthermore, p62 was reported to have neuroprotective effects in SH-SY5Y neuroblastoma cells against hydrogen peroxide-induced cell death [62]. This suggests that Protandim-mediated upregulation of p62 in primary rat OLs may have played a role in their survival against ROS, but this requires further research.
The high metabolic rate, large intracellular iron stores and relatively low levels of endogenous antioxidants make OPCs particularly vulnerable to oxidative and inflammation-driven cell death and injury. The low levels of antioxidant proteins in OPCs may potentially clarify why we could not detect NQO-1 protein levels in primary rat OPCs [14,15,23,24]. Furthermore, ROS are known to hamper OPC differentiation by enhanced expression of genes involved in inhibition of differentiation and decreased expression of genes known to promote OPC differentiation. In our study, ROS also reduced OPC differentiation, which is in line with the report by French and colleagues [25].
We show that Protandim pre-treatment was able to counteract ROS-induced inhibition of OPC differentiation. Interestingly, even under normal conditions we observed a slight increase in the number of mature oligodendrocytes upon exposure to Protandim, suggesting that Protandim, even in the absence of an oxidative insult, might stimulate OPC maturation. Like free radicals, pro-inflammatory cytokines, such as TNF, contribute to OPC cell damage and we observed a reduction in OPC differentiation upon TNF exposure, which was in line with previous reports [30,31]. In contrast to the beneficial effects observed under oxidative conditions, Protandim treatment did not attenuate TNF-mediated block of OPC differentiation.
Athough Protandim showed high potency in our rat OL cultures, and demonstrated efficacy in animal models of cardiovascular disease [43,63], little is known about the pharmacodynamic and pharmacokinetic properties. Protandim contains five herbal ingredients, including ashwagandha, bacopa extract, green tea extract, silymarin, and curcumin, and several studies have shown that Protandim is able to potently activate Nrf2-driven gene expression [44,45]. The therapeutic properties of all five compounds have been reported previously [64,65,66], of which curcumin has been the most thoroughly investigated [67,68,69]. Further research into the mode of action of Protandim is therefore required in order to determine if the compound would be efficacious in animal models for MS and MS patients.
5. Conclusions
Taken together, our findings indicate that Protandim effectively prevented ROS-driven oligodendrocyte cell death and promoted OPC differentiation under oxidative conditions. Our in vitro data warrants future research to further explore the therapeutic efficacy of Protandim in experimental MS animal models.
Supplementary Materials
The following are available online at www.mdpi.com/2076-3921/5/3/30/s1. Table S1. Antibody characterization.
Acknowledgments
This study was funded by the Dutch MS Research Foundation and Biogen Idec.
Author Contributions
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Jack van Horssen, Joe M. McCord, Jamie L. Lim, Wia Baron. Acquisition of data: Jamie L. Lim, Susanne M.A. van der Pol. Analysis and interpretation of data: Jamie L. Lim, Susanne M.A. van der Pol, Joe M. McCord, Wia Baron, Helga E. de Vries, Jack van Horssen. Drafting of the manuscript: Jamie L. Lim, Jack van Horssen, Helga E. de Vries. Critical revision of the manuscript for important intellectual content: Jamie L. Lim, Susanne M.A. van der Pol, Joe M. McCord, Wia Baron, Helga E. de Vries, Jack van Horssen. Statistical analysis: Jamie L. Lim. Obtained funding: Jack van Horssen. Administrative, technical, and material support: Susanne M.A. van der Pol. Study supervision: Jack van Horssen, Helga E. de Vries.
Conflicts of Interest
Authors report no conflicts of interest. Joe M. McCord, McCord was previously associated with LifeVantage Corp., the manufacturer of Protandim, but currently has no affiliation with, nor financial interest in the company.
References
- Lassmann, H.; van Horssen, J. The molecular basis of neurodegeneration in multiple sclerosis. FEBS Lett. 2011, 585, 3715–3723. [Google Scholar] [CrossRef] [PubMed]
- Lassmann, H.; Mahad, D.; van Horssen, J. Progressive multiple sclerosis: Pathology and pathogenesis. Nat. Rev. Neurol. 2012, 8, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Brück, W.; Schmied, M.; Suchanek, G.; Brück, Y.; Breitschopf, H.; Poser, S.; Piddlesden, S.; Lassmann, H. Oligodendrocytes in the early course of multiple sclerosis. Ann. Neurol. 1994, 35, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, K.; Suchanek, G.; Breitschopf, H.; Brück, W.; Budka, H.; Jellinger, K.; Lassmann, H. Patterns of oligodendroglia pathology in multiple sclerosis. Brain 1994, 117, 1311–1322. [Google Scholar] [CrossRef] [PubMed]
- Lucchinetti, C.; Brück, W.; Parisi, J.; Scheithauer, B.; Rodriguez, M.; Lassmann, H. A quantitative analysis of oligodendrocytes in multiple sclerosis lesions. A study of 113 cases. Brain 1999, 122, 2279–2295. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.; Tourtellotte, W.W.; Rudick, R.; Trapp, B.D. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N. Engl. J. Med. 2002, 346, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Schreibelt, G.; van Horssen, J.; van Rossum, S.; Dijkstra, C.D.; Drukarch, B.; de Vries, H.E. Therapeutic potential and biological role of endogenous antioxidant enzymes in multiple sclerosis pathology. Brain Res. Rev. 2007, 56, 322–330. [Google Scholar] [CrossRef] [PubMed]
- De Vries, H.E.; Witte, M.; Hondius, D.; Rozemuller, A.J.; Drukarch, B.; Hoozemans, J.; van Horssen, J. Nrf2-induced antioxidant protection: A promising target to counteract ROS-mediated damage in neurodegenerative disease? Free Radic. Biol. Med. 2008, 45, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Van Horssen, J.; Witte, M.E.; Schreibelt, G.; de Vries, H.E. Radical changes in multiple sclerosis pathogenesis. Biochim. Biophys. Acta. 2011, 1812, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Haider, L.; Fischer, M.T.; Frischer, J.M.; Bauer, J.; Hoftberger, R.; Botond, G.; Esterbauer, H.; Binder, C.J.; Witztum, J.L.; Lassmann, H. Oxidative damage in multiple sclerosis lesions. Brain 2011, 134, 1914–1924. [Google Scholar] [CrossRef] [PubMed]
- Van Horssen, J.; Schreibelt, G.; Drexhage, J.; Hazes, T.; Dijkstra, C.D.; van der Valk, P.; de Vries, H.E. Severe oxidative damage in multiple sclerosis lesions coincides with enhanced antioxidant enzyme expression. Free Radic. Biol. Med. 2008, 45, 1729–1737. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.T.; Sharma, R.; Lim, J.L.; Haider, L.; Frischer, J.M.; Drexhage, J.; Mahad, D.; Bradl, M.; van Horssen, J.; Lassmann, H. NADPH oxidase expression in active multiple sclerosis lesions in relation to oxidative tissue damage and mitochondrial injury. Brain 2012, 135, 886–899. [Google Scholar] [CrossRef] [PubMed]
- Mitrovic, B.; Ignarro, L.J.; Montestruque, S.; Smoll, A.; Merrill, J.E. Nitric oxide as a potential pathological mechanism in demyelination: Its differential effects on primary glial cells in vitro. Neuroscience 1994, 61, 575–585. [Google Scholar] [CrossRef]
- Husain, J.; Juurlink, B.H. Oligodendroglial precursor cell susceptibility to hypoxia is related to poor ability to cope with reactive oxygen species. Brain Res. 1995, 698, 86–94. [Google Scholar] [CrossRef]
- Thorburne, S.K.; Juurlink, B.H. Low glutathione and high iron govern the susceptibility of oligodendroglial precursors to oxidative stress. J. Neurochem. 1996, 67, 1014–1122. [Google Scholar] [CrossRef] [PubMed]
- Griot, C.; Vandevelde, M.; Richard, A.; Peterhans, E.; Stocker, R. Selective degeneration of oligodendrocytes mediated by reactive oxygen species. Free Radic. Res. Commun. 1990, 11, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Mitrovic, B.; Parkinson, J.; Merrill, J.E. An in vitro model of oligodendrocyte destruction by nitric oxide and its relevance to multiple sclerosis. Methods 1996, 10, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Oka, A.; Belliveau, M.J.; Rosenberg, P.A.; Volpe, J.J. Vulnerability of oligodendroglia to glutamate: Pharmacology, mechanisms, and prevention. J. Neurosci. 1993, 13, 1441–1453. [Google Scholar] [PubMed]
- Yonezawa, M.; Back, S.A.; Gan, X.; Rosenberg, P.A.; Volpe, J.J. Cystine deprivation induces oligodendroglial death: Rescue by free radical scavengers and by a diffusible glial factor. J. Neurochem. 1996, 67, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Back, S.A.; Luo, N.L.; Borenstein, N.S.; Levine, J.M.; Volpe, J.J.; Kinney, H.C. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J. Neurosci. 2001, 21, 1302–1312. [Google Scholar] [PubMed]
- Baud, O.; Greene, A.E.; Li, J.; Wang, H.; Volpe, J.J.; Rosenberg, P.A. Glutathione peroxidase-catalase cooperativity is required for resistance to hydrogen peroxide by mature rat oligodendrocytes. J. Neurosci. 2004, 24, 1531–1540. [Google Scholar] [CrossRef] [PubMed]
- Fragoso, G.; Martínez-Bermúdez, A.K.; Liu, H.N.; Khorchid, A.; Chemtob, S.; Mushynski, W.E.; Almazan, G. Developmental differences in HO-induced oligodendrocyte cell death: Role of glutathione, mitogen activated protein kinases and caspase 3. J. Neurochem. 2004, 90, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Back, S.A.; Gan, X.; Li, Y.; Rosenberg, P.A.; Volpe, J.J. Maturation-dependent vulnerability of oligodendrocytes to oxidative stress-induced death caused by glutathione depletion. J. Neurosci. 1998, 18, 6241–6253. [Google Scholar] [PubMed]
- Juurlink, B.H.; Thorburne, S.K.; Hertz, L. Peroxide-scavenging deficit underlies oligodendrocyte susceptibility to oxidative stress. Glia 1998, 22, 371–378. [Google Scholar] [CrossRef]
- French, H.M.; Reid, M.; Mamontov, P.; Simmons, R.A.; Grinspan, J.B. Oxidative stress disrupts oligodendrocyte maturation. J. Neurosci. Res. 2009, 87, 3076–3087. [Google Scholar] [CrossRef] [PubMed]
- Selmaj, K.W.; Raine, C.S. Tumor necrosis factor mediates myelin and oligodendrocyte damage in vitro. Ann. Neurol. 1988, 23, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Baerwald, K.D.; Popko, B. Developing and mature oligodendrocytes respond differently to the immune cytokine interferon-gamma. J. Neurosci. Res. 1998, 52, 230–239. [Google Scholar] [CrossRef]
- Loui, J.C.; Magal, E.; Takayama, S.; Varon, S. CNTF protection of oligodendrocytes against natural and tumor necrosis factor-induced death. Science 1993, 259, 689–692. [Google Scholar]
- Ye, P.; D’Ercole, A.J. Insulin-like growth factor I protects oligodendrocytes from tumor necrosis factor-alpha-induced injury. Endocrinology 1999, 140, 3063–3072. [Google Scholar] [PubMed]
- Jana, M.; Pahan, K. Redox regulation of cytokine-mediated inhibition of myelin gene expression in human primary oligodendrocytes. Free Radic. Biol. Med. 2005, 39, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Bonora, M.; de Marchi, E.; Patergnani, S.; Suski, J.M.; Celsi, F.; Bononi, A.; Giorgi, C.; Marchi, S.; Rimessi, A.; Duszyński, J.; et al. Tumor necrosis factor-alpha impairs oligodendroglial differentiation through a mitochondria-dependent process. Cell. Death Differ. 2014, 21, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.L.; Wilhelmus, M.M.; de Vries, H.E.; Drukarch, B.; Hoozemans, J.J.; van Horssen, J. Antioxidative defense mechanisms controlled by Nrf2: State-of-the-art and clinical perspectives in neurodegenerative diseases. Arch. Toxicol. 2014, 88, 1773–1786. [Google Scholar] [CrossRef] [PubMed]
- Kraft, A.D.; Johnson, D.A.; Johnson, J.A. Nuclear factor E2-related factor 2-dependent antioxidant response element activation by tert-butylhydroquinone and sulforaphane occurring preferentially in astrocytes conditions neurons against oxidative insult. J. Neurosci. 2004, 24, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Moore, A.N.; Clifton, G.L.; Dash, P.K. Sulforaphane enhances aquaporin-4 expression and decreases cerebral edema following traumatic brain injury. J. Neurosci. Res. 2005, 82, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Moore, A.N.; Redell, J.B.; Dash, P.K. Enhancing expression of Nrf2-driven genes protects the blood brain barrier after brain injury. J. Neurosci. 2007, 27, 10240–10248. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, L.O.; Kipp, M.; Lucius, R.; Pufe, T.; Wruck, C.J. Sulforaphane suppresses LPS-induced inflammation in primary rat microglia. Inflamm. Res. 2010, 59, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, K.; Kume, T.; Muto, C.; Takada-Takatori, Y.; Izumi, Y.; Sugimoto, H.; Akaike, A. Glutathione biosynthesis via activation of the nuclear factor E2-related factor 2 (Nrf2)--antioxidant-response element (ARE) pathway is essential for neuroprotective effects of sulforaphane and 6-(methylsulfinyl) hexyl isothiocyanate. J. Pharmacol. Sci. 2011, 115, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Cui, W.; Liu, J.; Li, R.; Liu, Q.; Xie, X.H.; Ge, X.L.; Zhang, J.; Song, X.J.; Wang, Y.; et al. Sulforaphane ameliorates the development of experimental autoimmune encephalomyelitis by antagonizing oxidative stress and Th17-related inflammation in mice. Exp. Neurol. 2013, 250, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Linker, R.A.; Lee, D.H.; Ryan, S.; van Dam, A.M.; Conrad, R.; Bista, P.; Zeng, W.; Hronowsky, X.; Buko, A.; Chollate, S.; et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain 2011, 134, 678–692. [Google Scholar] [CrossRef] [PubMed]
- Fox, R.J.; Miller, D.H.; Phillips, J.T.; Hutchinson, M.; Havrdova, E.; Kita, M.; Yang, M.; Raghupathi, K.; Novas, M.; Sweetser, M.T.; et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N. Engl. J. Med. 2012, 367, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Gold, R.; Kappos, L.; Arnold, D.L.; Bar-Or, A.; Giovannoni, G.; Selmaj, K.; Tornatore, C.; Sweetser, M.T.; Yang, M.; Sheikh, S.I.; et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N. Engl. J. Med. 2012, 367, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Scannevin, R.H.; Chollate, S.; Jung, M.Y.; Shackett, M.; Patel, H.; Bista, P.; Zeng, W.; Ryan, S.; Yamamoto, M.; Lukashev, M.; et al. Fumarates promote cytoprotection of central nervous system cells against oxidative stress via the nuclear factor (erythroid-derived 2)-like 2 pathway. J. Pharmacol. Exp. Ther. 2012, 341, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Bogaard, H.J.; Natarajan, R.; Henderson, S.C.; Long, C.S.; Kraskauskas, D.; Smithson, L.; Ockaili, R.; McCord, J.M.; Voelkel, N.F. Chronic pulmonary artery pressure elevation is insufficient to explain right heart failure. Circulation 2009, 120, 1951–1960. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, K.; Alam, J.; McCord, J.M.; Pugazhenthi, S. Synergistic induction of heme oxygenase-1 by the components of the antioxidant supplement Protandim. Free Radic. Biol. Med. 2009, 46, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Reuland, D.J.; Khademi, S.; Castle, C.J.; Irwin, D.C.; McCord, J.M.; Miller, B.F.; Hamilton, K.L. Upregulation of phase II enzymes through phytochemical activation of Nrf2 protects cardiomyocytes against oxidant stress. Free Radic. Biol. Med. 2013, 56, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Richter-Landsberg, C.; Heinrich, M. OLN-93: A new permanent oligodendroglia cell line derived from primary rat brain glial cultures. J. Neurosci. Res. 1996, 45, 161–173. [Google Scholar] [CrossRef]
- Maier, O.; van der Heide, T.; van Dam, A.M.; Baron, W.; de Vries, H.E.; Hoekstra, D. Alteration of the extracellular matrix interferes with raft association of neurofascin in oligodendrocytes. Potential significance for multiple sclerosis? Mol. Cell Neurosci. 2005, 28, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Nijland, P.G.; Witte, M.E.; van het Hof, B.; van der Pol, S.; Bauer, J.; Lassmann, H.; van der Valk, P.; de Vries, H.E.; van Horssen, J. Astroglial PGC-1α increases mitochondrial antioxidant capacity and suppresses inflammation: Implications for multiple sclerosis. Acta. Neuropathol. Commun. 2014, 2, 170. [Google Scholar] [CrossRef] [PubMed]
- Witte, M.E.; Nijland, P.G.; Drexhage, J.A.; Gerritsen, W.; Geerts, D.; van het Hof, B.; Reijerkerk, A.; de Vries, H.E.; van der Valk, P.; van Horssen, J. Reduced expression of PGC-1α partly underlies mitochondrial changes and correlates with neuronal loss in multiple sclerosis cortex. Acta. Neuropathol. 2013, 125, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Relvas, J.B.; Setzu, A.; Baron, W.; Buttery, P.C.; LaFlamme, S.E.; Franklin, R.J.; ffrench-Constant, C. Expression of dominant-negative and chimeric subunits reveals an essential role for beta1 integrin during myelination. Curr. Biol. 2011, 11, 1039–1043. [Google Scholar] [CrossRef]
- Inui, T.; Chano, T.; Takikita-Suzuki, M.; Nishikawa, M.; Yamamoto, G.; Okabe, H. Association of p62/SQSTM1 excess and oral carcinogenesis. PLoS ONE 2013, 8, e74398. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shi, S.; Gu, Z.; Du, Y.; Liu, M.; Yan, S.; Gao, J.; Li, J.; Shao, Y.; Zhong, W.; et al. Impaired autophagic function in rat islets with aging. Age (Dordr.) 2013, 35, 1531–1544. [Google Scholar] [CrossRef] [PubMed]
- Cheishvili, D.; Dietrich, P.; Maayan, C.; Even, A.; Weil, M.; Dragatsis, I.; Razin, A. IKAP deficiency in an FD mouse model and in oligodendrocyte precursor cells results in downregulation of genes involved in oligodendrocyte differentiation and myelin formation. PLoS ONE 2014, 9, e94612. [Google Scholar] [CrossRef] [PubMed]
- Thiessen, A.; Schmidth, M.M.; Dringen, R. Fumaric acid dialkyl esters deprive cultured rat oligodendroglial cells of glutathione and upregulate the expression of heme oxygenase 1. Neurosci. Lett. 2010, 475, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Hybertson, B.M.; Goa, B.; Bose, K.B.; McCord, J.M. Oxidative stress in health and disease: The therapeutic potential of Nrf2 activation. Mol. Aspects Med. 2011, 32, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.P. Redefining oxidative stress. Antioxid. Redox Signal. 2006, 8, 1865–1879. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Cidlowski, J.A. Apoptosis and glutathione: beyond an antioxidant. Cell Death Differ. 2009, 16, 1303–1314. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.; Sah, S.; Nath, S.K. Interaction between glutathione and apoptosis in systemic lupus erythematosus. Autoimmun. Rev. 2013, 12, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Kaidery, N.A.; Banerjee, R.; Yang, L.; Smirnova, N.A.; Hushpulian, D.M.; Liby, K.T.; Williams, C.R.; Yamamoto, M.; Kensler, T.W.; Ratan, R.R.; et al. Targeting Nrf2-mediated gene transcription by extremely potent synthetic triterpenoids attenuate dopaminergic neurotoxicity in the MPTP mouse model of Parkinson’s disease. Antioxid. Redox Signal. 2013, 18, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Schilling, S.; Goelz, S.; Linker, R.; Luehder, F.; Gold, R. Fumaric acid esters are effective in chronic experimental autoimmune encephalomyelitis and suppress macrophage infiltration. Clin. Exp. Immunol. 2006, 145, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Licht-Mayer, S.; Wimmer, I.; Traffehn, S.; Metz, I.; Brück, W.; Bauer, J.; Bradl, M.; Lassmann, H. Cell type-specific Nrf2 expression in multiple sclerosis lesions. Acta. Neuropathol. 2015, 130, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.R.; Han, A.M.; Kwon, Y.K.; Joung, I. p62 protects SH-SY5Y neuroblastoma cells against H2O2-induced injury through the PDK1/Akt pathway. Neurosci. Lett. 2009, 450, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Voelkel, N.F.; Bogaard, H.J.; Al Husseini, A.; Farkas, L.; Gomez-Arroyo, J.; Natarajan, R. Antioxidants for the treatment of patients with severe angioproliferative pulmonary hypertension? Antioxid. Redox Signal. 2013, 18, 1810–1817. [Google Scholar] [CrossRef] [PubMed]
- Joe, B.; Vijaykumar, M.; Lokesh, B.R. Biological properties of curcumin-cellular and molecular mechanisms of action. Crit. Rev. Food Sci. Nutr. 2004, 44, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Mandel, S.; Weinreb, O.; Amit, T.; Youdim, M.B. Cell signaling pathways in the neuroprotective actions of the green tea polyphenol (−)-epigallocatechin-3-gallate: Implications for neurodegenerative diseases. J. Neurochem. 2004, 88, 1555–1569. [Google Scholar] [CrossRef] [PubMed]
- Kishore, K.; Singh, M. Effect of bacosides, alcoholic extract of Bacopa monniera Linn. (brahmi), on experimental amnesia in mice. Indian J. Exp. Biol. 2005, 43, 640–645. [Google Scholar] [PubMed]
- Xie, L.; Li, X.K.; Takahara, S. Curcumin has bright prospects for the treatment of multiple sclerosis. Int. Immunopharmacol. 2011, 11, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Li, X.K.; Funeshima-Fuji, N.; Kimura, H.; Matsumoto, Y.; Isaka, Y.; Takahara, S. Amelioration of experimental autoimmune encephalomyelitis by curcumin treatment through inhibition of IL-17 production. Int. Immunopharmacol. 2009, 9, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Kanakasabai, S.; Casalini, E.; Walline, C.C.; Mo, C.; Chearwae, W.; Bright, J.J. Differential regulation of CD4(+) T helper cell responses by curcumin in experimental autoimmune encephalomyelitis. J. Nutr. Biochem. 2012, 23, 1498–1507. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).