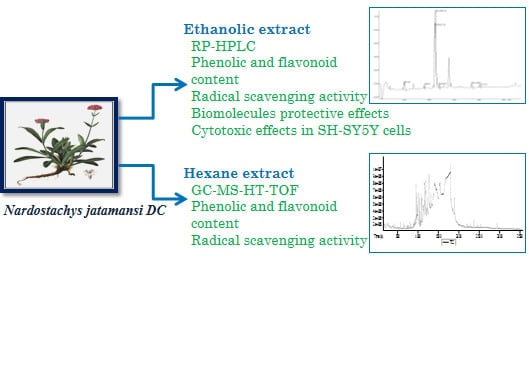
Antioxidant, Biomolecule Oxidation Protective Activities of Nardostachys jatamansi DC and Its Phytochemical Analysis by RP-HPLC and GC-MS
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Reagents
2.2. Plant Material and Preparation of the Extract
2.3. Metabolite Profile of N. jatamansi
2.3.1. Reversed Phase-HPLC Analysis of the 70% Ethanol Fraction
2.3.2. Gas Chromatography and Mass Spectrometry Analysis (GC-MS-HT-TOF) of the Hexane Fraction
2.4. Phytochemical Screening of the Plant Extract
2.4.1. Determination of Total Phenolic Content
2.4.2. Determination of Total Flavonoid Content
2.5. In Vitro Free Radical Scavenging and Antioxidant Activities
2.5.1. DPPH Radical Scavenging Activity
2.5.2. ABTS Radical Cation Decolorization Assay
2.5.3. Metal Chelating
2.5.4. Superoxide Radical Scavenging Activity
2.5.5. Ferric-Reducing Antioxidant Power Assay
2.6. Biomolecules Oxidation Assays
2.6.1. AAPH-Induced Plasmid Nick Assay
2.6.2. Protein Oxidation
2.6.3. Thiobarbituric Acid-Reactive Substances Assay
2.6.4. Estimation of Reactive Oxygen Species
2.6.5. Estimation of Protein Carbonyls
2.7. Cytoprotective Effects
LDH Leakage Assay
2.8. Statistical Analysis
3. Results and Discussion
3.1. Metabolite Profile of N. jatamansi
3.1.1. Reversed Phase-HPLC Analysis of Phenolic Compounds
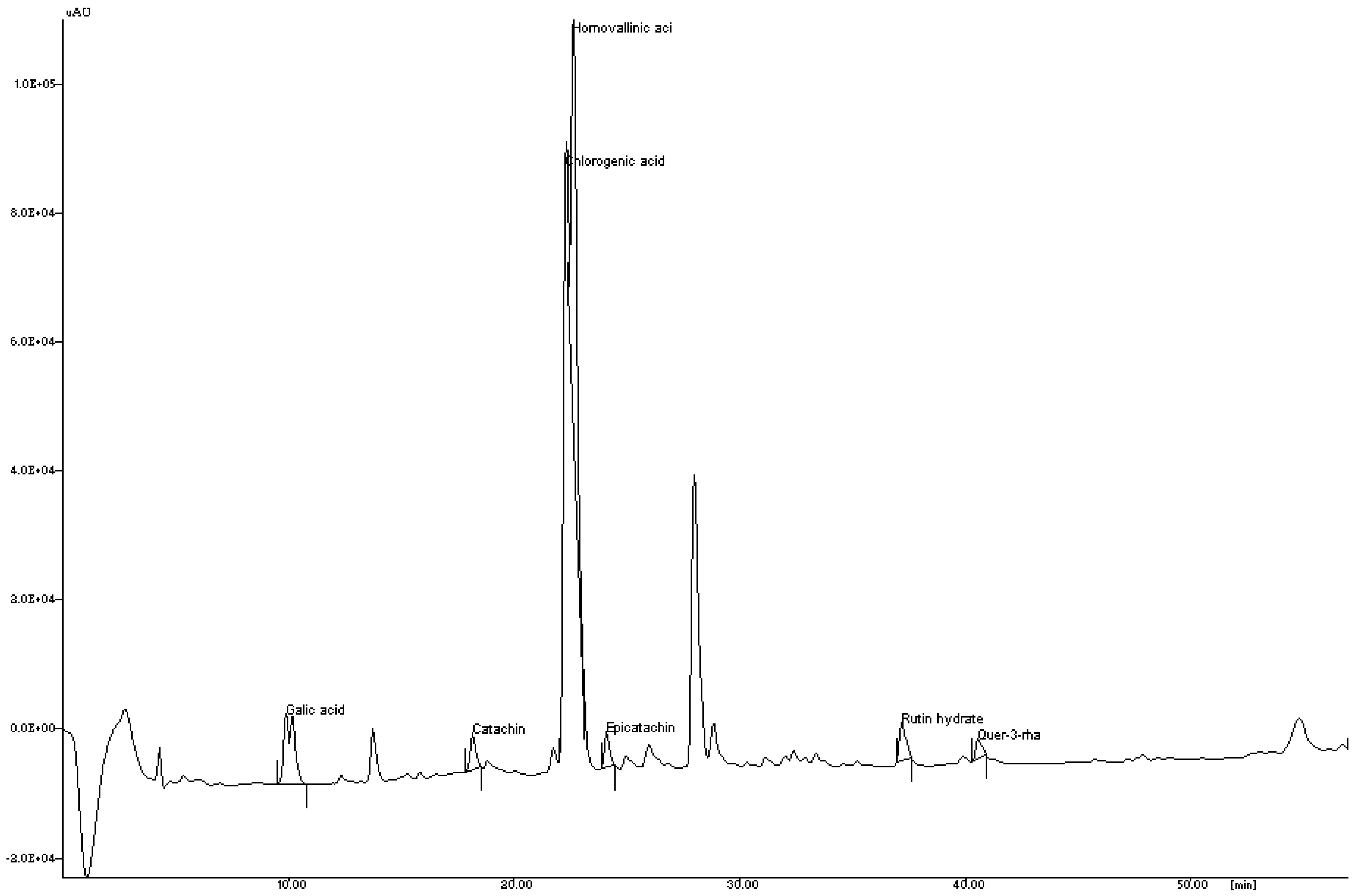
| No. | Polyphenols | RT (min) | Concentration (mg/g) |
|---|---|---|---|
| 1 | Gallic acid | 9.88 | 0.18 |
| 2 | Catechin | 18.16 | 4.37 |
| 3 | Chlorogenic acid | 22.27 | 19.90 |
| 4 | Homovanillin | 22.60 | 32.02 |
| 5 | Epicatechin | 24.09 | 4.23 |
| 6 | Rutin hydrate | 37.17 | 0.08 |
| 7 | Quercetin-3-rhamnoside | 40.56 | 7.13 |
3.1.2. GC-MS Analysis
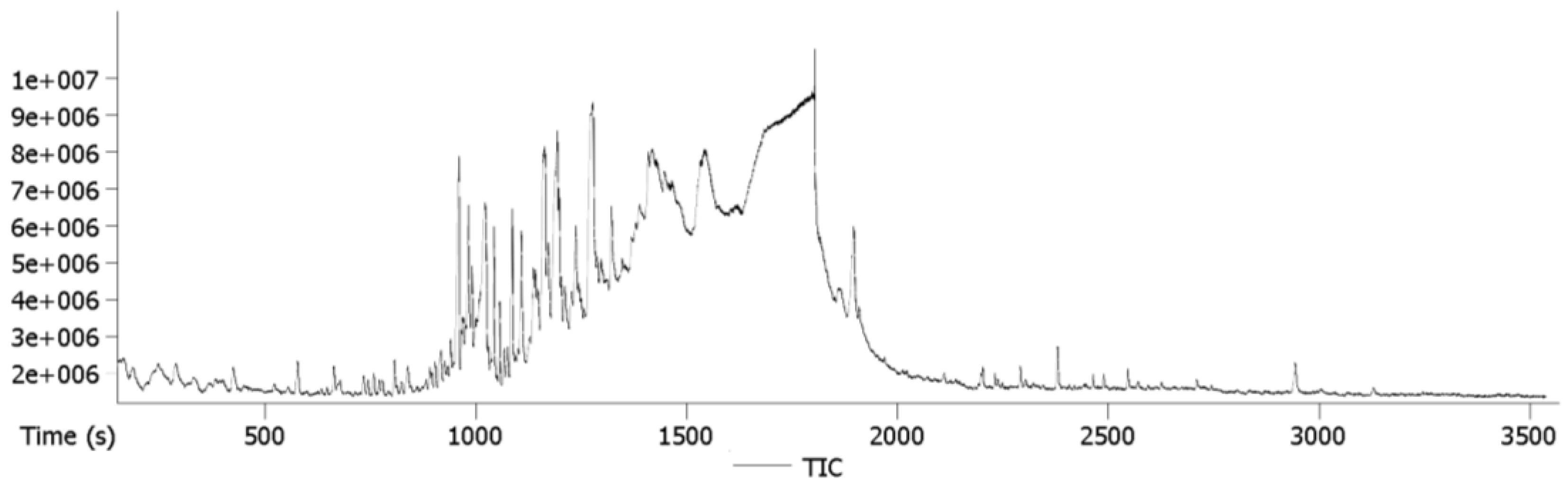
| No. | RT (min) | Compound Name | Chemical Formula | Mass | Area (%) | Hit |
|---|---|---|---|---|---|---|
| 1 | 9.62 | Dodecane | C12H26 | 170.204 | 1.615 | 1 |
| 2 | 11.22 | 2-Furanmethanol, tetrahydro-5-methyl-trans | C6H12O | 116.084 | 0.031 | 1 |
| 3 | 13.98 | Linalool | C10H18O | 154.135 | 0.362 | 1 |
| 4 | 15.30 | Benzenemethanol, a-methyl-propanoate | C11H14O2 | 178.099 | 0.705 | 1 |
| 5 | 15.68 | 1H-Cyclopropa(a)naphthalene, 1a,2,3,5,6,7,7a,7b-octahydro-1,1,7,7a-tetramethyl-(1aR-(1aa,7a,7aa,7ba)) | C15H24 | 204.188 | 1.061 | 1 |
| 6 | 16.02 | a-Muurolene | C15H24 | 204.188 | 14.071 | 1 |
| 7 | 16.39 | 1H-Cycloprop(e)azulene,1a,2,3,4,4a,5,6,7b-octahydro-1,1,4,7-tetramethyl-(1aR-(1aa,4a,4aa,7ba)) | C15H24 | 204.188 | 9.184 | 5 |
| 8 | 16.69 | l-calamenene | C15H22 | 202.172 | 0.065 | 1 |
| 9 | 17.11 | Α-ionone | C13H20O | 192.151 | 0.643 | 1 |
| 10 | 17.41 | Bicyclo(3.3.1)nonan-2-one,1-methyl-9-(1-methylethylidene) | C13H20O | 192.151 | 8.329 | 1 |
| 11 | 17.63 | Neoisolongifolene,8,9-dehydro | C15H22 | 202.172 | 2.002 | 1 |
| 12 | 17.81 | (−)-à-Panasinsen | C15H24 | 204.188 | 1.716 | 1 |
| 13 | 18.13 | Neoisolongifolene,8,9-dehydro | C15H22 | 202.172 | 10.86 | 1 |
| 14 | 18.96 | Bicyclo(2.2.2)octa-2,5-diene, 1,2,3,6-tetramethyl | C12H18 | 162.141 | 3.643 | 1 |
| 15 | 19.15 | Isolongifolene,4,5,9,10-dehydro | C15H20 | 200.157 | 1.24 | 1 |
| 16 | 19.76 | trans-Nerolidol | C15H26O | 222.198 | 4.781 | 1 |
| 17 | 19.88 | para-methoxyphenylpiperazine | C11H16N2O | 192.1263 | 5.576 | 1 |
| 18 | 20.2 | Cyclolongifolene oxide, dehydro | C15H22O | 218.167 | 1.368 | 1 |
| 19 | 21.51 | 1(2H)-Naphthalenone,octahydro-4a,8a-dimethyl-7-(1-ethylethyl)-, (4aR-(4aa,7a,8aa)) | C15H26O | 222.198 | 0.329 | 1 |
| 20 | 22.05 | 2-tetradecenal | C14H26O | 210.1984 | 4.873 | 1 |
| 21 | 30.1 | Palmitic acid | C16H32O2 | 256.24 | 1.537 | 1 |
| 22 | 31.64 | Oleic acid | C18H34O2 | 282.256 | 5.499 | 1 |
| 23 | 32.86 | 2,8,9-Trioxa-5-aza-1-silabicyclo(3.3.3)undecane,1-methoxy | C7H15NO4Si | 205.077 | 0.165 | 1 |
| 24 | 37.22 | Tridecanoic acid, methyl ester | C14H28O2 | 228.209 | 0.625 | 1 |
| 25 | 39.71 | Heptacosane | C27H56 | 380.438 | 2.664 | 1 |
3.2. In Vitro Antioxidant and Free Radical Scavenging Activities
| Assay | Ethanolic Extract (NJE) | Hexane Extract (NJH) |
|---|---|---|
| Total polyphenolic content | 53.06 ± 2.2 mg GAE/g of extract | 13.87 ± 1.3 mg GAE/g of extract |
| Total flavonoids | 25.303 ± 0.9 mg CE/g of extract | 4.58 ± 0.3 mg GAE/g of extract |
| DPPH radical scavenging assay (IC50) | 222.22 ± 7.4 μg/mL | 432.68 ± 13.7 μg/mL |
| Metal chelation (IC50) | 948 ± 21.1 μg/mL | 1211 ± 27.8 μg/mL |
| ABTS (IC50) | 13.90 ± 0.5 μg/mL | 23.57 ± 1.4 μg/mL |
| Superoxide (IC50) | 113.81 ± 4.2 μg/mL | 255.72 ± 9.7 μg/mL |
| Anti-lipid peroxidation (IC50) | 465.11 ± 14.3 μg/mL (brain) | 587.53 ± 17.6 μg/mL (brain) |
| 539.08 ± 18.9 μg/mL (liver) | 685.15 ± 13.4 μg/mL (liver) | |
| Ferric-reducing antioxidant power | 12.3 ± 0.43 mg FeSO4E/g of extract | 45.62 ± 1.34 mg FeSO4E/g of extract |
Polyphenol and Flavonoid Contents
3.3. Protective Properties against Biomolecules Oxidation
3.3.1. NJE Inhibits AAPH-Induced DNA Damage
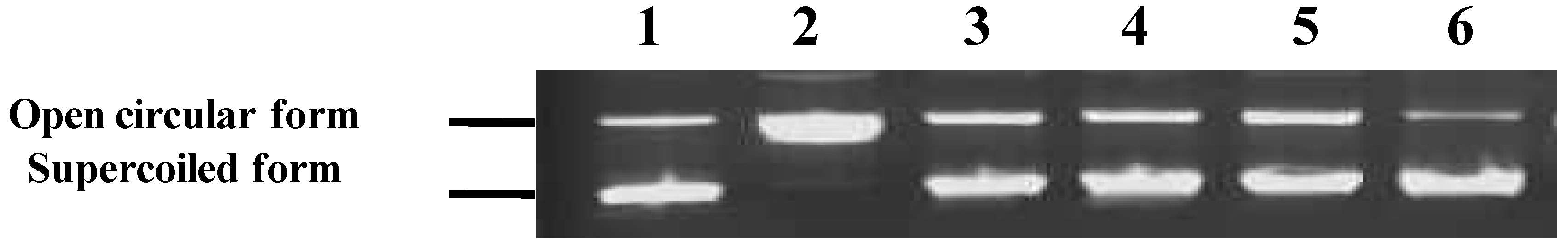
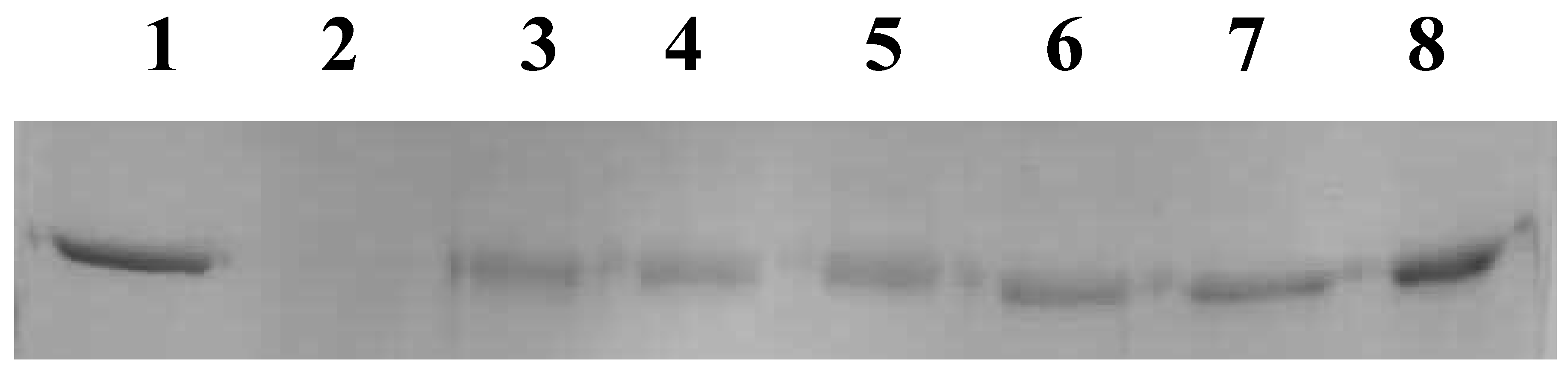
3.3.2. NJE Inhibits Protein Oxidation
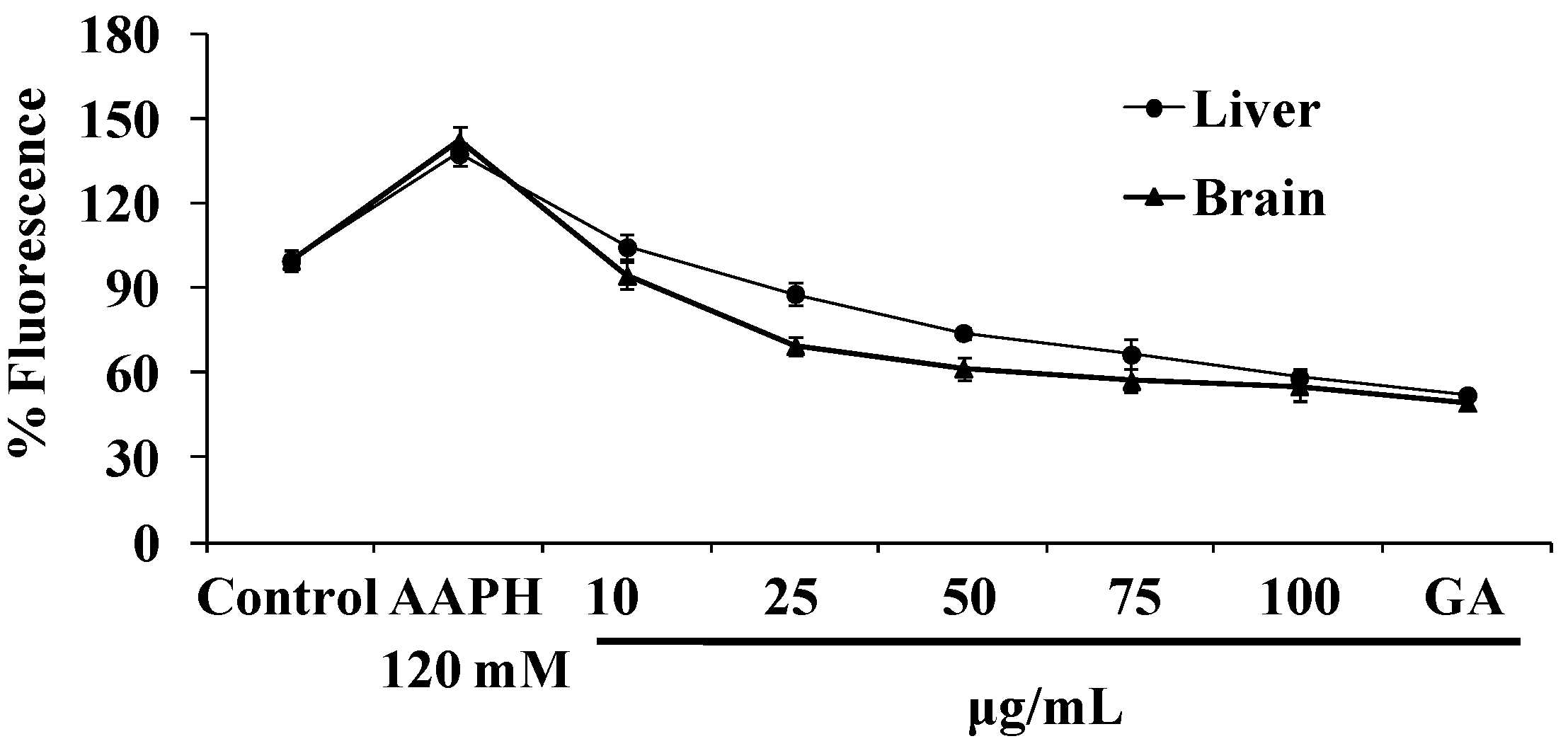
3.3.3. Inhibition of Lipid Peroxidation
3.3.4. Inhibition of Reactive Oxygen Species
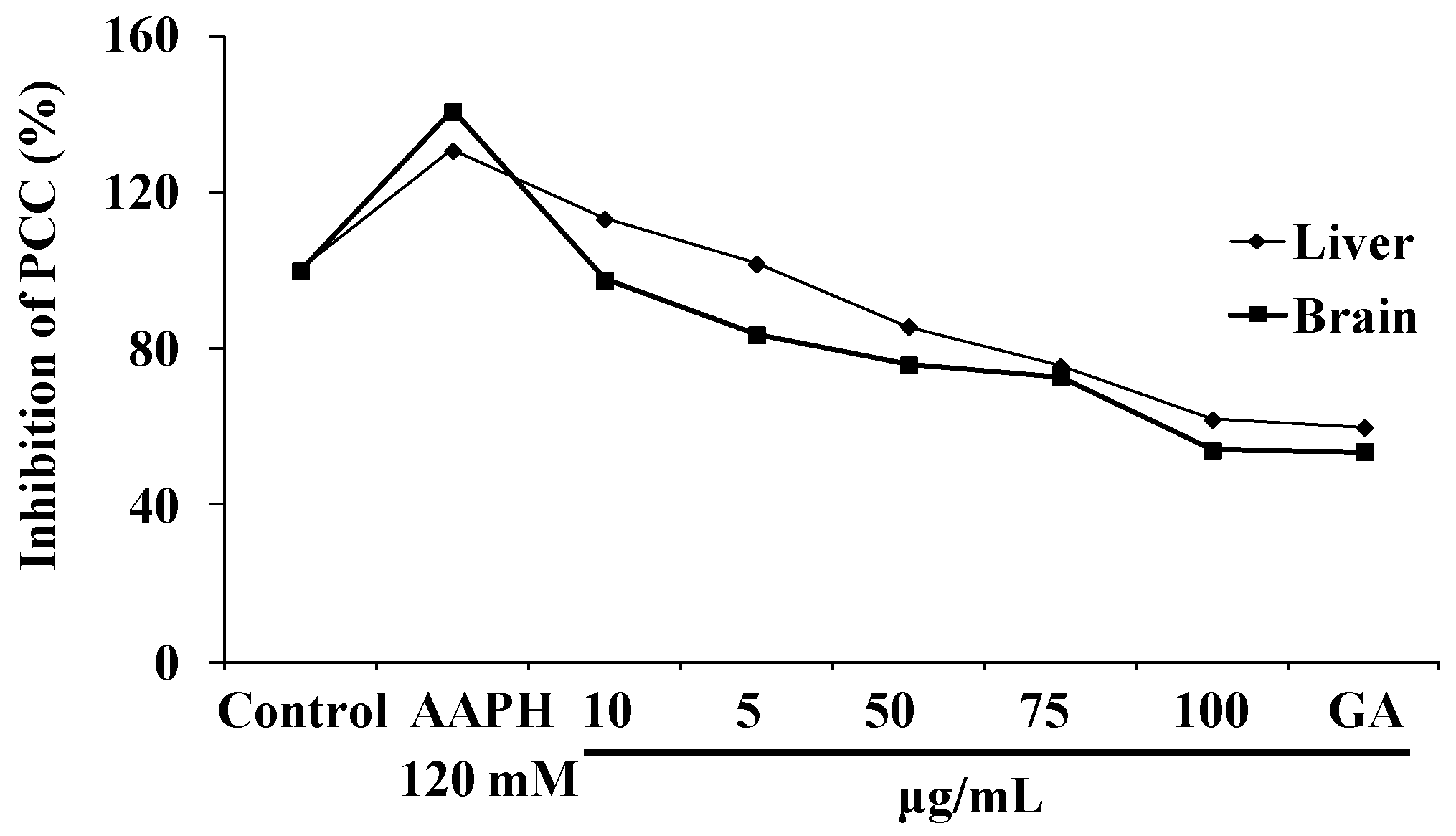
3.3.5. NJE Inhibits Protein Carbonyl Content
3.4. Cytotoxicity of NJE by the Plasma Membrane Leakage Assay

4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Halliwell, B.; Gutteridge, J.M. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Yildirim, A.; Mavi, A.; Oktay, M.; Kara, A.A.; Algur, O.F.; Bilaloglu, V. Comparison of antioxidant and antimicrobial activities of Tilia (Tilia argenta Desf Ex DC), sage (Salvia triloba L.) and black tea (Camellia sinensis) extracts. J. Agric. Food Chem. 2000, 48, 5030–5034. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, I.; Oktay, M.O.; Kufrevioglu, O.I.; Aslan, A. Determination of antioxidant activity of lichen Cetraria islandica (L.). J. Ethnopharmacol. 2002, 79, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Mittova, V.; Volokitam, M.; Guy, M.; Tal, M. Activities of SOD and the ascorbate-glutathione cycle enzymes in subcellular compartments in leaves and roots of the cultivated tomato and its wild salt-tolerant relative Lycopersicon pennellii. Physiol. Plant. 2000, 110, 45. [Google Scholar] [CrossRef]
- Dean, R.T.; Stocker, R.; Davies, M.J. Biochemistry and pathology of radical-mediated protein. J. Biochem. 1997, 324, 1–18. [Google Scholar] [CrossRef]
- Aruoma, O.I. Free radicals, oxidative stress and antioxidants in human health and disease. J. Am. Oil Chem. Soc. 1998, 75, 199–212. [Google Scholar] [CrossRef]
- Percival, M. Antioxidants. Clin. Nutr. Insight 1998, 31, 1–4. [Google Scholar]
- Ashokkumar, D.; Mazumder, U.K.; Gupta, M.; Senthilkumar, G.P.; Selvan, V.T. Evaluation of antioxidant and free radical scavenging activities of Oxystelma esculentum in various in vitro models. J. Compliment. Integr. Med. 2008, 5, 1553–3840. [Google Scholar]
- Veerapur, V.P.; Prabhakar, K.R.; Parihar, V.P.; Kandadi, M.R.; Ramakrishana, S.; Mishra, B.; Satish Rao, B.S.; Srinivasan, K.K.; Priyadarsini, K.I.; Unnikrishnan, M.K. Ficus racemosa stem bark extract: A potent antioxidant and a probable natural radioprotector. Evid. Based Complement. Alternat Med. 2009, 6, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Kitts, D.D.; Yuan, Y.V.; Wijewickreme, A.N.; Hu, C. Antioxidant properties of a North American gingseng extract. Mol. Cell. Biochem. 2000, 203, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Muselík, J.; García-Alonso, M.; Martín-López, M.P.; Želmièka, M.; Rivas-Gonzalo, J.C. Measurement of antioxidant activity of wine catechins, procyanidins, antocyanins and piranoantocyanins. Int. J. Mol. Sci. 2007, 8, 797–809. [Google Scholar] [CrossRef]
- Aviram, M. Review of human studies on oxidative damage and antioxidant protection related to cardiovascular diseases. Free Radic. Res. 2000, 33, 85–97. [Google Scholar]
- Baynes, J.W. From life to death-the struggle between chemistry and biology during aging: The Maillard reaction as an amplifier of genomic damage. Biogerontology 2000, 1, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Oxidative stress, nutrition and health. Experimental strategies for optimization of nutritional antioxidant intake in humans. Free Radic. Res. 1996, 25, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.S.; Rao, A.; Karanth, K.S. Anticonvulsant and neurotoxicity profile of Nardostachys jatamansi in rats. J. Ethnopharmacol. 2005, 102, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Yousuf, S.; Khan, M.B.; Hoda, M.N.; Ahmad, A.S.; Ansari, M.A.; Ishrata, T.; Agrawalb, A.K.; Islama, F. Attenuation by Nardostachys jatamansi of 6-hydroxydopamine-induced parkinsonism in rats: Behavioral, neurochemical, and immunohistochemical studies. Pharmacol. Biochem. Behav. 2006, 83, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Subashini, R.; Yogeeta, S.; Gnanapragasam, A.; Devaki, T. Protective effect of Nardostachys jatamansi on oxidative injury and cellular abnormalities during doxorubicin-induced cardiac damage in rats. J. Pharm. Pharmacol. 2006, 58, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Joshi, H.; Parle, M. Nardostachys jatamansi improves learning and memory in mice. J. Med. Food 2006, 9, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.P.; Chauhan, N.S.; Padh, H.; Rajani, M. Search for antibacterial and antifungal agents from selected Indian medicinal plants. J. Ethnopharmacol. 2006, 107, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, D.; Goyal, P.K. Inhibition of MAO and GABA: Probable mechanisms for antidepressant-like activity of Nardostachys jatamansi DC. in mice. Indian J. Exp. Biol. 2008, 46, 212–218. [Google Scholar] [PubMed]
- Dandagi, P.M.; Patil, M.B.; Mastiholimath, V.S.; Gadad, A.P.; Dhumansure, R.H. Development and evaluation of hepatoprotective polyherbal formulation containing some indigenous medicinal plants. Indian J. Pharm. Sci. 2008, 70, 265. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, V.M.; Thorat, R.M.; Kadam, V.J.; Kamble, S.S. Herbal anxiolyte: Nardostachys jatamansi. J. Pharm. Res. 2009, 2, 1208–1211. [Google Scholar]
- Khan, M.B.; Hoda, M.N.; Ishrat, T.; Ahmad, S.; Khan, M.M.; Ahmad, A.; Yusuf, S.; Islama, F. Neuroprotective efficacy of Nardostachys jatamansi and crocetin in conjunction with selenium in cognitive impairment. Neurol. Sci. 2012, 33, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Ross, K.A.; Beta, T.; Arntfield, S.D. A comparative study on the phenolic acids identified and quantified in dry beans using HPLC as affected by different extraction and hydrolysis methods. Food Chem. 2009, 113, 336–344. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic acid-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Sakanaka, S.; Tachibana, Y.; Okada, Y. Preparation and antioxidant properties of extracts of Japanese persimmon leaf tea (kakinoha-cha). Food Chem. 2005, 89, 569–575. [Google Scholar] [CrossRef]
- Eberhardt, M.V.; Lee, C.Y.; Liu, R.H. Antioxidant activity of fresh apple. Nature 2000, 405, 903–904. [Google Scholar] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Dinis, T.C.P.; Madeira, V.M.C.; Almeida, L.M. Action of phenolic derivatives (acetoaminophen, Salicylate and 5-aminosalicylate) as inhibitora of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.Y.; Wang, H.X.; Liu, F.; Ng, T.B. Ribonuclease, cell free transalation inhibitory and superoxide radical scavenging activities of the iron-binding protein lactoferrin from bovine mill. Int. J. Biochem. Cell Biol. 2000, 32, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.E.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Kim, H.R.; Kim, T.; Jang, Y.S. Antioxidant property of an ethanol extract of the stem of Opuntia ficus-indica Var. Saboten. J. Agric. Food Chem. 2002, 50, 6490–6496. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.Y.; Choi, S.Y.; Won, M.H.; Kang, T.C.; Kang, J.H. Oxidative modification and inactivation of Cu, Zn-superoxide dismutase by 2, 2′-azobis(2-amidinopropane) dihydrochloride. Biochim. Biophys. Acta 2000, 1543, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Bahramikia, S.; Ardestani, A.; Yazdanparast, R. Protective effects of four Iranian plants against free radical-mediated protein oxidation. Food Chem. 2009, 115, 37–42. [Google Scholar] [CrossRef]
- Kobayashi, D.; Kondo, K.; Uehara, N.; Otokozawa, S.; Tsuji, N.; Yagihashi, A.; Watanabe, N. Endogenous reactive oxygen species is an important endogenous reactive oxygen species is an important mediator of miconazole antifungal effect. Phytomedicine 2008, 15, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Reznick, A.Z.; Packer, L. Oxidative damage to proteins: Spectrophotometric method for carbonyl assay. Methods Enzymol. 1994, 233, 357–363. [Google Scholar] [PubMed]
- Cai, Y.J.; Fang, J.G.; Ma, L.P.; Li, Y.; Liu, Z.L. Inhibition of free radical induced peroxidation of rat liver microsomes by resveratrol and its analogues. Biochim. Biophys. Acta 2003, 1637, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Dalle-Donnea, I.; Rossib, R.; Giustarinib, D.; Milzania, A.; Colomboa, R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta 2003, 329, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Leeuwenburgh, C.; Heinecke, J.W. Oxidative stress and antioxidants in exercise. Curr. Med. Chem. 2001, 8, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.K.; Mori, A. Stress, aging, brain oxidative damage. Neurochem Res 1999, 24, 1479–1497. [Google Scholar] [CrossRef] [PubMed]
- Bouayed, J. Polyphenols: A potential new strategy for the prevention and treatment of anxiety and depression. Curr. Nutr. Food Sci. 2010, 6, 13–18. [Google Scholar] [CrossRef]
- Bouayed, J.; Rammal, H.; Dicko, A.; Younos, C.; Soulimani, R. Chlorogenic acid, a polyphenol from Prunus domestica (Mirabelle), with coupled anxiolytic and antioxidant effects. J. Neurol. Sci. 2007, 262, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Neganova, M.E.; Afanas’eva, S.V.; Klochkov, S.G.; Shevtsovs, E.F. Mechanisms of antioxidant effect of natural sesquiterpene lactone and alkaloid derivatives. Bull. Exp. Biol. Med. 2012, 152, 720–722. [Google Scholar] [CrossRef] [PubMed]
- Linck, V.M.; da Silva, A.L.; Figueiro, M.; Caramao, E.B.; Morena, P.R.H.; Elisabetsky, E. Effects of inhaled linalool in anxiety, social interaction and aggressive behaviour in mice. Phytomedicine 2010, 17, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Richard, D.; Kefi, K.; Barbe, U.; Bausero, P.; Visioli, F. Polyunsaturated fatty acids as antioxidants. Pharmacol. Res. 2008, 57, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Kalmijn, S.; Feskens, E.J.M.; Launer, L.J.; Kromhout, D. Polyunsaturated fatty acids, antioxidants, and cognitive function in very old men. Am. J. Epidemiol. 1997, 145, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Bramley, P.M.; Pridham, J.B. The relative antioxidant activity of plant derived polyphenolic flavonoids. Free Radic. Res. 1995, 22, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Hoerster, H.; Ruecker, G.; Tautges, J. Valeranone content in the roots of Nardostachys jatamansi and Valeriana officinalis. Phytochem 1977, 1, 1070–1071. [Google Scholar] [CrossRef]
- Rucker, G.; Tautges, J.; Sleck, A.; Wenzl, H.; Graf, E. Isolation and pharmacological activity of the sesquiterpene valeranone from Nardostachys jatamansi DC (in German). Arzneimittelforschung 1978, 28, 7–13. [Google Scholar] [PubMed]
- Aiyegoro, O.A.; Okoh, A.I. Prelimnary phytochemical screening and invitro antioxidant activities of the aqueous extract of Helichrysum longifolium DC. BMC Complement. Altern. Med. 2010, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.H. The mechanism of antioxidant action in vitro. In Food Antioxidants; Hudson, B.J.F., Ed.; Elsevier Applied Science: London, UK, 1990; pp. 1–18. [Google Scholar]
- Dorman, H.J.D.; Peltoketo, A.; Hiltunen, R.; Tikkanen, M.J. Characterisation of the antioxidant properties of deodorized aqueous extracts from selected Lamiaceae herbs. Food Chem. 2003, 83, 255–262. [Google Scholar] [CrossRef]
- Kumar, R.S.; Rajkapoor, B.; Perumal, P. Antioxidant activities of Indigofera cassioides Rottl. Ex. DC. using various in vitro assay models. Asian Pac. J. Trop. Biomed. 2012, 2, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Hemanth Kumar, K.; Razack, S.; Nallamuthu, I.; Khanum, F. Phytochemical analysis and biological properties of Cyperus rotundus L. Ind. Crops Prod. 2014, 52, 815–826. [Google Scholar] [CrossRef]
- Yang, J.L.; Weissman, L.; Bohr, V.A.; Mattson, M.P. Mitochondrial DNA damage and repair in neurodegenerative disorders. DNA Repair 2008, 7, 1110–1120. [Google Scholar] [CrossRef] [PubMed]
- Barrera, G.; Pizzimenti, S.; Dianzani, M.U. Lipid peroxidation: Control of cell proliferation cell differentiation and cell death. Mol. Aspects Med. 2008, 29, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Roche, M.; Tarnus, E.; Rondeau, P.; Bourdon, E. Effects of nutritional antioxidants on AAPH- or AGEs-induced oxidative stress in human SW872 liposarcoma cells. Cell Biol. Toxicol. 2009, 25, 635–644. [Google Scholar] [CrossRef] [PubMed]
- LeBel, C.P.; Ischiropoulos, H.; Bondys, S.C. Evaluation of the probe 2′,7′-dichiorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem. Res. Toxicol. 1992, 5, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R.; Levin, R.L. Protein oxidation. Ann. N. Y. Acad. Sci. 2000, 899, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Ardestani, A.; Yazdanparast, R. Antioxidant and free radical scavenging potential of Achillea santolina extracts. Food Chem. 2007, 104, 21–29. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razack, S.; Kumar, K.H.; Nallamuthu, I.; Naika, M.; Khanum, F. Antioxidant, Biomolecule Oxidation Protective Activities of Nardostachys jatamansi DC and Its Phytochemical Analysis by RP-HPLC and GC-MS. Antioxidants 2015, 4, 185-203. https://doi.org/10.3390/antiox4010185
Razack S, Kumar KH, Nallamuthu I, Naika M, Khanum F. Antioxidant, Biomolecule Oxidation Protective Activities of Nardostachys jatamansi DC and Its Phytochemical Analysis by RP-HPLC and GC-MS. Antioxidants. 2015; 4(1):185-203. https://doi.org/10.3390/antiox4010185
Chicago/Turabian StyleRazack, Sakina, Kandikattu Hemanth Kumar, Ilaiyaraja Nallamuthu, Mahadeva Naika, and Farhath Khanum. 2015. "Antioxidant, Biomolecule Oxidation Protective Activities of Nardostachys jatamansi DC and Its Phytochemical Analysis by RP-HPLC and GC-MS" Antioxidants 4, no. 1: 185-203. https://doi.org/10.3390/antiox4010185
APA StyleRazack, S., Kumar, K. H., Nallamuthu, I., Naika, M., & Khanum, F. (2015). Antioxidant, Biomolecule Oxidation Protective Activities of Nardostachys jatamansi DC and Its Phytochemical Analysis by RP-HPLC and GC-MS. Antioxidants, 4(1), 185-203. https://doi.org/10.3390/antiox4010185