Ceruloplasmin and Hypoferremia: Studies in Burn and Non-Burn Trauma Patients
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
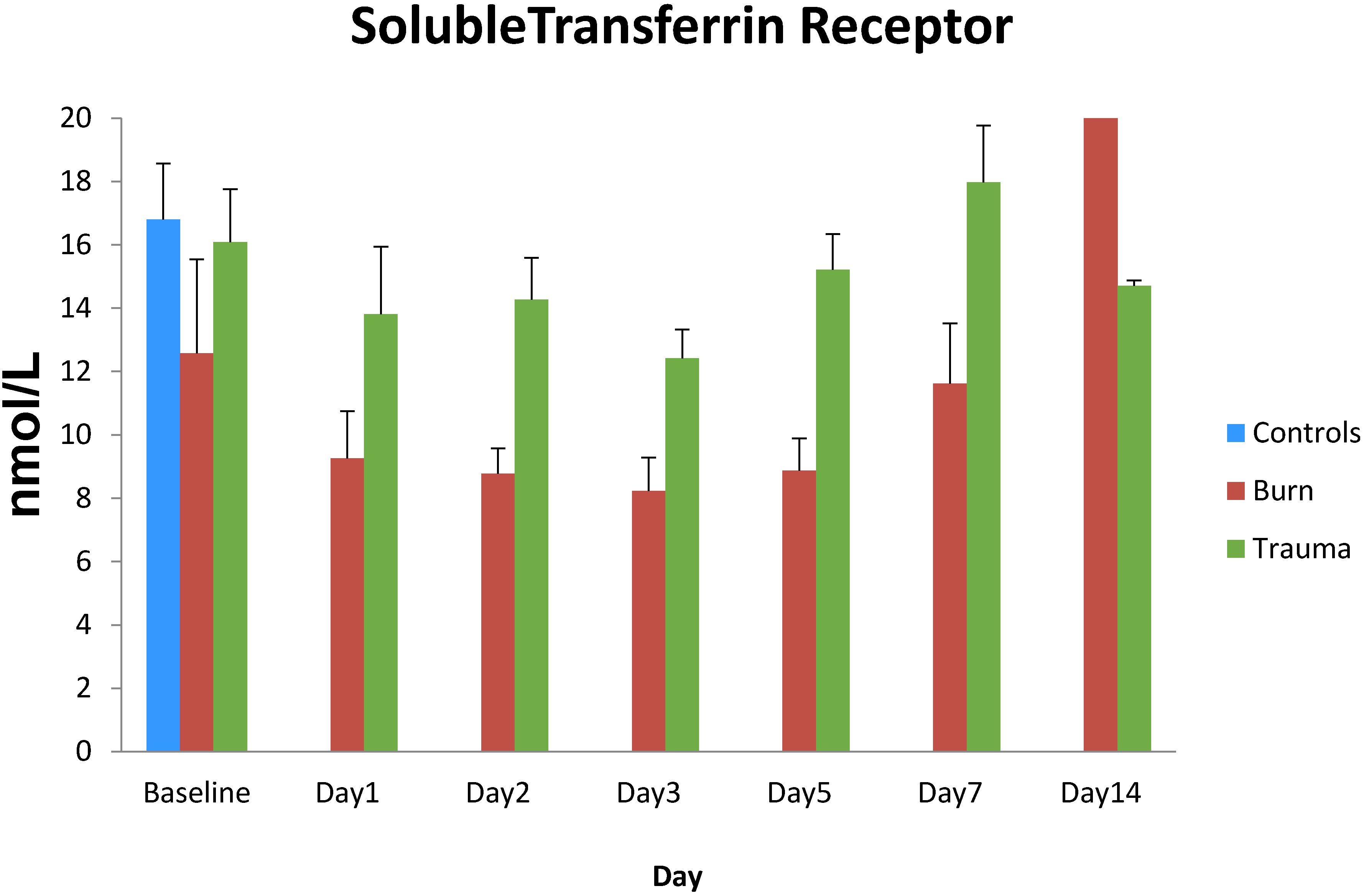
3. Results
| Gender: Male n = 10, Female n = 0 |
|---|
| Age: Range 20–50 years; Mean ± SE: 36.8 ± 3.0 years |
| Total Body Surface Area Burn: Range 19%–76%; Mean ± SE: 36.8% ± 5.3% |
| Total Full Thickness Burn: Range 0%–56%; Mean ± SE:20% ± 5.7% |
| Total ICU Time: Range 9–42 days; Mean ± SE: 24.9 ± 4.1 days |
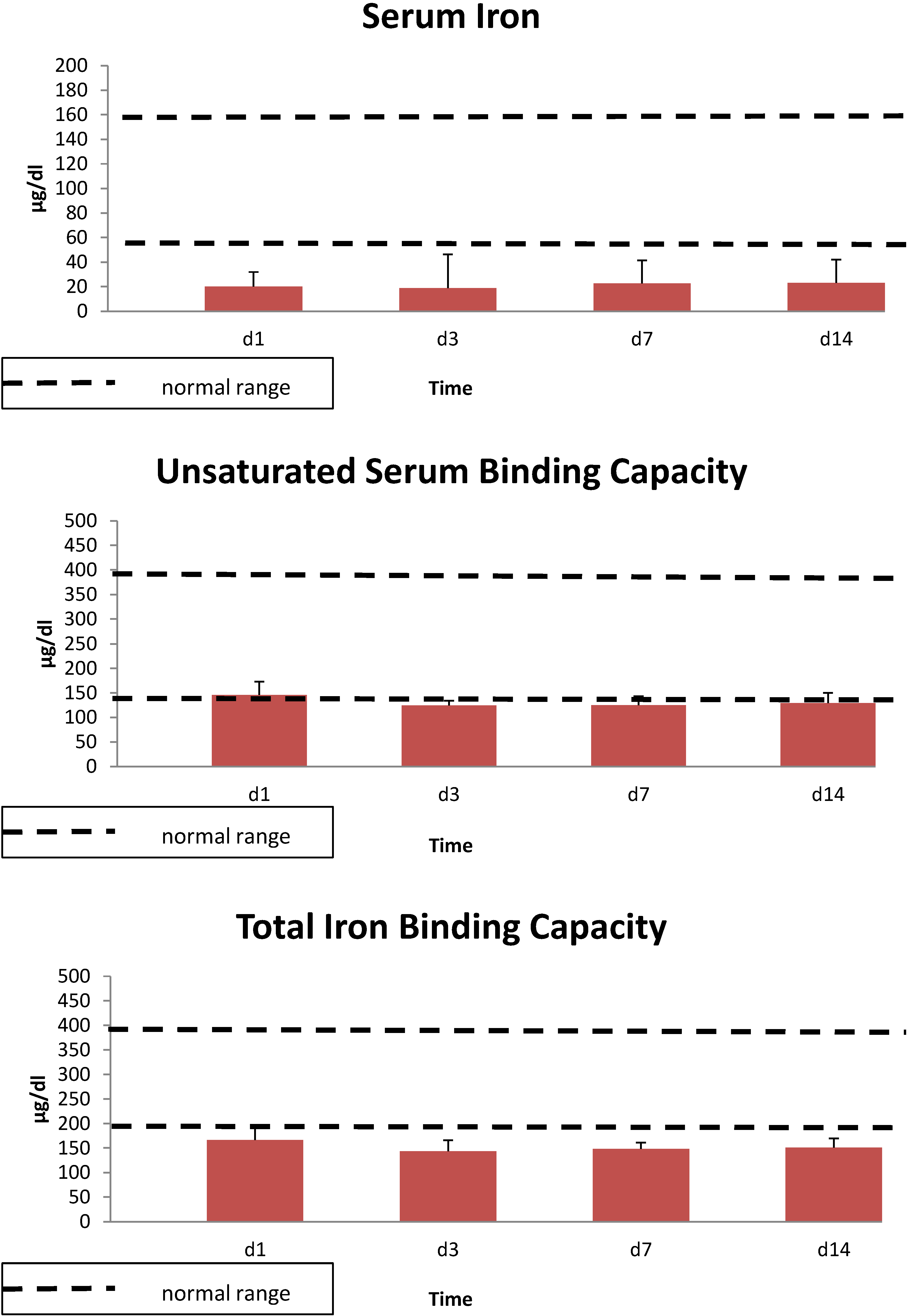
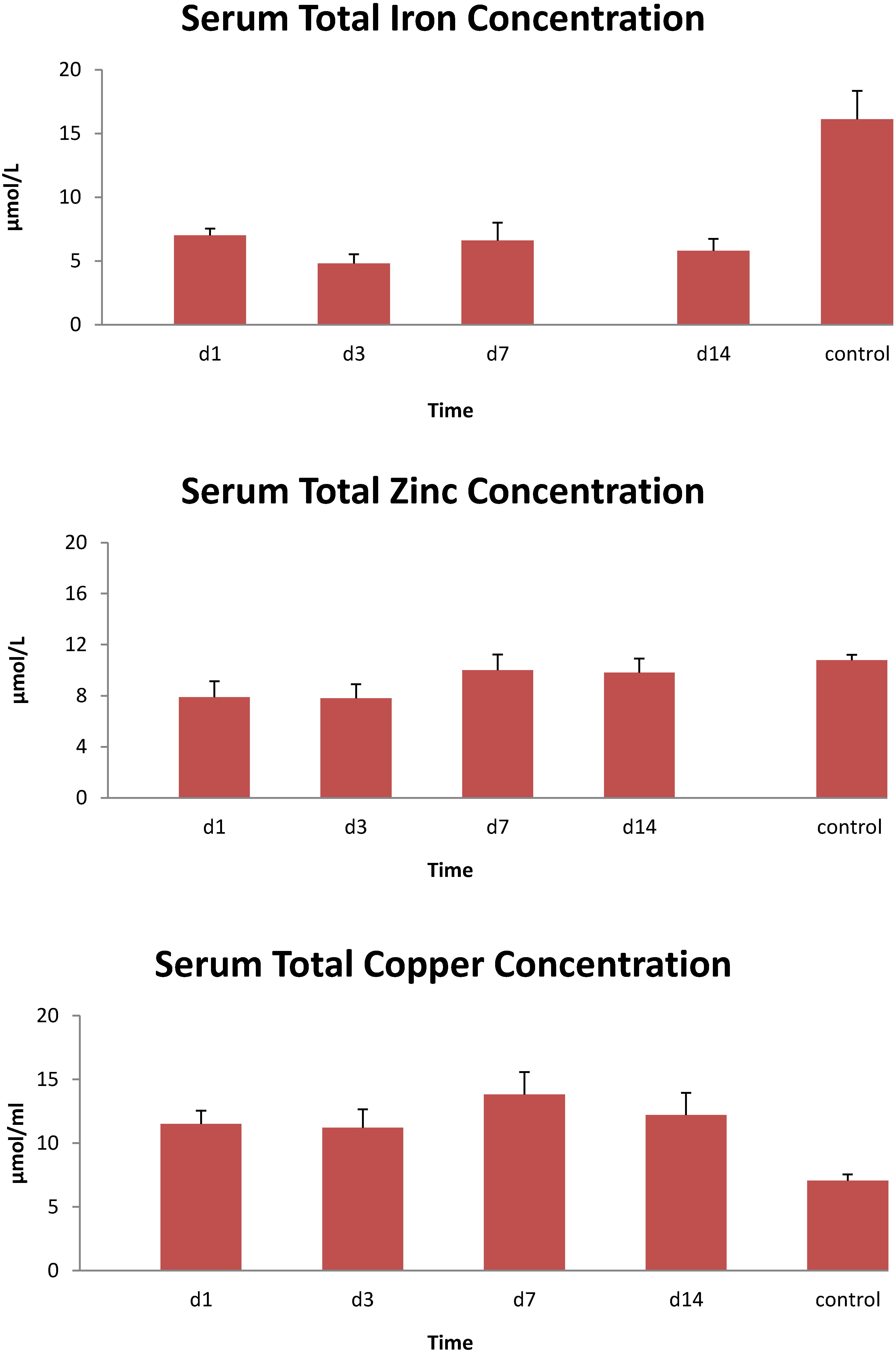

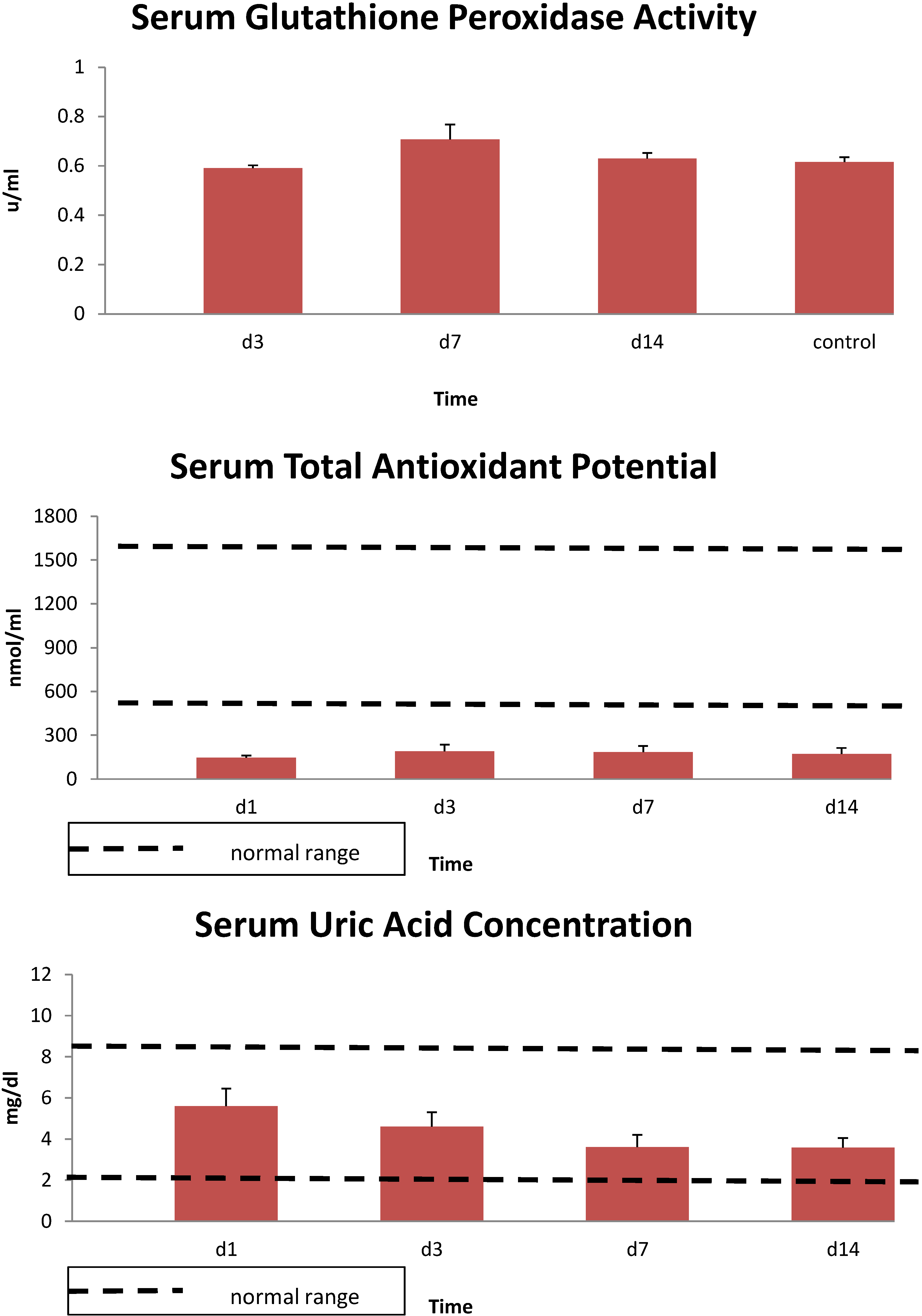
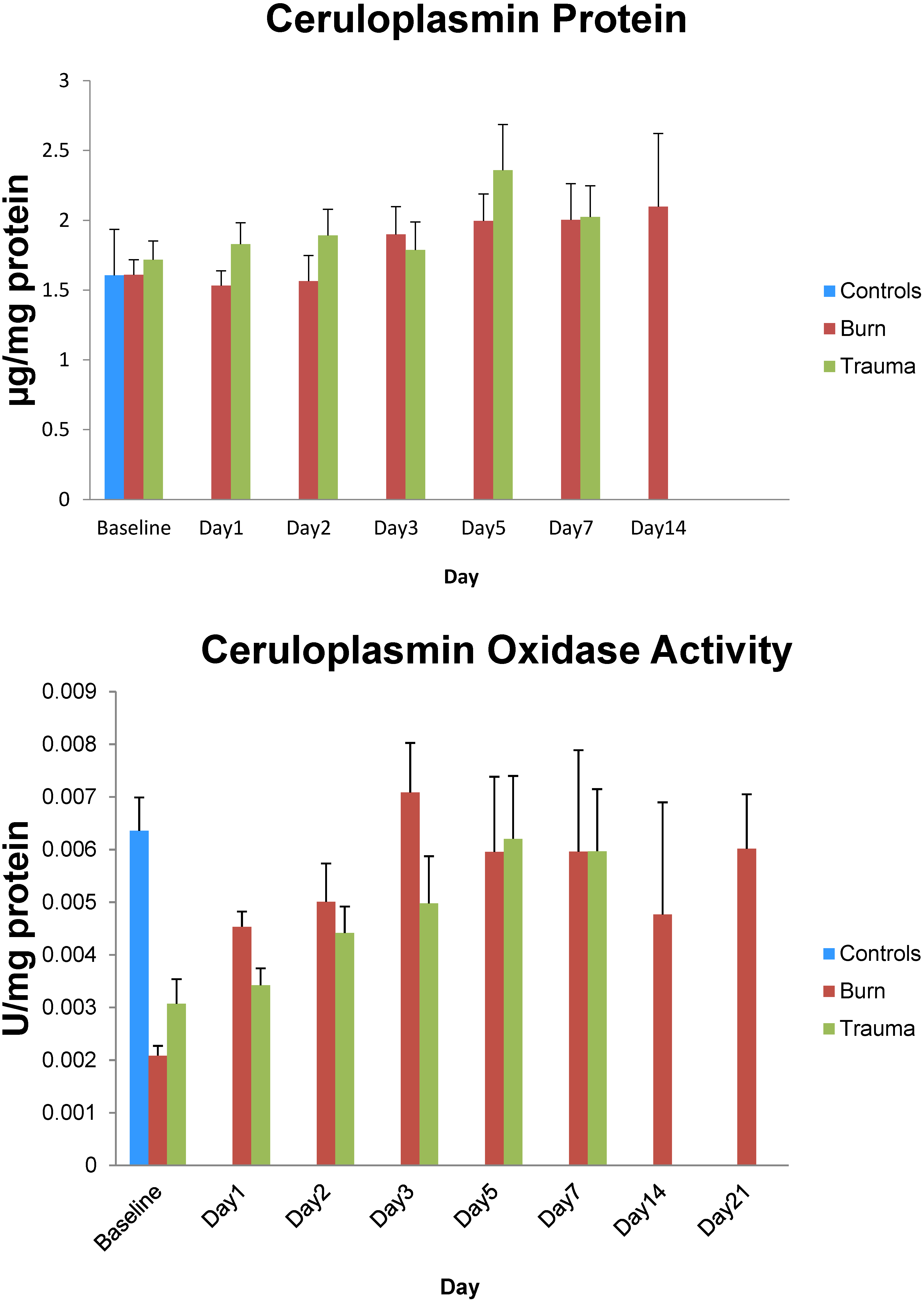
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Anderson, G.J.; Frazer, D.M. Hepatic iron metabolism. Semin. Liver Dis. 2005, 25, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.J.; Wang, F. Essential but toxic: Controlling the flux of iron in the body. Clin. Exp. Pharmacol. Physiol. 2012, 39, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Frazer, D.M.; Anderson, G.J. The regulation of iron transport. BioFactors 2014, 40, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pantopoulos, K. Regulation of cellular iron metabolism. Biochem. J. 2011, 434, 365–381. [Google Scholar] [CrossRef] [PubMed]
- Fleming, R.E.; Ponka, P. Iron overload in human disease. N. Engl. J. Med. 2012, 366, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.S.; Pattanapanyasat, K.; Lamchiagdhase, P.; Siritongtaworn, P.; Thavichaigarn, P.; Jiarakul, N.; Chuntrasakul, C.; Komoltri, C.; Dheeradhada, C.; Pearce, F.C.; et al. Iron status following trauma, excluding burns. Br. J. Surg. 1996, 83, 982–985. [Google Scholar] [CrossRef] [PubMed]
- Wessling-Resnick, M. Iron homeostasis and the inflammatory response. Ann. Rev. Nutr. 2010, 30, 105–122. [Google Scholar] [CrossRef]
- Atkins, J.L.; Gorbunov, N.V.; Trabosh, V.; van Duyne, R.; Kashanchi, F.; Komarov, A.M. Ferrous iron is found in mesenteric lymph bound to TIMP-2 following hemorrhage/resuscitation. Biometals 2011, 24, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, R.A.; Donnelly, S.C.; Connelly, K.G.; Robertson, C.E.; Haslett, C.; Repine, J.E. Initial serum ferritin levels in patients with multiple trauma and the subsequent development of acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 1999, 159, 1506–1509. [Google Scholar] [CrossRef] [PubMed]
- Corwin, H.L.; Krantz, S.B. Anemia of the critically ill: “Acute” anemia of chronic disease. Crit. Care Med. 2000, 28, 3098–3099. [Google Scholar] [CrossRef] [PubMed]
- Berlin, T.; Meyer, A.; Rotman-Pikielny, P.; Natur, A.; Levy, Y. Soluble transferrin receptor as a diagnostic laboratory test for detection of iron deficiency anemia in acute illness of hospitalized patients. Isr. Med. Assoc. J. 2011, 13, 96–98. [Google Scholar] [PubMed]
- Pieracci, F.M.; Stovall, R.T.; Jaouen, B.; Rodil, M.; Cappa, A.; Burlew, C.C.; Holena, D.N.; Maier, R.; Berry, S.; Jurkovich, J.; et al. A multicenter, randomized clinical trial of IV iron supplementation for anemia of traumatic critical illness. Crit. Care Med. 2014, 42, 2048–2057. [Google Scholar] [CrossRef] [PubMed]
- Kemna, E.; Pickkers, P.; Nemeth, E.; van der Hoeven, H.; Swinkels, D. Time-course analysis of hepcidin, serum iron, and plasma cytokine levels in humans injected with LPS. Blood 2005, 106, 1864–1866. [Google Scholar] [CrossRef] [PubMed]
- Ward, D.M.; Kaplan, J. Ferroportin-mediated iron transport: Expression and regulation. Biochim. Biophys. Acta 2012, 1823, 1426–1433. [Google Scholar] [CrossRef] [PubMed]
- Deschemin, J.C.; Vaulont, S. Role of hepcidin in the setting of hypoferremia during acute inflammation. PLoS One 2013, 8, e61050. [Google Scholar] [CrossRef] [PubMed]
- Sihler, K.C.; Raghavendran, K.; Westerman, M.; Ye, W.; Napolitano, L.M. Hepcidin in trauma: Linking injury, inflammation, and anemia. J. Trauma 2010, 69, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Gorbunov, N.V.; Volgarev, A.P.; Brailovskaia, I.V.; Bykova, N.O.; Avrova, N.F.; Kiselev, O.I. Activation of free radicals reaction and changes in the state of antioxidant protection in blood in toxic experimental influenza infection. Biull. Eksp. Biol. Med. 1992, 114, 42–44. [Google Scholar] [PubMed]
- Cunningham, J.J.; Lydon, M.K.; Emerson, R.; Harmatz, P.R. Low ceruloplasmin levels during recovery from major burn injury: Influence of open wound size and copper supplementation. Nutrition 1996, 12, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Dauberschmidt, R.; Mrochen, H.; Forster, I.; Stumpe, C.; Dressler, C.; Grajetzki, H.; Meyer, M. Changes in ceruloplasmin activity and lactate concentration in patients at high risk of acute organ system failure. Clin. Chim. Acta 1991, 199, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S.; Martini, W.Z.; Dubick, M.A.; Salinas, J.; Butenas, S.; Kheirabadi, B.S.; Pusateri, A.E.; Vos, J.A.; Guymon, C.H.; Wolf, S.E.; et al. Thromboelastography as a better indicator of hypercoagulable state after injury than prothrombin time or activated partial thromboplastin time. J. Trauma 2009, 67, 266–275, discussion 275–276. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, R.A.; Burk, R.F. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem. Biophys. Res. Commun. 1976, 71, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The frap assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Golub, M.S.; Han, B.; Keen, C.L. Developmental patterns of aluminum and five essential mineral elements in the central nervous system of the fetal and infant guinea pig. Biol. Trace Elem. Res. 1996, 55, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Macintyre, G.; Gutfreund, K.S.; Martin, W.R.; Camicioli, R.; Cox, D.W. Value of an enzymatic assay for the determination of serum ceruloplasmin. J. Lab. Clin. Med. 2004, 144, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Gorbunov, N.V.; McFaul, S.J.; van Albert, S.; Morrissette, C.; Zaucha, G.M.; Nath, J. Assessment of inflammatory response and sequestration of blood iron transferrin complexes in a rat model of lung injury resulting from exposure to low-frequency shock waves. Crit. Care Med. 2004, 32, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Drakesmith, H.; Prentice, A.M. Hepcidin and the iron-infection axis. Science 2012, 338, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Postl, A.; Zifko, C.; Hartl, R.T.; Ebel, T.; Miller, I.; Moldzio, R.; Redl, H.; Kozlov, A.V.; Bahrami, S.; Duvigneau, J.C. Transient increase of free iron in rat livers following hemorrhagic-traumatic shock and reperfusion is independent of heme oxygenase 1 upregulation. Shock 2011, 36, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, A.V.; Duvigneau, J.C.; Hyatt, T.C.; Raju, R.; Behling, T.; Hartl, R.T.; Staniek, K.; Miller, I.; Gregor, W.; Redl, H.; et al. Effect of estrogen on mitochondrial function and intracellular stress markers in rat liver and kidney following trauma-hemorrhagic shock and prolonged hypotension. Mol. Med. 2010, 16, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Lawen, A.; Lane, D.J. Mammalian iron homeostasis in health and disease: Uptake, storage, transport and molecular mechanisms of action. Antioxid. Redox Signal. 2013, 18, 2473–2507. [Google Scholar] [CrossRef] [PubMed]
- Brissot, P.; Ropert, M.; le Lan, C.; Loreal, O. Non-transferrin bound iron: A key role in iron overload and iron toxicity. Biochim. Biophys. Acta 2012, 1820, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Hod, E.A.; Brittenham, G.M.; Billote, G.B.; Francis, R.O.; Ginzburg, Y.Z.; Hendrickson, J.E.; Jhang, J.; Schwartz, J.; Sharma, S.; Sheth, S.; et al. Transfusion of human volunteers with older, stored red blood cells produces extravascular hemolysis and circulating non-transferrin-bound iron. Blood 2011, 118, 6675–6682. [Google Scholar] [CrossRef] [PubMed]
- Edeleva, N.V.; Osipova, N.A.; Nemtsova, E.R.; Iakubovskaia, R.I.; Ivanova, L.M.; Chissov, V.I. Role of the antioxidant ceruloplasmin in complex intensive therapy for severe posthemorrhagic complications in cancer surgery. Anesteziol. Reanimatol. 2005, Sep–Oct, 49–51. [Google Scholar]
- Demling, R.; LaLonde, C.; Ikegami, K. Fluid resuscitation with deferoxamine hetastarch complex attenuates the lung and systemic response to smoke inhalation. Surgery 1996, 119, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.W.; Shapiro, M.J.; Ali, M.A.; Chang, Y.J.; Taylor, W.H. Deferoxamine and hespan complex as a resuscitative adjuvant in hemorrhagic shock rat model. Shock 2002, 17, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Lagan, A.L.; Melley, D.D.; Evans, T.W.; Quinlan, G.J. Pathogenesis of the systemic inflammatory syndrome and acute lung injury: Role of iron mobilization and decompartmentalization. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 294, L161–L174. [Google Scholar] [CrossRef] [PubMed]
- Van Iperen, C.E.; Gaillard, C.A.; Kraaijenhagen, R.J.; Braam, B.G.; Marx, J.J.; van de Wiel, A. Response of erythropoiesis and iron metabolism to recombinant human erythropoietin in intensive care unit patients. Crit. Care Med. 2000, 28, 2773–2778. [Google Scholar] [PubMed]
- Gabriel, A.; Kozek, S.; Chiari, A.; Fitzgerald, R.; Grabner, C.; Geissler, K.; Zimpfer, M.; Stockenhuber, F.; Bircher, N.G. High-dose recombinant human erythropoietin stimulates reticulocyte production in patients with multiple organ dysfunction syndrome. J. Trauma 1998, 44, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Zdravkovic, D. Changes in serum ferritin following surgical trauma. Eur. J. Haematol. 1987, 38, 60–62. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.Y.; Hybertson, B.M.; Wright, R.M.; Repine, J.E. Serum ferritin increases in hemorrhaged rats that develop acute lung injury: Effect of an iron-deficient diet. Inflammation 2003, 27, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Mace, J.E.; Park, M.S.; Mora, A.G.; Chung, K.K.; Martini, W.; White, C.E.; Holcomb, J.B.; Merrill, G.A.; Dubick, M.A.; Wolf, S.E.; et al. Differential expression of the immunoinflammatory response in trauma patients: Burn vs. Non-burn. Burns 2012, 38, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Gulec, S.; Collins, J.F. Molecular mediators governing iron-copper interactions. Ann. Rev. Nutr. 2014, 34, 95–116. [Google Scholar] [CrossRef]
- Khorasani, G.; Hosseinimehr, S.J.; Kaghazi, Z. The alteration of plasma’s zinc and copper levels in patients with burn injuries and the relationship to the time after burn injuries. Singap. Med. J. 2008, 49, 627–630. [Google Scholar]
- Agay, D.; Anderson, R.A.; Sandre, C.; Bryden, N.A.; Alonso, A.; Roussel, A.M.; Chancerelle, Y. Alterations of antioxidant trace elements (Zn, Se, Cu) and related metallo-enzymes in plasma and tissues following burn injury in rats. Burns 2005, 31, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Boosalis, M.G.; McCall, J.T.; Solem, L.D.; Ahrenholz, D.H.; McClain, C.J. Serum copper and ceruloplasmin levels and urinary copper excretion in thermal injury. Am. J. Clin. Nutr. 1986, 44, 899–906. [Google Scholar] [PubMed]
- Shakespeare, P.G. Studies on the serum levels of iron, copper and zinc and the urinary excretion of zinc after burn injury. Burns Incl. Therm. Inj. 1982, 8, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, S.; Conti, A.; Iannaccone, S.; Cannistraci, C.V.; Campanella, A.; Barbariga, M.; Codazzi, F.; Pelizzoni, I.; Magnani, G.; Pesca, M.; et al. Ceruloplasmin oxidation, a feature of parkinson’s disease CSF, inhibits ferroxidase activity and promotes cellular iron retention. J. Neurosci.: Off. J. Soc. Neurosci. 2011, 31, 18568–18577. [Google Scholar] [CrossRef]
- Gole, M.D.; Souza, J.M.; Choi, I.; Hertkorn, C.; Malcolm, S.; Foust, R.F., III; Finkel, B.; Lanken, P.N.; Ischiropoulos, H. Plasma proteins modified by tyrosine nitration in acute respiratory distress syndrome. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 278, L961–L967. [Google Scholar] [PubMed]
- Sokolov, A.V.; Ageeva, K.V.; Pulina, M.O.; Zakharova, E.T.; Vasilyev, V.B. Effect of lactoferrin on oxidative features of ceruloplasmin. Biometals 2009, 22, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Samygina, V.R.; Sokolov, A.V.; Bourenkov, G.; Petoukhov, M.V.; Pulina, M.O.; Zakharova, E.T.; Vasilyev, V.B.; Bartunik, H.; Svergun, D.I. Ceruloplasmin: Macromolecular assemblies with iron-containing acute phase proteins. PLoS One 2013, 8, e67145. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, I.M.; Kaplan, H.B.; Edelson, H.S.; Weissmann, G. Ceruloplasmin: An acute phase reactant that scavenges oxygen-derived free radicals. Ann. N. Y. Acad. Sci. 1982, 389, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.M.; Shenkin, A. Trace elements in trauma and burns. Curr. Opin. Clin. Nutr. Metab. Care 1998, 1, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.M. Antioxidant micronutrients in major trauma and burns: Evidence and practice. Nutr. Clin. Pract. 2006, 21, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Dubick, M.A.; Carden, S.C.; Jordan, B.S.; Langlinais, P.C.; Mozingo, D.W. Indices of antioxidant status in rats subjected to wood smoke inhalation and/or thermal injury. Toxicology 2002, 176, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, K.; Nakazawa, H.; Traber, M.G.; Traber, D.L.; Nozaki, M. Plasma and tissue vitamin E depletion in sheep with burn and smoke inhalation injury. Burns 2008, 34, 1137–1141. [Google Scholar] [CrossRef] [PubMed]
- Parihar, A.; Parihar, M.S.; Milner, S.; Bhat, S. Oxidative stress and anti-oxidative mobilization in burn injury. Burns 2008, 34, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Atkins, J.L.; Day, B.W.; Handrigan, M.T.; Zhang, Z.; Pamnani, M.B.; Gorbunov, N.V. Brisk production of nitric oxide and associated formation of S-nitrosothiols in early hemorrhage. J. Appl. Physiol. 2006, 100, 1267–1277. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubick, M.A.; Barr, J.L.; Keen, C.L.; Atkins, J.L. Ceruloplasmin and Hypoferremia: Studies in Burn and Non-Burn Trauma Patients. Antioxidants 2015, 4, 153-169. https://doi.org/10.3390/antiox4010153
Dubick MA, Barr JL, Keen CL, Atkins JL. Ceruloplasmin and Hypoferremia: Studies in Burn and Non-Burn Trauma Patients. Antioxidants. 2015; 4(1):153-169. https://doi.org/10.3390/antiox4010153
Chicago/Turabian StyleDubick, Michael A., Johnny L. Barr, Carl L. Keen, and James L. Atkins. 2015. "Ceruloplasmin and Hypoferremia: Studies in Burn and Non-Burn Trauma Patients" Antioxidants 4, no. 1: 153-169. https://doi.org/10.3390/antiox4010153
APA StyleDubick, M. A., Barr, J. L., Keen, C. L., & Atkins, J. L. (2015). Ceruloplasmin and Hypoferremia: Studies in Burn and Non-Burn Trauma Patients. Antioxidants, 4(1), 153-169. https://doi.org/10.3390/antiox4010153





