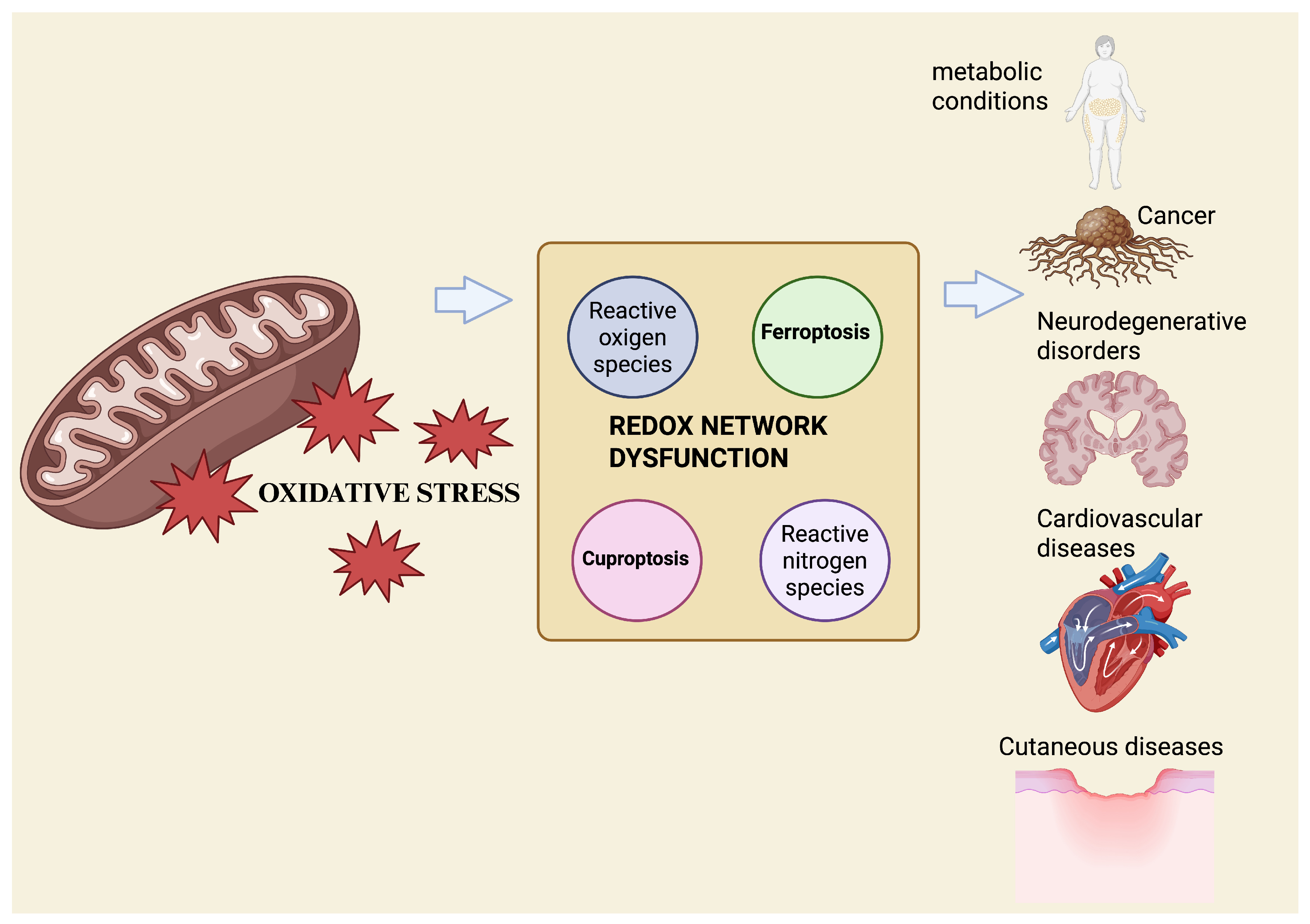
Redox Network Dysfunction: Integrating Ferroptosis and Cuproptosis Across Human Diseases
Abstract
1. Introduction
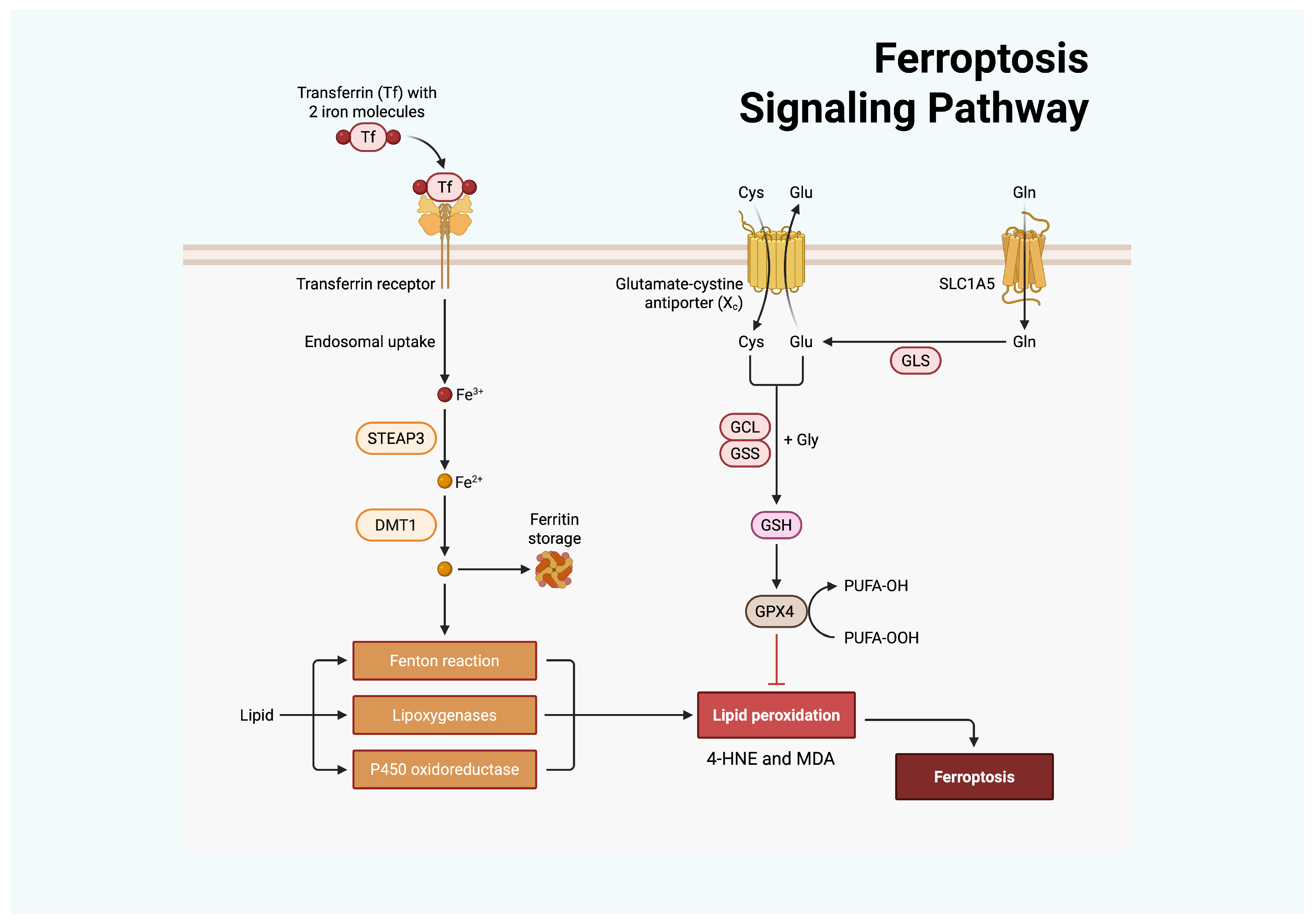
2. Molecular Mechanisms of Cell Death Pathways: Ferroptosis and Cuproptosis
3. Role in Human Diseases
3.1. Autoimmune and Endocrine–Metabolic Diseases
3.2. Cutaneous Disorders
3.3. Cancer and Chronic Inflammation
3.4. Neurodegenerative Disorders
3.5. Cardiovascular Diseases
3.6. Infectious Diseases
3.7. Systemic Redox Crosstalk
4. Clinical and Therapeutic Possible Applications
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li Pomi, F.; Gammeri, L.; Borgia, F.; Di Gioacchino, M.; Gangemi, S. Oxidative Stress and Skin Diseases: The Role of Lipid Peroxidation. Antioxidants 2025, 14, 555. [Google Scholar] [CrossRef]
- Morabito, F.; Cristani, M.; Saija, A.; Stelitano, C.; Callea, V.; Tomaino, A.; Minciullo, P.L.; Gangemi, S. Lipid Peroxidation and Protein Oxidation in Patients Affected by Hodgkin′s Lymphoma. Mediat. Inflamm. 2004, 13, 381–383. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive Oxygen Species (ROS) as Pleiotropic Physiological Signalling Agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative Eustress: On Constant Alert for Redox Homeostasis. Redox Biol. 2021, 41, 101867. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Sies, H.; Mailloux, R.J.; Jakob, U. Fundamentals of Redox Regulation in Biology. Nat. Rev. Mol. Cell Biol. 2024, 25, 701–719. [Google Scholar] [CrossRef]
- Lismont, C.; Nordgren, M.; Van Veldhoven, P.P.; Fransen, M. Redox Interplay between Mitochondria and Peroxisomes. Front. Cell Dev. Biol. 2015, 3, 35. [Google Scholar] [CrossRef]
- Punziano, C.; Trombetti, S.; Cesaro, E.; Grosso, M.; Faraonio, R. Antioxidant Systems as Modulators of Ferroptosis: Focus on Transcription Factors. Antioxidants 2024, 13, 298. [Google Scholar] [CrossRef] [PubMed]
- Artusi, I.; Rubin, M.; Cravin, G.; Cozza, G. Ferroptosis in Human Diseases: Fundamental Roles and Emerging Therapeutic Perspectives. Antioxidants 2025, 14, 1411. [Google Scholar] [CrossRef]
- Li, J.; Cao, F.; Yin, H.; Huang, Z.; Lin, Z.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, Present and Future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Colombo, R.; Giustarini, D.; Milzani, A. Biomarkers of Oxidative Damage in Human Disease. Clin. Chem. 2006, 52, 601–623. [Google Scholar] [CrossRef]
- Cordiano, R.; Di Gioacchino, M.; Mangifesta, R.; Panzera, C.; Gangemi, S.; Minciullo, P.L. Malondialdehyde as a Potential Oxidative Stress Marker for Allergy-Oriented Diseases: An Update. Molecules 2023, 28, 5979. [Google Scholar] [CrossRef]
- Ghezzi, P.; Floridi, L.; Boraschi, D.; Cuadrado, A.; Manda, G.; Levic, S.; D’Acquisto, F.; Hamilton, A.; Athersuch, T.J.; Selley, L. Oxidative Stress and Inflammation Induced by Environmental and Psychological Stressors: A Biomarker Perspective. Antioxid. Redox Signal. 2018, 28, 852–872. [Google Scholar] [CrossRef]
- Münzel, T.; Daiber, A. Environmental Stressors and Their Impact on Health and Disease with Focus on Oxidative Stress. Antioxid. Redox Signal. 2018, 28, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Pandics, T.; Major, D.; Fazekas-Pongor, V.; Szarvas, Z.; Peterfi, A.; Mukli, P.; Gulej, R.; Ungvari, A.; Fekete, M.; Tompa, A.; et al. Exposome and Unhealthy Aging: Environmental Drivers from Air Pollution to Occupational Exposures. Geroscience 2023, 45, 3381–3408. [Google Scholar] [CrossRef]
- Lan, Y.; Wu, S. Impacts of Environmental Insults on Cardiovascular Aging. Curr. Environ Health Rep. 2022, 9, 11–28. [Google Scholar] [CrossRef]
- Peterle, L.; Sanfilippo, S.; Borgia, F.; Li Pomi, F.; Vadalà, R.; Costa, R.; Cicero, N.; Gangemi, S. The Role of Nutraceuticals and Functional Foods in Skin Cancer: Mechanisms and Therapeutic Potential. Foods 2023, 12, 2629. [Google Scholar] [CrossRef]
- Daiber, A.; Rajagopalan, S.; Kuntic, M.; Münzel, T. Cardiovascular Risk Posed by the Exposome. Atherosclerosis 2025, 405, 119222. [Google Scholar] [CrossRef]
- Kareem, M.A.; Ashwini, A.; Sunil, T. The Role of the Exposome in Aging and Age-Related Diseases: A Comprehensive Review. J. Pharm. BioAllied Sci. 2025, 17, S2–S5. [Google Scholar] [CrossRef] [PubMed]
- Ngo, V.; Duennwald, M.L. Nrf2 and Oxidative Stress: A General Overview of Mechanisms and Implications in Human Disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef] [PubMed]
- Cannavò, S.P.; Riso, G.; Casciaro, M.; Di Salvo, E.; Gangemi, S. Oxidative Stress Involvement in Psoriasis: A Systematic Review. Free. Radic. Res. 2019, 53, 829–840. [Google Scholar] [CrossRef]
- Macca, L.; Li Pomi, F.; Ingrasciotta, Y.; Morrone, P.; Trifirò, G.; Guarneri, C. Hidradenitis Suppurativa and Psoriasis: The Odd Couple. Front. Med. 2023, 10, 1208817. [Google Scholar] [CrossRef] [PubMed]
- Borgia, F.; Li Pomi, F.; Vaccaro, M.; Alessandrello, C.; Papa, V.; Gangemi, S. Oxidative Stress and Phototherapy in Atopic Dermatitis: Mechanisms, Role, and Future Perspectives. Biomolecules 2022, 12, 1904. [Google Scholar] [CrossRef]
- Wang, L.; Liu, R.; Tang, Y.; Ma, Y.; Wang, G.; Ruan, Q.; Zheng, S.J. Advances in Psoriasis Research: Decoding Immune Circuits and Developing Novel Therapies. Int. J. Mol. Sci. 2025, 26, 9233. [Google Scholar] [CrossRef]
- Ruggeri, R.M.; Campennì, A.; Giuffrida, G.; Casciaro, M.; Barbalace, M.C.; Hrelia, S.; Trimarchi, F.; Cannavò, S.; Gangemi, S. Oxidative Stress as a Key Feature of Autoimmune Thyroiditis: An Update. Minerva Endocrinol. 2021, 45, 326–344. [Google Scholar] [CrossRef]
- da Silva, G.B.; Yamauchi, M.A.; Bagatini, M.D. Oxidative Stress in Hashimoto’s Thyroiditis: Possible Adjuvant Therapies to Attenuate Deleterious Effects. Mol. Cell. Biochem. 2023, 478, 949–966. [Google Scholar] [CrossRef]
- Rybakova, A.; Platonova, N.; Troshina, E. Oxidative Stress and Its Role in the Development of Autoimmune Thyroid Diseases. Probl. Endocrinol. 2020, 65, 451–457. [Google Scholar] [CrossRef]
- Cabrera-Serrano, A.J.; Sánchez-Maldonado, J.M.; González-Olmedo, C.; Carretero-Fernández, M.; Díaz-Beltrán, L.; Gutiérrez-Bautista, J.F.; García-Verdejo, F.J.; Gálvez-Montosa, F.; López-López, J.A.; García-Martín, P.; et al. Crosstalk Between Autophagy and Oxidative Stress in Hematological Malignancies: Mechanisms, Implications, and Therapeutic Potential. Antioxidants 2025, 14, 264. [Google Scholar] [CrossRef]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting Cancer Cells by ROS-Mediated Mechanisms: A Radical Therapeutic Approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, J.; Liu, J.; Chan, K.-Y.; Lee, H.-S.; Lin, K.N.; Wang, C.-C.; Lau, T.-S. Interplay of Ferroptosis and Cuproptosis in Cancer: Dissecting Metal-Driven Mechanisms for Therapeutic Potentials. Cancers 2024, 16, 512. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of Ferroptotic Cancer Cell Death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef]
- Huang, X. A Concise Review on Oxidative Stress-Mediated Ferroptosis and Cuproptosis in Alzheimer’s Disease. Cells 2023, 12, 1369. [Google Scholar] [CrossRef]
- Wang, D.; Tian, Z.; Zhang, P.; Zhen, L.; Meng, Q.; Sun, B.; Xu, X.; Jia, T.; Li, S. The Molecular Mechanisms of Cuproptosis and Its Relevance to Cardiovascular Disease. Biomed. Pharmacother. 2023, 163, 114830. [Google Scholar] [CrossRef]
- O’Neill, L.A.J.; Kishton, R.J.; Rathmell, J. A Guide to Immunometabolism for Immunologists. Nat. Rev. Immunol. 2016, 16, 553–565. [Google Scholar] [CrossRef]
- Sena, L.A.; Chandel, N.S. Physiological Roles of Mitochondrial Reactive Oxygen Species. Mol. Cell 2012, 48, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Sun, L.; Guo, J.; Ma, J. The Crosstalk between Ferroptosis and Anti-tumor Immunity in the Tumor Microenvironment: Molecular Mechanisms and Therapeutic Controversy. Cancer Commun. 2023, 43, 1071–1096. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Ji, Q.; Wu, M.; Tu, Z.; Lei, K.; Luo, M.; Liu, J.; Lin, L.; Li, K.; Li, J.; et al. Ferroptosis in Tumor Immunity and Therapy. J. Cell. Mol. Med. 2022, 26, 5565–5579. [Google Scholar] [CrossRef]
- Tyurina, Y.Y.; Tyurin, V.A.; Anthonymuthu, T.; Amoscato, A.A.; Sparvero, L.J.; Nesterova, A.M.; Baynard, M.L.; Sun, W.; He, R.; Khaitovich, P.; et al. Redox Lipidomics Technology: Looking for a Needle in a Haystack. Chem. Phys. Lipids 2019, 221, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Criscuolo, A.; Nepachalovich, P.; Garcia-del Rio, D.F.; Lange, M.; Ni, Z.; Baroni, M.; Cruciani, G.; Goracci, L.; Blüher, M.; Fedorova, M. Analytical and Computational Workflow for In-Depth Analysis of Oxidized Complex Lipids in Blood Plasma. Nat. Commun. 2022, 13, 6547. [Google Scholar] [CrossRef]
- Chen, H.; Wang, C.; Liu, Z.; He, X.; Tang, W.; He, L.; Feng, Y.; Liu, D.; Yin, Y.; Li, T. Ferroptosis and Its Multifaceted Role in Cancer: Mechanisms and Therapeutic Approach. Antioxidants 2022, 11, 1504. [Google Scholar] [CrossRef]
- Rochette, L.; Dogon, G.; Rigal, E.; Zeller, M.; Cottin, Y.; Vergely, C. Lipid Peroxidation and Iron Metabolism: Two Corner Stones in the Homeostasis Control of Ferroptosis. Int. J. Mol. Sci. 2022, 24, 449. [Google Scholar] [CrossRef] [PubMed]
- Ursini, F.; Maiorino, M. Lipid Peroxidation and Ferroptosis: The Role of GSH and GPx4. Free Radic. Biol. Med. 2020, 152, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jia, B.; Cheng, Y.; Song, Y.; Li, Q.; Luo, C. Targeting Molecular Mediators of Ferroptosis and Oxidative Stress for Neurological Disorders. Oxid. Med. Cell. Longev. 2022, 2022, 3999083. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, M.; Sun, M. ACSL4 at the Helm of the Lipid Peroxidation Ship: A Deep-Sea Exploration towards Ferroptosis. Front. Pharmacol. 2025, 16, 1594419. [Google Scholar] [CrossRef]
- Hu, Y.; He, B.; Cao, Q.; Li, Y.; Tang, Y.; Cao, T.; Peng, B.; Zhou, X.; Liu, S. Crosstalk of Ferroptosis and Oxidative Stress in Infectious Diseases. Front. Mol. Biosci. 2023, 10, 1315935. [Google Scholar] [CrossRef]
- Mortensen, M.S.; Ruiz, J.; Watts, J.L. Polyunsaturated Fatty Acids Drive Lipid Peroxidation during Ferroptosis. Cells 2023, 12, 804. [Google Scholar] [CrossRef]
- Jiang, Y.; Glandorff, C.; Sun, M. GSH and Ferroptosis: Side-by-Side Partners in the Fight against Tumors. Antioxidants 2024, 13, 697. [Google Scholar] [CrossRef]
- von Krusenstiern, A.N.; Robson, R.N.; Qian, N.; Qiu, B.; Hu, F.; Reznik, E.; Smith, N.; Zandkarimi, F.; Estes, V.M.; Dupont, M.; et al. Identification of Essential Sites of Lipid Peroxidation in Ferroptosis. Nat. Chem. Biol. 2023, 19, 719–730. [Google Scholar] [CrossRef]
- Noguchi, N.; Saito, Y.; Niki, E. Lipid Peroxidation, Ferroptosis, and Antioxidants. Free Radic. Biol. Med. 2025, 237, 228–238. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Yu, Q.; Xiao, Y.; Guan, M.; Zhang, X.; Yu, J.; Han, M.; Li, Z. Copper Metabolism in Osteoarthritis and Its Relation to Oxidative Stress and Ferroptosis in Chondrocytes. Front. Mol. Biosci. 2024, 11, 1472492. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Chen, Z.; Wu, D.; Chen, L. Ferritinophagy/Ferroptosis: Iron-related Newcomers in Human Diseases. J. Cell. Physiol. 2018, 233, 9179–9190. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Wang, Y.; Min, J.; Wang, F. Iron Metabolism and Ferroptosis in Human Health and Disease. BMC Biol. 2025, 23, 263. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yu, C.; Kang, R.; Tang, D. Iron Metabolism in Ferroptosis. Front. Cell Dev. Biol. 2020, 8, 590226. [Google Scholar] [CrossRef]
- Chiang, S.-K.; Chen, S.-E.; Chang, L.-C. A Dual Role of Heme Oxygenase-1 in Cancer Cells. Int. J. Mol. Sci. 2018, 20, 39. [Google Scholar] [CrossRef]
- Ryter, S.W. Heme Oxygenase-1: An Anti-Inflammatory Effector in Cardiovascular, Lung, and Related Metabolic Disorders. Antioxidants 2022, 11, 555. [Google Scholar] [CrossRef]
- Zou, X.; Cai, L.; Zhang, J.; Yuan, Y.; Song, J.; Hu, Z.; Ruan, X.; Peng, R.; Zhang, X. Mechanism of Heme Oxygenase-1 Regulation of Ferroptosis in Vascular Dementia. Front. Mol. Neurosci. 2025, 18, 1585079. [Google Scholar] [CrossRef]
- Liang, D.; Minikes, A.M.; Jiang, X. Ferroptosis at the Intersection of Lipid Metabolism and Cellular Signaling. Mol. Cell 2022, 82, 2215–2227. [Google Scholar] [CrossRef]
- Cañeque, T.; Baron, L.; Müller, S.; Carmona, A.; Colombeau, L.; Versini, A.; Solier, S.; Gaillet, C.; Sindikubwabo, F.; Sampaio, J.L.; et al. Activation of Lysosomal Iron Triggers Ferroptosis in Cancer. Nature 2025, 642, 492–500. [Google Scholar] [CrossRef]
- Sassano, M.L.; Tyurina, Y.Y.; Diokmetzidou, A.; Vervoort, E.; Tyurin, V.A.; More, S.; La Rovere, R.; Giordano, F.; Bultynck, G.; Pavie, B.; et al. Endoplasmic Reticulum–Mitochondria Contacts Are Prime Hotspots of Phospholipid Peroxidation Driving Ferroptosis. Nat. Cell Biol. 2025, 27, 902–917. [Google Scholar] [CrossRef]
- Han, J.; Zheng, D.; Liu, P.-S.; Wang, S.; Xie, X. Peroxisomal Homeostasis in Metabolic Diseases and Its Implication in Ferroptosis. Cell Commun. Signal. 2024, 22, 475. [Google Scholar] [CrossRef]
- Chen, X.; Tsvetkov, A.S.; Shen, H.-M.; Isidoro, C.; Ktistakis, N.T.; Linkermann, A.; Koopman, W.J.H.; Simon, H.-U.; Galluzzi, L.; Luo, S.; et al. International Consensus Guidelines for the Definition, Detection, and Interpretation of Autophagy-Dependent Ferroptosis. Autophagy 2024, 20, 1213–1246. [Google Scholar] [CrossRef]
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D. Ferroptosis in Infection, Inflammation, and Immunity. J. Exp. Med. 2021, 218, e20210518. [Google Scholar] [CrossRef]
- Bell, H.N.; Stockwell, B.R.; Zou, W. Ironing out the Role of Ferroptosis in Immunity. Immunity 2024, 57, 941–956. [Google Scholar] [CrossRef]
- Tsvetkov, P.; Coy, S.; Petrova, B.; Dreishpoon, M.; Verma, A.; Abdusamad, M.; Rossen, J.; Joesch-Cohen, L.; Humeidi, R.; Spangler, R.D.; et al. Copper Induces Cell Death by Targeting Lipoylated TCA Cycle Proteins. Science 2022, 375, 1254–1261. [Google Scholar] [CrossRef]
- Chen, L.; Min, J.; Wang, F. Copper Homeostasis and Cuproptosis in Health and Disease. Signal Transduct. Target. Ther. 2022, 7, 378. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Chen, M. Crosstalk between Ferroptosis and Cuproptosis: From Mechanism to Potential Clinical Application. Biomed. Pharmacother. 2024, 171, 116115. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Li, D.; Liang, Q.; Yang, M.; Pan, Y.; Li, H. Cross-Talk between Cuproptosis and Ferroptosis Regulators Defines the Tumor Microenvironment for the Prediction of Prognosis and Therapies in Lung Adenocarcinoma. Front. Immunol. 2023, 13, 1029092. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Du, Y.; Zhou, Y.; Chen, Q.; Luo, Z.; Ren, Y.; Chen, X.; Chen, G. Iron and Copper: Critical Executioners of Ferroptosis, Cuproptosis and Other Forms of Cell Death. Cell Commun. Signal. 2023, 21, 327. [Google Scholar] [CrossRef]
- Li, K.; Fan, C.; Chen, J.; Xu, X.; Lu, C.; Shao, H.; Xi, Y. Role of Oxidative Stress-induced Ferroptosis in Cancer Therapy. J. Cell Mol. Med. 2024, 28, e18399. [Google Scholar] [CrossRef]
- Lei, G.; Zhuang, L.; Gan, B. Targeting Ferroptosis as a Vulnerability in Cancer. Nat. Rev. Cancer 2022, 22, 381–396. [Google Scholar] [CrossRef]
- Zhao, W.-K.; Zhou, Y.; Xu, T.-T.; Wu, Q. Ferroptosis: Opportunities and Challenges in Myocardial Ischemia-Reperfusion Injury. Oxid. Med. Cell. Longev. 2021, 2021, 9929687. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, X.; Liu, X.; Shen, L.; Chen, Q.; Shu, Q. Targeting Ferroptosis as a Promising Therapeutic Strategy for Ischemia-Reperfusion Injury. Antioxidants 2022, 11, 2196. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kroemer, G.; Kang, R. Targeting Cuproplasia and Cuproptosis in Cancer. Nat. Rev. Clin. Oncol. 2024, 21, 370–388. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, T.; Li, L. Targeting Cuproptosis for Cancer Therapy: Mechanistic Insights and Clinical Perspectives. J. Hematol. Oncol. 2024, 17, 68. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M. Molecular Pathways Associated with Oxidative Stress in Diabetes Mellitus. Biomed. Pharmacother. 2018, 108, 656–662. [Google Scholar] [CrossRef]
- Darenskaya, M.A.; Kolesnikova, L.I.; Kolesnikov, S.I. Oxidative Stress: Pathogenetic Role in Diabetes Mellitus and Its Complications and Therapeutic Approaches to Correction. Bull. Exp. Biol. Med. 2021, 171, 179–189. [Google Scholar] [CrossRef]
- Weinberg Sibony, R.; Segev, O.; Dor, S.; Raz, I. Overview of Oxidative Stress and Inflammation in Diabetes. J. Diabetes 2024, 16, e70014. [Google Scholar] [CrossRef] [PubMed]
- Sha, W.; Hu, F.; Xi, Y.; Chu, Y.; Bu, S. Mechanism of Ferroptosis and Its Role in Type 2 Diabetes Mellitus. J. Diabetes Res. 2021, 2021, 9999612. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, Z.; Cai, Y.; Li, Z.; Zhou, Q.; Chen, Q. Ferroptosis: Mechanisms and Role in Diabetes Mellitus and Its Complications. Ageing Res. Rev. 2024, 94, 102201. [Google Scholar] [CrossRef]
- Pan, Z.; Huang, L.; Gan, Y.; Xia, Y.; Yu, W. The Molecular Mechanisms of Cuproptosis and Small-Molecule Drug Design in Diabetes Mellitus. Molecules 2024, 29, 2852. [Google Scholar] [CrossRef]
- Miao, R.; Fang, X.; Zhang, Y.; Wei, J.; Zhang, Y.; Tian, J. Iron Metabolism and Ferroptosis in Type 2 Diabetes Mellitus and Complications: Mechanisms and Therapeutic Opportunities. Cell Death Dis. 2023, 14, 186. [Google Scholar] [CrossRef]
- Liu, S.; Chen, X.; Qi, X.; Bai, J.; Tong, B.; Zhang, D.; Yin, X.; Yu, P. The Role of Metal Ion Metabolism in the Pathogenesis of Diabetes and Associated Complications. Front. Endocrinol. 2025, 16, 1541809. [Google Scholar] [CrossRef]
- Wang, C.; Wu, J.; Wang, Y.; Huang, C.; Wei, M.; Zhang, Y.; Shen, R.; Wang, J. The Role of Dysregulated Copper Metabolism in Diabetes and Its Complications: A Review. Front. Endocrinol. 2025, 16, 1681001. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lu, K.; Jiang, X.; Wei, Q.; Zhu, L.; Wang, X.; Jin, H.; Feng, L. Ferroptosis Inducers Enhanced Cuproptosis Induced by Copper Ionophores in Primary Liver Cancer. J. Exp. Clin. Cancer Res. 2023, 42, 142. [Google Scholar] [CrossRef] [PubMed]
- Karanikas, G.; Schuetz, M.; Wahl, K.; Paul, M.; Kontur, S.; Pietschmann, P.; Kletter, K.; Dudczak, R.; Willheim, M. Relation of Anti-TPO Autoantibody Titre and T-lymphocyte Cytokine Production Patterns in Hashimoto’s Thyroiditis. Clin. Endocrinol. 2005, 63, 191–196. [Google Scholar] [CrossRef]
- Yang, S.L.; Chung, K. The NADPH Oxidase-mediated Production of Hydrogen Peroxide (H2O2) and Resistance to Oxidative Stress in the Necrotrophic Pathogen Alternaria alternata of Citrus. Mol. Plant Pathol. 2012, 13, 900–914. [Google Scholar] [CrossRef]
- Ates, I.; Yilmaz, F.M.; Altay, M.; Yilmaz, N.; Berker, D.; Güler, S. The Relationship between Oxidative Stress and Autoimmunity in Hashimoto’s Thyroiditis. Eur. J. Endocrinol. 2015, 173, 791–799. [Google Scholar] [CrossRef]
- Ruggeri, R.M.; Barbalace, M.C.; Cristani, M.T.; Alibrandi, A.; Giovinazzo, S.; Giuffrida, G.; Trimarchi, F.; Cannavò, S.; Campennì, A. Serum Levels of Advanced Glycation End Products (AGEs) Are Increased and Their Soluble Receptor (SRAGE) Reduced in Hashimoto’s Thyroiditis. J. Endocrinol. Investig. 2020, 43, 1337–1342. [Google Scholar] [CrossRef]
- Kochman, J.; Jakubczyk, K.; Bargiel, P.; Janda-Milczarek, K. The Influence of Oxidative Stress on Thyroid Diseases. Antioxidants 2021, 10, 1442. [Google Scholar] [CrossRef] [PubMed]
- Papa, V.; Li Pomi, F.; Borgia, F.; Vaccaro, M.; Pioggia, G.; Gangemi, S. Immunosenescence and Skin: A State of Art of Its Etiopathogenetic Role and Crucial Watershed for Systemic Implications. Int. J. Mol. Sci. 2023, 24, 7956. [Google Scholar] [CrossRef]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.; Trost, A.; Richter, K. Oxidative Stress in Aging Human Skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef]
- Raimondo, A.; Serio, B.; Lembo, S. Oxidative Stress in Atopic Dermatitis and Possible Biomarkers: Present and Future. Indian J. Dermatol. 2023, 68, 657–660. [Google Scholar] [CrossRef] [PubMed]
- Almela, R.M.; Rubio, C.P.; Cerón, J.J.; Ansón, A.; Tichy, A.; Mayer, U. Selected Serum Oxidative Stress Biomarkers in Dogs with Non-food-induced and Food-induced Atopic Dermatitis. Vet. Dermatol. 2018, 29, 229. [Google Scholar] [CrossRef]
- Sutanto, H.; Pratiwi, L.; Fetarayani, D. Exploring Ferroptosis in Allergic Inflammatory Diseases: Emerging Mechanisms and Therapeutic Perspectives. Cell Biol. Int. 2025, 49, 739–756. [Google Scholar] [CrossRef]
- Wen, X.; Jia, S.; Wu, J.; Wang, S.; Yu, T.; Xu, H. Decoding Cuproptosis-Sphingolipid-Immune Crosstalk in Atopic Dermatitis: A Multi-Omics Network Analysis. Biomedicines 2025, 13, 1349. [Google Scholar] [CrossRef]
- Wang, L.; Wang, B.; Peng, M.; Yang, X.; Huang, S.; Wang, R.; Du, L.; Yao, R.; Wang, W.; Dong, B.; et al. Identification and Analysis of Cuproptosis Associated Molecular Clusters and Immunological Profiles in Atopic Dermatitis. Front. Immunol. 2025, 16, 1545457. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Li, K.; Luo, S.; Yan, R.; Yi, X.; Fang, C.; Ma, S.; Wang, G.; Luo, F.; Chen, X.; et al. Cuproptosis Facilitates Chronic Skin Inflammation by Regulating the A-Ketoglutarate/H3K4me3/Ferritin Heavy Chain 1 Signaling Pathway-Mediated Ferroptosis. MedComm 2025, 6, e70425. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Yang, L.; Li, T.; Wang, K.; Huang, X.; Shi, J.; Wang, Y. Mechanisms and Inhibitors of Ferroptosis in Psoriasis. Front. Mol. Biosci. 2022, 9, 1019447. [Google Scholar] [CrossRef]
- Le, J.; Meng, Y.; Wang, Y.; Li, D.; Zeng, F.; Xiong, Y.; Chen, X.; Deng, G. Molecular and Therapeutic Landscape of Ferroptosis in Skin Diseases. Chin. Med. J. 2024, 137, 1777–1789. [Google Scholar] [CrossRef]
- Lin, Q.; Zhu, J.; Chen, J.; Jia, S.; Nie, S. Significance of Cuproptosis- Related Genes in the Diagnosis and Classification of Psoriasis. Front. Mol. Biosci. 2023, 10, 1115091. [Google Scholar] [CrossRef]
- Chen, C.-H.; Wu, N.-L.; Tsai, T.-F. How Cells Die in Psoriasis? Int. J. Mol. Sci. 2025, 26, 3747. [Google Scholar] [CrossRef]
- Liu, L.; Lian, N.; Shi, L.; Hao, Z.; Chen, K. Ferroptosis: Mechanism and Connections with Cutaneous Diseases. Front. Cell Dev. Biol. 2023, 10, 1079548. [Google Scholar] [CrossRef]
- Białczyk, A.; Wełniak, A.; Kamińska, B.; Czajkowski, R. Oxidative Stress and Potential Antioxidant Therapies in Vitiligo: A Narrative Review. Mol. Diagn. Ther. 2023, 27, 723–739. [Google Scholar] [CrossRef]
- Wu, X.; Jin, S.; Yang, Y.; Lu, X.; Dai, X.; Xu, Z.; Zhang, C.; Xiang, L.F. Altered Expression of Ferroptosis Markers and Iron Metabolism Reveals a Potential Role of Ferroptosis in Vitiligo. Pigment. Cell Melanoma Res. 2022, 35, 328–341. [Google Scholar] [CrossRef]
- Zhang, J.; Xiang, F.; Ding, Y.; Hu, W.; Wang, H.; Zhang, X.; Lei, Z.; Li, T.; Wang, P.; Kang, X. Identification and Validation of RNA-Binding Protein SLC3A2 Regulates Melanocyte Ferroptosis in Vitiligo by Integrated Analysis of Single-Cell and Bulk RNA-Sequencing. BMC Genom. 2024, 25, 236. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Green, M.; Choi, J.E.; Gijón, M.; Kennedy, P.D.; Johnson, J.K.; Liao, P.; Lang, X.; Kryczek, I.; Sell, A.; et al. CD8+ T Cells Regulate Tumour Ferroptosis during Cancer Immunotherapy. Nature 2019, 569, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; You, C.; Zhao, L.; Wang, J.; Ye, X.; Yang, T.; Wan, C.; Deng, L. Ferroptosis-Associated Classifier and Indicator for Prognostic Prediction in Cutaneous Melanoma. J. Oncol. 2021, 2021, 3658196. [Google Scholar] [CrossRef]
- Meng, Y.; Zhou, Q.; Dian, Y.; Zeng, F.; Deng, G.; Chen, X. Ferroptosis: A Targetable Vulnerability for Melanoma Treatment. J. Investig. Dermatol. 2025, 145, 1323–1344. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Liu, X.; Zeng, X.; Liu, Y.; Zhang, C.; Zhang, Q.; Xu, J. Comprehensive Analysis of Cuproptosis-Related Genes in Immune Infiltration and Prognosis in Melanoma. Front. Pharmacol. 2022, 13, 930041. [Google Scholar] [CrossRef]
- Sun, Y.; Lei, S.; Luo, X.; Jiang, C.; Li, Z. The Value of Cuproptosis-Related Differential Genes in Guiding Prognosis and Immune Status in Patients with Skin Cutaneous Melanoma. Front. Pharmacol. 2023, 14, 1129544. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, X.; Wang, X. Identification of a Prognostic Model Using Cuproptosis-Related Genes in Uveal Melanoma. Front. Cell Dev. Biol. 2022, 10, 973073. [Google Scholar] [CrossRef] [PubMed]
- Vats, K.; Tian, H.; Singh, K.; Tyurina, Y.Y.; Sparvero, L.J.; Tyurin, V.A.; Kruglov, O.; Chang, A.; Wang, J.; Green, F.; et al. Ferroptosis of Select Skin Epithelial Cells Initiates and Maintains Chronic Systemic Immune-Mediated Psoriatic Disease. J. Clin. Investig. 2025, 135, e183219. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Huang, Q.; Ji, T.; Li, Q.; Hu, C. Copper Homeostasis and Copper-Induced Cell Death in Tumor Immunity: Implications for Therapeutic Strategies in Cancer Immunotherapy. Biomark. Res. 2024, 12, 130. [Google Scholar] [CrossRef]
- Zhou, Q.; Meng, Y.; Li, D.; Yao, L.; Le, J.; Liu, Y.; Sun, Y.; Zeng, F.; Chen, X.; Deng, G. Ferroptosis in Cancer: From Molecular Mechanisms to Therapeutic Strategies. Signal Transduct. Target. Ther. 2024, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhao, Y.; Guo, D. Application and Potential of the Tumor Microenvironment, Ferroptosis, Cuproptosis, and Disulfidptosis in Cancer Treatment and Monitoring. Crit. Rev. Oncol. Hematol. 2025, 218, 105066. [Google Scholar] [CrossRef]
- Badr, O.I.; Mustafa, F.A.; Radwan, B.A.; Abdulfatah, A.M.; Ragab, A.N.; Sleem, H.M. Ferritinophagy as a Double-Edged Sword in Cancer: Novel Insights into Therapeutic Targeting of Iron-Driven Ferroptosis. Cell Biochem. Biophys. 2025, 1–19. [Google Scholar] [CrossRef]
- Caforio, M.; Iacovelli, S.; Locatelli, F.; Folgiero, V. Inducing Ferroptosis to Improve Cancer Therapy: A Promising Tool for Enhancing Immunotherapy. J. Exp. Clin. Cancer Res. 2025. [Google Scholar] [CrossRef]
- Mou, Y.; Wang, J.; Wu, J.; He, D.; Zhang, C.; Duan, C.; Li, B. Ferroptosis, a New Form of Cell Death: Opportunities and Challenges in Cancer. J. Hematol. Oncol. 2019, 12, 34. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, J.; Wan, A.; Zhang, Y.; Qi, X. Epigenetic Regulation of Diverse Cell Death Modalities in Cancer: A Focus on Pyroptosis, Ferroptosis, Cuproptosis, and Disulfidptosis. J. Hematol. Oncol. 2024, 17, 22. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, X.; Liu, Y.; Gu, X. Ferroptosis in Immune Cells: Implications for Tumor Immunity and Cancer Therapy. Cytokine Growth Factor Rev. 2025, 84, 59–73. [Google Scholar] [CrossRef]
- Liu, J.; Dong, R.; Yuan, B.; Xie, Y.; Feng, Z.; Zhou, S.; Luan, F.; Chen, Y. Immune Cells Dying from Ferroptosis: Mechanisms and Therapeutic Opportunities. Cell Death Dis. 2025, 16, 878. [Google Scholar] [CrossRef]
- Tong, X.; Tang, R.; Xiao, M.; Xu, J.; Wang, W.; Zhang, B.; Liu, J.; Yu, X.; Shi, S. Targeting Cell Death Pathways for Cancer Therapy: Recent Developments in Necroptosis, Pyroptosis, Ferroptosis, and Cuproptosis Research. J. Hematol. Oncol. 2022, 15, 174. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Lin, W.; Yan, L.; Xu, W.; Yang, J. Copper Homeostasis and Cuproptosis in Cancer Immunity and Therapy. Immunol. Rev. 2024, 321, 211–227. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jia, B.; Li, J.; Li, Q.; Luo, C. The Interplay between Ferroptosis and Neuroinflammation in Central Neurological Disorders. Antioxidants 2024, 13, 395. [Google Scholar] [CrossRef] [PubMed]
- David, S.; Ryan, F.; Jhelum, P.; Kroner, A. Ferroptosis in Neurological Disease. Neuroscientist 2023, 29, 591–615. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Liao, Y.; Zhu, C.; Zou, Z. GPX4, Ferroptosis, and Diseases. Biomed. Pharmacother. 2024, 174, 116512. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, M.; Zhao, W. Research on Ferroptosis as a Therapeutic Target for the Treatment of Neurodegenerative Diseases. Ageing Res. Rev. 2023, 91, 102035. [Google Scholar] [CrossRef]
- Ryan, S.K.; Ugalde, C.L.; Rolland, A.-S.; Skidmore, J.; Devos, D.; Hammond, T.R. Therapeutic Inhibition of Ferroptosis in Neurodegenerative Disease. Trends Pharmacol. Sci. 2023, 44, 674–688. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, J.; Chen, T.; Song, S.; Hou, Y.; Feng, L.; Fan, C.; Li, M. Ferroptosis, Pyroptosis, and Cuproptosis in Alzheimer’s Disease. ACS Chem. Neurosci. 2023, 14, 3564–3587. [Google Scholar] [CrossRef]
- Tao, F.; Lin, M.; Meng, X.; Huang, L.; Zhuo, B.; Jiang, S.; Deng, S.; Meng, Z.; Shi, J. Copper Homeostasis and Cuproptosis: Implications for Neurodegenerative Diseases. Front. Aging Neurosci. 2025, 17, 1688554. [Google Scholar] [CrossRef]
- Li, X.; Chen, X.; Gao, X. Copper and Cuproptosis: New Therapeutic Approaches for Alzheimer’s Disease. Front. Aging Neurosci. 2023, 15, 172. [Google Scholar] [CrossRef]
- Cong, C.; Cong, H.; Yao, Y.; Bai, Y.; Xu, L. Copper Homeostasis and Cuproptosis in Alzheimer’s Disease (Review). Int. J. Mol. Med. 2025, 56, 1688554. [Google Scholar] [CrossRef]
- Meng, D.; Luo, G.; Liu, P. Copper Metabolism and Cuproptosis in Alzheimer’s Disease: Mechanisms and Therapeutic Potential. Biomed. Pharmacother. 2025, 190, 118354. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, X.; Wang, S.; Miao, R.; Zhong, J. Targeting Iron Metabolism and Ferroptosis as Novel Therapeutic Approaches in Cardiovascular Diseases. Nutrients 2023, 15, 591. [Google Scholar] [CrossRef]
- Fang, X.; Ardehali, H.; Min, J.; Wang, F. The Molecular and Metabolic Landscape of Iron and Ferroptosis in Cardiovascular Disease. Nat. Rev. Cardiol. 2023, 20, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, Y.; Meng, Q.; Wang, J.; Wang, K.; Yang, S. Copper Dyshomeostasis and Cardiovascular Disease: Molecular Mechanisms and New Strategies for Targeted Intervention with Cuproptosis (Review). Int. J. Mol. Med. 2025, 57, 19. [Google Scholar] [CrossRef]
- Gu, J.; Huang, W.; Duanmu, Z.; Zhuang, R.; Yang, X. Cuproptosis and Copper Deficiency in Ischemic Vascular Injury and Repair. Apoptosis 2024, 29, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Maung, M.T.; Carlson, A.; Olea-Flores, M.; Elkhadragy, L.; Schachtschneider, K.M.; Navarro-Tito, N.; Padilla-Benavides, T. The Molecular and Cellular Basis of Copper Dysregulation and Its Relationship with Human Pathologies. FASEB J. 2021, 35, e21810. [Google Scholar] [CrossRef]
- Chen, P.; He, P.; Rao, X.; Ding, M.; Liu, J.; Chu, Y.; Xiao, Y. Emerging Trends in Cardiovascular Diseases: The Impact of Ferroptosis and Cuproptosis on Cardiomyocyte Death. Mol. Cell. Biochem. 2025, 480, 5323–5344. [Google Scholar] [CrossRef]
- Dawi, J.; Affa, S.; Kafaja, K.; Misakyan, Y.; Kades, S.; Dayal, S.; Fardeheb, S.; Narasimhan, A.; Tumanyan, K.; Venketaraman, V. The Role of Ferroptosis and Cuproptosis in Tuberculosis Pathogenesis: Implications for Therapeutic Strategies. Curr. Issues Mol. Biol. 2025, 47, 99. [Google Scholar] [CrossRef]
- XI, L.; Gy, Z.; R, G.; N, C. Ferroptosis in Sepsis: The Mechanism, the Role and the Therapeutic Potential. Front. Immunol. 2022, 13, 956361. [Google Scholar] [CrossRef]
- Huang, Q.; Ding, Y.; Fang, C.; Wang, H.; Kong, L. The Emerging Role of Ferroptosis in Sepsis, Opportunity or Challenge? Infect Drug Resist. 2023, 16, 5551–5562. [Google Scholar] [CrossRef]
- Huo, L.; Liu, C.; Yuan, Y.; Liu, X.; Cao, Q. Pharmacological Inhibition of Ferroptosis as a Therapeutic Target for Sepsis-Associated Organ Damage. Eur. J. Med. Chem. 2023, 257, 115438. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.; Wen, K.; Zhao, T.; Guo, H.; Han, X.; Wang, J.; Ge, C.; Du, Q. Deciphering Cuproptosis in Sepsis: Mechanisms, Consequences, and Therapeutic Opportunities. J. Inflamm. Res. 2025, 18, 9879–9890. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qiu, X.; Liu, J.; Liu, X.; Pan, J.; Cai, J.; Liu, X.; Qu, S. Cuproptosis-Related Biomarkers and Characterization of Immune Infiltration in Sepsis. J. Inflamm. Res. 2024, 17, 2459–2478. [Google Scholar] [CrossRef]
- Cronin, S.J.F.; Woolf, C.J.; Weiss, G.; Penninger, J.M. The Role of Iron Regulation in Immunometabolism and Immune-Related Disease. Front. Mol. Biosci. 2019, 6, 116. [Google Scholar] [CrossRef]
- Pourhabib Mamaghani, M.; Mousavikia, S.N.; Azimian, H. Ferroptosis in Cancer: Mechanisms, Therapeutic Strategies, and Clinical Implications. Pathol. Res. Pract. 2025, 269, 155907. [Google Scholar] [CrossRef]
- Xu, G.; Wang, H.; Li, X.; Huang, R.; Luo, L. Recent Progress on Targeting Ferroptosis for Cancer Therapy. Biochem. Pharmacol. 2021, 190, 114584. [Google Scholar] [CrossRef] [PubMed]
- Elgendy, S.M.; Alyammahi, S.K.; Alhamad, D.W.; Abdin, S.M.; Omar, H.A. Ferroptosis: An Emerging Approach for Targeting Cancer Stem Cells and Drug Resistance. Crit. Rev. Oncol. Hematol. 2020, 155, 103095. [Google Scholar] [CrossRef]
- Lu, K.; Wijaya, C.S.; Yao, Q.; Jin, H.; Feng, L. Cuproplasia and Cuproptosis, Two Sides of the Coin. Cancer Commun. 2025, 45, 505–524. [Google Scholar] [CrossRef]
- Zheng, P.; Zhou, C.; Lu, L.; Liu, B.; Ding, Y. Elesclomol: A Copper Ionophore Targeting Mitochondrial Metabolism for Cancer Therapy. J. Exp. Clin. Cancer Res. 2022, 41, 271. [Google Scholar] [CrossRef]
- Chen, X.; Cai, Q.; Liang, R.; Zhang, D.; Liu, X.; Zhang, M.; Xiong, Y.; Xu, M.; Liu, Q.; Li, P.; et al. Copper Homeostasis and Copper-Induced Cell Death in the Pathogenesis of Cardiovascular Disease and Therapeutic Strategies. Cell Death Dis. 2023, 14, 105. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, W.-J.; McMillen, T.S.; LeBoeuf, R.C.; Frei, B. Copper Chelation by Tetrathiomolybdate Inhibits Vascular Inflammation and Atherosclerotic Lesion Development in Apolipoprotein E-Deficient Mice. Atherosclerosis 2012, 223, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Comish, P.B.; Tang, D.; Kang, R. Characteristics and Biomarkers of Ferroptosis. Front. Cell Dev. Biol. 2021, 9, 637162. [Google Scholar] [CrossRef]
- Bae, C.; Kim, H.; Kook, Y.-M.; Lee, C.; Kim, C.; Yang, C.; Park, M.H.; Piao, Y.; Koh, W.-G.; Lee, K. Induction of Ferroptosis Using Functionalized Iron-Based Nanoparticles for Anti-Cancer Therapy. Mater. Today Bio 2022, 17, 100457. [Google Scholar] [CrossRef]
- Guo, W.; Wang, X.; Wang, Y.; Zhu, S.; Zhu, R.; Zhu, L. Identification and Validation of Ferroptosis-Related DNA Methylation Signature for Predicting the Prognosis and Guiding the Treatment in Cutaneous Melanoma. Int. J. Mol. Sci. 2022, 23, 15677. [Google Scholar] [CrossRef]
- Yang, M.; Luo, H.; Yi, X.; Wei, X.; Jiang, D. The Epigenetic Regulatory Mechanisms of Ferroptosis and Its Implications for Biological Processes and Diseases. MedComm 2023, 4, e267. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Hong, M.; Kong, D.; Deng, J.; Zhong, Z.; Liang, J. Ferroptosis-Associated DNA Methylation Signature Predicts Overall Survival in Patients with Head and Neck Squamous Cell Carcinoma. BMC Genom. 2022, 23, 63. [Google Scholar] [CrossRef] [PubMed]
- Li Pomi, F.; Papa, V.; Borgia, F.; Vaccaro, M.; Pioggia, G.; Gangemi, S. Artificial Intelligence: A Snapshot of Its Application in Chronic Inflammatory and Autoimmune Skin Diseases. Life 2024, 14, 516. [Google Scholar] [CrossRef]
- Kazakov, Y.; Halperin, A.; Brainina, K. Artificial Intelligence as a Potential Tool for Oxidative Stress Estimation in Medicine. Explor. Digit. Health Technol. 2025, 3, 101157. [Google Scholar] [CrossRef]
- Peña-Bautista, C.; Durand, T.; Oger, C.; Baquero, M.; Vento, M.; Cháfer-Pericás, C. Assessment of Lipid Peroxidation and Artificial Neural Network Models in Early Alzheimer Disease Diagnosis. Clin. Biochem. 2019, 72, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Yan, J.; Zhang, D.; Zhou, X. Identification of Cuproptosis-Related Molecular Subtypes and a Novel Predictive Model of COVID-19 Based on Machine Learning. Front. Immunol. 2023, 14, 1152223. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Deng, J.; Zhou, X.; Wang, K.; Cai, H.; Yan, Y.; Jiang, J.; Yang, J.; Gu, J.; Zhang, Y.; et al. Comprehensive Analysis of Cuproptosis-Related Genes Involved in Immune Infiltration and Their Use in the Diagnosis of Hepatic Ischemia-Reperfusion Injury: An Experimental Study. Int. J. Surg. 2025, 111, 242–256. [Google Scholar] [CrossRef]
| Shared Mechanism | Contribution of Ferroptosis | Contribution of Cuproptosis | Link Between Ferroptosis and Cuproptosis |
|---|---|---|---|
| Mitochondrial Fe-S cluster instability | Oxidative degradation of Fe-S clusters increases mitochondrial Fe2+ and ROS | Cu+ binding destabilises Fe-S clusters and releases Fe2+ | Cuproptosis-induced Fe2+ release lowers the ferroptosis threshold; ferroptotic ROS exacerbate cuproptotic proteotoxicity [65,125] |
| GSH depletion/thiol-redox collapse | GSH loss → GPX4 inactivation → LPO | GSH loss reduces Cu+ buffering → more free copper to engage lipoylated proteins | Competition for GSH links the pathways: ferroptosis inducers sensitize cells to cuproptosis [30,31] |
| Fe/Cu homeostatic interplay | Iron overload drives Fenton ROS and enhances LPO | Copper overload impairs Fe-S cluster assembly, increasing mitochondrial Fe2+ | Copper-driven Fe2+ release amplifies ferroptosis, while iron dysregulation perturbs copper redox cycling [67,69] |
| TCA-cycle dependency and mitochondrial metabolism | Mitochondrial ROS and glutaminolysis potentiate LPO | Lipoylation-dependent TCA enzyme aggregation drives cuproptosis | Both require intact oxidative metabolism; inhibition of mitochondrial function protects from both [65] |
| Downstream oxidative/inflammatory signalling | DAMP release via oxidized lipids activates innate immunity | Proteotoxic stress activates mitochondrial danger pathways | Shared inflammatory amplification from distinct upstream triggers [32,70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Li Pomi, F.; Di Leo, G.; Genovese, S.; Borgia, F.; Gangemi, S. Redox Network Dysfunction: Integrating Ferroptosis and Cuproptosis Across Human Diseases. Antioxidants 2026, 15, 24. https://doi.org/10.3390/antiox15010024
Li Pomi F, Di Leo G, Genovese S, Borgia F, Gangemi S. Redox Network Dysfunction: Integrating Ferroptosis and Cuproptosis Across Human Diseases. Antioxidants. 2026; 15(1):24. https://doi.org/10.3390/antiox15010024
Chicago/Turabian StyleLi Pomi, Federica, Guglielmo Di Leo, Sara Genovese, Francesco Borgia, and Sebastiano Gangemi. 2026. "Redox Network Dysfunction: Integrating Ferroptosis and Cuproptosis Across Human Diseases" Antioxidants 15, no. 1: 24. https://doi.org/10.3390/antiox15010024
APA StyleLi Pomi, F., Di Leo, G., Genovese, S., Borgia, F., & Gangemi, S. (2026). Redox Network Dysfunction: Integrating Ferroptosis and Cuproptosis Across Human Diseases. Antioxidants, 15(1), 24. https://doi.org/10.3390/antiox15010024











