Advances and Perspectives in Curcumin Regulation of Systemic Metabolism: A Focus on Multi-Organ Mechanisms
Abstract
1. Introduction
2. Methods
3. Pharmacokinetics of Curcumin
4. Curcumin Regulation of Glucose Metabolism
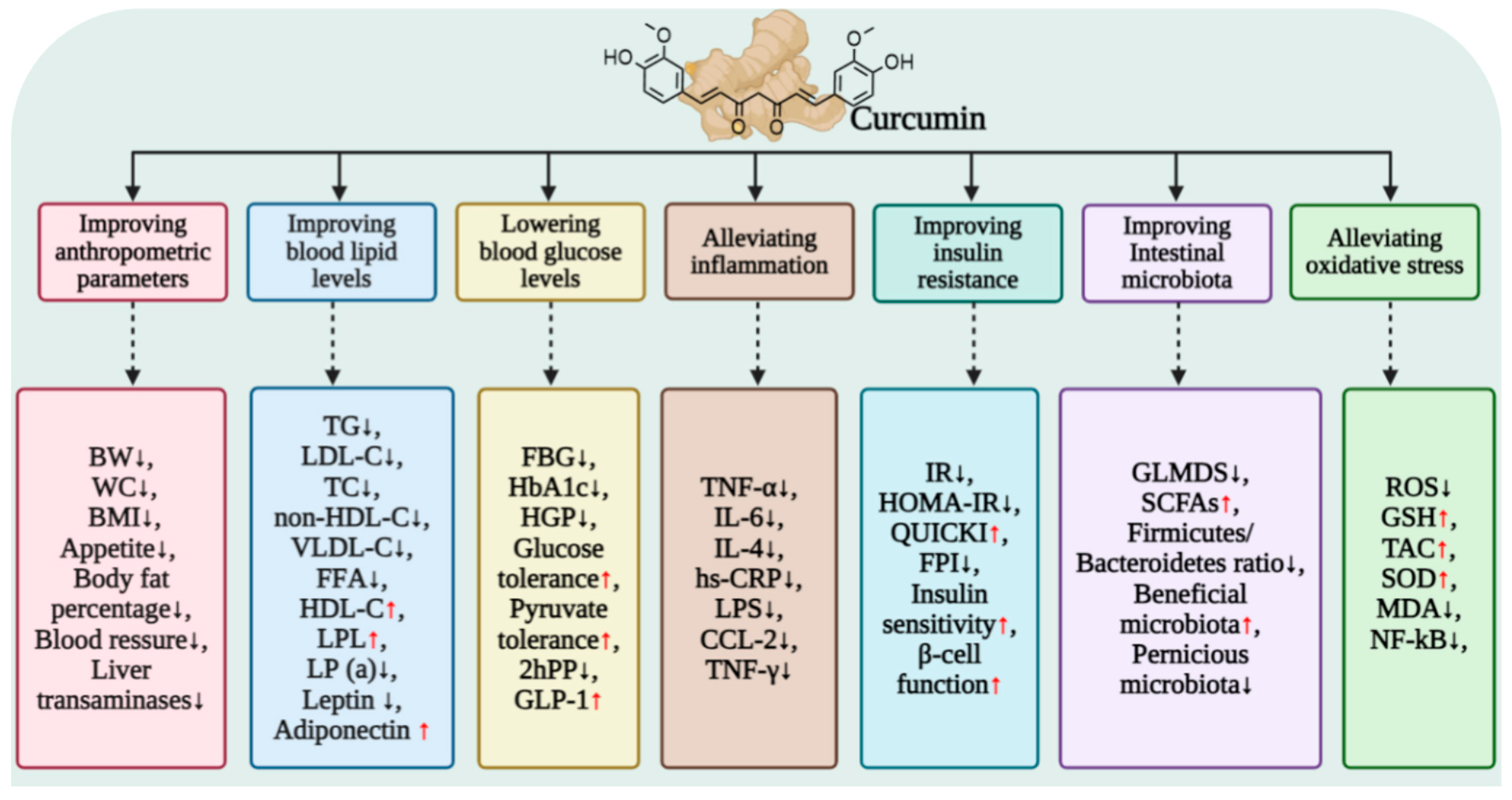
4.1. Overall Improvement in Glucose Homeostasis by Curcumin
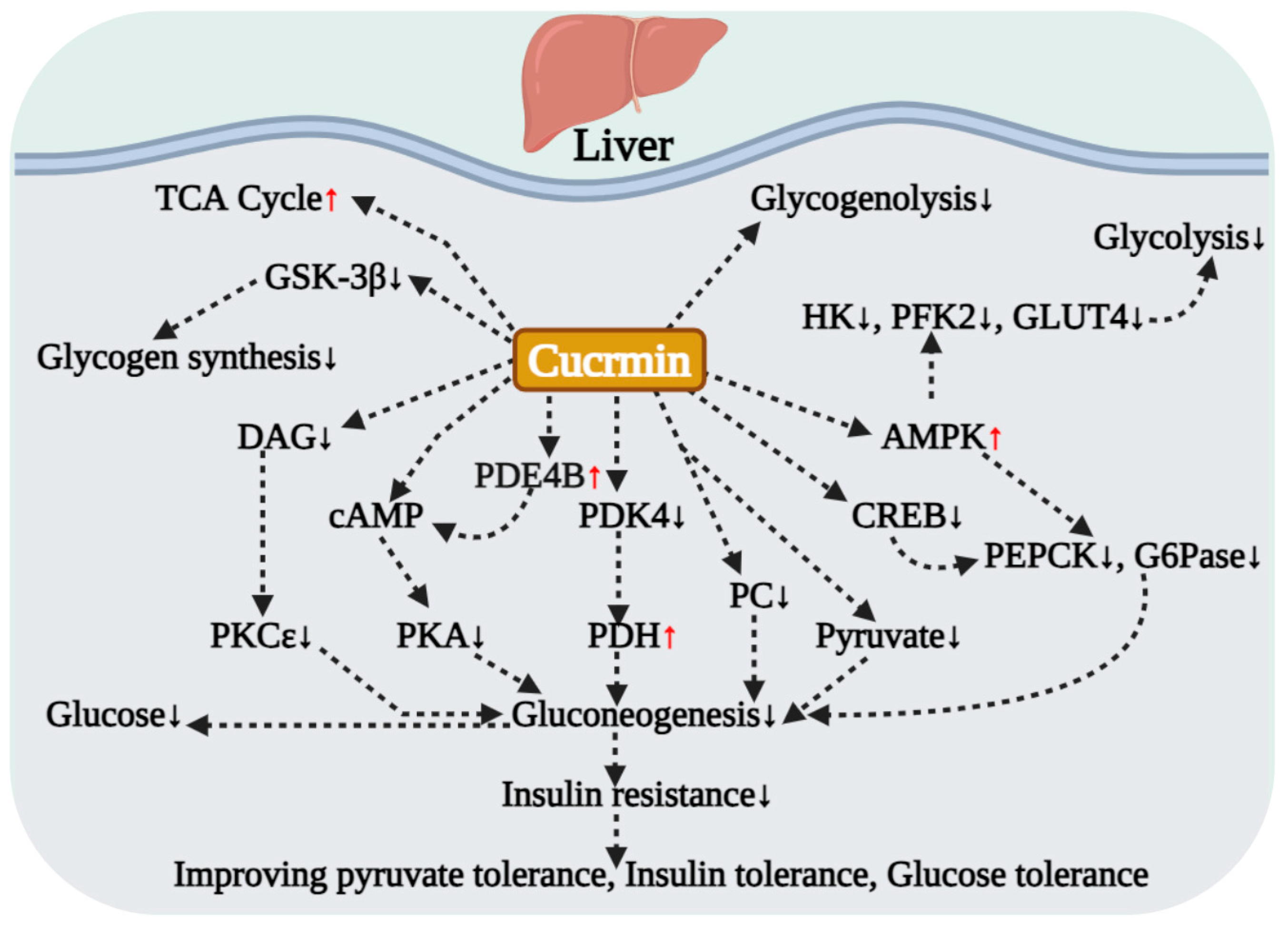
4.2. Core Mechanisms Regulating Hepatic Glucose Metabolism
4.3. Improving Hepatic Glucose Metabolism by Intervening in Glucose-Lipid Metabolic Crosstalk
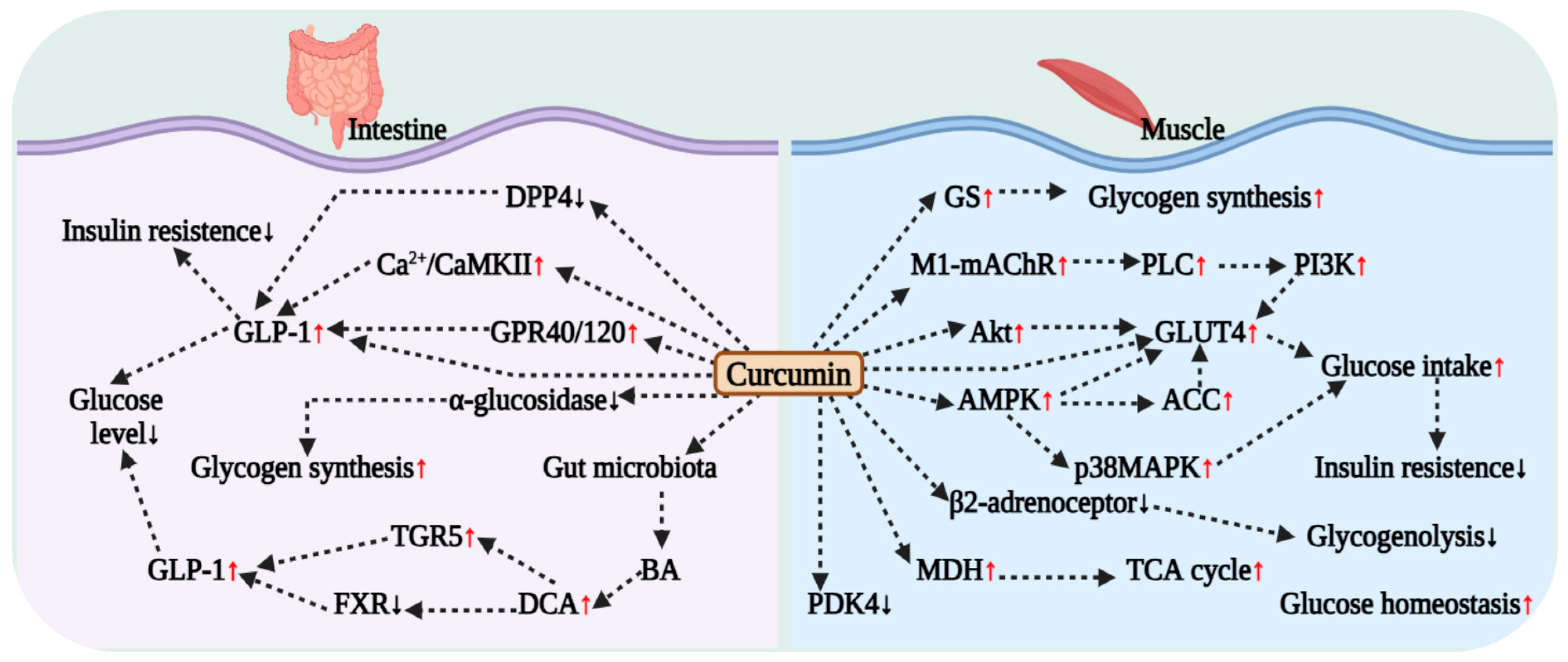
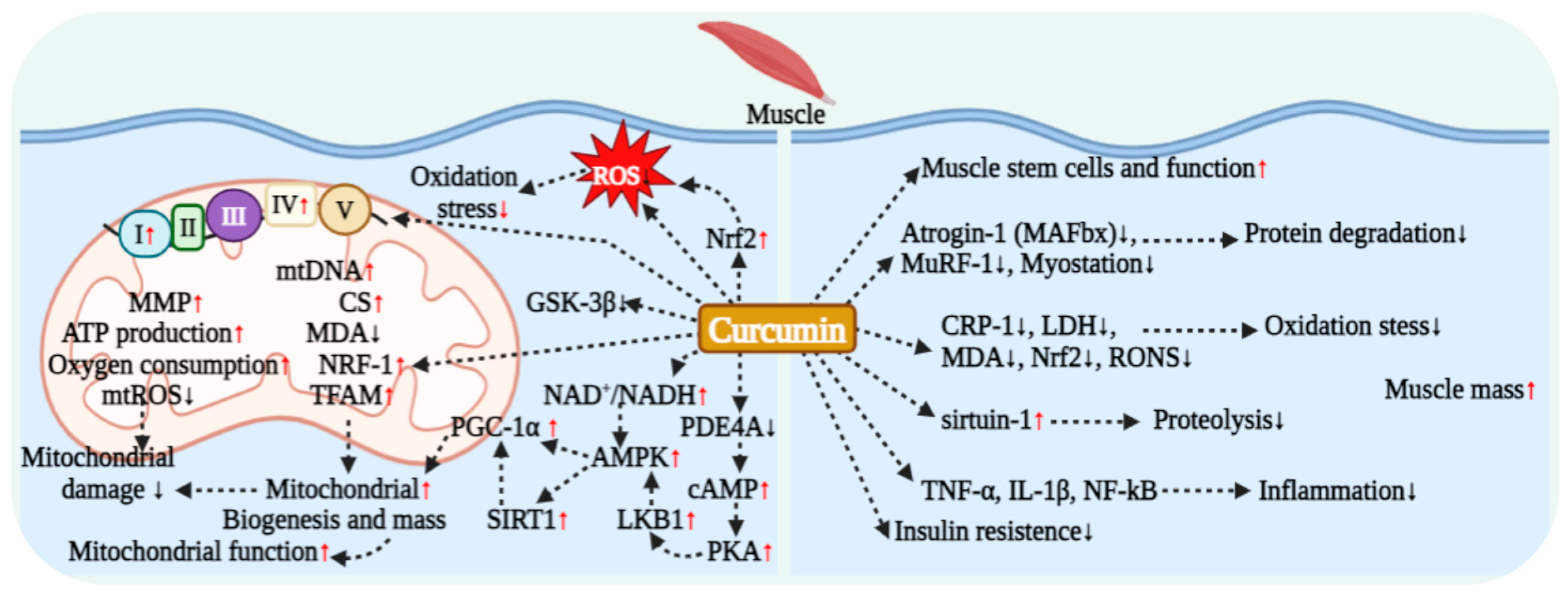
4.4. Curcumin Regulation and Improvement in Muscle Metabolic Function
4.5. Effect of Curcumin on Intestinal Glucose Metabolism
5. Curcumin Regulates Lipid Metabolism
5.1. Effect of Curcumin on Blood Lipid Metabolism
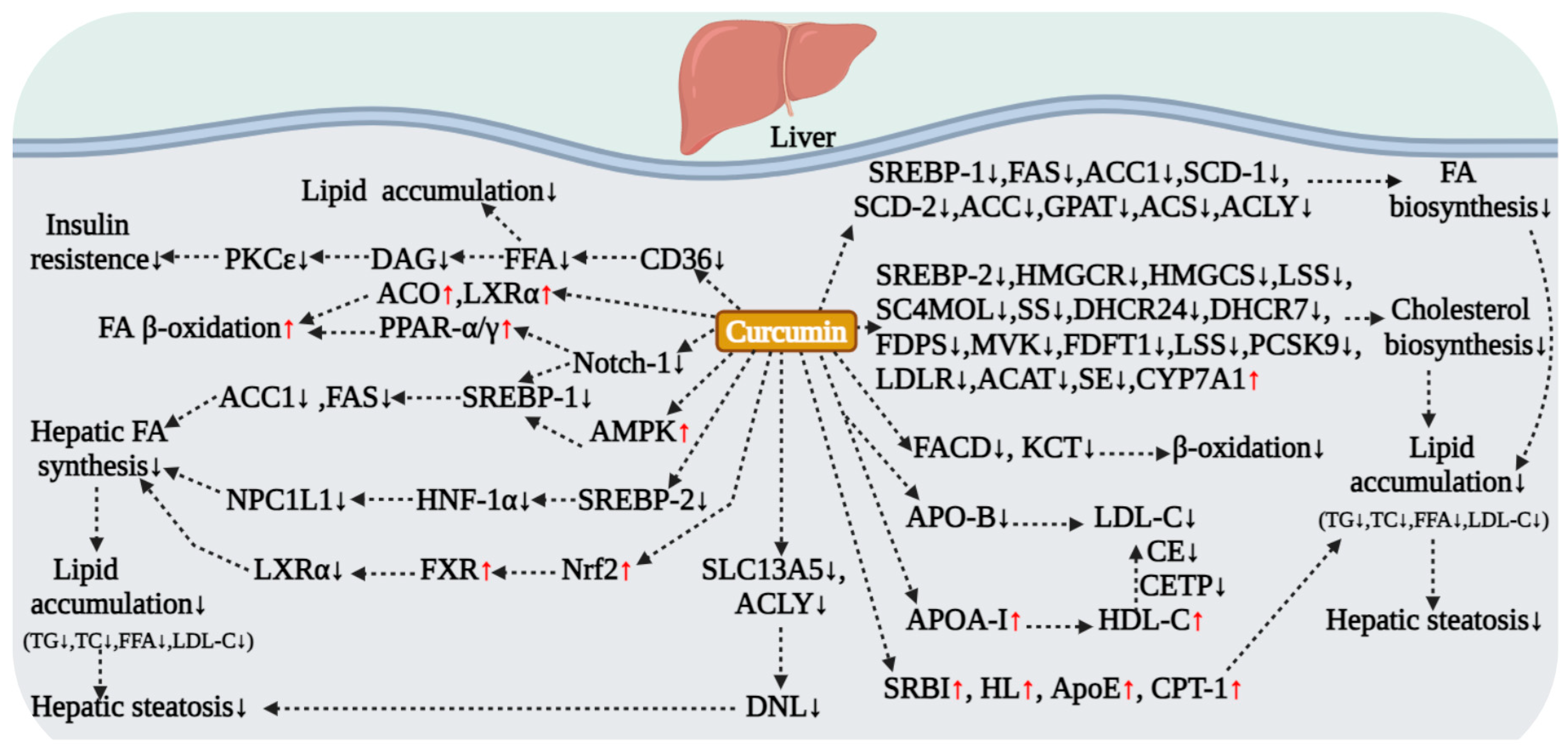
5.2. Curcumin Regulation of Hepatic Lipid Metabolism
5.2.1. Core Regulatory Strategies: Inhibiting Lipogenesis and Promoting Lipolysis
5.2.2. Inhibiting Lipogenesis via the AMPK/SREBP Signaling Axis
5.2.3. Regulating Fatty Acid Transport and Alleviating Lipotoxicity
5.2.4. Regulation of Lipid Metabolism via Multiple AMPK-Independent Pathways
5.2.5. Validation in NAFLD and Diabetic Models
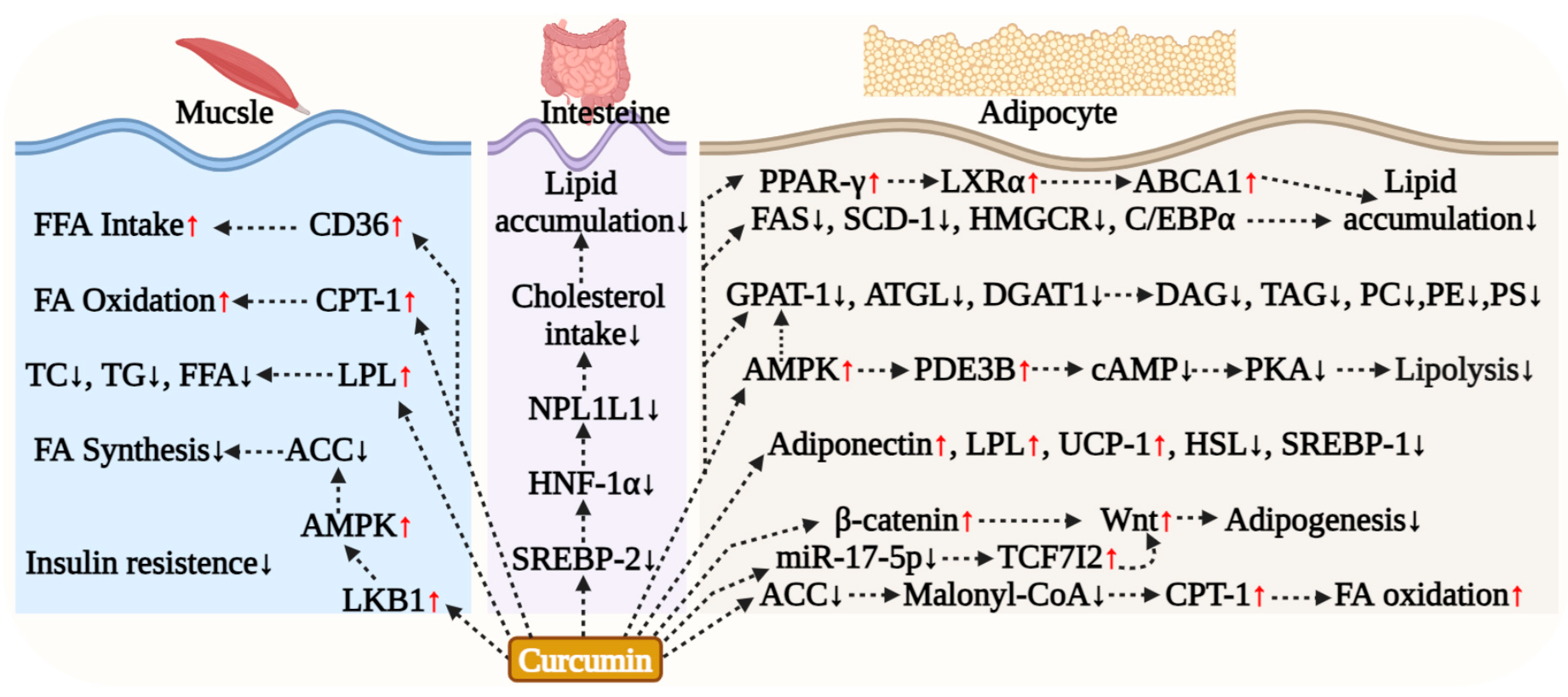
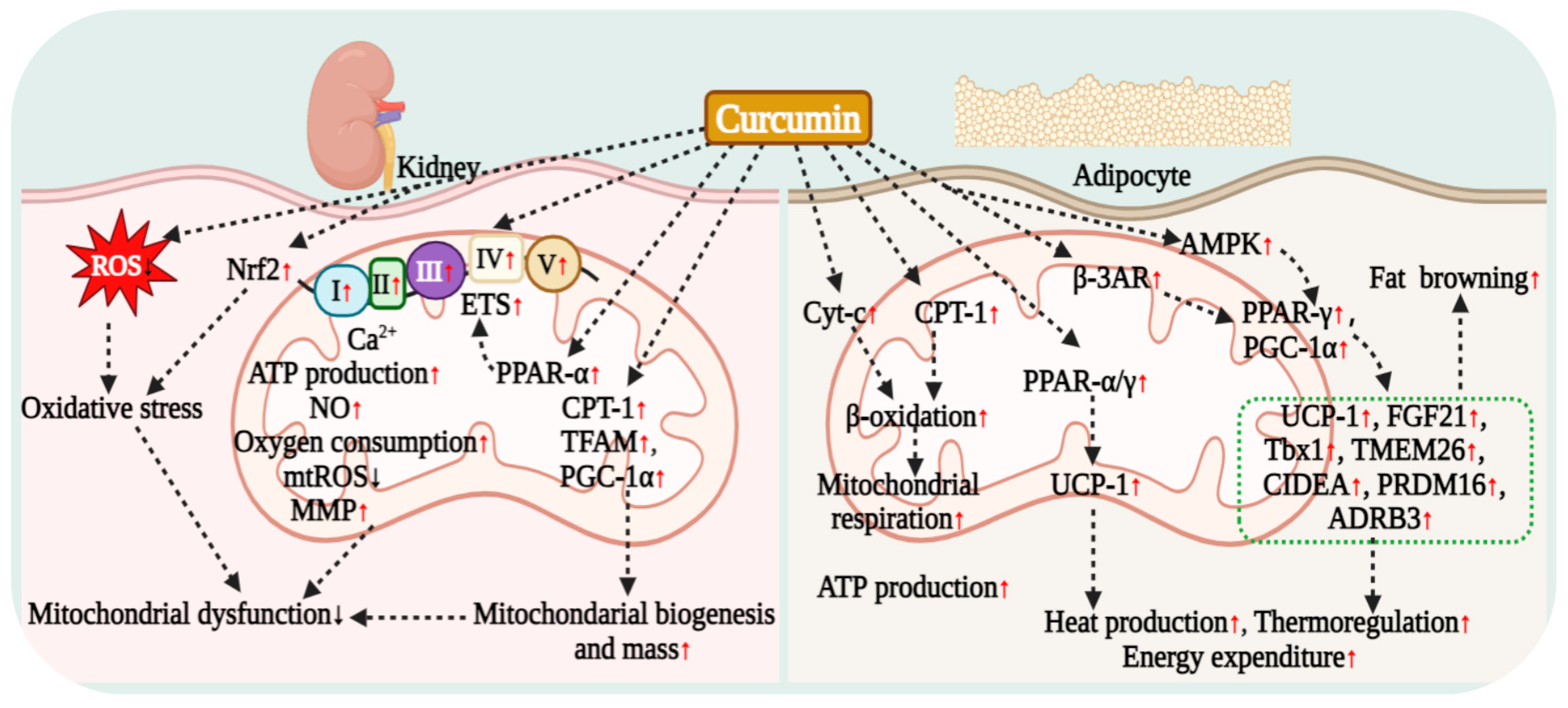
5.3. Curcumin Regulation of Adipocyte Lipid Metabolism
5.4. Curcumin Effect on Muscle Lipid Metabolism
5.5. Curcumin Effect on Intestinal Lipid Metabolism
6. Curcumin Modulation of Amino Acid and Protein Metabolism
6.1. Multi-Target Regulation of Amino Acid and Protein Metabolism by Curcumin
6.2. Regulatory Role and Evidence of Curcumin in Muscle Protein Metabolism
6.3. Antioxidant and Molecular Mechanisms of Curcumin’s Muscle Protective Effects
6.4. Clinical Research Evidence
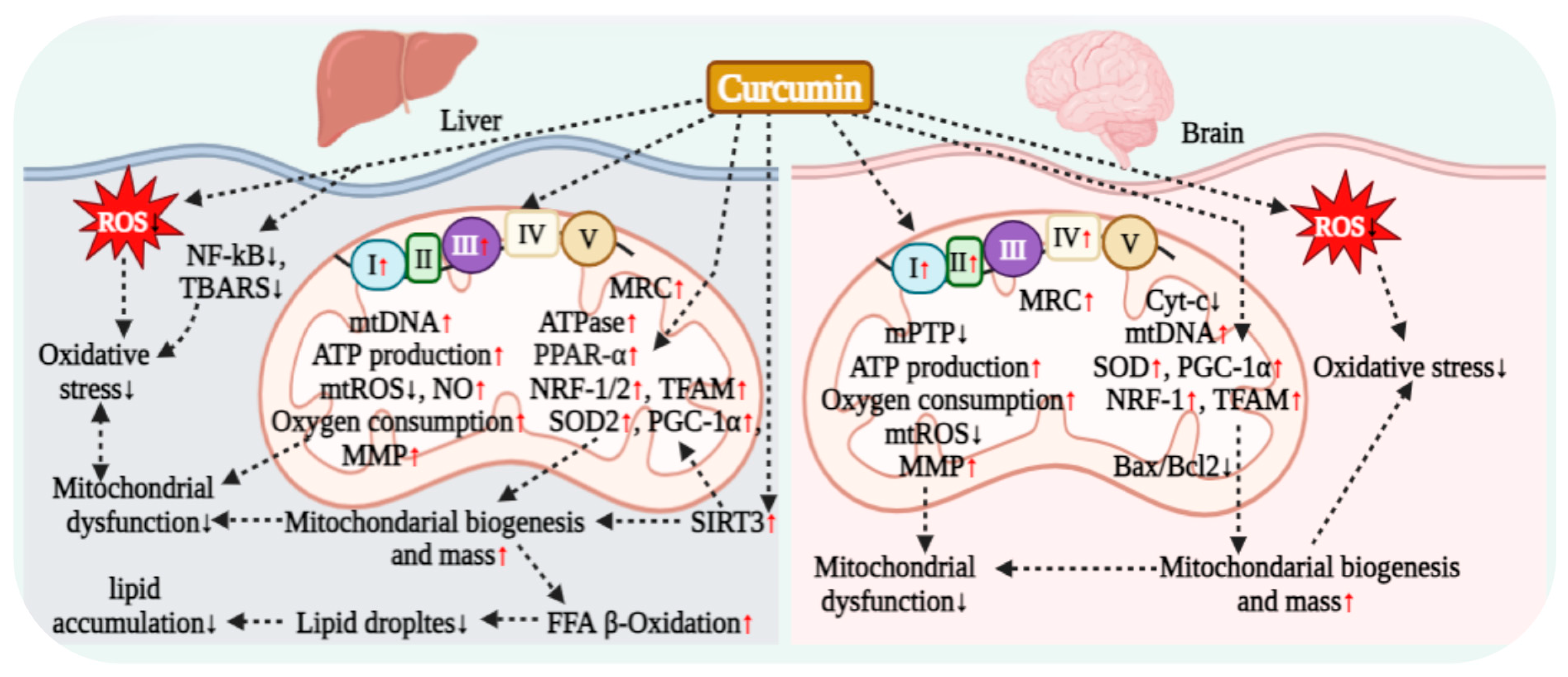
7. Curcumin Regulation of Mitochondrial Respiration
7.1. Curcumin Regulation of Hepatocyte Mitochondrial Respiration
7.2. Curcumin Regulation of Renal Cell Mitochondrial Respiration
7.3. Curcumin Regulation of Adipocyte Mitochondrial Respiration
7.4. Curcumin Regulation of Muscle Cell Mitochondrial Respiration
7.5. Curcumin Regulation of Neuronal Mitochondrial Respiration
8. Curcumin Regulation of Gut Microbiota and Maintenance of Intestinal Barrier Integrity
8.1. Curcumin Regulation of Gut Microbiota via Prebiotic-like Effects
8.2. Curcumin Ameliorates Obesity and Related Metabolic Diseases
8.3. Curcumin Enhances Intestinal Barrier Function and Exerts Anti-Inflammatory Effects
8.4. Curcumin Regulation of Bile Acid and Short-Chain Fatty Acid Metabolism
9. Curcumin Alleviates Inflammation and Oxidative Stress to Improve Systemic Insulin Resistance
| Compound | Disease | Dose | Duration | Outcome | Regulatory Ways | Conclusion | Refs |
|---|---|---|---|---|---|---|---|
| Curcumin | T2DM | 1500 mg/d | 10 weeks | BW ↓, BMI ↓, WC ↓, FBS ↓ | Glycometabolism, Lipid metabolism | Long-term intake of curcumin can effectively improve fasting blood glucose and body weight in patients with T2DM. | [5] |
| Curcumin | T2DM | 300 mg/d | 3 months | FBS ↓, HbA1c ↓, HOMA-IR ↓, FFAs ↓, TC ↓, LPL ↑ | Glycometabolism, Lipid metabolism | Effectively reduces blood glucose and serum free fatty acids in T2DM patients and promotes fatty acid oxidation. | [52] |
| Curcumin | T2DM | 1500 mg/d | 6 months | PWV ↓, adiponectin ↑, leptin ↓, HOMA-IR ↓, TC ↓, uric acid ↓, VF ↓, TBF ↓, LDL-C ↓, TG ↓, HDL-C ↑ | Lipid metabolism, Amino acid metabolism, Protein metabolism | Effectively improve atherosclerosis risk and related metabolic disorders in patients with T2DM. | [54] |
| Curcumin | T2DM | 500 mg/d | 3 months | FPG ↓, 2hpp ↓, HbA1c ↓, insulin sensitivity ↑, insulin ↓, IR ↓ | Glycometabolism | Significantly improve glycemic parameters in T2DM patients. | [6] |
| Curcumin | NASFL | 500 mg/d | 24 weeks | weight ↓, BMI ↓, FFAs ↓, TC ↓, FBS ↓, HbA1c ↓, insulin ↓, F/B ratio ↓, Bacteroides ↑, DCA ↑, GLP-1 ↑ | Glycometabolism, Lipid metabolism, Amino acid metabolism, Protein metabolism, Intestinal flora, Bile acid metabolism | Reduce liver fat content in NAFLD patients, regulate gut microbiota, and improve bile acid metabolism. | [40] |
| Curcumin | Obesity | 1000 mg/d | 30 days | TC ↓ | Lipid metabolism | Beneficial for reducing blood lipid levels in obese patients. | [84] |
| Curcumin | NAFLD | 70 mg/d | 8 weeks | Liver fat ↓, BMI ↓, TG ↓, HLD-C ↓, TC ↓, AST ↓, ALT ↓, Glucose ↓, HbA1c ↓ | Glycometabolism, Lipid metabolism, Amino acid metabolism, Protein metabolism | Short-term curcumin administration significantly improves NAFLD-related pathological features. | [87] |
| Curcumin | NAFLD | 1000 mg/d | 8 weeks | TG ↓, HDL-C ↓, TC ↓, non-HDL-C ↓, uric acid ↓ | Lipid metabolism, Amino acid metabolism | Significantly reduce blood lipid and uric acid levels in NAFLD patients and improve metabolic profiles. | [88] |
| Curcumin | NAFLD | 50 mg/d | 8 weeks | HDL-C ↑, adiponectin ↑, leptin ↓, Leptin/Adiponectin ratio ↓ | Lipid metabolism, Protein metabolism | Effectively improve serum adipokine levels in NAFLD patients. | [89] |
| Curcumin | Healthy people | 500 mg/d | 8 weeks | CRP ↓, LDH ↓, MDA ↓, VO2 max ↑ | Protein metabolism, Redox metabolism, Inflammation | Supplementation of curcumin during moderate-intensity exercise reduces markers of inflammation, oxidative stress, and muscle damage in the body. | [135] |
| Curcumin | T2MD | 1000 mg/d | 12 weeks | MDA ↓, TAC ↑, GSH ↑, PPAR-γ ↑ | Redox metabolism, Mitochondrial metabolism | Improving inflammation and oxidative stress in patients with T2DM. | [210] |
| Curcumin | CKD | 320 mg/d | 8 weeks | Lipid peroxidation ↓, TAC ↑ | Redox metabolism | Reduce oxidative stress levels in CKD patients. | [208] |
| Curcumin | T2DM | 1500 mg/d | 10 weeks | TG ↓, hs-CRP ↓, adiponectin ↑ | Lipid metabolism, Inflammation | Delaying the progression of diabetic complications by reducing triglyceride levels and inflammatory markers. | [35] |
| Curcumin | T2DM | 300 mg/d | 3 months | A-FABP ↓, CRP ↓, TNF-α ↓, IL-6 ↓, SOD ↑, Glucose ↓, FFAs ↓ | Lipid metabolism, Inflammation, Glycometabolism, Redox metabolism | Reducing serum A-FABP levels in T2DM patients, exerting anti-diabetic effects, and improving metabolic parameters. | [20] |
| Curcumin | T2DM | 1500 mg/d | 9 months | β-cell function ↑, HOMA-β ↑, C-peptide ↓, HOMA-IR ↓, adiponectin ↑ | Glycometabolism, Lipid metabolism, Protein metabolism | Long-term curcumin intake may delay the progression from prediabetes to T2DM, improve β-cell function, and reduce insulin resistance. | [211] |
| Nano curcumin | DFU | 80 mg/d | 12 weeks | FBS ↓, insulin ↓, IR ↓, insulin sensitivity ↑, TG ↓, LDL-C ↓, TAC ↑, GSH ↑ | Glycometabolism, Lipid metabolism, Redox metabolism | Improve glucose and lipid metabolism disorders in DFU patients, alleviate insulin resistance, and oxidative stress. | [15] |
| Nano curcumin | Diabetes on Hemodialysis | 80 mg/d | 12 weeks | FBS ↓, insulin ↓, TC ↓, VLDL-TG ↓, TG ↓, LDL-TG ↓, TG/HDL-TG ratio ↓, hs-CRP ↓, MDA ↓, TAC ↑, Total nitrite level ↑ | Glycometabolism, Lipid metabolism, Mitochondrial metabolism, Redox metabolism | Long-term administration of nano-curcumin can effectively improve the metabolic profile in diabetes on hemodialysis patients. | [51] |
| Nano curcumin | T2DM | 80 mg/d | 3 months | HbA1c ↓, FBG ↓, TG ↓, BMI ↓, eAG ↓, LDL-C ↓ | Glycometabolism, Lipid metabolism | Reduce serum HbA1C, LDL-C, and BMI levels in patients with T2DM. | [50] |
| Nano curcumin | NAFLD | 80 mg/d | 3 months | HDL ↑, QUICKI ↑, nesfatin ↑, WC ↓, FBS ↓, FBI ↓, HbA1c ↓, TG ↓, TC ↓, LDL ↓, HOMA-IR ↓, TNF-α, hs-CRP ↓, IL-6 ↓ | Glycometabolism, Lipid metabolism | Effectively improves blood glucose, blood lipids, inflammation, waist circumference, liver enzymes, and the degree of fatty liver in NAFLD patients. | [83] |
| Phospholipid curcumin | NAFLD | 1500 mg/d | 8 weeks | TG ↓, LDL-C ↓, HDL-C ↓, TC ↓, non-HDL-C ↓, Lipid profile ↑, AST ↓, ALT ↓, uric acid ↓ | Lipid metabolism, Protein metabolism | Alleviate the severity of NAFLD and improve disease progression-related indicators. | [90] |
| Phospholipid curcumin | NAFLD | 250 mg/d | 8 weeks | 3-methyl-2-oxovaleric acid ↓, 3-hydroxyisobutyric acid ↓, kynurenine ↓, succinate ↓, citrate ↓, α-ketoglutarate ↓, methylamine ↓, trimethylamine ↓, maleate ↓, indophenol sulfate ↓, CDCA ↓, taurocholic acid ↓, lithocholic acid ↓ | Amino acid metabolism, Bile acid metabolism, Glycometabolism (TCA Cycle) Intestinal flora | Significantly improve patients’ serum metabolic profiles and exert metabolic regulatory effects. | [7] |
| Curserin® (phytosomal curcumin) | Obesity | 400 mg/d | 8 weeks | FPI ↓, HOMA-IR ↓, waistline ↓, blood pressure ↓, TG ↓, HDL-C ↑, transaminase ↓, γ-GT ↓, HSI ↓, Serum cortisol ↓ | Glycometabolism, Lipid metabolism, Protein metabolism, Amino acid metabolism | Improving blood glucose, liver function, and serum cortisol levels in overweight patients with impaired fasting glucose. | [85] |
| Curcumin, Piperine | T2DM | Curcumin (500 mg/d), Piperine (5 mg/d) | 3 months | Glucose ↓, C-peptide ↓, HbA1c ↓, ALT ↓, AST ↓ | Glycometabolism, Lipid metabolism, Protein metabolism | Improvement of blood glucose and liver-related parameters in T2DM patients through combined use with piperine. | [55] |
| Curcumin, Piperine | T2DM | Curcumin (1000 mg/d), Piperine (10 mg/d) | 12 weeks | TC ↓, non-HDL-C ↓, Lp (a) ↓, HDL-C ↑ | Lipid metabolism | Reduce atherosclerotic lipid parameters and lipid levels in T2DM patients, and decrease the risk of cardiovascular diseases. | [86] |
| Curcumin, Piperine | T2DM | Curcumin (1000 mg/d), Piperine (10 mg/d) | 12 weeks | leptin ↓, TNF-α ↓, Leptin/Adiponectin ratio ↓, adiponectin ↑ | Inflammation, Lipid metabolism, Protein metabolism | Improving inflammatory factors and adipokine levels in patients with T2DM. | [209] |
| Curcumin, Piperine | T2DM | Curcumin (1000 mg/d), Piperine (10 mg/d) | 8 weeks | TAC ↑, SOD ↑, MDA ↓ | Redox metabolism | Significantly improve oxidative stress in T2DM patients. | [34] |
| Theracurmin® (curcumin preparation) | T2DM | 180 mg/d | 6 months | AT-LDL ↓, oxidized LDL ↓ | Glycometabolism, Lipid metabolism | Reduction of oxidized LDL levels in patients with impaired glucose tolerance or non-insulin-dependent diabetes mellitus. | [91] |
| Meriva® (curcumin preparation) | CKD | 1000 mg/d | 6 months | CCL-2 ↓, IFN-γ ↓, IL-4 ↓, Lipid peroxidation ↓, Escherichia-Shigella ↓, Lachnoclostridium ↑, Lactobacillaceae ↑ | Inflammation, Redox metabolism, Intestinal flora | Significantly reduce pro-inflammatory mediators and lipid peroxidation levels in CKD patients. | [185] |
| Meriva® (curcumin preparation) | Healthy older people | 500 mg/d | 3 months | Exercise capacity parameters (including grip strength, weight lifting, walking, cycling, etc.) and health-related indicators (including proteinuria, oxidative stress level, etc.) were significantly improved. | Protein metabolism, Redox metabolism | Combining a standard diet with moderate exercise helps improve physical strength and bodily functions in the elderly, with potential for preventing sarcopenia. | [134] |
| NCB-02 (curcumin preparation) | T2DM | 150 mg/d | 8 weeks | MDA ↓, ET-1 ↓, IL-6 ↓, TNF-α ↓ | Inflammation, Redox metabolism | Significantly improves endothelial dysfunction in T2DM patients, reduces inflammatory factors, and oxidative stress levels. | [36] |
10. Summary and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TCA | tricarboxylic acid cycle | APO-B | apolipoprotein B |
| GSK-3β | glycogen synthase kinase-3 beta | APOA-I | apolipoprotein A-I |
| DAG | diacylglycerol | SRBI | scavenger receptor class B type I |
| PKC | protein kinase C | HL | hepatic lipase |
| cAMP | cyclic adenosine monophosphate | ApoE | apolipoprotein E |
| PKA | protein kinase A | CPT-1 | carnitine palmitoyltransferase I |
| PDE4B | phosphodiesterase 4B | LPL | lipoprotein lipase |
| PDH | pyruvate dehydrogenase | LKB1 | liver Kinase B1 |
| PDK4 | pyruvate dehydrogenase kinase 4 | VO2 max | maximum oxygen uptake |
| PC | pyruvate carboxylase | UCP-1 | uncoupling protein 1 |
| CREB | cAMP response element-binding protein | TCF7I2 | transcription factor 7 like 2 |
| ABCA1 | ATP-binding cassette subfamily A member 1 | ROS | reactive oxygen species |
| HK | hexokinase | mtDNA | mitochondrial DNA |
| PFK2 | phosphofructokinase-2 | MRC | mitochondrial respiratory chain |
| GLUT4 | glucose transporter 4 | SOD2 | superoxide dismutase 2 |
| TFAM | mitochondrial transcription factor A | NRF-1/2 | nuclear respiratory factor 1/2 |
| PEPCK | phosphoenolpyruvate carboxykinase | SIRT3 | sirtuin 3 |
| mPTP | mitochondrial permeability transition pore | Cyt-c | cytochrome c |
| TGR5 | Takeda G protein-coupled receptor 5 | NO | nitric oxide |
| CIDEA | cell death-inducing DFFA-like effector A | ETS | electron transport system |
| BA | bile acid | β-3AR | beta-3 adrenergic receptor |
| GPR40/120 | G protein-coupled receptor 40/120 | Tbx1 | t-box transcription factor 1 |
| GS | glutamine synthetase | PRDM16 | PR domain containing 16 |
| PLC | phospholipase C | ADRB3 | adrenoceptor beta 3 |
| RONS | reactive oxygen and nitrogen species | FGF21 | fibroblast growth factor 21 |
| AMPK | AMP-activated protein kinase | TMEM26 | transmembrane protein 26 |
| ACC | acetyl-CoA carboxylase | MDA | malondialdehyde |
| MDH | malate dehydrogenase | SIRT1 | sirtuin 1 |
| FFA | free fatty acid | IL-1β | interleukin-1 beta |
| ACO | acyl-CoA oxidase | BW | body weight |
| LXRα | liver X receptor alpha | WC | waist circumference |
| VLDL-C | very low-density lipoprotein cholesterol | BMI | body mass index |
| PPAR-α | peroxisome proliferator-activated receptor α | LP (a) | lipoprotein (a) |
| 2hPP | 2-h postprandial plasma glucose | FBG | fasting blood glucose |
| ACC1 | acetyl-CoA carboxylase 1 | HbA1c | hemoglobin A1c |
| FAS | fatty acid synthase | HGP | hepatic glucose production |
| SREBP-1 | sterol regulatory element-binding protein-1 | GLP-1 | glucagon-;ike peptide-1 |
| NPC1L1 | niemann-pick c1-like 1 | IL-6 | interleukin-6 |
| HNF-1α | hepatocyte nuclear factor 1 alpha | CCL-2 | C-C motif chemokine ligand 2 |
| SREBP2 | sterol regulatory element-binding protein-2 | TNF-α/γ | tumor necrosis factor-α/γ |
| FXR | farnesoid X receptor | SCFA | short-chain fatty acid |
| SLC13A5 | solute carrier family 13 member 5 | TAC | total Antioxidant capacity |
| ACAT | acyl-CoA cholesterol acyltransferase | SOD | superoxide dismutase |
| LDL-C | low-density lipoprotein cholesterol | SE | cholesteryl ester |
| SCD-1/2 | stearoyl-CoA desaturase-1/2 | LDLR | low-density lipoprotein receptor |
| FADS1/2 | fatty acid desaturase 1/2 | MVK | mevalonate kinase |
| GPAT1 | glycerol-3-phosphate acyltransferase 1 | ACLY | ATP citrate lyase |
| ACS | acyl-CoA synthetase | DNL | de novo lipogenesis |
| HMGCR | 3-hydroxy-3-methylglutaryl-CoA reductase | TG | triglyceride |
| HMGCS | 3-hydroxy-3-methylglutaryl-CoA synthase | TC | total cholesterol |
| LSS | lanosterol synthase | DHCR24 | 24-dehydrocholesterol reductase |
| SC4MOL | sterol-C4-methyl oxidase-like | DHCR7 | 7-dehydrocholesterol reductase |
| SS | squalene synthase | FDPS | farnesyl diphosphate synthase |
| FDFT1 | farnesyl-diphosphate farnesyltransferase 1 | G6Pase | glucose-6-phosphatase |
| CYP7A1 | cytochrome P450 family 7 subfamily a member 1 | DPP4 | dipeptidyl peptidase-4 |
| HDL-C | high-density lipoprotein cholesterol | 2hpp | 2-h postprandial glucose |
| FBS | fasting blood sugar | DCA | deoxycholic acid |
| γ-GT | gamma glutamyl transpeptidase | ET-1 | Endothelin-1 |
| FPI | fasting plasma insulin | TBF | total body fat |
| DFU | diabetic foot ulce | PWV | pulse wave velocity |
| CKD | chronic kidney disease | VF | visceral fat |
| HSI | hepatic steatosis index | CDCA | chenodeoxycholic acid |
References
- Kunnumakkara, A.B.; Bordoloi, D.; Padmavathi, G.; Monisha, J.; Roy, N.K.; Prasad, S.; Aggarwal, B.B. Curcumin, the golden nutraceutical: Multitargeting for multiple chronic diseases. Br. J. Pharmacol. 2017, 174, 1325–1348. [Google Scholar] [CrossRef]
- Balakumar, P.; Venkatesan, K.; Abdulla Khan, N.; Raghavendra, N.M.; Venugopal, V.; Bharathi, D.R.; Fuloria, N.K. Mechanistic insights into the beneficial effects of curcumin on insulin resistance: Opportunities and challenges. Drug Discov. Today 2023, 28, 103627. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N.; Yki-Jarvinen, H.; Neuschwander-Tetri, B.A. Metabolic dysfunction-associated steatotic liver disease: Heterogeneous pathomechanisms and effectiveness of metabolism-based treatment. Lancet Diabetes Endocrinol. 2025, 13, 134–148. [Google Scholar] [CrossRef]
- He, S.; Wen, H.; Fu, Y.; Chen, C.; Xu, M.; Zhang, M.; Zhao, M.; Zhao, S. Uncovering Causal Links Between Dietary Habits and Cardiovascular Diseases. Food Sci. Nutr. 2025, 13, e70229. [Google Scholar] [CrossRef]
- Hodaei, H.; Adibian, M.; Nikpayam, O.; Hedayati, M.; Sohrab, G. The effect of curcumin supplementation on anthropometric indices, insulin resistance and oxidative stress in patients with type 2 diabetes: A randomized, double-blind clinical trial. Diabetol. Metab. Syndr. 2019, 11, 41. [Google Scholar] [CrossRef]
- Karandish, M.; Mozaffari-Khosravi, H.; Mohammadi, S.M.; Cheraghian, B.; Azhdari, M. The effect of curcumin and zinc co-supplementation on glycemic parameters in overweight or obese prediabetic subjects: A phase 2 randomized, placebo-controlled trial with a multi-arm, parallel-group design. Phytother. Res. 2021, 35, 4377–4387. [Google Scholar] [CrossRef] [PubMed]
- Chashmniam, S.; Mirhafez, S.R.; Dehabeh, M.; Hariri, M.; Azimi Nezhad, M.; Nobakht, M.G.B.F. A pilot study of the effect of phospholipid curcumin on serum metabolomic profile in patients with non-alcoholic fatty liver disease: A randomized, double-blind, placebo-controlled trial. Eur. J. Clin. Nutr. 2019, 73, 1224–1235. [Google Scholar] [CrossRef]
- Hassaninasab, A.; Hashimoto, Y.; Tomita-Yokotani, K.; Kobayashi, M. Discovery of the curcumin metabolic pathway involving a unique enzyme in an intestinal microorganism. Proc. Natl. Acad. Sci. USA 2011, 108, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- Scazzocchio, B.; Minghetti, L.; D’Archivio, M. Interaction between Gut Microbiota and Curcumin: A New Key of Understanding for the Health Effects of Curcumin. Nutrients 2020, 12, 2499. [Google Scholar] [CrossRef]
- Xu, X.; Wang, H.; Guo, D.; Man, X.; Liu, J.; Li, J.; Luo, C.; Zhang, M.; Zhen, L.; Liu, X. Curcumin modulates gut microbiota and improves renal function in rats with uric acid nephropathy. Ren. Fail. 2021, 43, 1063–1075. [Google Scholar] [CrossRef]
- Li, H.B.; Xu, M.L.; Du, M.M.; Yu, X.J.; Bai, J.; Xia, W.J.; Dai, Z.M.; Li, C.X.; Li, Y.; Su, Q.; et al. Curcumin ameliorates hypertension via gut-brain communication in spontaneously hypertensive rat. Toxicol. Appl. Pharmacol. 2021, 429, 115701. [Google Scholar] [CrossRef]
- Islam, T.; Koboziev, I.; Albracht-Schulte, K.; Mistretta, B.; Scoggin, S.; Yosofvand, M.; Moussa, H.; Zabet-Moghaddam, M.; Ramalingam, L.; Gunaratne, P.H.; et al. Curcumin Reduces Adipose Tissue Inflammation and Alters Gut Microbiota in Diet-Induced Obese Male Mice. Mol. Nutr. Food Res. 2021, 65, e2100274. [Google Scholar] [CrossRef]
- Alli-Oluwafuyi, A.M.; Luis, P.B.; Nakashima, F.; Gimenez-Bastida, J.A.; Presley, S.H.; Duvernay, M.T.; Iwalewa, E.O.; Schneider, C. Curcumin induces secretion of glucagon-like peptide-1 through an oxidation-dependent mechanism. Biochimie 2019, 165, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Zhu, X.; Zhou, N.; Zheng, W.; Zhou, W.; Li, X. Curcumin alleviates hepatic steatosis by improving mitochondrial function in postnatal overfed rats and fatty L02 cells through the SIRT3 pathway. Food Funct. 2022, 13, 2155–2171. [Google Scholar] [CrossRef]
- Mokhtari, M.; Razzaghi, R.; Momen-Heravi, M. The effects of curcumin intake on wound healing and metabolic status in patients with diabetic foot ulcer: A randomized, double-blind, placebo-controlled trial. Phytother. Res. 2021, 35, 2099–2107. [Google Scholar] [CrossRef] [PubMed]
- Manas-Garcia, L.; Bargallo, N.; Gea, J.; Barreiro, E. Muscle Phenotype, Proteolysis, and Atrophy Signaling During Reloading in Mice: Effects of Curcumin on the Gastrocnemius. Nutrients 2020, 12, 388. [Google Scholar] [CrossRef]
- Yu, T.; Dohl, J.; Elenberg, F.; Chen, Y.; Deuster, P. Curcumin induces concentration-dependent alterations in mitochondrial function through ROS in C2C12 mouse myoblasts. J. Cell Physiol. 2019, 234, 6371–6381. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, N.; He, H.; Tang, X. Pharmaceutical strategies of improving oral systemic bioavailability of curcumin for clinical application. J. Control. Release 2019, 316, 359–380. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Dohl, J.; Wang, L.; Chen, Y.; Gasier, H.G.; Deuster, P.A. Curcumin Ameliorates Heat-Induced Injury Through NADPH Oxidase-Dependent Redox Signaling and Mitochondrial Preservation in C2C12 Myoblasts and Mouse Skeletal Muscle. J. Nutr. 2020, 150, 2257–2267. [Google Scholar] [CrossRef]
- Na, L.X.; Yan, B.L.; Jiang, S.; Cui, H.L.; Li, Y.; Sun, C.H. Curcuminoids Target Decreasing Serum Adipocyte-fatty Acid Binding Protein Levels in Their Glucose-lowering Effect in Patients with Type 2 Diabetes. Biomed. Environ. Sci. 2014, 27, 902–906. [Google Scholar] [CrossRef]
- Santos-Parker, J.R.; Strahler, T.R.; Bassett, C.J.; Bispham, N.Z.; Chonchol, M.B.; Seals, D.R. Curcumin supplementation improves vascular endothelial function in healthy middle-aged and older adults by increasing nitric oxide bioavailability and reducing oxidative stress. Aging 2017, 9, 187–208. [Google Scholar] [CrossRef]
- Wahlang, B.; Pawar, Y.B.; Bansal, A.K. Identification of permeability-related hurdles in oral delivery of curcumin using the Caco-2 cell model. Eur. J. Pharm. Biopharm. 2011, 77, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T. Curcumin as a functional food-derived factor: Degradation products, metabolites, bioactivity, and future perspectives. Food Funct. 2018, 9, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.L.; Hsu, C.H.; Lin, J.K.; Hsu, M.M.; Ho, Y.F.; Shen, T.S.; Ko, J.Y.; Lin, J.T.; Lin, B.R.; Ming-Shiang, W.; et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001, 21, 2895–2900. [Google Scholar] [PubMed]
- Ravindranath, V.; Chandrasekhara, N. Absorption and tissue distribution of curcumin in rats. Toxicology 1980, 16, 259–265. [Google Scholar] [CrossRef]
- Wahlstrom, B.; Blennow, G. A study on the fate of curcumin in the rat. Acta Pharmacol. Toxicol. 1978, 43, 86–92. [Google Scholar] [CrossRef]
- Pan, M.H.; Huang, T.M.; Lin, J.K. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab. Dispos. 1999, 27, 486–494. [Google Scholar] [CrossRef]
- Holder, G.M.; Plummer, J.L.; Ryan, A.J. The metabolism and excretion of curcumin (1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) in the rat. Xenobiotica 1978, 8, 761–768. [Google Scholar] [CrossRef]
- Vareed, S.K.; Kakarala, M.; Ruffin, M.T.; Crowell, J.A.; Normolle, D.P.; Djuric, Z.; Brenner, D.E. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1411–1417. [Google Scholar] [CrossRef]
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Cooke, D.N.; Shafayat, A.; Hewitt, H.R.; Marczylo, T.H.; Morgan, B.; Hemingway, D.; Plummer, S.M.; et al. Phase I clinical trial of oral curcumin: Biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004, 10, 6847–6854. [Google Scholar] [CrossRef]
- Dhillon, N.; Aggarwal, B.B.; Newman, R.A.; Wolff, R.A.; Kunnumakkara, A.B.; Abbruzzese, J.L.; Ng, C.S.; Badmaev, V.; Kurzrock, R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin. Cancer Res. 2008, 14, 4491–4499. [Google Scholar] [CrossRef]
- Lao, C.D.; Ruffin, M.T.t.; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006, 6, 10. [Google Scholar] [CrossRef]
- Srivastava, P.; Yadav, R.S.; Chandravanshi, L.P.; Shukla, R.K.; Dhuriya, Y.K.; Chauhan, L.K.S.; Dwivedi, H.N.; Pant, A.B.; Khanna, V.K. Unraveling the mechanism of neuroprotection of curcumin in arsenic induced cholinergic dysfunctions in rats. Toxicol. Appl. Pharmacol. 2014, 279, 428–440. [Google Scholar] [CrossRef]
- Panahi, Y.; Khalili, N.; Sahebi, E.; Namazi, S.; Karimian, M.S.; Majeed, M.; Sahebkar, A. Antioxidant effects of curcuminoids in patients with type 2 diabetes mellitus: A randomized controlled trial. Inflammopharmacology 2017, 25, 25–31. [Google Scholar] [CrossRef]
- Adibian, M.; Hodaei, H.; Nikpayam, O.; Sohrab, G.; Hekmatdoost, A.; Hedayati, M. The effects of curcumin supplementation on high-sensitivity C-reactive protein, serum adiponectin, and lipid profile in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Phytother. Res. 2019, 33, 1374–1383. [Google Scholar] [CrossRef]
- Usharani, P.; Mateen, A.A.; Naidu, M.U.; Raju, Y.S.; Chandra, N. Effect of NCB-02, atorvastatin and placebo on endothelial function, oxidative stress and inflammatory markers in patients with type 2 diabetes mellitus: A randomized, parallel-group, placebo-controlled, 8-week study. Drugs R D 2008, 9, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Suzuki, K.; Kim, H.K.; Otsuka, Y.; Imaizumi, A.; Miyashita, M.; Sakamoto, S. Effects of curcumin supplementation on exercise-induced oxidative stress in humans. Int. J. Sports Med. 2014, 35, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Ahmed Nasef, N.; Loveday, S.M.; Golding, M.; Martins, R.N.; Shah, T.M.; Clarke, M.; Coad, J.; Moughan, P.J.; Garg, M.L.; Singh, H. Food matrix and co-presence of turmeric compounds influence bioavailability of curcumin in healthy humans. Food Funct. 2019, 10, 4584–4592. [Google Scholar] [CrossRef]
- Peterson, C.T.; Vaughn, A.R.; Sharma, V.; Chopra, D.; Mills, P.J.; Peterson, S.N.; Sivamani, R.K. Effects of Turmeric and Curcumin Dietary Supplementation on Human Gut Microbiota: A Double-Blind, Randomized, Placebo-Controlled Pilot Study. J. Evid. Based Integr. Med. 2018, 23, 2515690X18790725. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Chen, X.; Li, Y.; Liang, Y.; Hong, T.; Yang, J.; Cao, Z.; Mai, H.; Yao, J.; Zhang, T.; et al. Curcumin supplementation alleviates hepatic fat content associated with modulation of gut microbiota-dependent bile acid metabolism in patients with nonalcoholic simple fatty liver disease: A randomized controlled trial. Am. J. Clin. Nutr. 2024, 120, 66–79. [Google Scholar] [CrossRef]
- Servida, S.; Piontini, A.; Gori, F.; Tomaino, L.; Moroncini, G.; De Gennaro Colonna, V.; La Vecchia, C.; Vigna, L. Curcumin and Gut Microbiota: A Narrative Overview with Focus on Glycemic Control. Int. J. Mol. Sci. 2024, 25, 7710. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Chen, X.; Meng, J.; Guo, Q.; Xu, S.; Hou, S.; Yuan, Z.; Zhang, W. The role of curcumin in the liver-gut system diseases: From mechanisms to clinical therapeutic perspective. Crit. Rev. Food Sci. Nutr. 2024, 64, 8822–8851. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Cai, X.; Guan, Q.; Ou, Y.; Zheng, X.; Lin, X. Burden of type 1 and type 2 diabetes and high fasting plasma glucose in Europe, 1990–2019: A comprehensive analysis from the global burden of disease study 2019. Front. Endocrinol. 2023, 14, 1307432. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Yang, G.; Chai, X.; Zhou, Y.; Sun, X.; Xing, Z. Glycemic variability evaluated by HbA1c rather than fasting plasma glucose is associated with adverse cardiovascular events. Front. Endocrinol. 2024, 15, 1323571. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, B.; Huang, F.; Liu, B.; Xie, Y. Curcumin inhibits lipolysis via suppression of ER stress in adipose tissue and prevents hepatic insulin resistance. J. Lipid Res. 2016, 57, 1243–1255. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, D.; She, L.; Zhang, Y.; Wei, Q.; Aa, J.; Wang, G.; Liu, B.; Xie, Y. Curcumin restrains hepatic glucose production by blocking cAMP/PKA signaling and reducing acetyl CoA accumulation in high-fat diet (HFD)-fed mice. Mol. Cell Endocrinol. 2018, 474, 127–136. [Google Scholar] [CrossRef]
- Seo, K.I.; Choi, M.S.; Jung, U.J.; Kim, H.J.; Yeo, J.; Jeon, S.M.; Lee, M.K. Effect of curcumin supplementation on blood glucose, plasma insulin, and glucose homeostasis related enzyme activities in diabetic db/db mice. Mol. Nutr. Food Res. 2008, 52, 995–1004. [Google Scholar] [CrossRef]
- El-Moselhy, M.A.; Taye, A.; Sharkawi, S.S.; El-Sisi, S.F.; Ahmed, A.F. The antihyperglycemic effect of curcumin in high fat diet fed rats. Role of TNF-alpha and free fatty acids. Food Chem. Toxicol. 2011, 49, 1129–1140. [Google Scholar] [CrossRef]
- Zhong, Y.; Xiao, Y.; Gao, J.; Zheng, Z.; Zhang, Z.; Yao, L.; Li, D. Curcumin improves insulin sensitivity in high-fat diet-fed mice through gut microbiota. Nutr. Metab. 2022, 19, 76. [Google Scholar] [CrossRef]
- Rahimi, H.R.; Mohammadpour, A.H.; Dastani, M.; Jaafari, M.R.; Abnous, K.; Ghayour Mobarhan, M.; Kazemi Oskuee, R. The effect of nano-curcumin on HbA1c, fasting blood glucose, and lipid profile in diabetic subjects: A randomized clinical trial. Avicenna J. Phytomed. 2016, 6, 567–577. [Google Scholar]
- Shafabakhsh, R.; Asemi, Z.; Reiner, Z.; Soleimani, A.; Aghadavod, E.; Bahmani, F. The Effects of Nano-curcumin on Metabolic Status in Patients with Diabetes on Hemodialysis, a Randomized, Double Blind, Placebo-controlled Trial. Iran. J. Kidney Dis. 2020, 14, 290–299. [Google Scholar] [PubMed]
- Na, L.X.; Li, Y.; Pan, H.Z.; Zhou, X.L.; Sun, D.J.; Meng, M.; Li, X.X.; Sun, C.H. Curcuminoids exert glucose-lowering effect in type 2 diabetes by decreasing serum free fatty acids: A double-blind, placebo-controlled trial. Mol. Nutr. Food Res. 2013, 57, 1569–1577. [Google Scholar] [CrossRef]
- Tang, M.; Larson-Meyer, D.E.; Liebman, M. Effect of cinnamon and turmeric on urinary oxalate excretion, plasma lipids, and plasma glucose in healthy subjects. Am. J. Clin. Nutr. 2008, 87, 1262–1267. [Google Scholar] [CrossRef]
- Chuengsamarn, S.; Rattanamongkolgul, S.; Phonrat, B.; Tungtrongchitr, R.; Jirawatnotai, S. Reduction of atherogenic risk in patients with type 2 diabetes by curcuminoid extract: A randomized controlled trial. J. Nutr. Biochem. 2014, 25, 144–150. [Google Scholar] [CrossRef]
- Panahi, Y.; Khalili, N.; Sahebi, E.; Namazi, S.; Simental-Mendia, L.E.; Majeed, M.; Sahebkar, A. Effects of Curcuminoids Plus Piperine on Glycemic, Hepatic and Inflammatory Biomarkers in Patients with Type 2 Diabetes Mellitus: A Randomized Double-Blind Placebo-Controlled Trial. Drug Res. 2018, 68, 403–409. [Google Scholar] [CrossRef]
- Yuan, F.; Wu, W.; Ma, L.; Wang, D.; Hu, M.; Gong, J.; Fang, K.; Xu, L.; Dong, H.; Lu, F. Turmeric and curcuminiods ameliorate disorders of glycometabolism among subjects with metabolic diseases: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2022, 177, 106121. [Google Scholar] [CrossRef]
- Han, L.; Xu, S.; Chen, R.; Zheng, Z.; Ding, Y.; Wu, Z.; Li, S.; He, B.; Bao, M. Causal associations between HbA1c and multiple diseases unveiled through a Mendelian randomization phenome-wide association study in East Asian populations. Medicine 2025, 104, e41861. [Google Scholar] [CrossRef]
- Fujiwara, H.; Hosokawa, M.; Zhou, X.; Fujimoto, S.; Fukuda, K.; Toyoda, K.; Nishi, Y.; Fujita, Y.; Yamada, K.; Yamada, Y.; et al. Curcumin inhibits glucose production in isolated mice hepatocytes. Diabetes Res. Clin. Pract. 2008, 80, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Al-Saud, N.B.S. Impact of curcumin treatment on diabetic albino rats. Saudi J. Biol. Sci. 2020, 27, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.F.; Chen, X.; Tang, Y.; Wu, J.M.; Qin, D.L.; Yu, L.; Yu, C.L.; Zhou, X.G.; Wu, A.G. The Therapeutic Potential of Plant Polysaccharides in Metabolic Diseases. Pharmaceuticals 2022, 15, 1329. [Google Scholar] [CrossRef]
- Liang, H.; Tantiwong, P.; Sriwijitkamol, A.; Shanmugasundaram, K.; Mohan, S.; Espinoza, S.; Defronzo, R.A.; Dube, J.J.; Musi, N. Effect of a sustained reduction in plasma free fatty acid concentration on insulin signalling and inflammation in skeletal muscle from human subjects. J. Physiol. 2013, 591, 2897–2909. [Google Scholar] [CrossRef] [PubMed]
- Lian, N.; Jin, H.; Zhang, F.; Wu, L.; Shao, J.; Lu, Y.; Zheng, S. Curcumin inhibits aerobic glycolysis in hepatic stellate cells associated with activation of adenosine monophosphate-activated protein kinase. Iubmb Life 2016, 68, 589–596. [Google Scholar] [CrossRef]
- Li, Z.Y.; Ding, L.L.; Li, J.M.; Xu, B.L.; Yang, L.; Bi, K.S.; Wang, Z.T. 1H-NMR and MS based metabolomics study of the intervention effect of curcumin on hyperlipidemia mice induced by high-fat diet. PLoS ONE 2015, 10, e0120950. [Google Scholar] [CrossRef]
- Shoghi, K.I.; Gropler, R.J.; Sharp, T.; Herrero, P.; Fettig, N.; Su, Y.; Mitra, M.S.; Kovacs, A.; Finck, B.N.; Welch, M.J. Time course of alterations in myocardial glucose utilization in the Zucker diabetic fatty rat with correlation to gene expression of glucose transporters: A small-animal PET investigation. J. Nucl. Med. 2008, 49, 1320–1327. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, Y.C.; Guo, F.J.; Meng, Y.; Li, M.L. Anti-diabetic effects of cinnamaldehyde and berberine and their impacts on retinol-binding protein 4 expression in rats with type 2 diabetes mellitus. Chin. Med. J. 2008, 121, 2124–2128. [Google Scholar] [CrossRef]
- Higashida, K.; Higuchi, M.; Terada, S. Dissociation between PGC-1alpha and GLUT-4 expression in skeletal muscle of rats fed a high-fat diet. J. Nutr. Sci. Vitaminol. 2009, 55, 486–491. [Google Scholar] [CrossRef]
- Badr, G.A.; Tang, J.; Ismail-Beigi, F.; Kern, T.S. Diabetes downregulates GLUT1 expression in the retina and its microvessels but not in the cerebral cortex or its microvessels. Diabetes 2000, 49, 1016–1021. [Google Scholar] [CrossRef]
- Hou, W.K.; Xian, Y.X.; Zhang, L.; Lai, H.; Hou, X.G.; Xu, Y.X.; Yu, T.; Xu, F.Y.; Song, J.; Fu, C.L.; et al. Influence of blood glucose on the expression of glucose trans-porter proteins 1 and 3 in the brain of diabetic rats. Chin. Med. J. 2007, 120, 1704–1709. [Google Scholar] [CrossRef]
- Kang, C.; Kim, E. Synergistic effect of curcumin and insulin on muscle cell glucose metabolism. Food Chem. Toxicol. 2010, 48, 2366–2373. [Google Scholar] [CrossRef] [PubMed]
- Na, L.X.; Zhang, Y.L.; Li, Y.; Liu, L.Y.; Li, R.; Kong, T.; Sun, C.H. Curcumin improves insulin resistance in skeletal muscle of rats. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.T.; Chang, T.W.; Lee, M.S.; Lin, J.K. Suppression of free fatty acid-induced insulin resistance by phytopolyphenols in C2C12 mouse skeletal muscle cells. J. Agric. Food Chem. 2012, 60, 1059–1066. [Google Scholar] [CrossRef]
- Cheng, T.C.; Lin, C.S.; Hsu, C.C.; Chen, L.J.; Cheng, K.C.; Cheng, J.T. Activation of muscarinic M-1 cholinoceptors by curcumin to increase glucose uptake into skeletal muscle isolated from Wistar rats. Neurosci. Lett. 2009, 465, 238–241. [Google Scholar] [CrossRef]
- Arai, T.; Nakamura, M.; Magori, E.; Fukuda, H.; Sako, T. Decrease in malate dehydrogenase activities in peripheral leucocytes of type 1 diabetic dogs. Res. Vet. Sci. 2003, 74, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Xavier, S.; Sadanandan, J.; George, N.; Paulose, C.S. beta(2)-adrenoceptor and insulin receptor expression in the skeletal muscle of streptozotocin induced diabetic rats: Antagonism by vitamin D(3) and curcumin. Eur. J. Pharmacol. 2012, 687, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, A.P.; Watanabe, K.; Thandavarayan, R.A.; Sari, F.R.; Meilei, H.; Soetikno, V.; Arumugam, S.; Giridharan, V.V.; Suzuki, K.; Kodama, M. Curcumin attenuates hyperglycaemia-mediated AMPK activation and oxidative stress in cerebrum of streptozotocin-induced diabetic rat. Free Radic. Res. 2011, 45, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Takikawa, M.; Kurimoto, Y.; Tsuda, T. Curcumin stimulates glucagon-like peptide-1 secretion in GLUTag cells via Ca2+/calmodulin-dependent kinase II activation. Biochem. Biophys. Res. Commun. 2013, 435, 165–170. [Google Scholar] [CrossRef]
- Kato, M.; Nishikawa, S.; Ikehata, A.; Dochi, K.; Tani, T.; Takahashi, T.; Imaizumi, A.; Tsuda, T. Curcumin improves glucose tolerance via stimulation of glucagon-like peptide-1 secretion. Mol. Nutr. Food Res. 2017, 61, 1600471. [Google Scholar] [CrossRef]
- Tian, F.; Chen, T.; Xu, W.; Fan, Y.; Feng, X.; Huang, Q.; Chen, J. Curcumin Compensates GLP-1 Deficiency via the Microbiota-Bile Acids Axis and Modulation in Functional Crosstalk between TGR5 and FXR in ob/ob Mice. Mol. Nutr. Food Res. 2023, 67, e2300195. [Google Scholar] [CrossRef]
- Riyaphan, J.; Jhong, C.H.; Lin, S.R.; Chang, C.H.; Tsai, M.J.; Lee, D.N.; Sung, P.J.; Leong, M.K.; Weng, C.F. Hypoglycemic Efficacy of Docking Selected Natural Compounds against alpha-Glucosidase and alpha-Amylase. Molecules 2018, 23, 2260. [Google Scholar] [CrossRef]
- Huang, P.K.; Lin, S.R.; Chang, C.H.; Tsai, M.J.; Lee, D.N.; Weng, C.F. Natural phenolic compounds potentiate hypoglycemia via inhibition of Dipeptidyl peptidase IV. Sci. Rep. 2019, 9, 15585. [Google Scholar] [CrossRef]
- Cao, W.; Chen, X.; Chin, Y.; Zheng, J.; Lim, P.E.; Xue, C.; Tang, Q. Identification of curcumin as a potential alpha-glucosidase and dipeptidyl-peptidase 4 inhibitor: Molecular docking study, in vitro and in vivo biological evaluation. J. Food Biochem. 2022, 46, e13686. [Google Scholar] [CrossRef]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic roles of curcumin: Lessons learned from clinical trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef] [PubMed]
- Jazayeri-Tehrani, S.A.; Rezayat, S.M.; Mansouri, S.; Qorbani, M.; Alavian, S.M.; Daneshi-Maskooni, M.; Hosseinzadeh-Attar, M.J. Nano-curcumin improves glucose indices, lipids, inflammation, and Nesfatin in overweight and obese patients with non-alcoholic fatty liver disease (NAFLD): A double-blind randomized placebo-controlled clinical trial. Nutr. Metab. 2019, 16, 8. [Google Scholar] [CrossRef]
- Mohammadi, A.; Sahebkar, A.; Iranshahi, M.; Amini, M.; Khojasteh, R.; Ghayour-Mobarhan, M.; Ferns, G.A. Effects of supplementation with curcuminoids on dyslipidemia in obese patients: A randomized crossover trial. Phytother. Res. 2013, 27, 374–379. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Sahebkar, A.; Fogacci, F.; Bove, M.; Giovannini, M.; Borghi, C. Effects of phytosomal curcumin on anthropometric parameters, insulin resistance, cortisolemia and non-alcoholic fatty liver disease indices: A double-blind, placebo-controlled clinical trial. Eur. J. Nutr. 2020, 59, 477–483. [Google Scholar] [CrossRef]
- Panahi, Y.; Khalili, N.; Sahebi, E.; Namazi, S.; Reiner, Z.; Majeed, M.; Sahebkar, A. Curcuminoids modify lipid profile in type 2 diabetes mellitus: A randomized controlled trial. Complement. Ther. Med. 2017, 33, 1–5. [Google Scholar] [CrossRef]
- Rahmani, S.; Asgary, S.; Askari, G.; Keshvari, M.; Hatamipour, M.; Feizi, A.; Sahebkar, A. Treatment of Non-alcoholic Fatty Liver Disease with Curcumin: A Randomized Placebo-controlled Trial. Phytother. Res. 2016, 30, 1540–1548. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Kianpour, P.; Mohtashami, R.; Jafari, R.; Simental-Mendia, L.E.; Sahebkar, A. Curcumin Lowers Serum Lipids and Uric Acid in Subjects With Nonalcoholic Fatty Liver Disease: A Randomized Controlled Trial. J. Cardiovasc. Pharmacol. 2016, 68, 223–229. [Google Scholar] [CrossRef]
- Mirhafez, S.R.; Farimani, A.R.; Dehhabe, M.; Bidkhori, M.; Hariri, M.; Ghouchani, B.F.; Abdollahi, F. Effect of Phytosomal Curcumin on Circulating Levels of Adiponectin and Leptin in Patients with Non-Alcoholic Fatty Liver Disease: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J. Gastrointest. Liver Dis. 2019, 28, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Kianpour, P.; Mohtashami, R.; Soflaei, S.S.; Sahebkar, A. Efficacy of phospholipidated curcumin in nonalcoholic fatty liver disease: A clinical study. J. Asian Nat. Prod. Res. 2019, 21, 798–805. [Google Scholar] [CrossRef]
- Funamoto, M.; Shimizu, K.; Sunagawa, Y.; Katanasaka, Y.; Miyazaki, Y.; Kakeya, H.; Yamakage, H.; Satoh-Asahara, N.; Wada, H.; Hasegawa, K.; et al. Effects of Highly Absorbable Curcumin in Patients with Impaired Glucose Tolerance and Non-Insulin-Dependent Diabetes Mellitus. J. Diabetes Res. 2019, 2019, 8208237. [Google Scholar] [CrossRef]
- Jang, E.M.; Choi, M.S.; Jung, U.J.; Kim, M.J.; Kim, H.J.; Jeon, S.M.; Shin, S.K.; Seong, C.N.; Lee, M.K. Beneficial effects of curcumin on hyperlipidemia and insulin resistance in high-fat-fed hamsters. Metabolism 2008, 57, 1576–1583. [Google Scholar] [CrossRef]
- Ding, L.; Li, J.; Song, B.; Xiao, X.; Zhang, B.; Qi, M.; Huang, W.; Yang, L.; Wang, Z. Curcumin rescues high fat diet-induced obesity and insulin sensitivity in mice through regulating SREBP pathway. Toxicol. Appl. Pharmacol. 2016, 304, 99–109. [Google Scholar] [CrossRef]
- Um, M.Y.; Hwang, K.H.; Ahn, J.; Ha, T.Y. Curcumin attenuates diet-induced hepatic steatosis by activating AMP-activated protein kinase. Basic Clin. Pharmacol. Toxicol. 2013, 113, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, M.; Huang, N.; Guan, F.; Luo, H.; Chen, L.; Wei, G.; Li, M.; Lin, Z.; Su, Z.; et al. Curcumin Alleviates High-fat Diet-induced Nonalcoholic Steatohepatitis via Improving Hepatic Endothelial Function with Microbial Biotransformation in Rats. J. Agric. Food Chem. 2023, 71, 10338–10348. [Google Scholar] [CrossRef] [PubMed]
- Kuo, J.J.; Chang, H.H.; Tsai, T.H.; Lee, T.Y. Positive effect of curcumin on inflammation and mitochondrial dysfunction in obese mice with liver steatosis. Int. J. Mol. Med. 2012, 30, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.K.; Ha, T.Y.; McGregor, R.A.; Choi, M.S. Long-term curcumin administration protects against atherosclerosis via hepatic regulation of lipoprotein cholesterol metabolism. Mol. Nutr. Food Res. 2011, 55, 1829–1840. [Google Scholar] [CrossRef]
- Zhao, N.J.; Liao, M.J.; Wu, J.J.; Chu, K.X. Curcumin suppresses Notch-1 signaling: Improvements in fatty liver and insulin resistance in rats. Mol. Med. Rep. 2018, 17, 819–826. [Google Scholar] [CrossRef]
- Asai, A.; Miyazawa, T. Dietary curcuminoids prevent high-fat diet-induced lipid accumulation in rat liver and epididymal adipose tissue. J. Nutr. 2001, 131, 2932–2935. [Google Scholar] [CrossRef]
- Babu, P.S.; Srinivasan, K. Hypolipidemic action of curcumin, the active principle of turmeric (Curcuma longa) in streptozotocin induced diabetic rats. Mol. Cell Biochem. 1997, 166, 169–175. [Google Scholar] [CrossRef]
- Kim, M.; Kim, Y. Hypocholesterolemic effects of curcumin via up-regulation of cholesterol 7a-hydroxylase in rats fed a high fat diet. Nutr. Res. Pract. 2010, 4, 191–195. [Google Scholar] [CrossRef]
- Yang, J.; Zou, J.; Mai, H.; Hong, T.; Liu, H.; Feng, D. Curcumin protects against high-fat diet-induced nonalcoholic simple fatty liver by inhibiting intestinal and hepatic NPC1L1 expression via down-regulation of SREBP-2/HNF1alpha pathway in hamsters. J. Nutr. Biochem. 2023, 119, 109403. [Google Scholar] [CrossRef]
- Soetikno, V.; Sari, F.R.; Sukumaran, V.; Lakshmanan, A.P.; Harima, M.; Suzuki, K.; Kawachi, H.; Watanabe, K. Curcumin decreases renal triglyceride accumulation through AMPK-SREBP signaling pathway in streptozotocin-induced type 1 diabetic rats. J. Nutr. Biochem. 2013, 24, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Zhang, Y.; Zhang, X.; Aa, J.; Wang, G.; Xie, Y. Curcumin regulates endogenous and exogenous metabolism via Nrf2-FXR-LXR pathway in NAFLD mice. Biomed. Pharmacother. 2018, 105, 274–281. [Google Scholar] [CrossRef]
- Sun, Q.; Niu, Q.; Guo, Y.; Zhuang, Y.; Li, X.; Liu, J.; Li, N.; Li, Z.; Huang, F.; Qiu, Z. Regulation on Citrate Influx and Metabolism through Inhibiting SLC13A5 and ACLY: A Novel Mechanism Mediating the Therapeutic Effects of Curcumin on NAFLD. J. Agric. Food Chem. 2021, 69, 8714–8725. [Google Scholar] [CrossRef]
- Ejaz, A.; Wu, D.; Kwan, P.; Meydani, M. Curcumin inhibits adipogenesis in 3T3-L1 adipocytes and angiogenesis and obesity in C57/BL mice. J. Nutr. 2009, 139, 919–925. [Google Scholar] [CrossRef]
- Zhao, J.; Sun, X.B.; Ye, F.; Tian, W.X. Suppression of fatty acid synthase, differentiation and lipid accumulation in adipocytes by curcumin. Mol. Cell Biochem. 2011, 351, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Song, Z.; Shao, W.; Du, W.W.; Zhao, L.R.; Zeng, K.; Yang, B.B.; Jin, T. Curcumin represses mouse 3T3-L1 cell adipogenic differentiation via inhibiting miR-17-5p and stimulating the Wnt signalling pathway effector Tcf7l2. Cell Death Dis. 2017, 8, e2559. [Google Scholar] [CrossRef] [PubMed]
- Kobori, M.; Takahashi, Y.; Takeda, H.; Takahashi, M.; Izumi, Y.; Akimoto, Y.; Sakurai, M.; Oike, H.; Nakagawa, T.; Itoh, M.; et al. Dietary Intake of Curcumin Improves eIF2 Signaling and Reduces Lipid Levels in the White Adipose Tissue of Obese Mice. Sci. Rep. 2018, 8, 9081. [Google Scholar] [CrossRef]
- Dong, S.Z.; Zhao, S.P.; Wu, Z.H.; Yang, J.; Xie, X.Z.; Yu, B.L.; Nie, S. Curcumin promotes cholesterol efflux from adipocytes related to PPARgamma-LXRalpha-ABCA1 passway. Mol. Cell Biochem. 2011, 358, 281–285. [Google Scholar] [CrossRef]
- Altmann, S.W.; Davis, H.R., Jr.; Zhu, L.J.; Yao, X.; Hoos, L.M.; Tetzloff, G.; Iyer, S.P.; Maguire, M.; Golovko, A.; Zeng, M.; et al. Niemann-Pick C1 like 1 protein is critical for intestinal cholesterol absorption. Science 2004, 303, 1201–1204. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Malhotra, P.; Ma, K.; Singla, A.; Hedroug, O.; Saksena, S.; Dudeja, P.K.; Gill, R.K.; Alrefai, W.A. SREBP2 mediates the modulation of intestinal NPC1L1 expression by curcumin. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G148–G155. [Google Scholar] [CrossRef]
- Feng, D.; Zou, J.; Zhang, S.; Li, X.; Lu, M. Hypocholesterolemic Activity of Curcumin Is Mediated by Down-regulating the Expression of Niemann-Pick C1-like 1 in Hamsters. J. Agric. Food Chem. 2017, 65, 276–280. [Google Scholar] [CrossRef]
- Hong, T.; Zou, J.; Jiang, X.; Yang, J.; Cao, Z.; He, Y.; Feng, D. Curcumin Supplementation Ameliorates Bile Cholesterol Supersaturation in Hamsters by Modulating Gut Microbiota and Cholesterol Absorption. Nutrients 2022, 14, 1828. [Google Scholar] [CrossRef]
- Hong, T.; Zou, J.; Yang, J.; Liu, H.; Cao, Z.; He, Y.; Feng, D. Curcumin protects against bisphenol A-induced hepatic steatosis by inhibiting cholesterol absorption and synthesis in CD-1 mice. Food Sci. Nutr. 2023, 11, 5091–5101. [Google Scholar] [CrossRef]
- Zou, J.; Zhang, S.; Li, P.; Zheng, X.; Feng, D. Supplementation with curcumin inhibits intestinal cholesterol absorption and prevents atherosclerosis in high-fat diet-fed apolipoprotein E knockout mice. Nutr. Res. 2018, 56, 32–40. [Google Scholar] [CrossRef]
- Yao, P.; Cao, S.; Zhu, Z.; Wen, Y.; Guo, Y.; Liang, W.; Xie, J. Cellular Signaling of Amino Acid Metabolism in Prostate Cancer. Int. J. Mol. Sci. 2025, 26, 776. [Google Scholar] [CrossRef]
- Gupta, S.C.; Prasad, S.; Kim, J.H.; Patchva, S.; Webb, L.J.; Priyadarsini, I.K.; Aggarwal, B.B. Multitargeting by curcumin as revealed by molecular interaction studies. Nat. Prod. Rep. 2011, 28, 1937–1955. [Google Scholar] [CrossRef]
- Cui, C.; Han, Y.; Li, H.; Yu, H.; Zhang, B.; Li, G. Curcumin-driven reprogramming of the gut microbiota and metabolome ameliorates motor deficits and neuroinflammation in a mouse model of Parkinson’s disease. Front. Cell Infect. Microbiol. 2022, 12, 887407. [Google Scholar] [CrossRef] [PubMed]
- Receno, C.N.; Liang, C.; Korol, D.L.; Atalay, M.; Heffernan, K.S.; Brutsaert, T.D.; DeRuisseau, K.C. Effects of Prolonged Dietary Curcumin Exposure on Skeletal Muscle Biochemical and Functional Responses of Aged Male Rats. Int. J. Mol. Sci. 2019, 20, 1178. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Chun, Y.S.; Kim, J.K.; Lee, J.O.; Ku, S.K.; Shim, S.M. Curcumin Attenuates Sarcopenia in Chronic Forced Exercise Executed Aged Mice by Regulating Muscle Degradation and Protein Synthesis with Antioxidant and Anti-inflammatory Effects. J. Agric. Food Chem. 2021, 69, 6214–6228. [Google Scholar] [CrossRef]
- Hou, Y.; Xiang, J.; Wang, B.; Duan, S.; Song, R.; Zhou, W.; Tan, S.; He, B. Pathogenesis and comprehensive treatment strategies of sarcopenia in elderly patients with type 2 diabetes mellitus. Front. Endocrinol. 2023, 14, 1263650. [Google Scholar] [CrossRef]
- Ono, T.; Takada, S.; Kinugawa, S.; Tsutsui, H. Curcumin ameliorates skeletal muscle atrophy in type 1 diabetic mice by inhibiting protein ubiquitination. Exp. Physiol. 2015, 100, 1052–1063. [Google Scholar] [CrossRef] [PubMed]
- Saud Gany, S.L.; Chin, K.Y.; Tan, J.K.; Aminuddin, A.; Makpol, S. Curcumin as a Therapeutic Agent for Sarcopenia. Nutrients 2023, 15, 2526. [Google Scholar] [CrossRef]
- Liang, Y.J.; Yang, I.H.; Lin, Y.W.; Lin, J.N.; Wu, C.C.; Chiang, C.Y.; Lai, K.H.; Lin, F.H. Curcumin-Loaded Hydrophobic Surface-Modified Hydroxyapatite as an Antioxidant for Sarcopenia Prevention. Antioxidants 2021, 10, 616. [Google Scholar] [CrossRef]
- Kregel, K.C.; Zhang, H.J. An integrated view of oxidative stress in aging: Basic mechanisms, functional effects, and pathological considerations. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R18–R36. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, K.; Park, J.; Jun, W. Curcuma longa L. Water Extract Improves Dexamethasone-Induced Sarcopenia by Modulating the Muscle-Related Gene and Oxidative Stress in Mice. Antioxidants 2021, 10, 1000. [Google Scholar] [CrossRef]
- He, H.J.; Wang, G.Y.; Gao, Y.; Ling, W.H.; Yu, Z.W.; Jin, T.R. Curcumin attenuates Nrf2 signaling defect, oxidative stress in muscle and glucose intolerance in high fat diet-fed mice. World J. Diabetes 2012, 3, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Sahin, K.; Pala, R.; Tuzcu, M.; Ozdemir, O.; Orhan, C.; Sahin, N.; Juturu, V. Curcumin prevents muscle damage by regulating NF-kappaB and Nrf2 pathways and improves performance: An in vivo model. J. Inflamm. Res. 2016, 9, 147–154. [Google Scholar] [CrossRef]
- Gorza, L.; Germinario, E.; Tibaudo, L.; Vitadello, M.; Tusa, C.; Guerra, I.; Bondi, M.; Salmaso, S.; Caliceti, P.; Vitiello, L.; et al. Chronic Systemic Curcumin Administration Antagonizes Murine Sarcopenia and Presarcopenia. Int. J. Mol. Sci. 2021, 22, 11789. [Google Scholar] [CrossRef]
- Ray Hamidie, R.D.; Yamada, T.; Ishizawa, R.; Saito, Y.; Masuda, K. Curcumin treatment enhances the effect of exercise on mitochondrial biogenesis in skeletal muscle by increasing cAMP levels. Metabolism 2015, 64, 1334–1347. [Google Scholar] [CrossRef]
- Zhang, M.; Tang, J.; Li, Y.; Xie, Y.; Shan, H.; Chen, M.; Zhang, J.; Yang, X.; Zhang, Q.; Yang, X. Curcumin attenuates skeletal muscle mitochondrial impairment in COPD rats: PGC-1alpha/SIRT3 pathway involved. Chem. Biol. Interact. 2017, 277, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yang, Y.; Zou, X.; Zheng, Z.; Zhang, J. Curcumin ameliorates CKD-induced mitochondrial dysfunction and oxidative stress through inhibiting GSK-3beta activity. J. Nutr. Biochem. 2020, 83, 108404. [Google Scholar] [CrossRef]
- Franceschi, F.; Feregalli, B.; Togni, S.; Cornelli, U.; Giacomelli, L.; Eggenhoffner, R.; Belcaro, G. A novel phospholipid delivery system of curcumin (Meriva(R)) preserves muscular mass in healthy aging subjects. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 762–766. [Google Scholar] [PubMed]
- Salehi, M.; Mashhadi, N.S.; Esfahani, P.S.; Feizi, A.; Hadi, A.; Askari, G. The Effects of Curcumin Supplementation on Muscle Damage, Oxidative Stress, and Inflammatory Markers in Healthy Females with Moderate Physical Activity: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Int. J. Prev. Med. 2021, 12, 94. [Google Scholar] [CrossRef]
- de Mello, A.H.; Costa, A.B.; Engel, J.D.G.; Rezin, G.T. Mitochondrial dysfunction in obesity. Life Sci. 2018, 192, 26–32. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed]
- Peoples, J.N.; Saraf, A.; Ghazal, N.; Pham, T.T.; Kwong, J.Q. Mitochondrial dysfunction and oxidative stress in heart disease. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef]
- He, K.; Chen, R.; Xu, S.; Ding, Y.; Wu, Z.; Bao, M.; He, B.; Li, S. Environmental endocrine disruptor-induced mitochondrial dysfunction: A potential mechanism underlying diabetes and its complications. Front. Endocrinol. 2024, 15, 1422752. [Google Scholar] [CrossRef]
- Kuo, J.J.; Chang, H.H.; Tsai, T.H.; Lee, T.Y. Curcumin ameliorates mitochondrial dysfunction associated with inhibition of gluconeogenesis in free fatty acid-mediated hepatic lipoapoptosis. Int. J. Mol. Med. 2012, 30, 643–649. [Google Scholar] [CrossRef]
- Ceja-Galicia, Z.A.; Garcia-Arroyo, F.E.; Aparicio-Trejo, O.E.; El-Hafidi, M.; Gonzaga-Sanchez, G.; Leon-Contreras, J.C.; Hernandez-Pando, R.; Guevara-Cruz, M.; Tovar, A.R.; Rojas-Morales, P.; et al. Therapeutic Effect of Curcumin on 5/6Nx Hypertriglyceridemia: Association with the Improvement of Renal Mitochondrial beta-Oxidation and Lipid Metabolism in Kidney and Liver. Antioxidants 2022, 11, 2195. [Google Scholar] [CrossRef] [PubMed]
- Soto-Urquieta, M.G.; Lopez-Briones, S.; Perez-Vazquez, V.; Saavedra-Molina, A.; Gonzalez-Hernandez, G.A.; Ramirez-Emiliano, J. Curcumin restores mitochondrial functions and decreases lipid peroxidation in liver and kidneys of diabetic db/db mice. Biol. Res. 2014, 47, 74. [Google Scholar] [CrossRef] [PubMed]
- Molina-Jijon, E.; Tapia, E.; Zazueta, C.; El Hafidi, M.; Zatarain-Barron, Z.L.; Hernandez-Pando, R.; Medina-Campos, O.N.; Zarco-Marquez, G.; Torres, I.; Pedraza-Chaverri, J. Curcumin prevents Cr(VI)-induced renal oxidant damage by a mitochondrial pathway. Free Radic. Biol. Med. 2011, 51, 1543–1557. [Google Scholar] [CrossRef]
- Avila-Rojas, S.H.; Aparicio-Trejo, O.E.; Briones-Herrera, A.; Medina-Campos, O.N.; Reyes-Fermin, L.M.; Martinez-Klimova, E.; Leon-Contreras, J.C.; Hernandez-Pando, R.; Tapia, E.; Pedraza-Chaverri, J. Alterations in mitochondrial homeostasis in a potassium dichromate model of acute kidney injury and their mitigation by curcumin. Food Chem. Toxicol. 2020, 145, 111774. [Google Scholar] [CrossRef]
- Li, L.; Liu, S.; Zhou, Y.; Zhao, M.; Wang, Y.; Wang, C.; Lou, P.; Huang, R.; Ma, L.; Lu, Y.; et al. Indispensable role of mitochondria in maintaining the therapeutic potential of curcumin in acute kidney injury. J. Cell Mol. Med. 2021, 25, 9863–9877. [Google Scholar] [CrossRef]
- Negrette-Guzman, M.; Garcia-Nino, W.R.; Tapia, E.; Zazueta, C.; Huerta-Yepez, S.; Leon-Contreras, J.C.; Hernandez-Pando, R.; Aparicio-Trejo, O.E.; Madero, M.; Pedraza-Chaverri, J. Curcumin Attenuates Gentamicin-Induced Kidney Mitochondrial Alterations: Possible Role of a Mitochondrial Biogenesis Mechanism. Evid.-Based Complement. Altern. Med. 2015, 2015, 917435. [Google Scholar] [CrossRef]
- Lone, J.; Choi, J.H.; Kim, S.W.; Yun, J.W. Curcumin induces brown fat-like phenotype in 3T3-L1 and primary white adipocytes. J. Nutr. Biochem. 2016, 27, 193–202. [Google Scholar] [CrossRef]
- Zhao, D.; Pan, Y.; Yu, N.; Bai, Y.; Ma, R.; Mo, F.; Zuo, J.; Chen, B.; Jia, Q.; Zhang, D.; et al. Curcumin improves adipocytes browning and mitochondrial function in 3T3-L1 cells and obese rodent model. R. Soc. Open Sci. 2021, 8, 200974. [Google Scholar] [CrossRef]
- Song, Z.; Revelo, X.; Shao, W.; Tian, L.; Zeng, K.; Lei, H.; Sun, H.S.; Woo, M.; Winer, D.; Jin, T. Dietary Curcumin Intervention Targets Mouse White Adipose Tissue Inflammation and Brown Adipose Tissue UCP1 Expression. Obesity 2018, 26, 547–558. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X.; Ye, Z.; Xu, C.; Zhang, M.; Ruan, B.; Wei, M.; Jiang, Y.; Zhang, Y.; Wang, L.; et al. Curcumin promotes browning of white adipose tissue in a norepinephrine-dependent way. Biochem. Biophys. Res. Commun. 2015, 466, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, S.; Kamiya, M.; Aoyama, H.; Nomura, M.; Hyodo, T.; Ozeki, A.; Lee, H.; Takahashi, T.; Imaizumi, A.; Tsuda, T. Highly Dispersible and Bioavailable Curcumin but not Native Curcumin Induces Brown-like Adipocyte Formation in Mice. Mol. Nutr. Food Res. 2018, 62, 1700731. [Google Scholar] [CrossRef]
- Zhu, X.; Du, S.; Yan, Q.; Min, C.; Zhou, N.; Zhou, W.; Li, X. Dietary curcumin supplementation promotes browning and energy expenditure in postnatal overfed rats. Nutr. Metab. 2021, 18, 97. [Google Scholar] [CrossRef]
- Santos, A.C.C.; Amaro, L.B.R.; Batista Jorge, A.H.; Lelis, S.F.; Lelis, D.F.; Guimaraes, A.L.S.; Santos, S.H.S.; Andrade, J.M.O. Curcumin improves metabolic response and increases expression of thermogenesis-associated markers in adipose tissue of male offspring from obese dams. Mol. Cell Endocrinol. 2023, 563, 111840. [Google Scholar] [CrossRef]
- Tanahashi, K.; Kato, D.; Kojima, T.; Tsuda, T. Low Dose of Curcumin Combined with Exercise Synergistically Induces Beige Adipocyte Formation in Mice. J. Nutr. Sci. Vitaminol. 2023, 69, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Chen, E.; Xie, X.; Guo, Y.; Zhang, M.; Zhu, Y.; Wang, Y.; Fang, F.; Yan, L.; Liu, X. Flll32, a curcumin analog, improves adipose tissue thermogenesis. Biochem. Biophys. Res. Commun. 2024, 737, 150919. [Google Scholar] [CrossRef]
- Hamidie, R.D.R.; Shibaguchi, T.; Yamada, T.; Koma, R.; Ishizawa, R.; Saito, Y.; Hosoi, T.; Masuda, K. Curcumin induces mitochondrial biogenesis by increasing cyclic AMP levels via phosphodiesterase 4A inhibition in skeletal muscle. Br. J. Nutr. 2021, 126, 1642–1650. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.G.; Chen, X.C.; Chen, Z.Z.; Zeng, Y.Q.; Shi, G.B.; Su, Y.H.; Peng, X. Curcumin protects mitochondria from oxidative damage and attenuates apoptosis in cortical neurons. Acta Pharmacol. Sin. 2004, 25, 1606–1612. [Google Scholar] [PubMed]
- Motaghinejad, M.; Karimian, M.; Motaghinejad, O.; Shabab, B.; Yazdani, I.; Fatima, S. Protective effects of various dosage of Curcumin against morphine induced apoptosis and oxidative stress in rat isolated hippocampus. Pharmacol. Rep. 2015, 67, 230–235. [Google Scholar] [CrossRef]
- Xie, C.J.; Gu, A.P.; Cai, J.; Wu, Y.; Chen, R.C. Curcumin protects neural cells against ischemic injury in N2a cells and mouse brain with ischemic stroke. Brain Behav. 2018, 8, e00921. [Google Scholar] [CrossRef]
- Jia, N.; Sun, Q.; Su, Q.; Chen, G. SIRT1-mediated deacetylation of PGC1alpha attributes to the protection of curcumin against glutamate excitotoxicity in cortical neurons. Biochem. Biophys. Res. Commun. 2016, 478, 1376–1381. [Google Scholar] [CrossRef]
- Hagl, S.; Kocher, A.; Schiborr, C.; Kolesova, N.; Frank, J.; Eckert, G.P. Curcumin micelles improve mitochondrial function in neuronal PC12 cells and brains of NMRI mice—Impact on bioavailability. Neurochem. Int. 2015, 89, 234–242. [Google Scholar] [CrossRef]
- Sood, P.K.; Nahar, U.; Nehru, B. Curcumin attenuates aluminum-induced oxidative stress and mitochondrial dysfunction in rat brain. Neurotox. Res. 2011, 20, 351–361. [Google Scholar] [CrossRef]
- Liu, Z.J.; Liu, H.Q.; Xiao, C.; Fan, H.Z.; Huang, Q.; Liu, Y.H.; Wang, Y. Curcumin protects neurons against oxygen-glucose deprivation/reoxygenation-induced injury through activation of peroxisome proliferator-activated receptor-gamma function. J. Neurosci. Res. 2014, 92, 1549–1559. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, W.; Wang, L.; Li, Y.; Tan, B.; Lu, X.; Deng, Y.; Zhang, Y.; Guo, X.; Mu, J.; et al. Curcumin prevents cerebral ischemia reperfusion injury via increase of mitochondrial biogenesis. Neurochem. Res. 2014, 39, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Xu, K.; Jiang, Z.F. Curcumin-mediated neuroprotection against amyloid-beta-induced mitochondrial dysfunction involves the inhibition of GSK-3beta. J. Alzheimer’s Dis. 2012, 32, 981–996. [Google Scholar] [CrossRef] [PubMed]
- Banji, O.J.; Banji, D.; Ch, K. Curcumin and hesperidin improve cognition by suppressing mitochondrial dysfunction and apoptosis induced by D-galactose in rat brain. Food Chem. Toxicol. 2014, 74, 51–59. [Google Scholar] [CrossRef]
- Eckert, G.P.; Schiborr, C.; Hagl, S.; Abdel-Kader, R.; Muller, W.E.; Rimbach, G.; Frank, J. Curcumin prevents mitochondrial dysfunction in the brain of the senescence-accelerated mouse-prone 8. Neurochem. Int. 2013, 62, 595–602. [Google Scholar] [CrossRef]
- Yang, L.; Gao, Y.; Gong, J.; Su, Q.; Guo, Z.; Farag, M.A.; Xiao, J. Myricetin ameliorates prediabetes through gut microbiota-SCFAs-Gpr43 axis. Crit. Rev. Food Sci. Nutr. 2025, 65, 4225–4242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, Y.; Li, W.; Wang, Y.; Zhang, H.; Xu, D.; Chen, R.; Tang, L.; Tang, H. Association between serum vitamin D level and cardiovascular disease in Chinese patients with type 2 diabetes mellitus: A cross-sectional study. Sci. Rep. 2025, 15, 6454. [Google Scholar] [CrossRef]
- Zam, W. Gut Microbiota as a Prospective Therapeutic Target for Curcumin: A Review of Mutual Influence. J. Nutr. Metab. 2018, 2018, 1367984. [Google Scholar] [CrossRef]
- Li, S.; You, J.; Wang, Z.; Liu, Y.; Wang, B.; Du, M.; Zou, T. Curcumin alleviates high-fat diet-induced hepatic steatosis and obesity in association with modulation of gut microbiota in mice. Food Res. Int. 2021, 143, 110270. [Google Scholar] [CrossRef]
- Wang, K.; Ma, J.; Li, Y.; Han, Q.; Yin, Z.; Zhou, M.; Luo, M.; Chen, J.; Xia, S. Effects of essential oil extracted from Artemisia argyi leaf on lipid metabolism and gut microbiota in high-fat diet-fed mice. Front. Nutr. 2022, 9, 1024722. [Google Scholar] [CrossRef]
- Chu, H.; Du, C.; Yang, Y.; Feng, X.; Zhu, L.; Chen, J.; Yang, F. MC-LR Aggravates Liver Lipid Metabolism Disorders in Obese Mice Fed a High-Fat Diet via PI3K/AKT/mTOR/SREBP1 Signaling Pathway. Toxins 2022, 14, 833. [Google Scholar] [CrossRef]
- Li, R.; Yao, Y.; Gao, P.; Bu, S. The Therapeutic Efficacy of Curcumin vs. Metformin in Modulating the Gut Microbiota in NAFLD Rats: A Comparative Study. Front. Microbiol. 2020, 11, 555293. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Guan, B.; Lin, L.; Wang, Y. Improvement of intestinal barrier function, gut microbiota, and metabolic endotoxemia in type 2 diabetes rats by curcumin. Bioengineered 2021, 12, 11947–11958. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, T.; Hao, X.; Hu, Y.; Chen, M.; Zhang, D.; Cai, H.; Luo, J.; Kong, L.; Huang, S.; et al. Mapping the regulatory effects of herbal organic compounds on gut bacteria. Pharmacol. Res. 2023, 193, 106804. [Google Scholar] [CrossRef]
- Hong, T.; Jiang, X.; Zou, J.; Yang, J.; Zhang, H.; Mai, H.; Ling, W.; Feng, D. Hepatoprotective effect of curcumin against bisphenol A-induced hepatic steatosis via modulating gut microbiota dysbiosis and related gut-liver axis activation in CD-1 mice. J. Nutr. Biochem. 2022, 109, 109103. [Google Scholar] [CrossRef]
- Salarolli, R.T.; Alvarenga, L.; Cardozo, L.; Teixeira, K.T.R.; Moreira, L.d.S.G.; Lima, J.D.; Rodrigues, S.D.; Nakao, L.S.; Fouque, D.; Mafra, D. Can curcumin supplementation reduce plasma levels of gut-derived uremic toxins in hemodialysis patients? A pilot randomized, double-blind, controlled study. Int. Urol. Nephrol. 2021, 53, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.S.; Bie, J.; Wang, J.; Ghosh, S. Oral supplementation with non-absorbable antibiotics or curcumin attenuates western diet-induced atherosclerosis and glucose intolerance in LDLR−/− mice--role of intestinal permeability and macrophage activation. PLoS ONE 2014, 9, e108577. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Wang, H.; Zhang, P.; Gao, C.; Tao, J.; Ge, Z.; Zhu, D.; Bi, Y. Modulation of gut microbiota contributes to curcumin-mediated attenuation of hepatic steatosis in rats. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1801–1812. [Google Scholar] [CrossRef]
- Editors, P.O. Expression of Concern: Nanoparticle curcumin ameliorates experimental colitis via modulation of gut microbiota and induction of regulatory T cells. PLoS ONE 2023, 18, e0280115. [Google Scholar] [CrossRef]
- Geng, Q.; Xu, Y.; Huang, W.; Hu, Y.; Jin, H.; Hua, H.; Kong, D. The Potential Mechanism of the Anti-Liver Fibrotic Effect of Curcumin in the Gut-Liver Axis. J. Med. Food 2024, 27, 404–418. [Google Scholar] [CrossRef]
- Kang, Z.P.; Xiao, Q.P.; Huang, J.Q.; Wang, M.X.; Huang, J.; Wei, S.Y.; Cheng, N.; Wang, H.Y.; Liu, D.Y.; Zhong, Y.B.; et al. Curcumin Attenuates Dextran Sodium Sulfate Induced Colitis in Obese Mice. Mol. Nutr. Food Res. 2024, 68, e2300598. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, W.; Men, Q.; Ren, X.; Song, S.; Ai, C. Curcumin-loaded polysaccharide microparticles alleviated DSS-induced ulcerative colitis by improving intestinal microecology and regulating MAPK/NF-kappaB/Nrf2/NLRP3 pathways. Int. J. Biol. Macromol. 2024, 281, 136687. [Google Scholar] [CrossRef] [PubMed]
- Pivari, F.; Mingione, A.; Piazzini, G.; Ceccarani, C.; Ottaviano, E.; Brasacchio, C.; Dei Cas, M.; Vischi, M.; Cozzolino, M.G.; Fogagnolo, P.; et al. Curcumin Supplementation (Meriva((R))) Modulates Inflammation, Lipid Peroxidation and Gut Microbiota Composition in Chronic Kidney Disease. Nutrients 2022, 14, 231. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Xu, S.; Chen, R.; Ding, Y.; Fu, Q.; He, B.; Jiang, T.; Zeng, B.; Bao, M.; Li, S. Assessing causal associations of bile acids with obesity indicators: A Mendelian randomization study. Medicine 2024, 103, e38610. [Google Scholar] [CrossRef]
- Han, Z.; Yao, L.; Zhong, Y.; Xiao, Y.; Gao, J.; Zheng, Z.; Fan, S.; Zhang, Z.; Gong, S.; Chang, S.; et al. Gut microbiota mediates the effects of curcumin on enhancing Ucp1-dependent thermogenesis and improving high-fat diet-induced obesity. Food Funct. 2021, 12, 6558–6575. [Google Scholar] [CrossRef]
- Sun, Z.Z.; Li, X.Y.; Wang, S.; Shen, L.; Ji, H.F. Bidirectional interactions between curcumin and gut microbiota in transgenic mice with Alzheimer’s disease. Appl. Microbiol. Biotechnol. 2020, 104, 3507–3515. [Google Scholar] [CrossRef]
- Lamichhane, G.; Liu, J.; Lee, S.J.; Lee, D.Y.; Zhang, G.; Kim, Y. Curcumin Mitigates the High-Fat High-Sugar Diet-Induced Impairment of Spatial Memory, Hepatic Metabolism, and the Alteration of the Gut Microbiome in Alzheimer’s Disease-Induced (3xTg-AD) Mice. Nutrients 2024, 16, 240. [Google Scholar] [CrossRef] [PubMed]
- Heshmati, J.; Golab, F.; Morvaridzadeh, M.; Potter, E.; Akbari-Fakhrabadi, M.; Farsi, F.; Tanbakooei, S.; Shidfar, F. The effects of curcumin supplementation on oxidative stress, Sirtuin-1 and peroxisome proliferator activated receptor gamma coactivator 1alpha gene expression in polycystic ovarian syndrome (PCOS) patients: A randomized placebo-controlled clinical trial. Diabetes Metab. Syndr. 2020, 14, 77–82. [Google Scholar] [CrossRef]
- Jamilian, M.; Foroozanfard, F.; Kavossian, E.; Aghadavod, E.; Shafabakhsh, R.; Hoseini, A.; Asemi, Z. Effects of curcumin on body weight, glycemic control and serum lipids in women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. ESPEN 2020, 36, 128–133. [Google Scholar] [CrossRef]
- Boesing, F.; Patino, J.S.; da Silva, V.R.; Moreira, E.A. The interface between obesity and periodontitis with emphasis on oxidative stress and inflammatory response. Obes. Rev. 2009, 10, 290–297. [Google Scholar] [CrossRef]
- Reho, J.J.; Rahmouni, K. Oxidative and inflammatory signals in obesity-associated vascular abnormalities. Clin. Sci. 2017, 131, 1689–1700. [Google Scholar] [CrossRef] [PubMed]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef] [PubMed]
- Mokgalaboni, K.; Ntamo, Y.; Ziqubu, K.; Nyambuya, T.M.; Nkambule, B.B.; Mazibuko-Mbeje, S.E.; Gabuza, K.B.; Chellan, N.; Tiano, L.; Dludla, P.V. Curcumin supplementation improves biomarkers of oxidative stress and inflammation in conditions of obesity, type 2 diabetes and NAFLD: Updating the status of clinical evidence. Food Funct. 2021, 12, 12235–12249. [Google Scholar] [CrossRef]
- Cox, F.F.; Misiou, A.; Vierkant, A.; Ale-Agha, N.; Grandoch, M.; Haendeler, J.; Altschmied, J. Protective Effects of Curcumin in Cardiovascular Diseases-Impact on Oxidative Stress and Mitochondria. Cells 2022, 11, 342. [Google Scholar] [CrossRef]
- Yaikwawong, M.; Jansarikit, L.; Jirawatnotai, S.; Chuengsamarn, S. Curcumin Reduces Depression in Obese Patients with Type 2 Diabetes: A Randomized Controlled Trial. Nutrients 2024, 16, 2414. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Ding, F.; Li, L.; Dai, C.; Sun, X.; Xu, J.; Chen, F.; Li, M.; Li, X. Exploring the role of curcumin in mitigating oxidative stress to alleviate lipid metabolism disorders. Front. Pharmacol. 2025, 16, 1517174. [Google Scholar] [CrossRef]
- Satoskar, R.R.; Shah, S.J.; Shenoy, S.G. Evaluation of anti-inflammatory property of curcumin (diferuloyl methane) in patients with postoperative inflammation. Int. J. Clin. Pharmacol. Ther. Toxicol. 1986, 24, 651–654. [Google Scholar]
- Allegri, P.; Mastromarino, A.; Neri, P. Management of chronic anterior uveitis relapses: Efficacy of oral phospholipidic curcumin treatment. Long-term follow-up. Clin. Ophthalmol. 2010, 4, 1201–1206. [Google Scholar] [CrossRef]
- Kurd, S.K.; Smith, N.; VanVoorhees, A.; Troxel, A.B.; Badmaev, V.; Seykora, J.T.; Gelfand, J.M. Oral curcumin in the treatment of moderate to severe psoriasis vulgaris: A prospective clinical trial. J. Am. Acad. Dermatol. 2008, 58, 625–631. [Google Scholar] [CrossRef]
- Holt, P.R.; Katz, S.; Kirshoff, R. Curcumin therapy in inflammatory bowel disease: A pilot study. Dig. Dis. Sci. 2005, 50, 2191–2193. [Google Scholar] [CrossRef]
- Hanai, H.; Iida, T.; Takeuchi, K.; Watanabe, F.; Maruyama, Y.; Andoh, A.; Tsujikawa, T.; Fujiyama, Y.; Mitsuyama, K.; Sata, M.; et al. Curcumin maintenance therapy for ulcerative colitis: Randomized, multicenter, double-blind, placebo-controlled trial. Clin. Gastroenterol. Hepatol. 2006, 4, 1502–1506. [Google Scholar] [CrossRef]
- Asawanonda, P.; Klahan, S.O. Tetrahydrocurcuminoid cream plus targeted narrowband UVB phototherapy for vitiligo: A preliminary randomized controlled study. Photomed. Laser Surg. 2010, 28, 679–684. [Google Scholar] [CrossRef]
- Belcaro, G.; Cesarone, M.R.; Dugall, M.; Pellegrini, L.; Ledda, A.; Grossi, M.G.; Togni, S.; Appendino, G. Product-evaluation registry of Meriva(R), a curcumin-phosphatidylcholine complex, for the complementary management of osteoarthritis. Panminerva Med. 2010, 52, 55–62. [Google Scholar] [PubMed]
- Durgaprasad, S.; Pai, C.G.; Vasanthkumar; Alvres, J.F.; Namitha, S. A pilot study of the antioxidant effect of curcumin in tropical pancreatitis. Indian J. Med. Res. 2005, 122, 315–318. [Google Scholar] [PubMed]
- Koosirirat, C.; Linpisarn, S.; Changsom, D.; Chawansuntati, K.; Wipasa, J. Investigation of the anti-inflammatory effect of Curcuma longa in Helicobacter pylori-infected patients. Int. Immunopharmacol. 2010, 10, 815–818. [Google Scholar] [CrossRef]
- Jimenez-Osorio, A.S.; Garcia-Nino, W.R.; Gonzalez-Reyes, S.; Alvarez-Mejia, A.E.; Guerra-Leon, S.; Salazar-Segovia, J.; Falcon, I.; Montes de Oca-Solano, H.; Madero, M.; Pedraza-Chaverri, J. The Effect of Dietary Supplementation with Curcumin on Redox Status and Nrf2 Activation in Patients with Nondiabetic or Diabetic Proteinuric Chronic Kidney Disease: A Pilot Study. J. Ren. Nutr. 2016, 26, 237–244. [Google Scholar] [CrossRef]
- Panahi, Y.; Khalili, N.; Sahebi, E.; Namazi, S.; Atkin, S.L.; Majeed, M.; Sahebkar, A. Curcuminoids Plus Piperine Modulate Adipokines in Type 2 Diabetes Mellitus. Curr. Clin. Pharmacol. 2017, 12, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Shafabakhsh, R.; Mobini, M.; Raygan, F.; Aghadavod, E.; Ostadmohammadi, V.; Amirani, E.; Mansournia, M.A.; Asemi, Z. Curcumin administration and the effects on psychological status and markers of inflammation and oxidative damage in patients with type 2 diabetes and coronary heart disease. Clin. Nutr. ESPEN 2020, 40, 77–82. [Google Scholar] [CrossRef]
- Chuengsamarn, S.; Rattanamongkolgul, S.; Luechapudiporn, R.; Phisalaphong, C.; Jirawatnotai, S. Curcumin extract for prevention of type 2 diabetes. Diabetes Care 2012, 35, 2121–2127. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Sun, D.; Wang, J.; Li, X.; Peng, J.; Wang, S. Advances and Perspectives in Curcumin Regulation of Systemic Metabolism: A Focus on Multi-Organ Mechanisms. Antioxidants 2026, 15, 109. https://doi.org/10.3390/antiox15010109
Sun D, Wang J, Li X, Peng J, Wang S. Advances and Perspectives in Curcumin Regulation of Systemic Metabolism: A Focus on Multi-Organ Mechanisms. Antioxidants. 2026; 15(1):109. https://doi.org/10.3390/antiox15010109
Chicago/Turabian StyleSun, Dingya, Jialu Wang, Xin Li, Jun Peng, and Shan Wang. 2026. "Advances and Perspectives in Curcumin Regulation of Systemic Metabolism: A Focus on Multi-Organ Mechanisms" Antioxidants 15, no. 1: 109. https://doi.org/10.3390/antiox15010109
APA StyleSun, D., Wang, J., Li, X., Peng, J., & Wang, S. (2026). Advances and Perspectives in Curcumin Regulation of Systemic Metabolism: A Focus on Multi-Organ Mechanisms. Antioxidants, 15(1), 109. https://doi.org/10.3390/antiox15010109





