Sex- and Ethnic-Specific Associations of Serum Lipids with Risk of 12 Cancers: Findings from 506,381 Adults in Two Large Cohorts
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Study Population
2.2. Case Ascertainment
2.3. Assessment of Serum Lipid Indices and Serum Lipid Ratios
2.4. Covariables
2.5. Statistical Analysis
3. Results
3.1. Study Population and Cancer Incidence
| Characteristics | UK Biobank (n = 368,288) | KCPS-II Biobank (n = 138,093) | ||
|---|---|---|---|---|
| Women (n = 196,739) | Men (n = 171,549) | Women (n = 51,949) | Men (n = 86,144) | |
| Mean Age (SD), years | 56.7 (8.00) | 57.0 (8.19) | 39.9 (10.9) | 41.5 (9.55) |
| Age groups | ||||
| Under 40 years old | 73 (0.04%) | 79 (0.05%) | 28,610 (55.1%) | 41,660 (48.4%) |
| 40 to 50 years old | 47,898 (24.3%) | 41,167 (24.0%) | 13,318 (25.6%) | 27,545 (32.0%) |
| 50 to 60 years old | 68,474 (34.8%) | 55,751 (32.5%) | 7018 (13.5%) | 12,655 (14.7%) |
| Over 60 years old | 80,294 (40.8%) | 74,552 (43.5%) | 2578 (4.96%) | 3593 (4.17%) |
| Mean BMI (SD), kg/m2 | 27.0 (5.11) | 27.8 (4.19) | 22.1 (3.08) | 24.4 (2.90) |
| BMI groups | ||||
| Underweight | 1501 (0.76%) | 373 (0.22%) | 4899 (9.44%) | 1182 (1.37%) |
| Normal weight | 78,172 (39.7%) | 42,926 (25.0%) | 29,843 (57.5%) | 25,990 (30.2%) |
| Overweight | 72,281 (36.7%) | 85,364 (49.8%) | 13,627 (26.2%) | 44,396 (51.6%) |
| Obesity | 44,785 (22.8%) | 42,886 (25.0%) | 3550 (6.84%) | 14,538 (16.9%) |
| Type 2 Diabetes mellitus | ||||
| No | 186,566 (94.8%) | 155,564 (90.7%) | 50,567 (97.3%) | 81,105 (94.2%) |
| Yes | 10,173 (5.17%) | 15,985 (9.32%) | 1382 (2.66%) | 5039 (5.85%) |
| Hypertension | ||||
| No | 149,636 (76.1%) | 116,579 (68.0%) | 46,710 (89.9%) | 68,632 (79.7%) |
| Yes | 47,103 (23.9%) | 54,970 (32.0%) | 5239 (10.1%) | 17,512 (20.3%) |
| Cardiovascular diseases | ||||
| No | 181,038 (92.0%) | 142,265 (82.9%) | 51,685 (99.5%) | 85,726 (99.5%) |
| Yes | 15,701 (7.98%) | 29,284 (17.1%) | 264 (0.51%) | 418 (0.49%) |
| Smoking status | ||||
| Never | 117,465 (59.7%) | 84,861 (49.5%) | 46,543 (89.6%) | 19,725 (22.9%) |
| Former | 62,125 (31.6%) | 65,835 (38.4%) | 3232 (6.22%) | 27,943 (32.4%) |
| Current | 17,149 (8.72%) | 20,853 (12.2%) | 2174 (4.18%) | 38,476 (44.7%) |
| Alcohol consumption | ||||
| Under 1 times/week | 124,879 (63.5%) | 134,173 (78.2%) | 16,178 (31.1%) | 5056 (5.87%) |
| 1 to 2 times/week | 25,789 (13.1%) | 15,230 (8.88%) | 8653 (16.7%) | 7109 (8.25%) |
| Over 2 times/week | 46,071 (23.4%) | 22,146 (12.9%) | 27,118 (52.2%) | 73,979 (85.9%) |
| Physical activity | ||||
| Under 2 times/week | 39,535 (20.1%) | 37,056 (21.6%) | 14,267 (43.9%) | 37,870 (56.4%) |
| 2 to 4 times/week | 81,373 (41.4%) | 65,276 (38.1%) | 12,931 (39.8%) | 21,706 (32.3%) |
| Over 4 times/week | 75,831 (38.5%) | 69,217 (40.3%) | 5279 (16.3%) | 7571 (11.3%) |
| Mean Systolic blood pressure (SD), mmHg | 137 (20.2) | 143 (18.4) | 112 (14.2) | 121 (13.0) |
| Mean Diastolic blood pressure (SD), mmHg | 80.6 (10.5) | 84.1 (10.5) | 70.1 (9.65) | 76.5 (9.63) |
| Mean FBS, (SD), mmol/L | 5.05 (1.04) | 5.17 (1.35) | 4.86 (0.85) | 5.16 (1.12) |
| Mean HDL-C (SD), mmol/L | 1.60 (0.38) | 1.28 (0.31) | 1.49 (0.29) | 1.26 (0.24) |
| Mean LDL-C (SD), mmol/L | 3.63 (0.87) | 3.50 (0.86) | 2.76 (0.78) | 2.98 (0.81) |
| Mean TG (SD), mmol/L | 1.54 (0.85) | 1.97 (1.13) | 1.10 (0.66) | 1.78 (1.12) |
| Mean TC (SD), mmol/L | 5.88 (1.12) | 5.50 (1.12) | 4.74 (0.85) | 4.98 (0.85) |
| Mean non-HDL-C (SD) | 4.28 (1.07) | 4.22 (1.07) | 3.25 (0.88) | 3.71 (0.90) |
| Mean AIP (SD) | −0.14 (0.62) | 0.32 (0.67) | −0.40 (0.56) | 0.21 (0.63) |
| Mean AC (SD) | 2.83 (1.00) | 3.46 (1.15) | 2.30 (1.09) | 3.09 (1.18) |
| Mean CRI-I (SD) | 3.83 (1.00) | 4.46 (1.15) | 3.30 (1.09) | 4.09 (1.18) |
| Mean CRI-II (SD) | 2.39 (0.78) | 2.85 (0.87) | 1.94 (0.93) | 2.47 (0.98) |
| Mean LCI (SD) | 25.2 (25.3) | 35.6 (33.3) | 22.0 (15.2) | 25.0 (28.7) |
| Mean THDL (SD) | 1.07 (0.80) | 1.71 (1.27) | 0.80 (0.64) | 1.52 (1.18) |
3.2. Overall Cancer
3.3. Cancer Site–Specific Associations
| Cancer/Cohort | HDL-C | LDL-C | TG | TC | Non-HDL-C | AIP | AC | CRI-I | CRI-II | LCI | THDL |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall cancer | |||||||||||
| UKB | 0.982 (0.969–0.995) | 1.021 (1.009–1.034) | 1.020 (1.007–1.032) | 1.016 (1.004–1.029) | 1.022 (1.010–1.035) | 1.029 (1.015–1.043) | 1.031 (1.019–1.044) | 1.031 (1.019–1.044) | 1.032 (1.019–1.044) | 1.023 (1.012–1.035) | 1.022 (1.009–1.034) |
| KCPS-II | 0.974 (0.934–1.015) | 1.005 (0.966–1.046) | 0.992 (0.956–1.030) | 0.990 (0.951–1.030) | 0.999 (0.960–1.039) | 1.018 (0.977–1.061) | 1.018 (0.974–1.063) | 1.018 (0.974–1.063) | 1.023 (0.979–1.070) | 0.986 (0.946–1.029) | 1.003 (0.967–1.041) |
| Overall cancer * | |||||||||||
| UKB | 0.974 (0.959–0.990) | 1.024 (1.010–1.038) | 1.023 (1.009–1.037) | 1.018 (1.004–1.032) | 1.026 (1.012–1.040) | 1.035 (1.019–1.050) | 1.038 (1.024–1.053) | 1.038 (1.024–1.053) | 1.038 (1.024–1.053) | 1.025 (1.012–1.038) | 1.025 (1.012–1.039) |
| KCPS-II | 0.996 (0.938–1.057) | 1.023 (0.968–1.082) | 0.979 (0.931–1.029) | 1.006 (0.951–1.065) | 1.008 (0.952–1.067) | 1.009 (0.954–1.069) | 1.027 (0.964–1.093) | 1.027 (0.964–1.093) | 1.041 (0.977–1.109) | 1.007 (0.953–1.065) | 0.991 (0.943–1.042) |
| Lung cancer | |||||||||||
| UKB | 0.912 (0.861–0.966) | 0.976 (0.930–1.023) | 1.060 (1.015–1.107) | 0.967 (0.922–1.015) | 0.990 (0.945–1.038) | 1.110 (1.053–1.170) | 1.045 (0.995–1.096) | 1.045 (0.995–1.096) | 1.037 (0.987–1.089) | 1.020 (0.980–1.061) | 1.055 (1.013–1.099) |
| KCPS-II | 0.999 (0.815–1.226) | 1.068 (0.912–1.250) | 0.985 (0.807–1.204) | 1.045 (0.884–1.234) | 1.044 (0.885–1.231) | 0.967 (0.790–1.185) | 1.049 (0.875–1.257) | 1.049 (0.875–1.257) | 1.074 (0.900–1.280) | 1.095 (0.944–1.270) | 1.024 (0.864–1.213) |
| Colon cancer | |||||||||||
| UKB | 1.012 (0.961–1.066) | 1.046 (0.999–1.095) | 1.053 (1.010–1.098) | 1.048 (1.001–1.097) | 1.046 (0.999–1.095) | 1.045 (0.993–1.099) | 1.031 (0.984–1.081) | 1.031 (0.984–1.081) | 1.032 (0.985–1.083) | 1.049 (1.009–1.090) | 1.039 (0.997–1.084) |
| KCPS-II | 0.975 (0.799–1.190) | 1.114 (0.891–1.393) | 1.015 (0.888–1.159) | 1.095 (0.918–1.305) | 1.101 (0.930–1.303) | 1.071 (0.883–1.298) | 1.063 (0.883–1.279) | 1.063 (0.883–1.279) | 1.080 (0.884–1.320) | 1.012 (0.896–1.143) | 1.004 (0.867–1.161) |
| Rectal cancer | |||||||||||
| UKB | 1.064 (0.980–1.156) | 1.033 (0.956–1.117) | 1.033 (0.956–1.116) | 1.058 (0.979–1.144) | 1.041 (0.964–1.125) | 1.008 (0.924–1.099) | 0.981 (0.905–1.063) | 0.981 (0.905–1.063) | 0.972 (0.897–1.053) | 0.998 (0.930–1.070) | 1.011 (0.929–1.099) |
| KCPS-II | 0.934 (0.730–1.194) | 1.124 (0.844–1.497) | 1.036 (0.866–1.239) | 1.075 (0.845–1.367) | 1.096 (0.870–1.383) | 1.018 (0.773–1.341) | 1.126 (0.858–1.480) | 1.126 (0.858–1.480) | 1.152 (0.852–1.557) | 1.041 (0.889–1.219) | 1.034 (0.873–1.224) |
| Stomach cancer | |||||||||||
| UKB | 0.812 (0.693–0.952) | 0.969 (0.863–1.088) | 0.958 (0.851–1.078) | 0.930 (0.824–1.051) | 0.979 (0.872–1.098) | 1.077 (0.937–1.239) | 1.081 (0.962–1.216) | 1.081 (0.962–1.216) | 1.084 (0.963–1.221) | 0.947 (0.849–1.055) | 0.999 (0.898–1.111) |
| KCPS-II | 0.883 (0.739–1.055) | 1.046 (0.895–1.221) | 1.027 (0.929–1.136) | 0.974 (0.830–1.142) | 1.014 (0.869–1.182) | 1.116 (0.955–1.304) | 1.066 (0.911–1.248) | 1.066 (0.911–1.248) | 1.090 (0.933–1.273) | 1.017 (0.915–1.130) | 1.044 (0.949–1.147) |
| Liver cancer | |||||||||||
| UKB | 0.978 (0.830–1.152) | 0.945 (0.829–1.078) | 1.055 (0.929–1.198) | 0.957 (0.836–1.095) | 0.961 (0.841–1.098) | 1.092 (0.941–1.268) | 1.024 (0.883–1.188) | 1.024 (0.883–1.188) | 1.006 (0.869–1.165) | 1.030 (0.904–1.173) | 1.090 (0.964–1.232) |
| KCPS-II | 1.156 (0.739–1.809) | 1.002 (0.654–1.536) | 0.854 (0.655–1.113) | 1.046 (0.684–1.599) | 0.995 (0.651–1.523) | 0.922 (0.665–1.278) | 1.008 (0.630–1.615) | 1.008 (0.630–1.615) | 1.029 (0.620–1.709) | 0.936 (0.657–1.334) | 0.869 (0.655–1.154) |
| Breast cancer | |||||||||||
| UKB | 1.000 (0.979–1.023) | 1.027 (1.007–1.048) | 1.025 (1.005–1.046) | 1.027 (1.006–1.048) | 1.027 (1.007–1.048) | 1.027 (1.004–1.049) | 1.026 (1.004–1.047) | 1.026 (1.004–1.047) | 1.026 (1.005–1.048) | 1.033 (1.014–1.053) | 1.021 (1.001–1.042) |
| KCPS-II | 1.004 (0.924–1.092) | 1.030 (0.948–1.119) | 1.009 (0.931–1.093) | 1.028 (0.949–1.114) | 1.026 (0.946–1.113) | 1.017 (0.929–1.112) | 1.024 (0.933–1.123) | 1.024 (0.933–1.123) | 1.026 (0.934–1.127) | 0.993 (0.917–1.077) | 1.016 (0.935–1.105) |
| Cervix uteri cancer | |||||||||||
| UKB | 1.167 (1.019–1.337) | 1.007 (0.890–1.140) | 1.040 (0.922–1.172) | 1.066 (0.947–1.201) | 1.020 (0.902–1.154) | 0.980 (0.854–1.125) | 0.955 (0.834–1.093) | 0.955 (0.834–1.093) | 0.942 (0.822–1.080) | 1.008 (0.906–1.121) | 0.979 (0.867–1.105) |
| KCPS-II | 0.970 (0.670–1.404) | 1.524 (1.103–2.105) | 1.101 (1.003–1.209) | 1.190 (1.062–1.334) | 1.197 (1.067–1.344) | 1.279 (0.923–1.774) | 1.261 (1.076–1.478) | 1.261 (1.076–1.478) | 1.302 (1.094–1.549) | 1.126 (1.041–1.217) | 1.112 (1.011–1.224) |
| Thyroid cancer | |||||||||||
| UKB | 0.878 (0.769–1.002) | 1.015 (0.903–1.141) | 1.054 (0.944–1.176) | 1.002 (0.888–1.131) | 1.038 (0.923–1.167) | 1.093 (0.967–1.236) | 1.083 (0.969–1.210) | 1.083 (0.969–1.210) | 1.069 (0.958–1.194) | 1.062 (0.955–1.182) | 1.069 (0.963–1.186) |
| KCPS-II | 0.900 (0.833–0.972) | 0.962 (0.893–1.037) | 1.014 (0.942–1.091) | 0.933 (0.865–1.007) | 0.968 (0.898–1.043) | 1.047 (0.970–1.131) | 1.008 (0.935–1.087) | 1.008 (0.935–1.087) | 1.002 (0.925–1.086) | 0.939 (0.867–1.017) | 1.025 (0.961–1.094) |
| Pancreas cancer | |||||||||||
| UKB | 1.052 (0.953–1.161) | 1.050 (0.962–1.146) | 1.001 (0.913–1.099) | 1.061 (0.972–1.158) | 1.049 (0.962–1.143) | 0.996 (0.902–1.100) | 1.013 (0.924–1.111) | 1.013 (0.924–1.111) | 1.012 (0.923–1.110) | 1.017 (0.930–1.112) | 0.990 (0.900–1.090) |
| KCPS-II | 1.022 (0.688–1.517) | 0.948 (0.637–1.410) | 1.067 (0.934–1.219) | 1.092 (0.715–1.667) | 1.083 (0.708–1.656) | 1.134 (0.818–1.572) | 1.092 (0.716–1.665) | 1.092 (0.716–1.665) | 0.981 (0.625–1.539) | 1.100 (0.935–1.294) | 1.074 (0.897–1.287) |
| Ovarian cancer | |||||||||||
| UKB | 1.018 (0.953–1.088) | 1.063 (0.996–1.135) | 1.026 (0.964–1.091) | 1.069 (1.001–1.141) | 1.066 (0.998–1.138) | 1.018 (0.950–1.091) | 1.037 (0.971–1.107) | 1.037 (0.971–1.107) | 1.034 (0.969–1.103) | 1.049 (0.990–1.111) | 1.015 (0.953–1.082) |
| KCPS-II | 1.047 (0.803–1.365) | 1.130 (0.853–1.497) | 0.993 (0.729–1.352) | 1.107 (0.907–1.353) | 1.094 (0.862–1.388) | 0.924 (0.667–1.280) | 1.095 (0.854–1.406) | 1.095 (0.854–1.406) | 1.120 (0.898–1.397) | 1.098 (0.972–1.239) | 1.027 (0.755–1.395) |
| Cancer/Cohort | HDL-C | LDL-C | TG | TC | Non-HDL-C | AIP | AC | CRI-I | CRI-II | LCI | THDL |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall cancer | |||||||||||
| UKB | 1.006 (0.993–1.019) | 1.028 (1.016–1.041) | 0.999 (0.987–1.012) | 1.028 (1.016–1.040) | 1.028 (1.015–1.040) | 1.001 (0.988–1.014) | 1.026 (1.013–1.039) | 1.026 (1.013–1.039) | 1.027 (1.014–1.040) | 1.011 (0.998–1.023) | 1.001 (0.988–1.013) |
| KCPS-II | 1.017 (0.987–1.049) | 1.008 (0.977–1.039) | 0.982 (0.950–1.015) | 1.008 (0.978–1.040) | 1.003 (0.972–1.035) | 0.984 (0.951–1.017) | 0.991 (0.960–1.023) | 0.991 (0.960–1.023) | 0.995 (0.965–1.027) | 0.987 (0.951–1.024) | 0.981 (0.949–1.016) |
| Overall cancer * | |||||||||||
| UKB | 1.006 (0.993–1.019) | 1.029 (1.016–1.041) | 1.000 (0.987–1.012) | 1.028 (1.016–1.041) | 1.028 (1.016–1.040) | 1.001 (0.988–1.014) | 1.026 (1.013–1.039) | 1.026 (1.013–1.039) | 1.027 (1.014–1.040) | 1.011 (0.999–1.024) | 1.001 (0.989–1.014) |
| KCPS-II | 1.026 (0.993–1.060) | 1.013 (0.979–1.048) | 0.969 (0.934–1.006) | 1.014 (0.980–1.050) | 1.006 (0.972–1.042) | 0.969 (0.933–1.005) | 0.989 (0.954–1.024) | 0.989 (0.954–1.024) | 0.995 (0.961–1.030) | 0.978 (0.938–1.019) | 0.969 (0.932–1.007) |
| Lung cancer | |||||||||||
| UKB | 1.035 (0.980–1.092) | 0.948 (0.902–0.996) | 0.999 (0.950–1.051) | 0.961 (0.914–1.009) | 0.951 (0.905–0.999) | 0.987 (0.935–1.042) | 0.961 (0.913–1.012) | 0.961 (0.913–1.012) | 0.958 (0.909–1.008) | 0.976 (0.926–1.028) | 0.995 (0.947–1.045) |
| KCPS-II | 1.058 (0.970–1.154) | 1.047 (0.943–1.162) | 0.984 (0.874–1.109) | 1.047 (0.943–1.162) | 1.026 (0.925–1.139) | 0.958 (0.854–1.075) | 0.961 (0.861–1.071) | 0.961 (0.861–1.071) | 0.979 (0.880–1.090) | 0.977 (0.855–1.115) | 0.962 (0.850–1.088) |
| Colon cancer | |||||||||||
| UKB | 0.991 (0.945–1.038) | 1.072 (1.027–1.119) | 1.061 (1.017–1.106) | 1.079 (1.034–1.126) | 1.085 (1.039–1.132) | 1.046 (0.999–1.097) | 1.082 (1.036–1.130) | 1.082 (1.036–1.130) | 1.074 (1.028–1.122) | 1.077 (1.036–1.119) | 1.060 (1.018–1.103) |
| KCPS-II | 0.989 (0.868–1.127) | 1.150 (1.006–1.314) | 1.053 (0.928–1.194) | 1.200 (1.057–1.362) | 1.192 (1.048–1.357) | 1.088 (0.951–1.246) | 1.142 (1.012–1.290) | 1.142 (1.012–1.290) | 1.132 (0.998–1.283) | 1.068 (0.989–1.153) | 1.034 (0.918–1.165) |
| Rectal cancer | |||||||||||
| UKB | 1.087 (1.018–1.160) | 1.095 (1.030–1.165) | 1.022 (0.958–1.089) | 1.112 (1.046–1.183) | 1.093 (1.028–1.162) | 0.985 (0.922–1.053) | 1.037 (0.972–1.106) | 1.037 (0.972–1.106) | 1.036 (0.972–1.104) | 1.057 (0.993–1.124) | 1.006 (0.942–1.075) |
| KCPS-II | 1.032 (0.908–1.172) | 0.951 (0.819–1.105) | 1.068 (0.939–1.214) | 1.007 (0.868–1.168) | 0.998 (0.858–1.162) | 1.068 (0.913–1.248) | 0.962 (0.825–1.121) | 0.962 (0.825–1.121) | 0.930 (0.794–1.089) | 0.986 (0.863–1.126) | 1.031 (0.906–1.173) |
| Stomach cancer | |||||||||||
| UKB | 0.991 (0.892–1.101) | 0.973 (0.897–1.056) | 1.003 (0.924–1.089) | 0.973 (0.896–1.056) | 0.974 (0.899–1.055) | 1.023 (0.932–1.123) | 1.006 (0.925–1.093) | 1.006 (0.925–1.093) | 1.002 (0.922–1.090) | 0.958 (0.884–1.038) | 1.023 (0.943–1.110) |
| KCPS-II | 0.948 (0.877–1.025) | 1.043 (0.965–1.128) | 1.051 (0.978–1.129) | 1.061 (0.982–1.148) | 1.075 (0.995–1.161) | 1.061 (0.977–1.152) | 1.071 (0.998–1.150) | 1.071 (0.998–1.150) | 1.054 (0.980–1.133) | 1.030 (0.973–1.090) | 1.052 (0.984–1.124) |
| Liver cancer | |||||||||||
| UKB | 1.061 (0.945–1.190) | 0.807 (0.718–0.906) | 0.980 (0.880–1.091) | 0.833 (0.741–0.937) | 0.813 (0.723–0.915) | 0.955 (0.855–1.067) | 0.829 (0.736–0.934) | 0.829 (0.736–0.934) | 0.813 (0.725–0.912) | 0.894 (0.784–1.020) | 0.988 (0.889–1.098) |
| KCPS-II | 1.082 (0.959–1.221) | 0.860 (0.759–0.975) | 0.626 (0.477–0.820) | 0.765 (0.673–0.869) | 0.747 (0.649–0.861) | 0.650 (0.553–0.763) | 0.818 (0.680–0.983) | 0.818 (0.680–0.983) | 0.876 (0.757–1.013) | 0.694 (0.494–0.976) | 0.724 (0.518–1.012) |
| Bladder cancer | |||||||||||
| UKB | 0.957 (0.910–1.007) | 1.057 (1.011–1.106) | 1.019 (0.973–1.067) | 1.042 (0.995–1.090) | 1.055 (1.008–1.103) | 1.043 (0.994–1.095) | 1.066 (1.018–1.116) | 1.066 (1.018–1.116) | 1.075 (1.026–1.126) | 1.027 (0.982–1.074) | 1.011 (0.968–1.057) |
| KCPS-II | 0.921 (0.787–1.077) | 1.032 (0.855–1.247) | 1.125 (0.977–1.295) | 1.089 (0.903–1.312) | 1.113 (0.925–1.339) | 1.184 (0.963–1.455) | 1.066 (0.907–1.252) | 1.066 (0.907–1.252) | 1.018 (0.857–1.208) | 1.033 (0.909–1.174) | 1.096 (0.964–1.246) |
| Prostate cancer | |||||||||||
| UKB | 1.025 (1.001–1.049) | 1.056 (1.034–1.080) | 0.986 (0.963–1.010) | 1.056 (1.034–1.080) | 1.052 (1.029–1.075) | 0.980 (0.957–1.004) | 1.028 (1.005–1.052) | 1.028 (1.005–1.052) | 1.033 (1.010–1.057) | 1.014 (0.990–1.038) | 0.978 (0.954–1.003) |
| KCPS-II | 1.054 (0.972–1.143) | 1.079 (0.991–1.174) | 0.934 (0.839–1.041) | 1.069 (0.982–1.165) | 1.050 (0.962–1.146) | 0.941 (0.856–1.035) | 1.001 (0.915–1.096) | 1.001 (0.915–1.096) | 1.032 (0.945–1.126) | 0.979 (0.873–1.098) | 0.938 (0.840–1.048) |
| Thyroid cancer | |||||||||||
| UKB | 0.778 (0.604–1.001) | 1.022 (0.833–1.253) | 1.176 (1.008–1.373) | 1.012 (0.821–1.247) | 1.069 (0.877–1.302) | 1.277 (1.038–1.572) | 1.202 (1.009–1.431) | 1.202 (1.009–1.431) | 1.172 (0.980–1.400) | 1.140 (1.006–1.292) | 1.198 (1.050–1.367) |
| KCPS-II | 0.950 (0.873–1.034) | 1.000 (0.931–1.074) | 1.050 (0.977–1.129) | 1.002 (0.931–1.079) | 1.015 (0.943–1.092) | 1.078 (0.996–1.166) | 1.026 (0.956–1.101) | 1.026 (0.956–1.101) | 1.018 (0.950–1.091) | 1.032 (0.956–1.113) | 1.048 (0.977–1.123) |
| Pancreas Cancer | |||||||||||
| UKB | 1.014 (0.923–1.115) | 1.039 (0.955–1.130) | 0.999 (0.919–1.086) | 1.040 (0.955–1.132) | 1.038 (0.955–1.128) | 1.001 (0.918–1.093) | 1.025 (0.939–1.118) | 1.025 (0.939–1.118) | 1.028 (0.943–1.120) | 1.022 (0.939–1.112) | 0.993 (0.912–1.081) |
| KCPS-II | 1.086 (0.920–1.282) | 1.073 (0.908–1.269) | 0.933 (0.775–1.123) | 1.072 (0.900–1.277) | 1.044 (0.873–1.250) | 0.941 (0.769–1.150) | 0.949 (0.786–1.145) | 0.949 (0.786–1.145) | 0.976 (0.824–1.156) | 0.934 (0.769–1.134) | 0.887 (0.723–1.089) |
| Cancer Type | KCPS-II | UK Biobank | |||||
|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |||||
| HR (95% CI) | E-Value (CI) | HR (95% CI) | E-Value (CI) | HR (95% CI) | E-Value (CI) | ||
| Overall cancer | 1 | 1.185 (1.155–1.215) | 1.499 (1.446) | 1.256 (1.224–1.29) | 1.619 (1.566) | 1.302 (1.265–1.340) | 1.691 (1.633) |
| Overall cancer * | 1 | 1.450 (1.407–1.494) | 1.909 (1.847) | 1.536 (1.489–1.585) | 2.028 (1.964) | 1.608 (1.555–1.663) | 2.123 (2.053) |
| Lung cancer | 1 | 0.689 (0.627–0.758) | 1.910 (1.718) | 0.671 (0.607–0.742) | 1.965 (1.762) | 0.713 (0.632–0.805) | 1.840 (1.596) |
| Colon cancer | 1 | 1.562 (1.393–1.752) | 2.063 (1.827) | 1.663 (1.481–1.869) | 2.196 (1.952) | 1.718 (1.516–1.947) | 2.265 (2.001) |
| Rectal cancer | 1 | 0.946 (0.834–1.074) | NA | 1.028 (0.901–1.173) | NA | 1.016 (0.875–1.179) | NA |
| Stomach cancer | 1 | 0.127 (0.115–0.141) | 7.281 (6.796) | 0.125 (0.112–0.141) | 7.354 (6.823) | 0.120 (0.102–0.141) | 7.572 (6.820) |
| Liver cancer | 1 | 0.259 (0.224–0.300) | 4.440 (3.977) | 0.304 (0.260–0.355) | 3.934 (3.479) | 0.284 (0.232–0.348) | 4.144 (3.541) |
| Bladder cancer | 1 | 2.675 (2.210–3.237) | 3.338 (2.845) | 2.840 (2.335–3.454) | 3.504 (2.983) | 3.308 (2.691–4.066) | 3.950 (3.355) |
| Prostate cancer | 1 | 2.402 (2.192–2.632) | 3.054 (2.825) | 2.533 (2.307–2.780) | 3.192 (2.952) | 2.482 (2.247–2.741) | 3.138 (2.886) |
| Breast cancer | 1 | 2.190 (2.033–2.36) | 2.823 (2.645) | 2.299 (2.131–2.479) | 2.943 (2.757) | 2.349 (2.166–2.548) | 2.997 (2.796) |
| Cervix uteri cancer | 1 | 1.436 (0.966–2.136) | NA | 1.44 (0.960–2.161) | NA | 1.039 (0.659–1.637) | NA |
| Thyroid cancer | 1 | 0.072 (0.064–0.082) | 10.330 (9.599) | 0.073 (0.064–0.083) | 10.315 (9.547) | 0.062 (0.051–0.076) | 11.285 (10.040) |
| Pancreas cancer | 1 | 0.897 (0.748–1.074) | NA | 1.001 (0.831–1.205) | NA | 1.129 (0.916–1.390) | NA |
| Ovarian cancer | 1 | 2.292 (1.772–2.964) | 2.935 (2.332) | 2.405 (1.854–3.121) | 3.058 (2.433) | 2.276 (1.723–3.007) | 2.918 (2.271) |
3.4. Cross-Cohort, Sex-Specific Patterns
4. Discussion
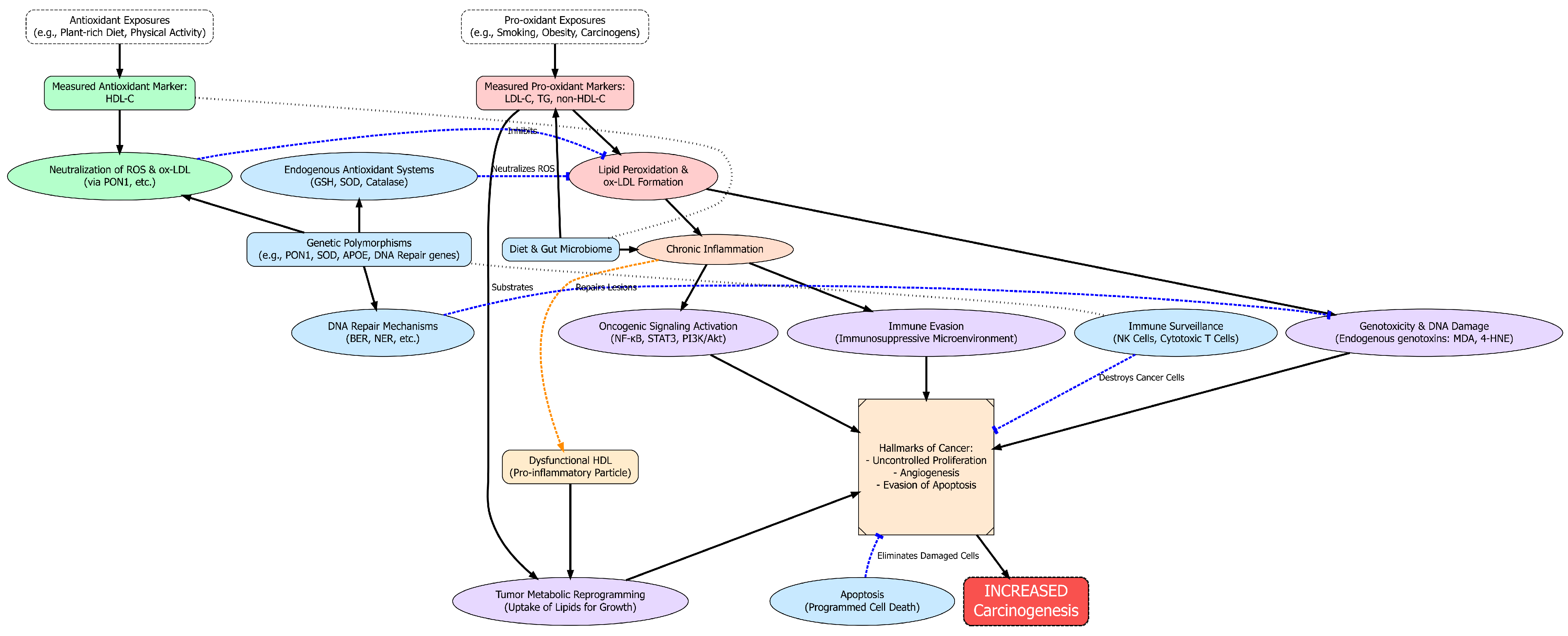
4.1. Strengths
4.2. Limitations
4.3. Practical Implications
4.4. Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 4-HNE | 4-hydroxynonenal |
| AC | Atherogenic coefficient |
| AIP | Atherogenic Index of Plasma |
| BMI | Body Mass Index |
| CI | Confidence Interval |
| CRI-I | Castelli Risk Index-I |
| CRI-II | Castelli Risk Index-II |
| CVD | Cardiovascular Disease |
| FBS | Fasting Blood Sugar |
| HDL-C | High-density Lipoprotein Cholesterol |
| HR | Hazard Ratio |
| ICD-10 | International Classification of Diseases, 10th Revision |
| KCPS-II | Korean Cancer Prevention Study-II |
| LCI | Lipoprotein Combination Index |
| LDL-C | Low-density Lipoprotein Cholesterol |
| MDA | Malondialdehyde |
| ox-LDL | Oxidized Low-density Lipoprotein |
| SD | Standard Deviation |
| SLIs | Serum Lipid Indicators |
| T2DM | Type 2 Diabetes Mellitus |
| TC | Total Cholesterol |
| TG | Triglycerides |
| THDL | Triglyceride HDL-C Ratio |
| UKB | UK Biobank |
References
- Gentile, F.; Arcaro, A.; Pizzimenti, S.; Daga, M.; Cetrangolo, G.P.; Dianzani, C.; Lepore, A.; Graf, M.; Ames, P.R.J.; Barrera, G. DNA damage by lipid peroxidation products: Implications in cancer, inflammation and autoimmunity. AIMS Genet. 2017, 4, 103–137. [Google Scholar] [CrossRef]
- Gasparovic, A.C.; Milkovic, L.; Sunjic, S.B.; Zarkovic, N. Cancer growth regulation by 4-hydroxynonenal. Free Radic. Biol. Med. 2017, 111, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Murdocca, M.; De Masi, C.; Pucci, S.; Mango, R.; Novelli, G.; Di Natale, C.; Sangiuolo, F. LOX-1 and cancer: An indissoluble liaison. Cancer Gene Ther. 2021, 28, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Soran, H.; Schofield, J.D.; Durrington, P.N. Antioxidant properties of HDL. Front. Pharmacol. 2015, 6, 222. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, Z.; Riwanto, M.; Gao, S.; Levison, B.S.; Gu, X.; Fu, X.; Wagner, M.A.; Besler, C.; Gerstenecker, G.; et al. Myeloperoxidase, paraoxonase-1, and HDL form a functional ternary complex. J. Clin. Investig. 2013, 123, 3815–3828. [Google Scholar] [CrossRef]
- Erdoğan, K.; Sanlier, N.T.; Özen, E.U.; Erol, S.; Kahyaoğlu, I.; Neselioglu, S.; Erel, Ö.; Akar, S.; Üstün, Y.E. Evaluation of Dysfunctional HDL by Myeloperoxidase/Paraoxonase Ratio in Unexplained Infertility Patients Undergoing IVF/ICSI. J. Clin. Med. 2023, 12, 1506. [Google Scholar] [CrossRef]
- Fang, Z.; He, M.; Song, M. Serum lipid profiles and risk of colorectal cancer: A prospective cohort study in the UK Biobank. Br. J. Cancer 2021, 124, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu, S.; Song, M.; Huang, W.; Yan, M.; Li, X. Association between blood lipid levels and the risk of liver cancer: A systematic review and meta-analysis. Cancer Causes Control 2024, 35, 943–953. [Google Scholar] [CrossRef]
- Pedersen, K.M.; Çolak, Y.; Bojesen, S.E.; Nordestgaard, B.G. Low high-density lipoprotein and increased risk of several cancers: 2 population-based cohort studies including 116,728 individuals. J. Hematol. Oncol. 2020, 13, 129. [Google Scholar] [CrossRef]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef]
- Jee, Y.H.; Emberson, J.; Jung, K.J.; Lee, S.J.; Lee, S.; Back, J.H.; Hong, S.; Kimm, H.; Sherliker, P.; Jee, S.H.; et al. Cohort Profile: The Korean Cancer Prevention Study-II (KCPS-II) Biobank. Int. J. Epidemiol. 2018, 47, 385–386f. [Google Scholar] [CrossRef] [PubMed]
- Elliott, P.; Peakman, T.C.; Biobank, U.K. The UK Biobank sample handling and storage protocol for the collection, processing and archiving of human blood and urine. Int. J. Epidemiol. 2008, 37, 234–244. [Google Scholar] [CrossRef]
- Supruniuk, E.; Baczewska, M.; Żebrowska, E.; Maciejczyk, M.; Lauko, K.K.; Dajnowicz-Brzezik, P.; Milewska, P.; Knapp, P.; Zalewska, A.; Chabowski, A. Redox Biomarkers and Matrix Remodeling Molecules in Ovarian Cancer. Antioxidants 2024, 13, 200. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yuan, H.; Li, L.; Li, Q.; Lin, P.; Li, K. Oxidative Stress and Reprogramming of Lipid Metabolism in Cancers. Antioxidants 2025, 14, 201. [Google Scholar] [CrossRef] [PubMed]
- Dalleau, S.; Baradat, M.; Guéraud, F.; Huc, L. Cell death and diseases related to oxidative stress:4-hydroxynonenal (HNE) in the balance. Cell Death Differ. 2013, 20, 1615–1630. [Google Scholar] [CrossRef]
- Duprat, F.; Robles, C.; Castillo, M.P.; Rivas, Y.; Mondaca, M.; Jara, N.; Roa, F.; Bertinat, R.; Toledo, J.; Paz, C.; et al. LOX-1 Activation by oxLDL Induces AR and AR-V7 Expression via NF-κB and STAT3 Signaling Pathways Reducing Enzalutamide Cytotoxic Effects. Int. J. Mol. Sci. 2023, 24, 5082. [Google Scholar] [CrossRef]
- Gordon, J.A.; Noble, J.W.; Midha, A.; Derakhshan, F.; Wang, G.; Adomat, H.H.; Tomlinson Guns, E.S.; Lin, Y.-Y.; Ren, S.; Collins, C.C.; et al. Upregulation of Scavenger Receptor B1 Is Required for Steroidogenic and Nonsteroidogenic Cholesterol Metabolism in Prostate Cancer. Cancer Res. 2019, 79, 3320–3331. [Google Scholar] [CrossRef]
- Traughber, C.A.; Opoku, E.; Brubaker, G.; Major, J.; Lu, H.; Lorkowski, S.W.; Neumann, C.; Hardaway, A.; Chung, Y.M.; Gulshan, K.; et al. Uptake of high-density lipoprotein by scavenger receptor class B type 1 is associated with prostate cancer proliferation and tumor progression in mice. J. Biol. Chem. 2020, 295, 8252–8261. [Google Scholar] [CrossRef]
- Taguchi, K.; Yamamoto, M. The KEAP1-NRF2 System in Cancer. Front. Oncol. 2017, 7, 85. [Google Scholar] [CrossRef]
- LeClair, K.; Bell, K.J.L.; Furuya-Kanamori, L.; Doi, S.A.; Francis, D.O.; Davies, L. Evaluation of Gender Inequity in Thyroid Cancer Diagnosis: Differences by Sex in US Thyroid Cancer Incidence Compared With a Meta-analysis of Subclinical Thyroid Cancer Rates at Autopsy. JAMA Intern. Med. 2021, 181, 1351–1358. [Google Scholar] [CrossRef]
- Arthur, R.S.; Dannenberg, A.J.; Rohan, T.E. The association of prediagnostic circulating levels of cardiometabolic markers, testosterone and sex hormone-binding globulin with risk of breast cancer among normal weight postmenopausal women in the UK Biobank. Int. J. Cancer 2021, 149, 42–57. [Google Scholar] [CrossRef]
- Watts, E.L.; Perez-Cornago, A.; Fensom, G.K.; Smith-Byrne, K.; Noor, U.; Andrews, C.D.; Gunter, M.J.; Holmes, M.V.; Martin, R.M.; Tsilidis, K.K.; et al. Circulating free testosterone and risk of aggressive prostate cancer: Prospective and Mendelian randomisation analyses in international consortia. Int. J. Cancer 2022, 151, 1033–1046. [Google Scholar] [CrossRef]
- Moosazadeh, M.; Ebrahimnejad, P.; Kheradmand, M.; Modanloo, M.; Mardanshah, F.; Mahboobi, S.; Rostamian, M.; Safajoo, A.; Dehghanzadegan, M.; Kianmehr, F. Association Between Smoking and Lipid Profile in Men Aged 35 to 70 Years: Dose-Response Analysis. Am. J. Mens. Health 2024, 18, 15579883241249655. [Google Scholar] [CrossRef] [PubMed]
- Bouras, E.; Gill, D.; Zuber, V.; Murphy, N.; Dimou, N.; Aleksandrova, K.; Lewis, S.J.; Martin, R.M.; Yarmolinsky, J.; Albanes, D.; et al. Identification of potential mediators of the relationship between body mass index and colorectal cancer: A Mendelian randomization analysis. Int. J. Epidemiol. 2024, 53, dyae067. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Wu, S.; Sharkey, C.; Tabatabaei, S.; Wu, C.-L.; Tao, Z.; Cheng, Z.; Strand, D.; Olumi, A.F.; Wang, Z. Obesity-associated inflammation induces androgenic to estrogenic switch in the prostate gland. Prostate Cancer Prostatic Dis. 2020, 23, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Doi, T.; Langsted, A.; Nordestgaard, B.G. Lipoproteins, Cholesterol, and Atherosclerotic Cardiovascular Disease in East Asians and Europeans. J. Atheroscler. Thromb. 2023, 30, 1525–1546. [Google Scholar] [CrossRef]
- Collatuzzo, G.; Boffetta, P. Cancers Attributable to Modifiable Risk Factors: A Road Map for Prevention. Annu. Rev. Public Health 2023, 44, 279–300. [Google Scholar] [CrossRef]
| Cancer Type | UK Biobank | KCPS-II Biobank | ||
|---|---|---|---|---|
| Women | Men | Women | Men | |
| OVERALL | ||||
| N | 196,739 | 171,549 | 51,949 | 86,144 |
| Overall cancer | 1238.28 | 1472.89 | 572.09 | 456.38 |
| Overall cancer * | 930.02 | 1467.45 | 274.53 | 380.04 |
| Lung cancer | 66.44 | 83.28 | 23.56 | 43.26 |
| Colon cancer | 80.27 | 111.06 | 17.49 | 23.92 |
| Rectal cancer | 27.36 | 53.12 | 13.36 | 22.25 |
| Stomach cancer | 10.33 | 26.25 | 31.98 | 73.70 |
| Liver cancer | 9.64 | 19.63 | 6.47 | 26.17 |
| Bladder cancer | 25.39 | 93.15 | 2.34 | 10.16 |
| Prostate cancer | 0 | 424.80 | 0 | 53.32 |
| Breast cancer | 437.16 | 4.67 | 130.08 | 0.42 |
| Cervix uteri cancer | 10.54 | 0 | 7.99 | 0 |
| Thyroid cancer | 12.82 | 5.76 | 168.47 | 77.01 |
| Pancreas cancer | 20.09 | 29.17 | 6.74 | 11.32 |
| Ovarian cancer | 43.72 | 0 | 10.74 | 0 |
| EARLY ONSET CANCER | ||||
| N | 47,971 | 41,246 | 42,971 | 71,191 |
| Overall cancer | 389.17 | 201.06 | 458.45 | 212.90 |
| Overall cancer * | 260.54 | 199.99 | 171.25 | 128.05 |
| Lung cancer | 7.46 | 4.77 | 6.52 | 6.81 |
| Colon cancer | 16.78 | 11.66 | 7.61 | 7.64 |
| Rectal cancer | 6.52 | 5.30 | 8.26 | 15.15 |
| Stomach cancer | 0.47 | 3.18 | 16.75 | 25.30 |
| Liver cancer | 0 | 1.06 | 0.87 | 9.03 |
| Bladder cancer | 2.80 | 5.83 | 1.09 | 2.22 |
| Prostate cancer | 0 | 12.19 | 0 | 2.92 |
| Breast cancer | 191.38 | 0 | 114.98 | 0.14 |
| Cervix uteri cancer | 9.32 | 0 | 8.48 | 0 |
| Thyroid cancer | 9.79 | 2.65 | 173.63 | 84.42 |
| Pancreas cancer | 2.80 | 4.24 | 1.09 | 2.08 |
| Ovarian cancer | 20.98 | 0 | 7.39 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thien, M.N.; Baek, J.W.; Yang, Y.S.; Jee, S.H. Sex- and Ethnic-Specific Associations of Serum Lipids with Risk of 12 Cancers: Findings from 506,381 Adults in Two Large Cohorts. Antioxidants 2025, 14, 1135. https://doi.org/10.3390/antiox14091135
Thien MN, Baek JW, Yang YS, Jee SH. Sex- and Ethnic-Specific Associations of Serum Lipids with Risk of 12 Cancers: Findings from 506,381 Adults in Two Large Cohorts. Antioxidants. 2025; 14(9):1135. https://doi.org/10.3390/antiox14091135
Chicago/Turabian StyleThien, Minh Nguyen, Ji Woo Baek, Yeun Soo Yang, and Sun Ha Jee. 2025. "Sex- and Ethnic-Specific Associations of Serum Lipids with Risk of 12 Cancers: Findings from 506,381 Adults in Two Large Cohorts" Antioxidants 14, no. 9: 1135. https://doi.org/10.3390/antiox14091135
APA StyleThien, M. N., Baek, J. W., Yang, Y. S., & Jee, S. H. (2025). Sex- and Ethnic-Specific Associations of Serum Lipids with Risk of 12 Cancers: Findings from 506,381 Adults in Two Large Cohorts. Antioxidants, 14(9), 1135. https://doi.org/10.3390/antiox14091135






