Applications of Antioxidant Nanoparticles in Immune-Mediated Inflammatory Diseases
Abstract
1. Introduction
2. Methods
3. Nano-Antioxidants for Autoimmune Diseases
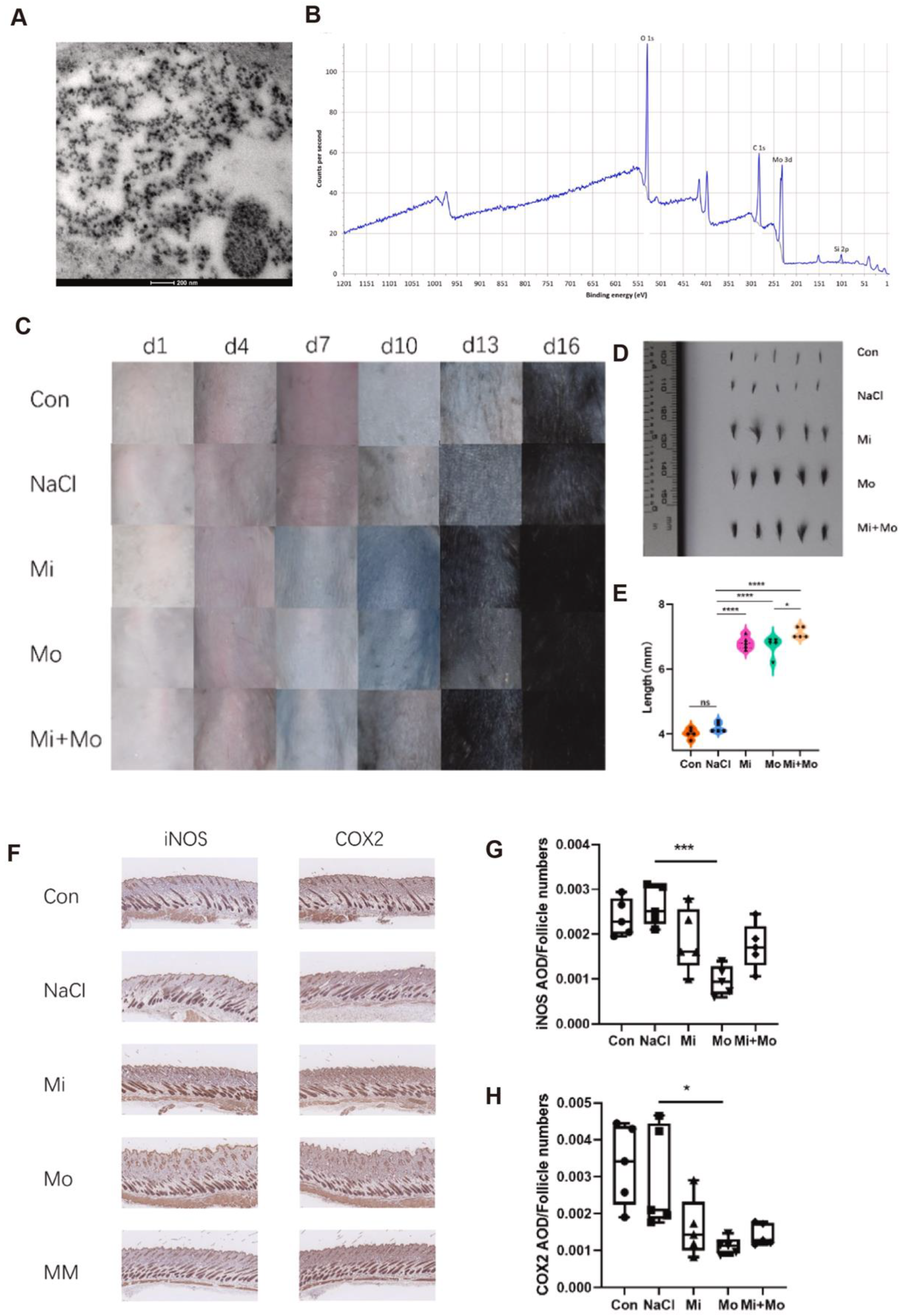
3.1. Alopecia Areata
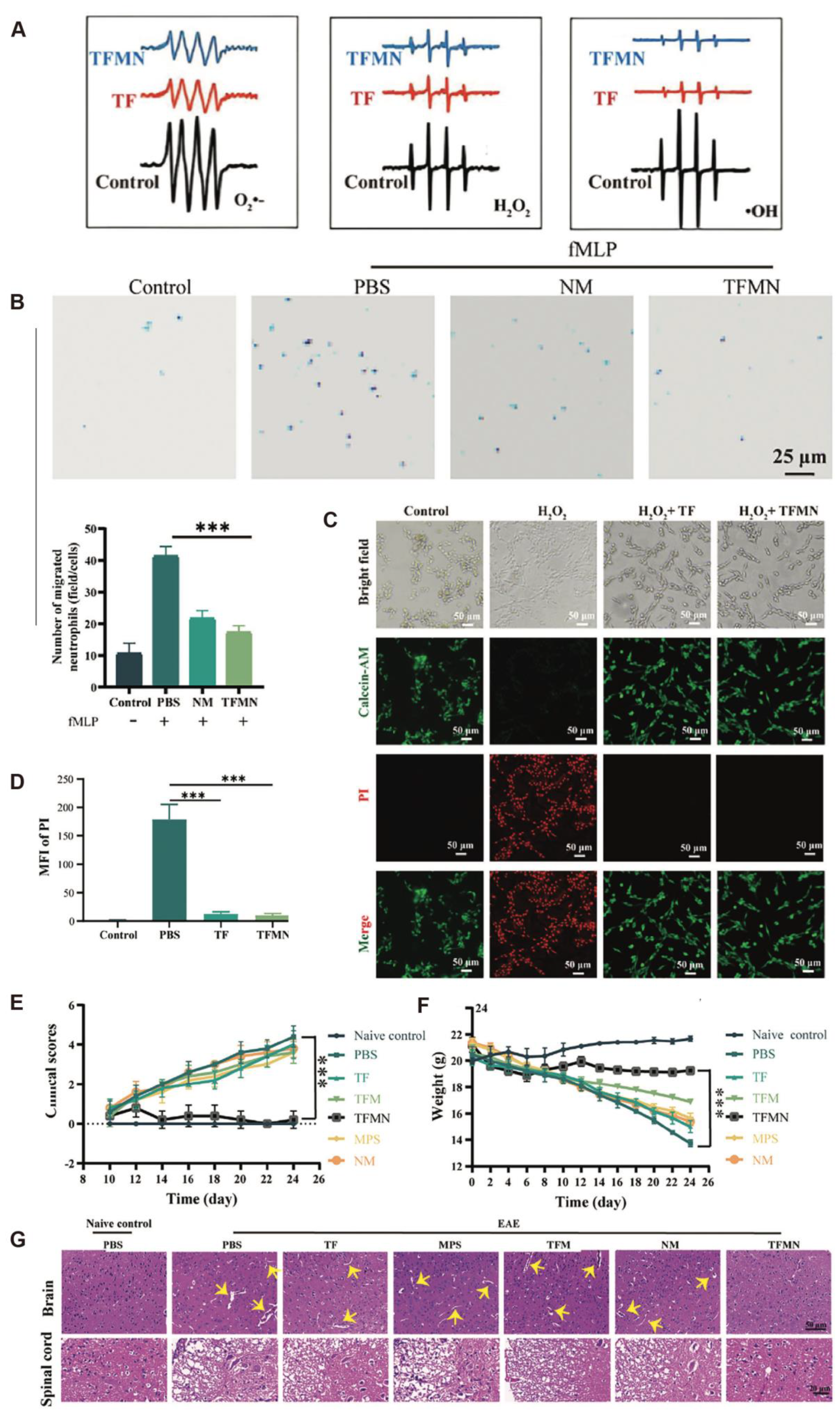
3.2. Multiple Sclerosis
4. Nano-Antioxidants for Acute Inflammatory Diseases
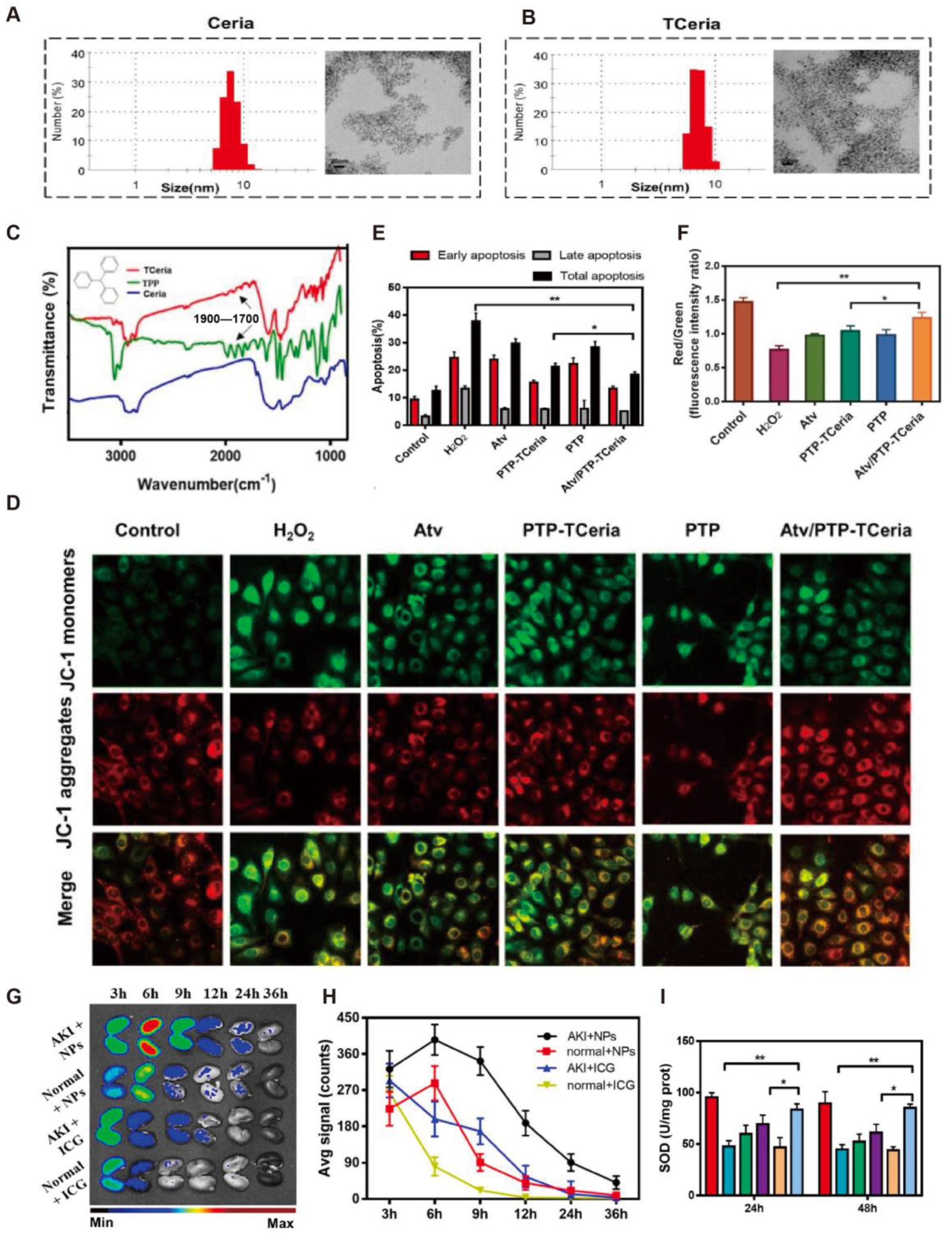
4.1. Acute Kidney Injury
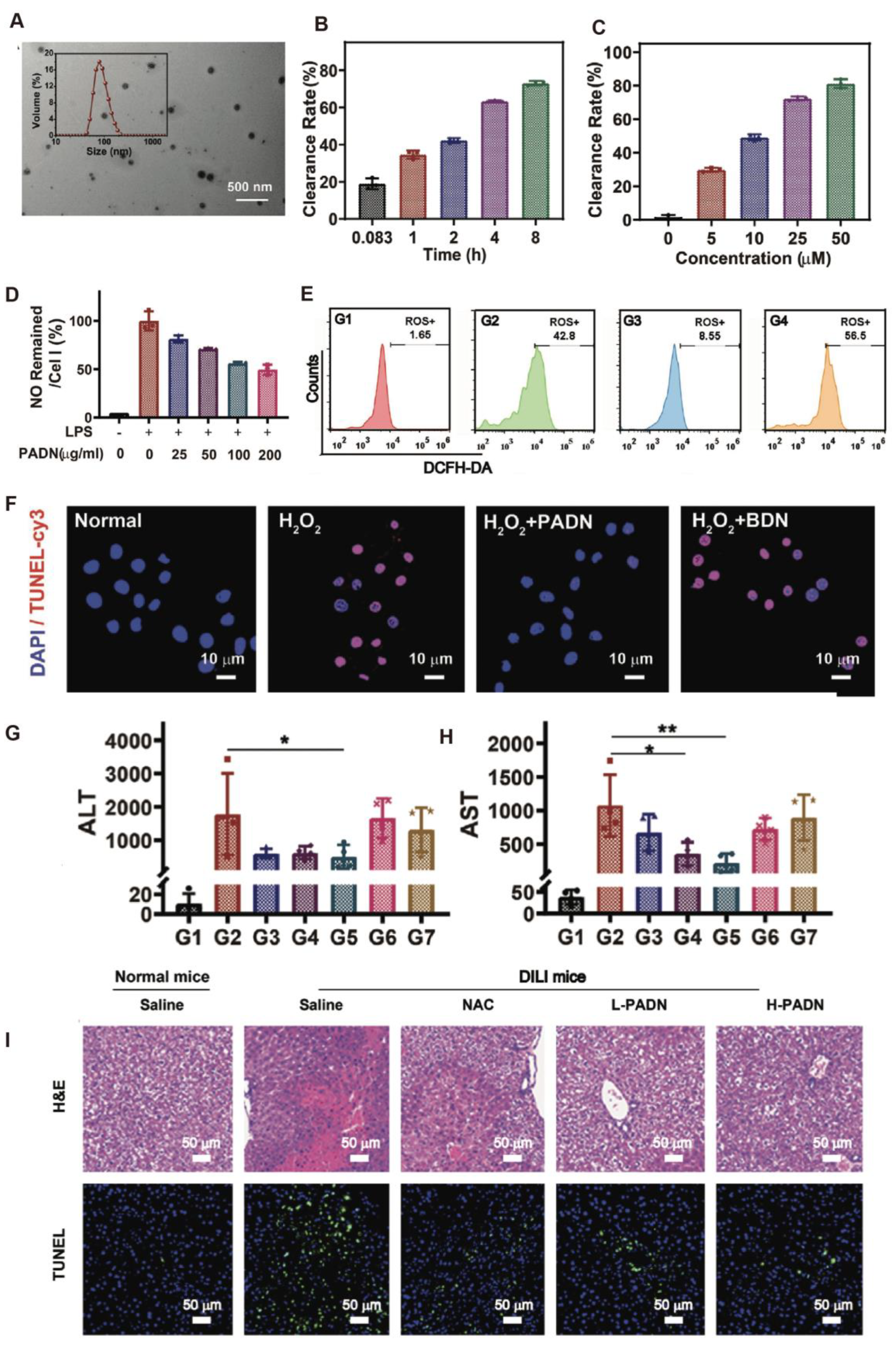
4.2. Acute Liver Injury
5. Nano-Antioxidants for Chronic Inflammatory Diseases
5.1. Periodontitis
5.2. Ulcerative Colitis
5.3. Crohn’s Disease
6. Others
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, H. Global burden and cross-country inequalities in six major immune-mediated inflammatory diseases from 1990 to 2021: A systemic analysis of the Global Burden of Disease Study 2021. Autoimmun. Rev. 2024, 23, 103639. [Google Scholar] [CrossRef]
- Dobrica, E.C.; Cozma, M.A.; Gaman, M.A.; Voiculescu, V.M.; Gaman, A.M. The Involvement of Oxidative Stress in Psoriasis: A Systematic Review. Antioxidants 2022, 11, 282. [Google Scholar] [CrossRef] [PubMed]
- Muro, P.; Zhang, L.; Li, S.; Zhao, Z.; Jin, T.; Mao, F.; Mao, Z. The emerging role of oxidative stress in inflammatory bowel disease. Front. Endocrinol. 2024, 15, 1390351. [Google Scholar] [CrossRef]
- Abot, A.; Fried, S.; Cani, P.D.; Knauf, C. Reactive Oxygen Species/Reactive Nitrogen Species as Messengers in the Gut: Impact on Physiology and Metabolic Disorders. Antioxid. Redox Signal. 2022, 37, 394–415. [Google Scholar] [CrossRef]
- Lapenna, D. Glutathione and glutathione-dependent enzymes: From biochemistry to gerontology and successful aging. Ageing Res. Rev. 2023, 92, 102066. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ren, X.; Nie, Z.; You, Y.; Zhu, Y.; Chen, H.; Yu, H.; Mo, G.P.; Su, L.; Peng, Z.; et al. Dual-responsive renal injury cells targeting nanoparticles for vitamin E delivery to treat ischemia reperfusion-induced acute kidney injury. J. Nanobiotechnol. 2024, 22, 626. [Google Scholar] [CrossRef]
- Akanchise, T.; Angelova, A. Potential of Nano-Antioxidants and Nanomedicine for Recovery from Neurological Disorders Linked to Long COVID Syndrome. Antioxidants 2023, 12, 393. [Google Scholar] [CrossRef] [PubMed]
- Bardestani, A.; Ebrahimpour, S.; Esmaeili, A.; Esmaeili, A. Quercetin attenuates neurotoxicity induced by iron oxide nanoparticles. J. Nanobiotechnol. 2021, 19, 327. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, L.; Zeng, H.; Meng, W.; Liu, G.; Zhang, W.; Zhao, P.; Zhang, Q.; Chen, M.; Chen, J. Manganese-Based Immunomodulatory Nanocomposite with Catalase-Like Activity and Microwave-Enhanced ROS Elimination Ability for Efficient Rheumatoid Arthritis Therapy. Small 2023, 19, e2304610. [Google Scholar] [CrossRef]
- Laurindo, L.F.; de Carvalho, G.M.; de Oliveira Zanuso, B.; Figueira, M.E.; Direito, R.; de Alvares Goulart, R.; Buglio, D.S.; Barbalho, S.M. Curcumin-Based Nanomedicines in the Treatment of Inflammatory and Immunomodulated Diseases: An Evidence-Based Comprehensive Review. Pharmaceutics 2023, 15, 229. [Google Scholar] [CrossRef]
- Indriyani, N.; Nur’aeny, N. The Therapeutic Effects of Curcumin on Oral Disease: A Systematic Review. Clin. Pharmacol. 2025, 17, 13–24. [Google Scholar] [CrossRef]
- Mohammadi, S.; Farjam, H.; Hosseini, S.; Larijani, K. Inhibitory effects of saffron compounds on multiple sclerosis: Molecular docking and dynamic, enhancing properties with gold nanoparticles and evaluating antioxidant, antibacterial, cytotoxicity. Heliyon 2025, 11, e42693. [Google Scholar] [CrossRef] [PubMed]
- Bemidinezhad, A.; Mirzavi, F.; Gholamhosseinian, H.; Gheybi, F.; Soukhtanloo, M. Gold-containing liposomes and glucose-coated gold nanoparticles enhances the radiosensitivity of B16F0 melanoma cells via increasing apoptosis and ROS production. Life Sci. 2023, 318, 121495. [Google Scholar] [CrossRef]
- Liu, X.; Ou, X.; Zhang, T.; Li, X.; Qiao, Q.; Jia, L.; Xu, Z.; Zhang, F.; Tian, T.; Lan, H.; et al. In situ neutrophil apoptosis and macrophage efferocytosis mediated by Glycyrrhiza protein nanoparticles for acute inflammation therapy. J. Control. Release 2024, 369, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Jin, F.; Liu, D.; Shu, G.; Wang, X.; Qi, J.; Sun, M.; Yang, P.; Jiang, S.; Ying, X.; et al. ROS-responsive nano-drug delivery system combining mitochondria-targeting ceria nanoparticles with atorvastatin for acute kidney injury. Theranostics 2020, 10, 2342–2357. [Google Scholar] [CrossRef]
- Hu, Q.; Li, J.; Wang, T.; Xu, X.; Duan, Y.; Jin, Y. Polyphenolic Nanoparticle-Modified Probiotics for Microenvironment Remodeling and Targeted Therapy of Inflammatory Bowel Disease. ACS Nano 2024, 18, 12917–12932. [Google Scholar] [CrossRef]
- Xiao, Q.; Lu, Y.; Yao, W.; Gong, C.; Jia, C.; Gao, J.; Guo, J.; Qiu, T.; Jiang, Y.; Huang, M.; et al. Molybdenum nanoparticles as a potential topical medication for alopecia treatment through antioxidant pathways that differ from minoxidil. J. Trace Elem. Med. Biol. 2024, 82, 127368. [Google Scholar] [CrossRef]
- Motavaf, M.; Sadeghizadeh, M.; Babashah, S.; Zare, L.; Javan, M. Dendrosomal nanocurcumin promotes remyelination through induction of oligodendrogenesis in experimental demyelination animal model. J. Tissue Eng. Regen. Med. 2020, 14, 1449–1464. [Google Scholar] [CrossRef]
- Ibrahim Fouad, G.; Mabrouk, M.; El-Sayed, S.A.M.; Abdelhameed, M.F.; Rizk, M.Z.; Beherei, H.H. Berberine-loaded iron oxide nanoparticles alleviate cuprizone-induced astrocytic reactivity in a rat model of multiple sclerosis. Biometals 2025, 38, 203–229. [Google Scholar] [CrossRef]
- Li, Z.; Chen, K.; Shao, Q.; Lu, H.; Zhang, X.; Pu, Y.; Sun, X.; He, H.; Cao, L. Nanoparticulate MgH(2) ameliorates anxiety/depression-like behaviors in a mouse model of multiple sclerosis by regulating microglial polarization and oxidative stress. J. Neuroinflamm. 2023, 20, 16. [Google Scholar] [CrossRef] [PubMed]
- Abdelalim, L.R.; Elnaggar, Y.S.R.; Abdallah, O.Y. Lactoferrin, chitosan double-coated oleosomes loaded with clobetasol propionate for remyelination in multiple sclerosis: Physicochemical characterization and in-vivo assessment in a cuprizone-induced demyelination model. Int. J. Biol. Macromol. 2024, 277 Pt 1, 134144. [Google Scholar] [CrossRef] [PubMed]
- Rezaeimanesh, N.; Rafiee, P.; Saeedi, R.; Khosravian, P.; Sahraian, M.A.; Eskandarieh, S.; Moghadasi, A.N.; Jahromi, S.R. The effect of crocin-selenium nanoparticles on the cognition and oxidative stress markers of multiple sclerosis patients: A randomized triple-blinded placebo-controlled clinical trial. Biometals 2024, 37, 305–319. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, Y.; Liu, H.; Wu, X.; Guo, M.; Xie, L.; Wang, G.; Shi, J.; Yu, W.; Dong, G. Inflammation-Targeted Biomimetic Nano-Decoys via Inhibiting the Infiltration of Immune Cells and Effectively Delivering Glucocorticoids for Enhanced Multiple Sclerosis Treatment. Adv. Healthc. Mater. 2025, 14, e2402965. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Zhang, P.; Li, H.; Wang, Y.; Huang, Y.; Fan, L.; Ma, X.; Qian, X.; Xi, J. Cerium–Luteolin Nanocomplexes in Managing Inflammation-Related Diseases by Antioxidant and Immunoregulation. ACS Nano 2024, 18, 6229–6242. [Google Scholar] [CrossRef]
- Zheng, Y.; Yi, H.; Zhan, Z.; Xue, S.S.; Tang, G.; Yu, X.; Zhang, D.Y. Reactive oxygen/nitrogen species scavenging and inflammatory regulation by renal-targeted bio-inspired rhodium nanozymes for acute kidney injury theranostics. J. Colloid. Interface Sci. 2024, 662, 413–425. [Google Scholar] [CrossRef]
- Yan, R.; Cui, W.; Ma, W.; Li, J.; Liu, Z.; Lin, Y. Typhaneoside-Tetrahedral Framework Nucleic Acids System: Mitochondrial Recovery and Antioxidation for Acute Kidney Injury treatment. ACS Nano 2023, 17, 8767–8781. [Google Scholar] [CrossRef]
- Pan, Q.; Xie, L.; Cai, P.; Wu, D.; Zhu, H.; Xu, L.; Liu, R.; Luo, K.; He, B.; Pu, Y. Acid-Resistant Nano-antioxidants Based on Epigallocatechin Gallate Alleviate Acute Intestinal and Kidney Inflammation. ACS Appl. Mater. Interfaces 2024, 16, 46090–46101. [Google Scholar] [CrossRef]
- Jia, F.; Yu, B.; Li, J.; Cai, F.; Fu, G.; Jin, Q.; Ji, J. Supramolecular Nano-Assembly of Caffeate-Strengthened Phenylboronic Ester with Multistep ROS Scavenging Ability for Targeted Therapy of Acute Kidney Injury. Adv. Healthc. Mater. 2023, 12, e2301615. [Google Scholar] [CrossRef]
- Luo, F.; Zhu, B.; Wu, D.; Xu, Y.; Chen, T.; Li, Y.; Hu, J. Construction of Phlorotannin-Based Nanoparticles for Alleviating Acute Liver Injury. ACS Appl. Mater. Interfaces 2023, 15, 47338–47349. [Google Scholar] [CrossRef]
- Jang, D.; Choi, H.; Lee, J.; Chun, Y.; Heo, Y.H.; Lee, L.P.; Ahn, D.J.; Shin, I.S.; Kim, D.H.; Seo, Y.H.; et al. Inflamed Tissue-Targeting Polyphenol-Condensed Antioxidant Nanoparticles with Therapeutic Potential. Adv. Healthc. Mater. 2025, 14, e2500495. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Li, K.; Wang, X.; Zhang, X.; Qian, D.; Meng, X.; Yu, L.; Yan, X.; He, Z. Self-Sulfhydrated, Nitro-Fixed Albumin Nanoparticles as a Potent Therapeutic Agent for the Treatment of Acute Liver Injury. ACS Nano 2024, 18, 20772–20791. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Li, Z.; Chen, J.; Su, L.; Wang, J.; Chen, D.S.; Ye, J.; Liao, N.; Yang, H.; Song, J.; et al. Site-Specific Biomimicry of Antioxidative Melanin Formation and Its Application for Acute Liver Injury Therapy and Imaging. Adv. Mater. 2021, 33, e2102391. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; Hu, Y.; Zhao, R.; Zhou, Y.N.; Zhuang, Y.; Zhu, Y.; Ge, X.L.; Lu, T.W.; Lin, K.L.; Xu, Y.J. Quercetin-loaded mesoporous nano-delivery system remodels osteoimmune microenvironment to regenerate alveolar bone in periodontitis via the miR-21a-5p/PDCD4/NF-kappaB pathway. J. Nanobiotechnol. 2024, 22, 94. [Google Scholar] [CrossRef]
- Xin, X.; Liu, J.; Liu, X.; Xin, Y.; Hou, Y.; Xiang, X.; Deng, Y.; Yang, B.; Yu, W. Melatonin-Derived Carbon Dots with Free Radical Scavenging Property for Effective Periodontitis Treatment via the Nrf2/HO-1 Pathway. ACS Nano 2024, 18, 8307–8324. [Google Scholar] [CrossRef]
- Cao, B.; Zhang, J.; Ma, Y.; Wang, Y.; Li, Y.; Wang, R.; Cao, D.; Yang, Y.; Zhang, R. Dual-Polymer Functionalized Melanin-AgNPs Nanocomposite with Hydroxyapatite Binding Ability to Penetrate and Retain in Biofilm Sequentially Treating Periodontitis. Small 2024, 20, e2400771. [Google Scholar] [CrossRef]
- Cao, B.; Da, X.; Wu, W.; Xie, J.; Li, X.; Wang, X.; Xu, H.; Gao, J.; Yang, H.; Su, J. Multifunctional human serum albumin-crosslinked and self-assembling nanoparticles for therapy of periodontitis by anti-oxidation, anti-inflammation and osteogenesis. Mater. Today Bio. 2024, 28, 101163. [Google Scholar] [CrossRef]
- Ye, R.; Guo, J.; Yang, Z.; Wang, Z.; Chen, Y.; Huang, J.; Dong, Y. Somatostatin and Mannooligosaccharide Modified Selenium Nanoparticles with Dual-Targeting for Ulcerative Colitis Treatment. ACS Nano 2025, 19, 14914–14930. [Google Scholar] [CrossRef]
- Kanika; Ahmad, A.; Kumar, A.; Rahul; Mishra, R.K.; Ali, N.; Navik, U.; Parvez, S.; Khan, R. Leveraging thiol-functionalized biomucoadhesive hybrid nanoliposome for local therapy of ulcerative colitis. Biomaterials 2025, 312, 122747. [Google Scholar] [CrossRef]
- Cai, W.Q.; Liang, W.; Li, D.; Dai, W.; Li, Z.; Wei, X.; Cheng, L.; Zhang, B.B.; Yang, Q. Reactive Oxygen Species-Responsive Polymer Drug Delivery System Targeted Oxidative Stressed Colon Cells to Ameliorate Colitis. ACS Nano 2025, 19, 17287–17308. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Meng, L.; Zhang, X.; Deng, Z.; Gao, B.; Zhang, Y.; Yang, M.; Ma, Y.; Zhang, Y.; Tu, K.; et al. Reactive oxygen species-responsive nanocarrier ameliorates murine colitis by intervening colonic innate and adaptive immune responses. Mol. Ther. 2023, 31, 1383–1401. [Google Scholar] [CrossRef]
- Guo, H.; Guo, H.; Xie, Y.; Chen, Y.; Lu, C.; Yang, Z.; Zhu, Y.; Ouyang, Y.; Zhang, Y.; Wang, X. Mo3Se4 nanoparticle with ROS scavenging and multi-enzyme activity for the treatment of DSS-induced colitis in mice. Redox Biol. 2022, 56, 102441. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Zhang, Y.; Li, R.; Cao, Y.; Yan, X.; Ma, Y.; Zhang, Y.; Yang, M.; Zhang, M. Oral Delivery of Pterostilbene by L-Arginine-Mediated “Nano-Bomb” Carrier for the Treatment of Ulcerative Colitis. Int. J. Nanomed. 2022, 17, 603–616. [Google Scholar] [CrossRef]
- Wu, T.; Bai, X.; Zhang, Y.; Dai, E.; Ma, J.; Yu, C.; He, C.; Li, Q.; Yang, Y.; Kong, H.; et al. Natural medicines-derived carbon dots as novel oral antioxidant administration strategy for ulcerative colitis therapy. J. Nanobiotechnol. 2024, 22, 511. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Wang, E.H.C.; Petukhova, L.; Chang, Y.; Lee, E.Y.; Christiano, A.M. Blockade of IL-7 signaling suppresses inflammatory responses and reverses alopecia areata in C3H/HeJ mice. Sci. Adv. 2021, 7, eabd1866. [Google Scholar] [CrossRef]
- Zhou, C.; Li, X.; Wang, C.; Zhang, J. Alopecia Areata: An Update on Etiopathogenesis, Diagnosis, and Management. Clin. Rev. Allergy Immunol. 2021, 61, 403–423. [Google Scholar] [CrossRef]
- McMichael, A.J.; Roberson, M.L. Characterizing Epidemiology and Burden of Disease in Alopecia Areata-Making It Count. JAMA Dermatol. 2023, 159, 369–370. [Google Scholar] [CrossRef]
- Ma, Y.Q.; Sun, Z.; Li, Y.M.; Xu, H. Oxidative stress and alopecia areata. Front. Med. 2023, 10, 1181572. [Google Scholar] [CrossRef]
- Shakoei, S.; Mirmiranpoor, H.; Nakhjavani, M.; Nasimi, M.; Bakhshi, G.; Azizpour, A. Oxidative stress and antioxidant markers in patients with alopecia areata: A comparative cross-sectional study. Indian J. Dermatol. Venereol. Leprol. 2023, 89, 411–415. [Google Scholar] [CrossRef]
- Sachdeva, S.; Khurana, A.; Goyal, P.; Sardana, K. Does oxidative stress correlate with disease activity and severity in alopecia areata? An analytical study. J. Cosmet. Dermatol. 2022, 21, 1629–1634. [Google Scholar] [CrossRef] [PubMed]
- Peterle, L.; Sanfilippo, S.; Borgia, F.; Cicero, N.; Gangemi, S. Alopecia Areata: A Review of the Role of Oxidative Stress, Possible Biomarkers, and Potential Novel Therapeutic Approaches. Antioxidants 2023, 12, 135. [Google Scholar] [CrossRef]
- Taskin, S.; Celik, H.; Cakirca, G.; Manav, V.; Taskin, A. Nitric oxide synthase activity: A novel potential biomarker for predicting Alopecia areata. J. Cosmet. Dermatol. 2022, 21, 7075–7080. [Google Scholar] [CrossRef]
- Perlmutter, J.; Akouris, P.P.; Fremont, S.; Yang, B.; Toth, E.; Eze, M.; Wiseman, M. The Role of Reactive Oxygen Species in the Pathogenesis of Alopecia Areata: A Systematic Review and Meta-Analysis. Skin. Pharmacol. Physiol. 2025, 38, 59–67. [Google Scholar] [CrossRef]
- Younis, N.; Puigmal, N.; El Kurdi, A.; Badaoui, A.; Zhang, D.; Morales-Garay, C.; Saad, A.; Cruz, D.; Al Rahy, N.; Daccache, A.; et al. Microneedle-Mediated Delivery of Immunomodulators Restores Immune Privilege in Hair Follicles and Reverses Immune-Mediated Alopecia. Adv. Mater. 2024, 36, e2312088. [Google Scholar] [CrossRef]
- Jeong, D.I.; Lee, S.Y.; Kim, S.; Jo, S.-M.; Yang, H.; You, J.; Hwang, C.; Huh, J.W.; Choi, Y.E.; Jo, W.; et al. Baricitinib-loaded separable microneedle array patch (S-MAP) based on hyaluronic acid for alopecia areata therapy. Carbohydr. Polym. 2025, 364, 123789. [Google Scholar] [CrossRef]
- Hindam, M.O.; Ahmed, L.A.; El Sayed, N.S.; Khattab, M.; Sallam, N.A. Repositioning of baricitinib for management of memory impairment in ovariectomized/D-galactose treated rats: A potential role of JAK2/STAT3-PI3K/AKT/mTOR signaling pathway. Life Sci. 2024, 351, 122838. [Google Scholar] [CrossRef]
- McGinley, M.P.; Goldschmidt, C.H.; Rae-Grant, A.D. Diagnosis and Treatment of Multiple Sclerosis. JAMA 2021, 325, 765–779. [Google Scholar] [CrossRef]
- Graf, J.; Akmatov, M.K.; Meuth, S.G.; Tremlett, H.; Holstiege, J. Updated Multiple Sclerosis Incidence, 2015–2022. JAMA Neurol 2024, 81, 1100–1102. [Google Scholar] [CrossRef] [PubMed]
- Dash, U.C.; Bhol, N.K.; Swain, S.K.; Samal, R.R.; Nayak, P.K.; Raina, V.; Panda, S.K.; Kerry, R.G.; Duttaroy, A.K.; Jena, A.B. Oxidative stress and inflammation in the pathogenesis of neurological disorders: Mechanisms and implications. Acta Pharm. Sin. B 2025, 15, 15–34. [Google Scholar] [CrossRef]
- Kasprzak-Drozd, K.; Nizinski, P.; Hawryl, A.; Gancarz, M.; Hawryl, D.; Oliwa, W.; Palka, M.; Markowska, J.; Oniszczuk, A. Potential of Curcumin in the Management of Skin Diseases. Int. J. Mol. Sci. 2024, 25, 3617. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Qi, S.; Chen, Y.; Luo, H.; Huang, S.; Yu, X.; Luo, Q.; Zhang, Z. Targeted immunomodulation of inflammatory monocytes across the blood-brain barrier by curcumin-loaded nanoparticles delays the progression of experimental autoimmune encephalomyelitis. Biomaterials 2020, 245, 119987. [Google Scholar] [CrossRef] [PubMed]
- Sanadgol, N.; Barati, M.; Houshmand, F.; Hassani, S.; Clarner, T.; Shahlaei, M.; Golab, F. Metformin accelerates myelin recovery and ameliorates behavioral deficits in the animal model of multiple sclerosis via adjustment of AMPK/Nrf2/mTOR signaling and maintenance of endogenous oligodendrogenesis during brain self-repairing period. Pharmacol. Rep. 2020, 72, 641–658. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Huang, J.; Zhang, M.; Xu, M.; He, J.; Liu, Q. Nanotechnology-Based Drug Delivery Systems for Treating Acute Kidney Injury. ACS Biomater. Sci. Eng. 2024, 10, 6078–6096. [Google Scholar] [CrossRef]
- Jensen, S.K.; Rasmussen, T.B.; Jacobsen, B.H.; Heide-Jørgensen, U.; Sawhney, S.; Gammelager, H.; Birn, H.; Johnsen, S.P.; Christiansen, C.F. Regional variation in incidence and prognosis of acute kidney injury. Nephrol. Dial. Transpl. 2024, 39, 1171–1180. [Google Scholar] [CrossRef]
- Levey, A.S.; Eckardt, K.-U.; Dorman, N.M.; Christiansen, S.L.; Hoorn, E.J.; Ingelfinger, J.R.; Inker, L.A.; Levin, A.; Mehrotra, R.; Palevsky, P.M.; et al. Nomenclature for kidney function and disease: Report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int. 2020, 97, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Zhou, D.; Pu, Z.; Chen, S.; Shen, Y.; Zhao, S.; Qian, X.; Hu, Q.; Wu, X.; Xie, Z.; et al. Cellular Senescence in Acute Liver Injury: What Happens to the Young Liver? Aging Dis. 2024, 16, 1347–1362. [Google Scholar] [CrossRef]
- Yuan, X.; Zhou, Y.; Sun, J.; Wang, S.; Hu, X.; Li, J.; Huang, J.; Chen, N. Preventing acute liver injury via hepatocyte-targeting nano-antioxidants. Cell Prolif. 2023, 56, e13494. [Google Scholar] [CrossRef]
- Song, B.; Zhang, C.; Hu, W.; Guo, C.; Xia, Z.; Hu, W.; Qin, M.; Jiang, W.; Lv, J.; Xu, D.; et al. Nano-designed carbon monoxide donor SMA/CORM2 exhibits protective effect against acetaminophen induced liver injury through macrophage reprograming and promoting liver regeneration. J. Control. Release 2021, 331, 350–363. [Google Scholar] [CrossRef]
- Liu, M.; Wu, H.; Li, Q.; Liu, H.; Chen, C.; Yin, F.; Wang, H.; Zha, Z.; Wang, F. Mn3O4 nanozymes prevent acetaminophen-induced acute liver injury by attenuating oxidative stress and countering inflammation. J. Colloid Interface Sci. 2024, 654 Pt A, 83–95. [Google Scholar] [CrossRef]
- Aslam, M.; Fatima, B.; Batool, R.; Imran, M.; Hussain, D.; Najam-Ul-Haq, M.; Mehmood, R. Saponin Gallate-Loaded Gd-Doped Zinc-Gallium Layered Double Hydroxides (Zn/Ga@Gd-LDH) Nanocarrier for Attenuating NF-kappaB-Mediated Inflammation. ACS Appl. Mater. Interfaces 2025, 17, 26263–26281. [Google Scholar] [CrossRef]
- Trindade, D.; Carvalho, R.; Machado, V.; Chambrone, L.; Mendes, J.J.; Botelho, J. Prevalence of periodontitis in dentate people between 2011 and 2020: A systematic review and meta-analysis of epidemiological studies. J. Clin. Periodontol. 2023, 50, 604–626. [Google Scholar] [CrossRef] [PubMed]
- Kassebaum, N.J.; Bernabe, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.; Marcenes, W. Global burden of severe periodontitis in 1990–2010: A systematic review and meta-regression. J. Dent. Res. 2014, 93, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.X.; Zhong, Y.J.; Dong, Q.Q.; Wong, H.M.; Wen, Y.F. Global, regional, and national burden of severe periodontitis, 1990–2019: An analysis of the Global Burden of Disease Study 2019. J. Clin. Periodontol. 2021, 48, 1165–1188. [Google Scholar] [CrossRef]
- Sczepanik, F.S.C.; Grossi, M.L.; Casati, M.; Goldberg, M.; Glogauer, M.; Fine, N.; Tenenbaum, H.C. Periodontitis is an inflammatory disease of oxidative stress: We should treat it that way. Periodontol. 2000 2020, 84, 45–68. [Google Scholar] [CrossRef]
- Li, L.; Qin, W.; Ye, T.; Wang, C.; Qin, Z.; Ma, Y.; Mu, Z.; Jiao, K.; Tay, F.R.; Niu, W.; et al. Bioactive Zn-V-Si-Ca Glass Nanoparticle Hydrogel Microneedles with Antimicrobial and Antioxidant Properties for Bone Regeneration in Diabetic Periodontitis. ACS Nano 2025, 19, 7981–7995. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Wan, Y.; Qi, M.; Chen, Q.; Sun, Y.; Sun, X.; Fang, J.; Fu, L.; Xu, L.; et al. Quercetin-Loaded Ceria Nanocomposite Potentiate Dual-Directional Immunoregulation via Macrophage Polarization against Periodontal Inflammation. Small 2021, 17, 2101505. [Google Scholar] [CrossRef]
- Zhao, M.; Zhai, H.; Li, H.; Wei, F.; Ma, H.; Liu, Y.; Li, W.; Wei, P. Age-standardized incidence, prevalence, and mortality rates of autoimmune diseases in adolescents and young adults (15–39 years): An analysis based on the global burden of disease study 2021. BMC Public Health 2024, 24, 1800. [Google Scholar] [CrossRef]
- Ye, R.; Guo, Q.; Huang, J.; Wang, Z.; Chen, Y.; Dong, Y. Eucommia ulmoides polysaccharide modified nano-selenium effectively alleviated DSS-induced colitis through enhancing intestinal mucosal barrier function and antioxidant capacity. J. Nanobiotechnol. 2023, 21, 222. [Google Scholar] [CrossRef]
- Gonczi, L.; Lakatos, L.; Kurti, Z.; Golovics, P.A.; Pandur, T.; David, G.; Erdelyi, Z.; Szita, I.; Lakatos, P.L. Incidence, Prevalence, Disease Course, and Treatment Strategy of Crohn’s Disease Patients from the Veszprem Cohort, Western Hungary: A Population-based Inception Cohort Study Between 2007 and 2018. J. Crohns Colitis 2023, 17, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Roda, G.; Chien Ng, S.; Kotze, P.G.; Argollo, M.; Panaccione, R.; Spinelli, A.; Kaser, A.; Peyrin-Biroulet, L.; Danese, S. Crohn’s disease. Nat. Rev. Dis. Primers 2020, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Li, X.; Zhang, S.; Qi, C.; Zhang, Z.; Ma, R.; Xiang, L.; Chen, L.; Zhu, Y.; Tang, C.; et al. Oxidative stress gene expression, DNA methylation, and gut microbiota interaction trigger Crohn’s disease: A multi-omics Mendelian randomization study. BMC Med. 2023, 21, 179. [Google Scholar] [CrossRef]
- Tratensek, A.; Locatelli, I.; Grabnar, I.; Drobne, D.; Vovk, T. Oxidative stress-related biomarkers as promising indicators of inflammatory bowel disease activity: A systematic review and meta-analysis. Redox Biol. 2024, 77, 103380. [Google Scholar] [CrossRef]
- Jarmakiewicz-Czaja, S.; Ferenc, K.; Filip, R. Antioxidants as Protection against Reactive Oxidative Stress in Inflammatory Bowel Disease. Metabolites 2023, 13, 573. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ruan, X.; Yuan, S.; Deng, M.; Zhang, H.; Sun, J.; Yu, L.; Satsangi, J.; Larsson, S.C.; Therdoratou, E.; et al. Antioxidants, minerals and vitamins in relation to Crohn’s disease and ulcerative colitis: A Mendelian randomization study. Aliment. Pharmacol. Ther. 2023, 57, 399–408. [Google Scholar] [CrossRef]
- Chen, W.; Xu, Z.; Jiang, J.; Feng, W.; Li, B. Mitochondrial Protease AFG3L2 Inhibits Ferroptosis of Intestinal Epithelial Cells through PPARA/GPX4 Signaling Pathway to Improve Experimental Enteritis. Am. J. Pathol. 2025, 195, 1443–1456. [Google Scholar] [CrossRef]
- Zhong, H.; Luo, X.; Abdullah; Liu, X.; Hussain, M.; Guan, R. Nano-targeted delivery system: A promising strategy of anthocyanin encapsulation for treating intestinal inflammation. Crit. Rev. Food Sci. Nutr. 2025, online ahead of print. [Google Scholar] [CrossRef]
- Ibrahim, D.; Khater, S.I.; Sherkawy, H.S.; Elgamal, A.; Hasan, A.A.; Muhammed, A.A.; Farag, M.F.M.; Eissa, S.A.; Ismail, T.A.; Eissa, H.M.; et al. Protective Role of Nano-encapsulated Bifidobacterium breve, Bacilllus coagulans, and Lactobacillus plantarum in Colitis Model: Insights Toward Propagation of Short-Chain Fatty Acids and Reduction of Exaggerated Inflammatory and Oxidative Response. Probiotics Antimicrob. Proteins 2025, online ahead of print. [Google Scholar] [CrossRef]
- Mohanbhai, S.J.; Sardoiwala, M.N.; Gupta, S.; Shrimali, N.; Choudhury, S.R.; Sharma, S.S.; Guchhait, P.; Karmakar, S. Colon targeted chitosan-melatonin nanotherapy for preclinical Inflammatory Bowel Disease. Biomater. Adv. 2022, 136, 212796. [Google Scholar] [CrossRef]
- Zhu, D.; Wu, H.; Jiang, K.; Xu, Y.; Miao, Z.; Wang, H.; Ma, Y. Zero-Valence Selenium-Enriched Prussian Blue Nanozymes Reconstruct Intestinal Barrier against Inflammatory Bowel Disease via Inhibiting Ferroptosis and T Cells Differentiation. Adv. Healthc. Mater. 2023, 12, e2203160. [Google Scholar] [CrossRef]
- Shah, S.U.; Socha, M.; Fries, I.; Gibaud, S. Synthesis of S-nitrosoglutathione-alginate for prolonged delivery of nitric oxide in intestines. Drug Deliv. 2016, 23, 2927–2935. [Google Scholar] [CrossRef] [PubMed]
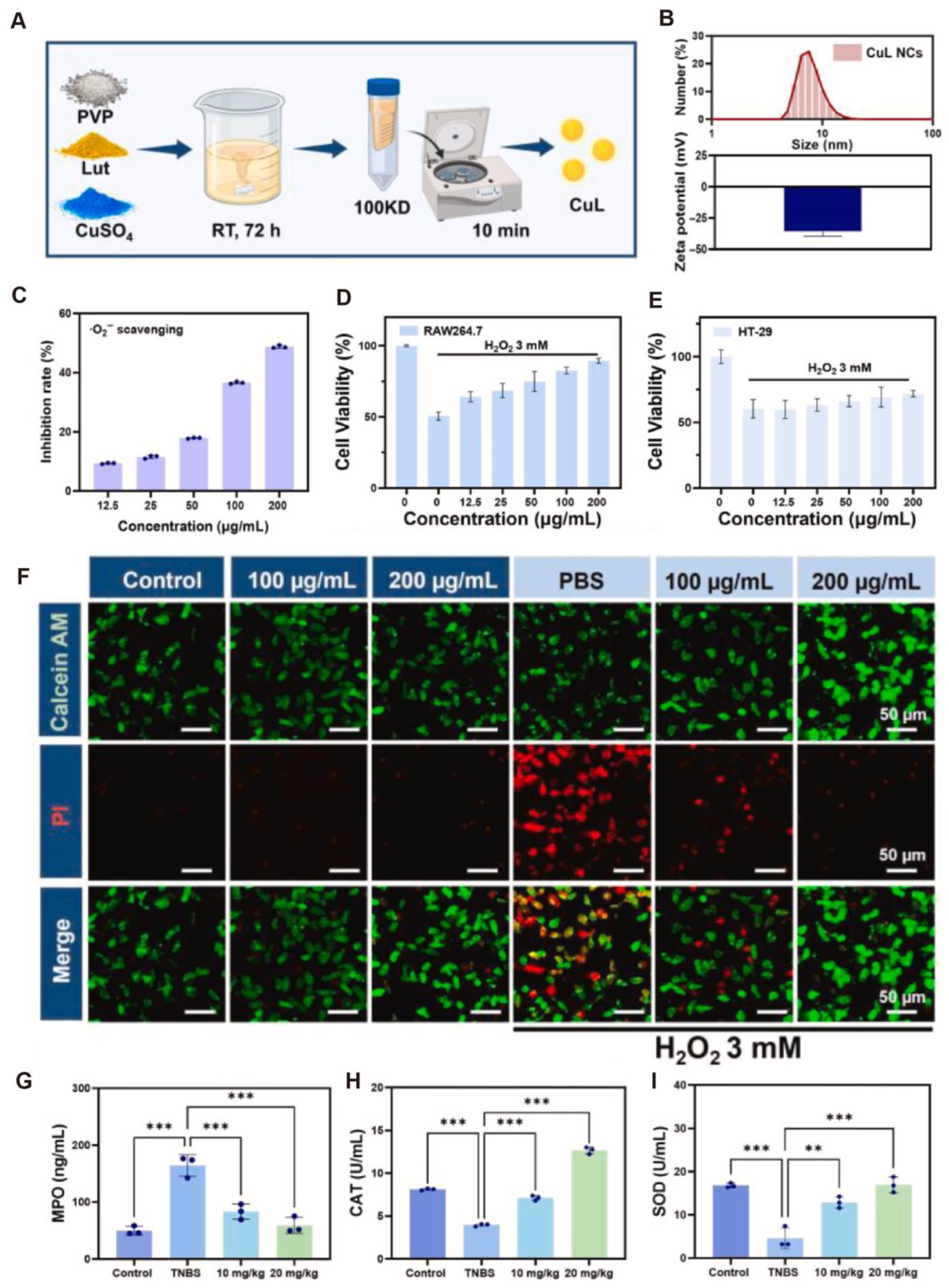
- Fu, W.; Huang, Z.; Li, W.; Xu, L.; Yang, M.; Ma, Y.; Liu, H.; Qian, H.; Wang, W. Copper-luteolin nanocomplexes for Mediating multifaceted regulation of oxidative stress, intestinal barrier, and gut microbiota in inflammatory bowel disease. Bioact. Mater. 2025, 46, 118–133. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Wang, T.; Liu, J.P.; Chen, Q.; Dai, X.L.; Su, M.; Cheng, Y.H.; Chu, C.C.; Ren, Y.Q. Recent Trends in the Development and Application of Nano-Antioxidants for Skin-Related Disease. Antioxidants 2024, 14, 27. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, Y.; Shi, S.; Liang, M.; Yang, D.; Sui, N.; Yu, W.W.; Wang, L.; Zhu, Z. Machine Learning Guided Discovery of Superoxide Dismutase Nanozymes for Androgenetic Alopecia. Nano Lett. 2022, 22, 8592–8600. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, P.; Gong, Y.; Yu, Z.; Gan, Y.; Li, P.; Liu, W.; Wang, X. Curcumin-zinc framework encapsulated microneedle patch for promoting hair growth. Theranostics 2023, 13, 3675–3688. [Google Scholar] [CrossRef]
- Yang, W.; Lv, Y.; Wang, B.; Luo, S.; Le, Y.; Tang, M.; Zhao, R.; Li, Y.; Kong, X. Polydopamine Synergizes with Quercetin Nanosystem to Reshape the Perifollicular Microenvironment for Accelerating Hair Regrowth in Androgenetic Alopecia. Nano Lett. 2024, 24, 6174–6182. [Google Scholar] [CrossRef] [PubMed]
- Speeckaert, R.; Bulat, V.; Speeckaert, M.M.; van Geel, N. The Impact of Antioxidants on Vitiligo and Melasma: A Scoping Review and Meta-Analysis. Antioxidants 2023, 12, 2082. [Google Scholar] [CrossRef]
- Li, C.; Wang, W.; Shao, J.; Zhou, S.; Ji, X.; Xi, Y.; Xu, Q.; Huang, Y.; Wang, J.; Wan, Y.; et al. Biomimetic polydopamine loaded with janus kinase inhibitor for synergistic vitiligo therapy via hydrogel microneedles. J. Nanobiotechnol. 2025, 23, 63. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Gao, X.; Wang, Y.; Cheng, S.; Zhong, X.; Xu, Y.; Zhou, X.; Zhang, Z.; Liu, Z.; Cheng, L. Ultrasmall iron-quercetin metal natural product nanocomplex with antioxidant and macrophage regulation in rheumatoid arthritis. Acta Pharm. Sin. B 2023, 13, 1726–1739. [Google Scholar] [CrossRef]
- McInnes, I.B.; Gravallese, E.M. Immune-mediated inflammatory disease therapeutics: Past, present and future. Nat. Rev. Immunol. 2021, 21, 680–686. [Google Scholar] [CrossRef]
- Jing, W.; Liu, C.; Su, C.; Liu, L.; Chen, P.; Li, X.; Zhang, X.; Yuan, B.; Wang, H.; Du, X. Role of reactive oxygen species and mitochondrial damage in rheumatoid arthritis and targeted drugs. Front. Immunol. 2023, 14, 1107670. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.; Wang, K.; Li, J.; Li, Y.; Zhu, H.; Lu, M.; Zhang, Y.; Fan, Q.; Han, L.; Wang, K.; et al. ROS Scavenging and inflammation-directed polydopamine nanoparticles regulate gut immunity and flora therapy in inflammatory bowel disease. Acta Biomater. 2023, 161, 250–264. [Google Scholar] [CrossRef]
- Asif, N.; Amir, M.; Fatma, T. Recent advances in the synthesis, characterization and biomedical applications of zinc oxide nanoparticles. Bioprocess. Biosyst. Eng. 2023, 46, 1377–1398. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defense against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Kim, Y.G.; Lee, Y.; Lee, N.; Soh, M.; Kim, D.; Hyeon, T. Ceria-Based Therapeutic Antioxidants for Biomedical Applications. Adv. Mater. 2024, 36, e2210819. [Google Scholar] [CrossRef]
- Awasthi, N.; Liongue, C.; Ward, A.C. STAT proteins: A kaleidoscope of canonical and non-canonical functions in immunity and cancer. J. Hematol. Oncol. 2021, 14, 198. [Google Scholar] [CrossRef]
- Summer, M.; Ashraf, R.; Ali, S.; Bach, H.; Noor, S.; Noor, Q.; Riaz, S.; Khan, R.R.M. Inflammatory response of nanoparticles: Mechanisms, consequences, and strategies for mitigation. Chemosphere 2024, 363, 142826. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri, M.; Sadeghizadeh, M.; Najafi, F.; Javan, M. Polymerized nano-curcumin attenuates neurological symptoms in EAE model of multiple sclerosis through down regulation of inflammatory and oxidative processes and enhancing neuroprotection and myelin repair. Neuropharmacology 2015, 99, 156–167. [Google Scholar] [CrossRef]
- Hou, J.; Wang, H.; Ge, Z.; Zuo, T.; Chen, Q.; Liu, X.; Mou, S.; Fan, C.; Xie, Y.; Wang, L. Treating Acute Kidney Injury with Antioxidative Black Phosphorus Nanosheets. Nano Lett. 2020, 20, 1447–1454. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Duan, D.; Peng, J.; Li, R.; Cao, Q.; Li, X.; Guo, Y.; Li, J.; Liu, K.; Li, Y.; et al. Oral enzyme-responsive nanoprobes for targeted theranostics of inflammatory bowel disease. J. Nanobiotechnol. 2024, 22, 484. [Google Scholar] [CrossRef]

| Diseases | Active Ingredient | Nanomaterial | Side Effect | Benefit | Ref. |
|---|---|---|---|---|---|
| Alopecia areata (AA) | Minoxidil | Molybdenum nanoparticles (Mo NPs) | Mo-NPs may cause liver and kidney toxicity, oxidative imbalance, and potential inflammatory responses | Offering a novel direction and translational potential for antioxidant-based treatment of AA | [17] |
| Multiple Sclerosis (MS) | Curcumin | Dendritic nanocarriers (DNCs) | High doses (20 µM) reduce neural stem cell viability and increase oxidative stress, shifting differentiation toward astrocytes instead of oligodendrocytes | Enhancing remyelination capacity in vivo | [18] |
| BBN-IONPs | Iron oxide nanoparticles | May not adequately reflect long-term effects, with the absence of human trials restricting therapeutic relevance | Elevated glutathione levels and total antioxidant capacity in brain tissues, reduced lipid peroxidation and inflammatory mediators | [19] | |
| MgH2 | Low-toxicity magnesium hydride nanoparticles (MgH2) | Uncertainties remain regarding mechanism, brain region sensitivity, and variable microglial polarization effects | Significantly alleviating anxiety- and depression-like behaviors and attenuating inflammatory demyelinating lesions | [20] | |
| Clobetasol propionate (CP) | Double-layered nanoemulsion | Serious side effects include osteoporosis, glaucoma, and adrenal suppression | Improving brain-targeted delivery and reducing systemic toxicity | [21] | |
| Selenium and crocin | Crocin–selenium nanoparticles (Cor@SeNs) | Headache in two patients; no severe adverse events observed, generally well tolerated | Ameliorating MS-associated cognitive impairment | [22] | |
| Methylprednisolone, neutrophil membranes | Biomimetic nanosystem (TFMN) | Negligible cytotoxicity up to 200 μg/mL; no severe adverse effects observed in vivo, but long-term safety unknown | Indicating excellent safety and therapeutic potential | [23] |
| Diseases | Active Ingredient | Nanomaterial | Side Effect | Benefit | Ref. |
|---|---|---|---|---|---|
| Acute kidney injury | Cerium and luteolin | Cerium–luteolin coordination nanocomposite (CeLutNCs) | Excellent biocompatibility with no significant toxicity or adverse effects in vitro and in vivo | Significantly improved organ function and reduced oxidative stress and inflammation | [24] |
| L-serine | L-serine (Rh-Ser, 2–4 nm) | Rh-Ser showed no obvious adverse reactions or systemic toxicity in the experiment | Mimicked multiple antioxidant enzymes to efficiently eliminate RONS | [25] | |
| Tetrahedral framework nucleic acid (tFNA) | Nanodrug delivery system (TTC) | tFNAs showed good biocompatibility with no nephrotoxicity or significant side effects in vitro and in vivo | Significantly ameliorated renal injury and restored renal function, indicating promising antioxidant-mediated therapeutic effects | [26] | |
| Epigallocatechin gallate (EGCG) and 5-aminosalicylic acid (5-ASA) | pH-stable antioxidant nanoparticle system (EGA NPs) | EGCG shows poor stability in acidic environments, limiting oral bioavailability | Effectively scavenged ROS and mitigated oxidative stress | [27] | |
| ethyl caffeate and 4-hydroxybenzyl alcohol | Supramolecular nanoplatform (Ser-HPEC) | No significant systemic side effects in vivo in the experiment | Preserved renal structure and function | [28] | |
| Acute liver injury | Polyphenol | Phlorotannin-based nanoparticles (PT NPs) | Traditional polyphenols face bioavailability and immunogenicity issues | Effectively alleviated oxidative and inflammatory damage | [29] |
| Catechin | CCN150 | CCN150 showed negligible acute toxicity, but potential long-term risks like | Reduced systemic inflammation driven by ROS | [30] | |
| NO | BSA-SNO NPs | No significant toxicity observed; potential risks include immune response, NO over-release, or thiol oxidation/cross-linking | Alleviated oxidative stress, improved hepatic microcirculation | [31] | |
| phenylboronic-acid-protected L-DOPA precursor (PAD) | Responsive biomimetic antioxidant nanoplatform (PADN) | Potential risks include batch variability, incomplete H2O2 scavenging, and reactive group-related off-target effects | Integrated efficient ROS scavenging, inflammation suppression, and hepatocyte protection | [32] |
| Diseases | Active Ingredient | Nanomaterial | Side Effect | Benefit | Ref. |
|---|---|---|---|---|---|
| Periodontitis | quercetin | A mesoporous bioactive glass-based nanocarrier system | No significant toxicity observed; potential risks include initial burst release and uncertain long-term local effects. | Promoted both osteogenesis and angiogenesis in experimental periodontitis-related bone defects | [33] |
| melatonin (MT) | melatonin-derived carbon dots (MT-CDs) | No significant side effects were observed during the experiment | Reduced alveolar bone resorption and osteoclast activity | [34] | |
| melanin | P/D-MNP-Ag | No significant organ inflammation observed in the experiment | Disrupted biofilms, attenuated inflammation, and reduced alveolar bone loss | [35] | |
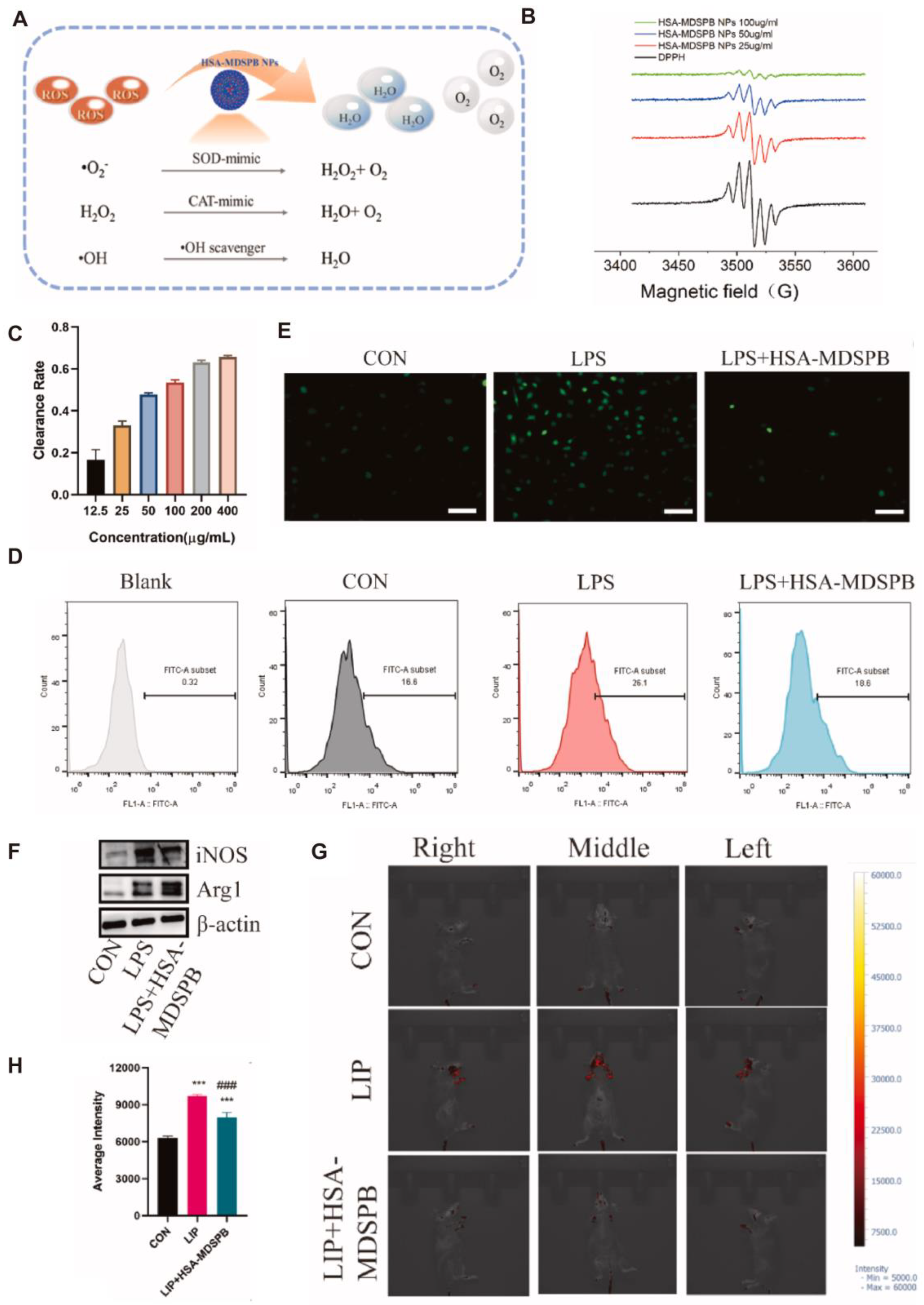
| HAS, Mn2+, [Fe(CN)6]4− | HSA-MDSPB NPs | No significant side effects were observed during the experiment | Broadly scavenged ROS species | [36] | |
| Ulcerative colitis (UC) | somatostatin (SST) and manno-oligosaccharide (MOS) | EUP-SeNPs | No obvious toxicity observed, potential risks include selenium accumulation | Improved oral stability and intestinal localization | [37] |
| gallic acid | thiolated anionic nanoliposomes | No apparent toxicity observed | Prolonged drug residence time and enhanced local therapeutic effects | [38] | |
| curcumin | CMC | No obvious toxicity or systemic side effects were observed in this experiment | Effectively scavenged ROS, restored mucosal integrity, and regulated gut microbiota composition to mitigate UC pathology | [39] | |
| PSB | PSB@NP-FA | Minimized systemic toxicity compared to free DEX | Modulated dendritic cell function, promoted M2 polarization of macrophages, and regulated T cell infiltration | [40] | |
| TMCs and PEG | PMNFs | No significant side effects | Increased junction protein and MUC2 expression, thereby preserving intestinal barrier function | [41] | |
| pterostilbene | HA-L-Arg-CO2@NPs | No significant side effects | Downregulated inflammatory mediators, restored barrier permeability, and effectively alleviated UC symptoms | [42] | |
| carbon dots | MML-CDs | No significant side effects were observed during the experiment | Demonstrated promising therapeutic efficacy | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, H.-W.; Yang, B.-C.; Ren, Y.-Q.; Xue, Y. Applications of Antioxidant Nanoparticles in Immune-Mediated Inflammatory Diseases. Antioxidants 2025, 14, 1128. https://doi.org/10.3390/antiox14091128
Shi H-W, Yang B-C, Ren Y-Q, Xue Y. Applications of Antioxidant Nanoparticles in Immune-Mediated Inflammatory Diseases. Antioxidants. 2025; 14(9):1128. https://doi.org/10.3390/antiox14091128
Chicago/Turabian StyleShi, Hong-Wei, Bo-Cheng Yang, Yun-Qing Ren, and Yi Xue. 2025. "Applications of Antioxidant Nanoparticles in Immune-Mediated Inflammatory Diseases" Antioxidants 14, no. 9: 1128. https://doi.org/10.3390/antiox14091128
APA StyleShi, H.-W., Yang, B.-C., Ren, Y.-Q., & Xue, Y. (2025). Applications of Antioxidant Nanoparticles in Immune-Mediated Inflammatory Diseases. Antioxidants, 14(9), 1128. https://doi.org/10.3390/antiox14091128








